Abstract
In order to establish the extraction technology of flavonoids from Dendrobium officinale leaves, a method combining Plackett–Burman design (PBD), steepest ascent design, and central composite design was developed to optimize the extraction of flavonoids. In addition, the tyrosinase activity inhibition of flavonoids was further tested in vitro. PBD results showed that ethanol concentration and number of extractions were key factors. Response surface methodology (RSM) indicated that the optimal extraction conditions were 78% ethanol concentration, six extraction times, 2 h, and 1:50 solid-liquid ratio. Under these conditions, the total flavonoid content could reach 35 mg/50 mL. In vitro tyrosinase experiment, the extracted total flavonoids had better inhibitory effect on tyrosinase activity than β-arbutin, and its inhibition rate for monophenolase and diphenolase exceeded 100% and 70%, respectively. These results indicate that RSM can effectively improve the extraction of flavonoids from Dendrobium officinale leaves and the flavonoids have the prospect of being applied to foods and cosmetics.
1. Introduction
Flavonoids, which are a class of compound with a 2-phenylchromanthone structure and C6-C3-C6 as basic carbon scaffold, are usually combined with sugars to form glycosides in plants and a small part in the form of free (glycosides) [1]. Flavonoids have been proven to possess a variety of biological activities, such as antioxidant, anti-inflammatory, and antiapoptosis activities [2, 3]. In the nutraceutical industry, most of the recently discovered natural tyrosinase inhibitors are flavonoids, which are safer and more efficient than certain synthetic tyrosinase inhibitors [4]. Tyrosinase inhibitors can slow down food browning during processing and storage by inhibiting the role of tyrosinase and the growth of a variety of microorganisms [5]. Flavonoids can also be used as whitening agent to achieve whitening efficacy. As the most potential tyrosinase inhibitors, flavonoids have been widely used in foods and cosmetics; hence, finding new and effective tyrosinase inhibitors among natural flavonoids is necessary [6].
Dendrobium officinale is a perennial herb in the biological classification of Orchidaceae [7]. Medical history records show that this herb can nourish yin, increase body fluid production, enhance gastric motility, lower blood sugar, and prolong life [8]. As a precious Chinese herb, the stems of D. officinale are edible nationally, and the leaves and flowers are also used as food in Zhejiang and Yunnan, respectively. In 2018, the leaves and flowers were included in the “Food Safety Law” for management. With the continuous development of the D. officinale industry, the number of people who enjoy eating the leaves and flowers is growing. Our research team has been testing the efficacy of various parts of D. officinale for several years, and results show that the chemical composition of each part of D. officinale is similar; in particular, the flavonoid content in leaves is higher than that in stems [9]. Flavonoids extracted from the leaves of D. officinale have also been found to have antioxidant and antihypertensive effects. In addition, flavonoids may restrain the action of tyrosinase [10], which means that flavonoids can be fully utilized in foods and cosmetics as a tyrosinase inhibitor. However, a universal extraction protocol of flavonoids in the leaves of D. officinale does not exist, and its extraction rate is relatively low.
Thus, single-factor experiments were designed to improve the extraction rate of flavonoids in D. officinale leaves. On the basis of the results, the extraction process was further optimized by Plackett–Burman design (PBD), steepest ascent design, and response surface methodology. In addition, the inhibition rate of flavonoids on tyrosinase activity was determined in vitro.
2. Materials and Methods
2.1. Compounds, Materials, and Reagents
D. officinale was provided by Zhejiang Senyu Industrial Co., Ltd. (Jinghua, China). Tyrosine, β-arbutin, and L-3,4-dihydroxyphenylalanine (L-DOPA) were purchased from Source Leaf Biotechnology Co., Ltd. (Shanghai, China). Ethanol was provided by East China Pharmaceutical Co., Ltd. (Hangzhou, China). A microplate reader was procured from Molecular Devices (Silicon Valley, USA). A UV spectrophotometer was obtained from Shimadzu (Kyoto, Japan). A constant temperature incubator was provided by Shanghai Jinghong Experimental Equipment Co., Ltd. (Shanghai, China), and a rotary evaporator was provided by Henan Yuhua Instrument Co., Ltd. (Henan, China).
2.2. Content Determination
Preparation of rutin standard solution was as follows: approximately 10 mg of rutin standard was accurately weighed, placed in a 50 mL volumetric flask, diluted with 70% ethanol to constant volume, and shaken to obtain the mass concentration of 0.2 mg/mL rutin standard solution.
Drawing of the standard curve was as follows: rutin standard solutions of 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 mL were precisely drawn and placed in 10 mL plugged test volumetric flasks. Subsequently, 70% ethanol was added to 5ml; then 0.4 mL of 5% NaNO2 solution was added and placed for 6 min. Approximately 0.4 mL of 10% Al (NO3)3 solution was added and then shaken for 6 min, and 4 mL of 4% NaOH solution was added and placed for 15 min. Finally, the reagent blank was used as reference to measure the absorbance at 510 nm. A standard curve was drawn to obtain a linear regression equation for the rutin standard curve.
Determination of total flavonoids was as follows: the sample was accurately obtained and processed a similar method to the standard. The result was then brought into the standard [11].
2.3. Experimental Design
2.3.1. Single-Factor Test
A single-factor experiment was executed to estimate the optimal range of each influencing factor. The effects of the ethanol concentration (35%–85%), number of extractions (1–5), extraction time (0.5–2.5 h), and liquid ratio (1:10–1:60) on the extraction rate of flavonoids were investigated [12, 13]. The amounts of flavonoids were evaluated and analyzed to determine the best parameters for the PBD.
2.3.2. PBD
The effects of four factors on flavonoid extraction were evaluated by the PBD (Table 1). The results of the single-factor experiment confirmed that the selected level of material-to-liquid ratio was 1:40 and 1:60, the extraction time was 1.5 and 2.5 h, the number of extractions was 3 and 5, and the ethanol concentration was 70% and 80%. Twelve groups of experimental conditions were designed by Design Expert V.8.0.6 software [14, 15].
Table 1.
PBD and results.
| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response |
|---|---|---|---|---|---|
| Liquid Ratio (mL/g; X1) |
Time (h; X2) |
Number (X3) |
Ethanol (%; X4) |
Flavonoid (mg/50mL) |
|
| 1 | 1:60 | 2.5 | 3 | 80 | 24.58 |
| 2 | 1:40 | 2.5 | 5 | 70 | 24.96 |
| 3 | 1:60 | 1.5 | 5 | 80 | 32.28 |
| 4 | 1:40 | 2.5 | 3 | 80 | 26.18 |
| 5 | 1:40 | 1.5 | 5 | 70 | 24.45 |
| 6 | 1:40 | 1.5 | 3 | 80 | 22.04 |
| 7 | 1:60 | 1.5 | 3 | 70 | 22.58 |
| 8 | 1:60 | 2.5 | 3 | 70 | 20.98 |
| 9 | 1:60 | 2.5 | 5 | 70 | 27.31 |
| 10 | 1:40 | 2.5 | 5 | 80 | 30.68 |
| 11 | 1:60 | 1.5 | 5 | 80 | 32.28 |
| 12 | 1:40 | 1.5 | 3 | 70 | 21.98 |
2.3.3. Steepest Ascent Design
The steepest ascent design determined the best areas of these key factors. By analyzing the positive and negative effects on the results of the PBD, the step length and null point of the extraction concentration and number of extractions were 70% and 1.5 and 1 and 1, respectively (Table 2). These values increased the intensity of the experiment, and the area with the best effect was approached [15, 16].
Table 2.
Path of steepest ascent experimental design and results.
| Run | Factor 1 | Factor 2 | Response |
|---|---|---|---|
| Number | Ethanol (%) |
Flavonoid (mg/50mL) |
|
| null point(0) | 1 | 70 | |
| step length(Δ) | 1 | 1.5 | |
| 1 | 1 | 70 | 12.94 |
| 2 | 2 | 71.5 | 20.26 |
| 3 | 3 | 73 | 22.67 |
| 4 | 4 | 74.5 | 28.49 |
| 5 | 5 | 76 | 30.27 |
| 6 | 6 | 77.5 | 33.81 |
| 7 | 7 | 79 | 31.13 |
2.3.4. Central Composite Design (CCD)
On the basis of the results of the steepest ascent design, the optimal ethanol concentration was 77.5% and the number of extractions was six [17, 18]. A two-factor CCD, which involved five repetitions of the central point (Table 3), was devised. In this design, the flavonoid content was the response value, and data analysis was performed for each factor used in Design Expert V.8.0.6 software [19].
Table 3.
Central composite design and results.
| Run | Factor 1 | Factor 2 | Response |
|---|---|---|---|
| Number | Ethanol (%) |
Flavonoid (mg/50mL) |
|
| 1 | 5 | 76% | 30.22 |
| 2 | 5 | 79% | 31.68 |
| 3 | 7 | 76% | 26.27 |
| 4 | 7 | 79% | 29.18 |
| 5 | 6 | 75% | 26.40 |
| 6 | 6 | 80% | 29.90 |
| 7 | 5 | 78% | 31.40 |
| 8 | 7 | 78% | 28.40 |
| 9 | 6 | 78% | 36.45 |
| 10 | 6 | 78% | 36.95 |
| 11 | 6 | 78% | 34.13 |
| 12 | 6 | 78% | 33.81 |
| 13 | 6 | 78% | 34.95 |
2.3.5. Verification and Analysis of Flavonoids in Dendrobium
Three parallel experiments were conducted on the optimal extraction conditions determined by CCD experiments. The resulting values were averaged to obtain the final results.
2.4. Determination of Tyrosinase Inhibitory Activity
The obtained extract was concentrated to 0.4 g/mL crude drug, centrifuged for 30 min at 8000 r/min for clarification, and washed off with 50% ethanol by ADS-17 macroporous resin. The extract was concentrated to 2, 3, 4, 5, and 6 mg/mL, and β-arbutin was concentrated on the same concentration gradient.
L-Tyrosine and L-DOPA were used as substrates, and four groups of reaction liquids were accurately absorbed into 96-well plates according to the volume presented in Table 4 by using a pipette gun. Tyrosinase was added to each group at 30 μL of 0.25 mg/mL after 10 min of constant temperature in a 37°C constant-temperature incubator. The absorbance of A0, A1, Asample, and Ablank at 475 nm after reaction for 10 min, 30 min, 1 h, 2 h, and 4 h was measured by an enzyme labeling instrument. The inhibition rate of tyrosinase activity was calculated using the following formula [20]:
| (1) |
Table 4.
Loading table.
| Reagent /Number | A0 | A1 | ASample | Ablank |
|---|---|---|---|---|
| L-tyrosine /L-dopa | 100 | 0 | 100 | 0 |
| Tyrosinase | 30 | 30 | 30 | 30 |
| Inhibitor | 0 | 0 | 10 | 10 |
| Buffer | 70 | 170 | 60 | 160 |
2.5. Statistical Analysis
The results of the PBD, steepest ascent design, and CCD were analyzed by Design Expert.V.8.0.6 software. Other relevant data were analyzed through ANOVA by SPSS 21.0 and considered significant at p < 0.05 and highly significant at p < 0.01.
3. Results and Discussion
3.1. Single-Factor Experiment of Flavonoid Extraction
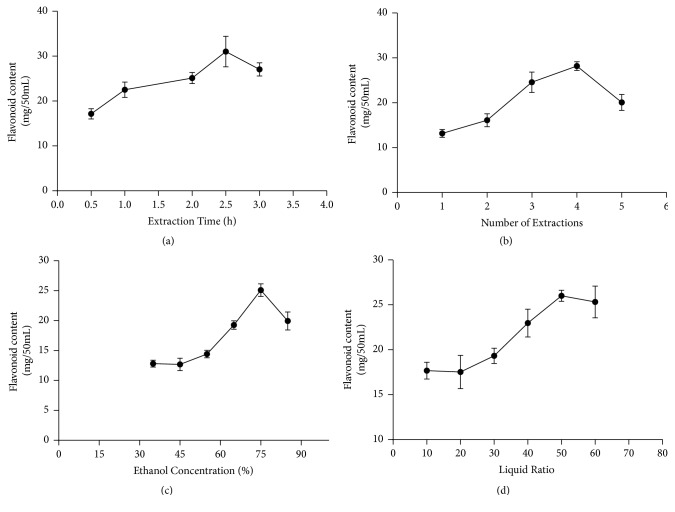
The results of single-factor optimization experiments are shown in Figure 1. The flavonoid content first increased and then decreased with the changes in four various factors. In line with the curve, the extraction times, ethanol concentration, and material-to-liquid ratio exerted evident effects on flavonoid extraction. The optimal conditions were an extraction time of 2.5 h, four extractions, ethanol concentration of 75%, and material-to-liquid ratio of 1:50 [21].
Figure 1.
Effect of reaction conditions on the extraction efficiency of flavonoids: (a) extraction time, (b) number of extractions, (c) ethanol concentration, and (d) liquid ratio.
3.2. Optimization of Flavonoid Extraction Factors by the PBD
On the basis of the results of single-factor optimization experiments and the influence of extraction conditions, four factors and two levels were used to filter the ethanol concentration, number of extractions, extraction time, and liquid ratio, as shown in Table 1. The ANOVA results are presented in Table 5. Given that a PBD was employed, quadratic polynomial equations with significant terms were obtained as follows:
Final equation in terms of coded factors:
Flavonoid = +25.86 + 2.80 × C + 2.15 × D
Final equation in terms of actual factors:
Flavonoid = −17.57651 + 2.80253 × Number + 0.42966 × Methanol
Table 5.
ANOVA and R-squared for selected factorial model.
| Source | Sum of squares | Mean | F | p-value | ||
|---|---|---|---|---|---|---|
| df | Square | Value | Prob>F | |||
| Model | 149.63 | 2 | 74.82 | 24.96 | 0.0002 | significant |
| C-number | 94.25 | 1 | 94.25 | 31.44 | 0.0003 | |
| D-Methanol | 55.38 | 1 | 55.38 | 18.48 | 0.002 | |
| Residual | 26.98 | 9 | 3.00 | |||
| Cor Total | 176.61 | 11 | ||||
| Std. Dev. | 1.73 | |||||
| Mean | 25.86 | |||||
| C.V.% | 6.70 | |||||
| PRESS | 47.96 | |||||
| R-Squared | 0.8472 | |||||
| Adj R-Squared | 0.8133 | |||||
| Pred R-Squared | 0.7284 | |||||
| Adeq Precisior | 11.438 |
In line with the results of the PBD experiment, the regression model obtained by software analysis showed a significant R-squared of 0.8472; thus, the equation was consistent with the actual situation [22]. The two tested factors, namely, the number of extractions and ethanol concentration, significantly (p < 0.01) influenced total flavonoid extraction, and the extraction time and material-to-liquid ratio had no significant effect on the flavonoid content. When the number of extractions was increased from 3 to 5, the flavonoid content significantly increased (p = 0.0003) possibly due to the comprehensive extraction of flavonoids. Changing the ethanol concentration from 70% to 80% may adjust the polarity of the system and alter the solubility of flavonoids [23]. The two factors were assessed as key factors in the steepest ascent design, and the results of single-factor experiment showed that the optimal material-liquid ratio was 1:50 at an extraction time of 2 h.
3.3. Optimization of Flavonoid Extraction by the Steepest Ascent Design
The path of steepest ascent was used to approach the optimal region of the two aforementioned factors [24]. On the basis of the experimental results of the previous step, the null point of the number of extractions was 1 (Table 2), the step length was 1, the null point of the ethanol concentration was 70%, and the step size was 1.5. The climbing experiment was set in accordance with the obtained step size and null point, and the results are shown in Table 2.
The results of climbing experiment showed that the flavonoid content gradually increased with the number of extractions and ethanol concentration. When the number of extractions was six and the ethanol concentration was 77.5%, the highest value was reached at 33.81 mg/mL, and the extracted flavonoid content further improved [25, 26].
3.4. Optimization of Flavonoid Extraction by the Response Surface Methodology
Through the PBD and steepest ascent design experiment, the significant parameters [27] were selected. Table 3 shows the design group and corresponding results, and Table 6 presents the ANOVA results. The findings suggested that the optimal number of extractions was six, and the ethanol concentration was 78%; the results obtained under the central conditions were similar. Moreover, in accordance with the findings of CCD, the quadratic polynomial equations are expressed as follows:
Final equation in terms of coded factors:
Flavonoid = +35.26 +1.16 × A −1.34 × B + 0.36 × A × B − 3.48 × A2 − 2.60 × B2
Final equation in terms of actual factors:
Flavonoid = −9278.13580 + 238.81473 × Methanol + 11.08481 × Number + 0.24249 × Methanol × Number − 1.54512 × Methanol2 − 2.60128 × Number2
Table 6.
ANOVA and R-squared for the selected factorial model.
| Source | Sum of squares | Mean | F | p-value | ||
|---|---|---|---|---|---|---|
| df | Square | Value | Prob>F | |||
| Model | 142.41 | 5 | 28.48 | 23.24 | 0.0003 | Significant |
| A- Methanol | 10.85 | 1 | 10.85 | 8.85 | 0.0207 | |
| B- number | 14.31 | 1 | 14.31 | 11.68 | 0.0112 | |
| AB | 0.53 | 1 | 0.53 | 0.43 | 0.5321 | |
| A2 | 84.08 | 1 | 84.08 | 68.59 | <0.0001 | |
| B2 | 47.07 | 1 | 47.07 | 38.40 | 0.0004 | |
| Residual | 8.58 | 7 | 1.23 | |||
| Lack of Fit | 0.84 | 3 | 0.28 | 0.15 | 0.9275 | Not Significant |
| Pure Error | 7.74 | 4 | 1.93 | |||
| Cor Total | 150.99 | 12 | ||||
| Std. Dev. | 1.11 | |||||
| Mean | 31.52 | |||||
| C.V.% | 3.51 | |||||
| PRESS | 18.09 | |||||
| R-Squared | 0.9432 | |||||
| Adj R-Squared | 0.9026 | a | ||||
| Pred R-Squared | 0.8802 | |||||
| Adeq Precisior | 11.890 |
ANOVA was used to evaluate the optimization results. A small p-value indicates a significant influence on the response variables [7]. The model had a correlation p value of 0.0003 < 0.01, which indicated that the optimization model was significant. Furthermore, p values of the number of extractions and ethanol concentration were 0.0207 < 0.05 and 0.0112 < 0.05, respectively, which showed that the two factors exerted significant effects [28]. The final equation in terms of actual factors revealed that the ethanol concentration and number of extractions increased the positive coefficient of the linear effect, whereas A2 and B2 both had the negative coefficient. The lack of fit was not significant, and the goodness of fit of the model was determined by estimating the variance of the coefficients (R2), which was observed as 0.9432; thus, 94.32% of this factor could be interpreted by fitting the model [29]. Moreover, the R-squared value of 0.9432 was close to the adjusted R-squared value of 0.9026, which implied a good statistical model [30].
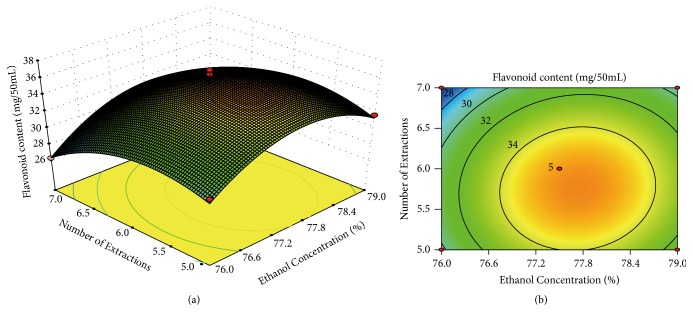
In this study, 3D curved surface (Figure 2(a)) and 2D contour plots (Figure 2(b)), which explained the relationship between each variable and the experimental level [29] of the corresponding response, were created with extraction times and ethanol concentration as X and Y coordinates, respectively, and flavonoid content as Z coordinates [31]. In the 3D curved surface and 2D contour plots, an interaction was observed between the two selected factors, but the effect was unremarkable [32].
Figure 2.
3D response surface plots (a) and 2D contour plots (b).
3.5. Validation of Optimal Conditions
A confirmatory experiment was performed based on the optimal extraction conditions obtained from the aforementioned experiments. A method was considered credible if the percentage error was less than 10% [7]. The average experimental value for the flavonoid extraction efficiency model was 35.45 ± 0.34 mg/50 mL. A 4.2% difference was observed between the predicted and confirmatory experimental values (Table 7), which confirmed the validity of the optimization model.
Table 7.
Verification of the experimental results.
| Category | Run | Yield (mg/50mL) | STDEV (%) | |
|---|---|---|---|---|
| Predictive | Experimental | |||
| Flavonoids | 1 | 35.51 | 35.41 | 4.2% |
| 2 | 35.51 | 35.13 | ||
| 3 | 35.51 | 35.81 | ||
In addition, our research group found that the flavonoids in D. officinale, which were mostly rutin derivatives, were abundant. As determined via fingerprint analysis, the leaves of D. officinale contained eight flavonoids [9], as shown in Table 8.
Table 8.
Flavonoids in D. officinale.
| Herb | Flavonoids |
|---|---|
| D. officinale | Apigenin-6, 8- two -C-β-D- glucoside |
| Apigenin-6-C-β-D-xylose-8-C-β-D-glucopyranoside | |
| Isophora | |
| Schaftoside | |
| Apigenin-6-C-β-D- glucoside -8-C-β-D-glucoside | |
| Apigenin-6-C-β-D-xylose-8-C-α-L-arabinoside | |
| Apigenin-6,8-di-C-α-L-pyranosin | |
| Apigenin-6-C-α-L-arabinose-8-C-β-D-xyloside |
3.6. Determination of Tyrosinase Inhibitory Activity
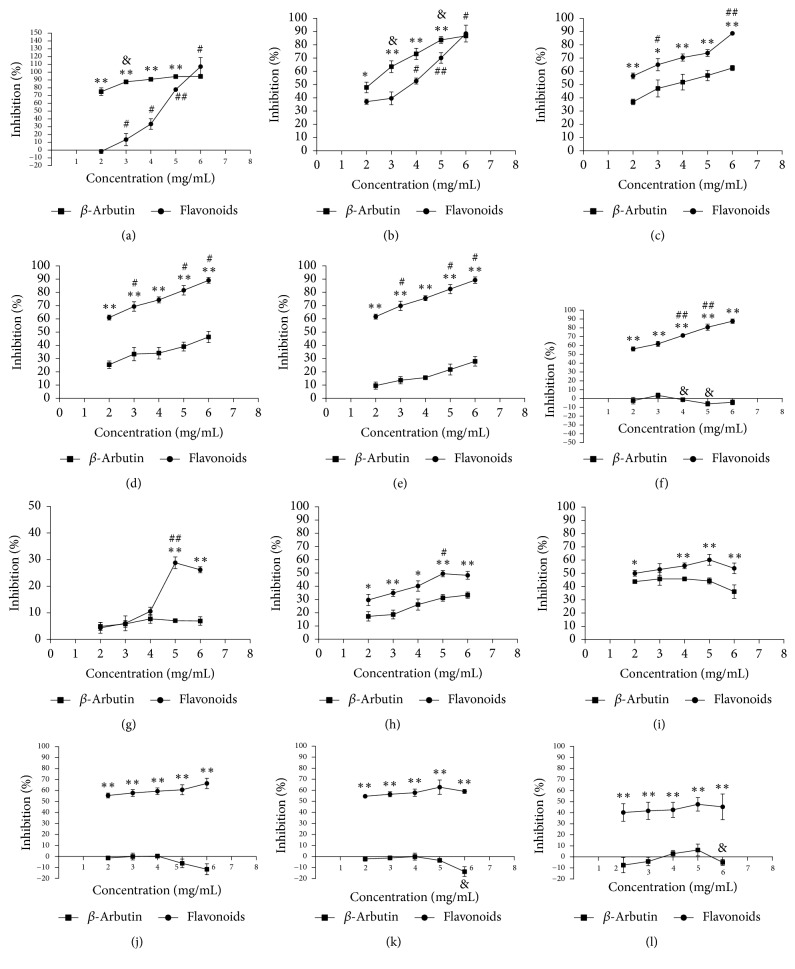
L-Tyrosine and L-dopa were used as substrates to detect the inhibitory activity of tyrosinase and β-arbutin as positive control [33, 34]. As shown in Figure 3, flavonoid extracts and β-arbutin had strong tyrosinase inhibitory activity. Overall, the inhibitory effect of flavonoids was better than that of β-arbutin. The trend of the curve revealed that tyrosinase activity was suppressed in a dose-dependent manner. When the concentration of total flavonoids was 6 mg/mL, the inhibitory rate of tyrosine monophenolase was nearly 100%, but the inhibitory rate of tyrosine diphenolase was only 70%. Therefore, compared with the inhibition rate of tyrosine diphenolase (Figures 3(a)–3(f)), the inhibitory effect of flavonoid extract on tyrosine monophenolase (Figures 3(i)–3(l)) was more significant [35].
Figure 3.
(a)–(f) Monophenolase activity inhibition rates at 10, 30, 60, 90, 120, and 240 min, respectively. (g)–(l) Diphenolase activity inhibition rates at 10, 30, 60, 90, 120, and 240 min, respectively. ∗∗P < 0.01 versus β-arbutin,∗P < 0.05 versus β-arbutin, ##P < 0.01, #P < 0.05 self-comparison of flavonoids, &&P < 0.01, &P < 0.05 self-comparison of β-arbutin.
Six time points were tested to comprehensively understand the inhibition of tyrosine monophenolase activity (Figures 3(a)–3(f)) and diphenolase activity (Figures 3(g)–3(l)) by flavonoid extracts. The inhibitory rate of β-arbutin on monophenolase gradually decreased with time, and a slight significant difference was observed among the concentrations. However, the inhibitory rate of flavonoid extracts on monophenolase slowly decreased with the increase in time, and the difference between different concentrations was evident. When the reaction time was 10 min (Figure 3(a)), the inhibition rate of β-arbutin was considerably higher than that of flavonoid extracts, except at 6 mg/mL. When the reaction time was 30 min (Figure 3(b)), the inhibition rate of flavonoid extracts was considerably higher than that of β-arbutin, and the gap widened with the increase in time; this phenomenon might be related to the strong antioxidant activity of flavonoid extracts for tyrosinase, catalyzing the role of L-tyrosine in the presence of free radicals [36].
Figures 3(g)–3(l) revealed that the effect of flavonoid extracts on diphenolase was more significant compared with that on β-arbutin. The best inhibitory effect was observed when the concentration of flavonoid extracts was 5 mg/mL (Figure 3(i)). Similar to the action of monophenolase inhibition, the inhibition rate of diphenolase slightly changed. Therefore, flavonoid extracts exerted better inhibitory effects than β-arbutin, and the inhibitory effect on monophenolase was better than that on diphenolase [37].
4. Conclusions
This study investigated the extraction of flavonoids by RSM from the leaves of D. officinale and analyzed the inhibitory effect of flavonoid extracts on tyrosinase. On the basis of the results of the PBD, steepest ascent design, and CCD, the optimal extraction conditions were determined as follows: ethanol concentration of 78%, six extractions, material-to-liquid ratio of 1:50, extraction time of 2 h, and flavonoid content of 35 mg/50 mL. The extraction of flavonoids from D. officinale leaves increased under these conditions.
The results showed that the flavonoid extracts from the leaves of D. officinale could effectively inhibit tyrosinase activity, and a dose–effect relationship was observed. At a certain concentration, flavonoid extracts demonstrated good inhibition effect on tyrosine monophenolase and diphenolase. In addition, the inhibition effect and intensity of these extracts were significantly better than those of β-arbutin, and their inhibition rate on monophenolase activity was higher than that on diphenolase. Therefore, as a tyrosinase inhibitor, the flavonoid extracts from the leaves of D. officinale may become a component in future hypopigmentation foods and cosmetics.
However, this experiment still had some limitations. The composition of flavonoid extracts can be further analyzed in the future, and the types of interaction between flavonoid extracts and tyrosinase can be further researched on cellular and overall levels.
Acknowledgments
This study was supported by the National Key Research and Development Program (no. 2017YFC1702200), the National Science Foundation of China (no. 81673638, no. 81874352, no. 81703772, no. 81803760, and no. 81803761), the Funding for Young Talents Project of Zhejiang University of Technology (no. GY17034148004), the National Science and Technology on New Drug Creation and Development Projects (no. 2011ZX09101-002-07), and the Zhejiang Provincial Key Laboratory Project (no. 2012E10002).
Contributor Information
Guiyuan Lv, Email: zjtcmlgy@163.com.
Suhong Chen, Email: chensuhong@zjut.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Haixia Lu and Ke Yang contributed equally to this work and are co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Liu M., Hansen P. E., Wang G., et al. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus) Molecules. 2015;20(1):754–779. doi: 10.3390/molecules20010754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oldoni T. L. C., Melo P. S., Massarioli A. P., et al. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chemistry. 2016;192:306–312. doi: 10.1016/j.foodchem.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Gontijo D. C., Brandão G. C., Gontijo P. C., et al. Identification of phenolic compounds and biologically related activities from Ocotea odorifera aqueous extract leaves. Food Chemistry. 2017;230:618–626. doi: 10.1016/j.foodchem.2017.03.087. [DOI] [PubMed] [Google Scholar]
- 4.Lee S. Y., Baek N., Nam T.-G. Natural, semisynthetic and synthetic tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31(1):1–13. doi: 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- 5.Du Y. J., Dou S. Q., Wu S. J. Efficacy of phytic acid as an inhibitor of enzymatic and non-enzymatic browning in apple juice. Food Chemistry. 2012;135(2):580–582. doi: 10.1016/j.foodchem.2012.04.131. [DOI] [PubMed] [Google Scholar]
- 6.Şöhretoğlu D., Sari S., Barut B., Özel A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorganic Chemistry. 2018;81:168–174. doi: 10.1016/j.bioorg.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Zheng S., Zhu Y., Jiao C., et al. Extraction and analysis of gigantol from Dendrobium officinale with response surface methodology. Molecules. 2018;23(1) doi: 10.3390/molecules23040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam Y., Ng T. B., Yao R. M., et al. Evaluation of chemical constituents and important mechanism of pharmacological biology in dendrobium plants. Evidence-based Complementary and Alternative Medicine. 2015;2015:16. doi: 10.1155/2015/841752.841752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou G.-F., Lü G.-Y., Chen S.-H. Study on HPLC fingerprints of flavone C -glycosides in dendrobium officinale leaves and determination ofindex component. Chinese Pharmaceutical Journal. 2012;47(11) [Google Scholar]
- 10.Ye Z., Dai J.-R., Zhang C.-G., et al. Chemical differentiation of dendrobium officinale and dendrobium devonianum by using HPLC fingerprints, HPLC-ESI-MS, and HPTLC analyses. Evidence-Based Complementary and Alternative Medicine. 2017;2017:15. doi: 10.1155/2017/8647212.8647212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X., Liu D., Zhang J., et al. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complementary and Alternative Medicine. 2016;16(1):p. 245. doi: 10.1186/s12906-016-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiran L., Ying L., Mingzhuo Z., Ye Y., Xiuming C. The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells. Food & Function. 2017;8(11):4251–4264. doi: 10.1039/c7fo00880e. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Wang H., Cai X. Optimization of the extraction of total flavonoids from Scutellaria baicalensis Georgi using the response surface methodology. Journal of Food Science and Technology. 2015;52(4):2336–2343. doi: 10.1007/s13197-014-1275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed E. M., El-Refai H. A. Cyclodextrin glucosyltransferase production by Bacillus megaterium NCR: Evaluation and optimization of culture conditions using factorial design. Indian Journal of Microbiology. 2010;50(3):303–308. doi: 10.1007/s12088-010-0009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belwal T., Giri L., Bhatt I. D., Rawal R. S., Pande V. An improved method for extraction of nutraceutically important polyphenolics from Berberis jaeschkeana C.K. Schneid. fruits. Food Chemistry. 2017;230:657–666. doi: 10.1016/j.foodchem.2017.03.086. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh S., Muthuvelayudham R., Rajesh Kannan R., Viruthagiri T. Enhanced production of xylitol from corncob by Pachysolen tannophilus using response surface methodology. International Journal of Food Science. 2013;2013:230–241. doi: 10.1155/2013/514676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A., Wu L., Li X. Optimization of ultrasonic-assisted preparation of dietary fiber from corn pericarp using response surface methodology. Journal of the Science of Food and Agriculture. 2013;93(12):2922–2926. doi: 10.1002/jsfa.6083. [DOI] [PubMed] [Google Scholar]
- 18.Pan H., Zhang Q., Cui K., Chen G., Liu X., Wang L. Optimization of extraction of linarin from Flos chrysanthemi indici by response surface methodology and artificial neural network. Journal of Separation Science. 2017;40(9):2062–2070. doi: 10.1002/jssc.201601259. [DOI] [PubMed] [Google Scholar]
- 19.Trindade A. S. N., Dantas A. F., Lima D. C., Ferreira S. L. C., Teixeira L. S. G. Multivariate optimization of ultrasound-assisted extraction for determination of Cu, Fe, Ni and Zn in vegetable oils by high-resolution continuum source atomic absorption spectrometry. Food Chemistry. 2015;185:145–150. doi: 10.1016/j.foodchem.2015.03.118. [DOI] [PubMed] [Google Scholar]
- 20.Kim S. B., Liu Q., Ahn J. H., et al. Polyamine derivatives from the bee pollen of Quercus mongolica with tyrosinase inhibitory activity. Bioorganic Chemistry. 2018;81:127–133. doi: 10.1016/j.bioorg.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y., Tian W., Pei F., et al. Simultaneous determination of pesticide residues and antioxidants in blended oil using a liquid-liquid extraction combined with dispersive solid phase extraction method. Food Chemistry. 2017;229:347–353. doi: 10.1016/j.foodchem.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 22.Surwase S. N., Jadhav S. B., Phugare S. S., Jadhav J. P. Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology. 3 Biotech. 2013;3(3):187–194. doi: 10.1007/s13205-012-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F., Long L., Sun X., Wu H., Li T., Xiang W. Optimization of medium using response surface methodology for lipid production by Scenedesmus sp. Marine Drugs. 2014;12(3):1245–1257. doi: 10.3390/md12031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrarca M. H., Rodrigues M. I., Rossi E. A., De Sylos C. M. Optimisation of a sample preparation method for the determination of fumonisin B(1) in rice. Food Chemistry. 2014;158:270–277. doi: 10.1016/j.foodchem.2014.02.126. [DOI] [PubMed] [Google Scholar]
- 25.Ji S., Li W., Xin H., Wang S., Cao B. Improved production of sublancin 168 biosynthesized by bacillus subtilis 168 using chemometric methodology and statistical experimental designs. BioMed Research International. 2015;2015(19):1–11. doi: 10.1155/2015/687915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H., Liu M., Liu J., et al. Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresource Technology. 2009;100(17):4012–4016. doi: 10.1016/j.biortech.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Jouki M., Mortazavi S. A., Yazdi F. T., Koocheki A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. International Journal of Biological Macromolecules. 2014;66:113–124. doi: 10.1016/j.ijbiomac.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q. H., Zhang X. W., Xu X. L., et al. Antioxidant and anticomplement functions of flavonoids extracted from Penthorum chinense Pursh. Food & Function. 2013;4(4):1811–1818. doi: 10.1039/c3fo60342c. [DOI] [PubMed] [Google Scholar]
- 29.Şahin S., Şamli R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrasonics Sonochemistry. 2013;20(1):595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Ramos L. R., Santos J. S., Dague H. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chemisty. 2017;221:950–958. doi: 10.1016/j.foodchem.2016.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Loewen A., Chan B., ECY L. C. Optimization of vitamins A and D3 loading in re-assembled casein micelles and effect of loading on stability of vitamin D3 during storage. Food Chemisty. 2018;240:472–481. doi: 10.1016/j.foodchem.2017.07.126. [DOI] [PubMed] [Google Scholar]
- 32.Bochi V. C., Barcia M. T., Rodrigues D., Speroni C. S., Giusti M. M., Godoy H. T. Polyphenol extraction optimisation from Ceylon gooseberry (Dovyalis hebecarpa) pulp. Food Chemistry. 2014;164:347–354. doi: 10.1016/j.foodchem.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Fan M., Zhang G., Pan J., Gong D. An inhibition mechanism of dihydromyricetin on tyrosinase and the joint effects of vitamins B6, D3 or E. Food & Function. 2017;8(7):2601–2610. doi: 10.1039/c7fo00236j. [DOI] [PubMed] [Google Scholar]
- 34.Goldfeder M., Kanteev M., Adir N., Fishman A. Influencing the monophenolase/diphenolase activity ratio in tyrosinase. Biochimica et Biophysica Acta. 2013;1834(3):629–633. doi: 10.1016/j.bbapap.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Peng H.-Y., Lai C.-C., Lin C.-C., Chou S.-T. Effect of vetiveria zizanioides essential oil on melanogenesis in melanoma cells: downregulation of tyrosinase expression and suppression of oxidative stress. The Scientific World Journal. 2014;2014:234–240. doi: 10.1155/2014/213013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furubayashi A., Hiradate S., Fujii Y. Role of catechol structure in the adsorption and transformation reactions of L-DOPA in soils. Journal of Chemical Ecology. 2007;33(2):239–250. doi: 10.1007/s10886-006-9218-5. [DOI] [PubMed] [Google Scholar]
- 37.Peritore C. S., Ho A., Yamamoto B. K., Schaus S. E. Resveratrol attenuates L-DOPA-induced hydrogen peroxide toxicity in neuronal cells. Neuroreport. 2012;23(17):989–994. doi: 10.1097/WNR.0b013e32835a4ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.