Abstract
Background:
Circadian disruption is a probable human carcinogen. From the eastern to western border of a timezone, social time is equal whereas solar time is progressively delayed, producing increased discrepancies between individuals’ social and biological circadian time. Accordingly, western timezone residents experience greater circadian disruption and may be at an increased risk of cancer.
Methods:
We examine associations between the position in a timezone (PTZ) and age-standardized county-level incidence rates for total cancers combined and 23 specific cancers by gender using the data of the Surveillance, Epidemiology, and End Results Program (2000-2012), including four million cancer diagnoses in white residents of 607 counties in 11 US states. Log-linear regression was conducted, adjusting for latitude, poverty, cigarette smoking, and state. Bonferroni corrected p-values were used as the significance criteria.
Results:
Risk increased from east to west within a timezone for total and many specific cancers, including chronic lymphocytic leukemia (both genders) and cancers of the stomach, liver, prostate, and non-Hodgkin lymphoma in men and cancers of the esophagus, colorectum, lung, breast, and corpus uteri in women.
Conclusions:
Risk increased from the east to the west in a timezone for total and many specific cancers, in accord with the circadian disruption hypothesis. Replication in analytic epidemiologic studies are warranted.
Impact:
Our findings suggest that circadian disruption may not be a rare phenomenon affecting only shift workers, but is widespread in the general population with broader implications for public health than generally appreciated.
Keywords: timezone, longitude, cancer risk, circadian, social jet lag
Introduction
Disturbances of circadian rhythm may produce health consequences including metabolic syndrome (1–3), psychiatric conditions (4) and cancer (5,6). Circadian rhythms are disrupted by night light exposure or night shift work, but a degree of disruption may also occur due to misalignment between environmental/social time and internal circadian timing, termed ‘social jet lag’(7), defined as the change of sleep/wake timing individuals experience between days when they have a free choice and those days when their sleep/wake timing is determined by school or work schedules. Since circadian rhythms are entrained and synchronized by light exposure (8), social jet lag may be more severe in the western region of a timezone where solar time and hence circadian time is delayed, resulting in increased exposure to light during later circadian ‘night’ similar to ‘late’ chronotypes (9). Shift work has been classified as a probable human carcinogen (Group 2A) by the International Agency for Research on Cancer (6) , based on sufficient evidence in experimental animals and epidemiological studies of breast cancer in female shift workers and flight attendants. However, health consequences of more subtle circadian misalignment due to the delayed circadian clock as would occur in the western part of a timezone have not been well studied. Social jetlag has been associated with increased body mass index (3) in the US population. In Russia and China, residence in the western border of a timezone was reported to have higher cancer incidence and mortality rates as well as lower life expectancy (9). Here we conducted the first investigation of timezone position in the United States in relation to incidence of total and specific cancers.
Materials and Methods
Cancer incidence data
We calculated county-specific age-adjusted (2000 US standard) cancer incidence rates by gender for malignant neoplasms diagnosed during the years 2000-2012 from the Surveillance, Epidemiology and End Results (SEER) program, using SEER*Stat software (10). We restricted analyses to whites to reduce confounding by race. We included 607 counties in 11 states of the continental United States: California, Connecticut, Georgia, Iowa, Kentucky, Louisiana, New Mexico, New Jersey, Utah, and the Detroit metropolitan area of Michigan and the Seattle-Puget Sound area of Washington. Seven counties that encompassed 2 timezones were excluded. Cancers were defined using ICD-O-3 site and morphology codes as used in SEER (10).
We included 23 types of cancer: 20 most common cancers among males and females in the United States (11) (breast, lung and bronchus, prostate, colon and rectum, urinary bladder, melanoma of the skin, non-Hodgkin lymphoma, thyroid, kidney and renal pelvis, corpus uteri and uterus NOS, pancreas, oral cavity and pharynx (excluding lip and salivary glands), liver and intrahepatic bile duct, myeloma, stomach, brain, ovary, acute myeloid leukemia, chronic lymphocytic leukemia, esophagus), as well as larynx, cervix uteri, and gallbladder cancers, which have been associated with cigarette smoking (12) , alcohol drinking (13), or obesity (14).
We also evaluated cancer subgroups for breast cancer (Hormone Receptive positive/negative (HR+/ HR−) (15), esophagus cancer (adenocarcinoma, squamous cell carcinoma) (16) and lung and bronchus cancer (adenocarcinoma, small cell carcinoma and squamous cell carcinoma) (17). Coding for subgroups of each cancer was described previously (15–17).
Position in a time zone data
Geographical coordinates of each county’s population centroid, which is defined as the latitude and longitude of the population balance point (official Census definition) of each county using 2010 U.S. Census data, were obtained from the United States Census Bureau (http://www.census.gov/geo/reference/centersofpop.htm). The position in a timezone (PTZ) is calculated as the distance (in degrees longitude) between the population centroid of each county and the central meridian of longitude of the respective timezone. In the United States, timezones correspond to approximately 15 degrees of longitude, and the central meridians of the Eastern, Central, Mountain and Pacific Timezones are 75, 90, 105, and 120 degrees longitude west, respectively.
Statistical methods
For total cancer and each specific cancer, we used a weighted least squares linear regression to examine the associations between the natural logarithm of age-adjusted county-level cancer rates and PTZ as continuous variable, where the weights were the county population sizes. The analyses adjusted for latitude, poverty percentage (the American Community Survey, 2008-2013- Census Planning Database http://www.census.gov/research/data/planning_database), prevalence of cigarette smoking (18), and state. Latitude was included as it is related to degree and intensity of solar exposure, which influences vitamin D levels, day length, solar zenith angle (in winter at high latitudes, more UVB radiation is absorbed) and sleep (19,20). Latitude also subsumes climatic factors such as temperature that influence overall mortality and may impact cancer (21). Smoking is related to diverse cancers. Poverty and state relate to health care quality and access. Covariate-adjusted rate ratios per 5 degrees difference in longitude, corresponding to 20 minutes, were estimated by exponentiating 5 times the regression coefficients estimated in the model. Analyses were conducted in men and women separately.
To check whether an association could be explained by obesity, urbanization, and Hispanic ethnicity, we further adjusted for obesity prevalence (BMI>30, from Centers for Disease Control and Prevention for 2010) (22), and an urban/rural index (2013 Rural-Urban Continuum Codes, http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx). Sensitivity analyses were conducted by restricting to non-Hispanic whites. To adjust for multiple comparisons, we used Bonferroni-corrected two-sided p-values as the significance criteria (i.e., p value < 1.8×10-3 (0.05/28) for men, and < 1.7×10-3 (0.05/30) for women). R software version 3.2.2 was used for statistical analysis. For each gender, age-adjusted cancer rates were calculated for position tertiles across the four timezones.
Results
After adjustment for age and county-level covariates, the total cancer incidence rates for counties increased significantly from eastern to western locations within timezones (Table 1, Figure 1, Figure 2). The rate ratio (RR) per five degrees of longitude toward the west (corresponding to 20 minutes’ delay of sunrise) was 1.029 (95% confidence intervals, CI, 1.017-1.041, p=2.7×10−6) for men and 1.039 (95% CI, 1.027-1.051, p=3.8 ×10−10) for women. Chronic lymphocytic leukemia rates also increased significantly among men (RR=1.134, 95% CI=1.076-1.196, p=3.1 ×10−6) and women (RR=1.118, 95%CI=1.052-1.189, p=3.7 ×10−4).
Table 1.
Association between position in a timezonea and age-adjusted county level cancer incidence in 11 states within the continental United States, SEER program 2000-2012b
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Cancerc | Cases | Rate Ratio (95% CI)f | P-value | Cases | Rate Ratio (95% CI)f | P-value |
| All Cancers | 2,095,394 | 1.029 (1.017-1.041) | 2.7×10−6 | 1,972,514 | 1.039 (1.027-1.051) | 3.8×10−10 |
| Oral Cavity and Pharynxd | 54,889 | 1.037 (0.997-1.078) | 0.07 | 23,199 | 1.086 (1.029-1.145) | 2.6×10−3 |
| Esophagus | 30,956 | 1.003 (0.960-1.049) | 0.89 | 8,751 | 1.163 (1.075-1.258) | 1.8×10−4 |
| Adenocarcinoma | 21,355 | 1.000 (0.947-1.056) | 1 | 3,448 | 1.054 (0.946-1.175) | 0.34 |
| Squamous Cell | 6,570 | 1.024 (0.942-1.114) | 0.57 | 4,150 | 1.071 (0.956-1.201) | 0.24 |
| Stomach | 35,990 | 1.087 (1.035-1.142) | 9.6×10−4 | 21,924 | 1.039 (0.974-1.107) | 0.24 |
| Colon and Rectum | 205,056 | 1.023 (1.002-1.044) | 0.03 | 193,349 | 1.045 (1.024-1.066) | 1.8×10−5 |
| Liver and Intrahepatic Bile Duct | 39,276 | 1.110 (1.046-1.177) | 6.0×10−4 | 15,796 | 1.099 (1.028-1.175) | 6.0×10−3 |
| Gallbladder | 2,834 | 0.875 (0.763-1.003) | 0.06 | 6,683 | 1.030 (0.943-1.124) | 0.51 |
| Pancreas | 51,150 | 1.041 (1.008-1.075) | 0.015 | 50,553 | 1.047 (1.013-1.083) | 0.01 |
| Larynx | 24,578 | 0.997 (0.951-1.045) | 0.9 | 6,158 | 1.077 (0.996-1.165) | 0.07 |
| Lung and Bronchus | 288,452 | 1.002 (0.977-1.028) | 0.87 | 256,618 | 1.046 (1.017-1.076) | 1.6×10−3 |
| Adenocarcinoma | 89,166 | 1.006 (0.967-1.047) | 0.75 | 94,771 | 1.058 (1.019-1.100) | 3.8×10−3 |
| Squamous Cell | 65,665 | 1.003 (0.965-1.043) | 0.87 | 38,324 | 1.029 (0.974-1.087) | 0.31 |
| Small Cell | 35,852 | 0.985 (0.941-1.031) | 0.51 | 35,834 | 1.037 (0.986-1.090) | 0.16 |
| Melanoma of the Skin | 119,304 | 1.050 (0.998-1.103) | 0.06 | 85,934 | 1.023 (0.967-1.083) | 0.43 |
| Breast | 4,463 | 1.074 (0.972-1.187) | 0.16 | 593,753 | 1.037 (1.019-1.055) | 6.5×10−5 |
| HR Positive | 3,713 | 1.038 (0.932-1.156) | 0.49 | 435,649 | 1.055 (1.026-1.084) | 1.8×10−4 |
| HR Negative | 141 | 0.450 (0.134-1.514) | 0.2 | 92,225 | 1.025 (0.995-1.056) | 0.1 |
| Cervix Uteri | 0 | - | - | 34,283 | 1.031 (0.985-1.080) | 0.19 |
| Corpus and Uterus NOSe | 0 | - | - | 113,344 | 1.100 (1.067-1.135) | 2.4×10−9 |
| Ovary | 0 | - | - | 62,958 | 1.011 (0.977-1.045) | 0.54 |
| Prostate | 578,119 | 1.042 (1.018-1.066) | 4.8×10−4 | 0 | - | - |
| Urinary Bladder | 147,266 | 1.012 (0.986-1.038) | 0.39 | 47,073 | 1.025 (0.983-1.070) | 0.24 |
| Kidney and Renal Pelvis | 80,453 | 1.020 (0.989-1.052) | 0.2 | 47,964 | 1.009 (0.972-1.047) | 0.64 |
| Brain | 32,043 | 1.019 (0.978-1.061) | 0.37 | 24,808 | 0.995 (0.949-1.042) | 0.82 |
| Thyroid | 24,274 | 1.042 (0.976-1.112) | 0.22 | 73,382 | 1.063 (1.008-1.121) | 0.02 |
| Non-Hodgkin Lymphoma | 95,143 | 1.059 (1.028-1.091) | 1.6×10−4 | 80,861 | 1.039 (1.010-1.070) | 0.01 |
| Myeloma | 26,959 | 0.980 (0.935-1.027) | 0.39 | 20,839 | 1.064 (1.013-1.118) | 0.01 |
| Chronic Lymphocytic Leukemia | 25,251 | 1.134 (1.076-1.196) | 3.1×10−6 | 17,072 | 1.118 (1.052-1.189) | 3.7×10−4 |
| Acute Myeloid Leukemia | 17,990 | 1.030 (0.975-1.089) | 0.28 | 14,815 | 1.045 (0.987-1.106) | 0.13 |
The position in the timezone (PTZ) is calculated as the distance (in degrees longitude) between the population centroid of each county and the middle meridian of the located time zone (EST:75, CST:90, MST:105, and WST:120).
A weighted (by population size) logarithmic linear regression was used between PTZ and age-adjusted (2000 US standard population) county-level cancer incidence rate. Model adjusted for latitude, poverty percentage, smoking and state.
Ordered by organ systems, based on SEER Statistics Review 1975-2013
Excluding lip and salivary glands
Not otherwise specified
Rate Ratios are per five degrees of longitude difference, equivalent to 20 minutes. CI: Confidence Interval.
P values less than 1.8×10−3 (0.05/28) for men and less than 1.7×10−3 (0.05/30) for women are in bold and indicate statistical significance using Bonferroni correction.
Figure 1.
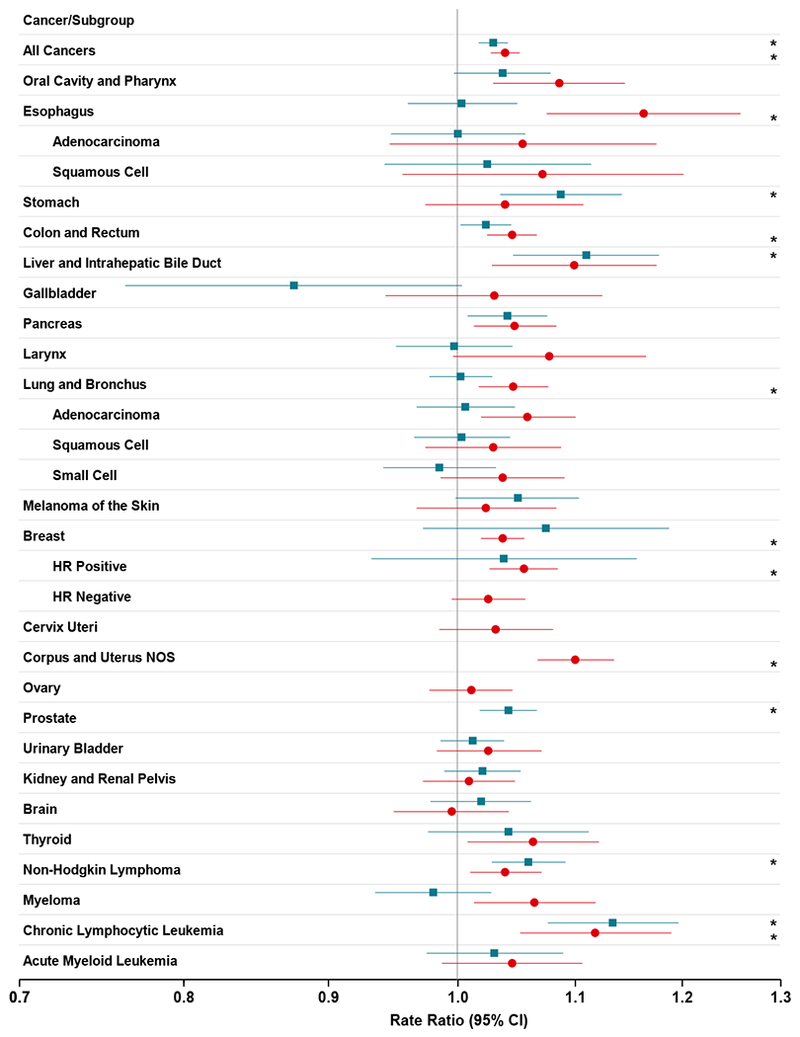
Adjusted cancer rate ratios and 95% confidence intervals in 11 states within the continental United States, SEER program 2000-2012, by gender and cancer type. The adjusted cancer rate ratios are per five degrees of longitude difference, equivalent to 20 minutes; *p-value less than 1.8×10−3 (0.05/28) for men and less than 1.7×10−3 (0.05/30) for women.
Figure 2.
Map of the Continental United States showing the 4 timezones (Pacific- purple, Mountain- peach, Central- yellow, Eastern-green) with each of the 11 SEER states (California, Connecticut, Georgia, Iowa, Kentucky, Louisiana, New Mexico, New Jersey, Utah) and registries (the Detroit metropolitan area of Michigan and the Seattle-Puget Sound area of Washington) shaded a darker shade. The 607 counties are also indicated.
In men only, the east- west gradients in risk within a timezone were elevated for stomach cancer (RR=1.087, 95% CI=1.035-1.142, p=9.6 ×10−4), liver cancer (RR=1.110, 95% CI=1.046-1.177, p=6.0 ×10−4), prostate cancer (RR=1.042, 95% CI=1.018-1.066, p=4.8 × 10−4) and non-Hodgkin lymphoma (RR=1.059, 95% CI=1.028-1.091, p=1.6 × 10−4).
Among women only, the east-west gradients in risk within a timezone were increased for breast cancer overall (RR=1.037, 95% CI=1.019-1.055, p=6.5 × 10−5) and specifically HR+ breast cancer (RR=1.055, 95% CI= (1.026-1.084, p=1.8 × 10−4). Also elevated were esophageal cancer (RR=1.163, 95% CI=1.075-1.258, p=1.8 × 10−4), colorectal cancer (RR=1.045, 95% CI= (1.024-1.066, p=1.8 × 10−5), lung cancer (RR=1.046, 95% CI=1.017-1.076, p=1.6×10−3), and corpus uteri cancer (RR=1.1, 95% CI=1.067-1.135, p=2.4 × 10−9) None of the subgroups for esophagus cancer was significantly associated with the position in a timezone.
The age-adjusted cancer incidence rates and 95% CI for each tertile of the PTZ combined across the four timezones are provided in Supplementary Table 1. A strong east-to-west gradient appears only for all cancers and chronic lymphocytic leukemia among both males and females.
Further adjusting the models for urban-rural characteristic and the prevalence of obesity, and restricting to non-Hispanic whites did not change the findings.
Discussion
In 607 counties of the continental United States, involving more than 4 million cancer diagnoses among whites, we found that residents in the western regions of timezones had increased rates of overall cancer and many specific cancers, in accord with the circadian disruption hypothesis. These observations might implicate a novel source of circadian disruption that occurs as one proceeds westward in each time zone due to the increased divergence between social time and internal circadian time.
Our findings on breast, corpus uteri and prostate cancer are consistent with a previous report based on a small ecologic database of 59 regions in an European population in Russia (9). We also identified new associations that will require follow-up: increased risk of chronic lymphocytic leukemia in both genders, stomach cancer, liver cancer and non-Hodgkin lymphoma in males, and esophageal, colorectal, and lung cancer in females associated with western timezone position. In particular, the association with chronic lymphocytic leukemia is the strongest and most robust; the association was consistent in each timezone, and in both men and women.
The associations with female breast cancer are only significant for HR+ breast cancer. The positive findings in the hormone-related malignancies are generally concordant with a body of previous work that reported elevated risk in shift workers of breast cancer (15,23), prostate cancer (24) and uterine corpus cancer (25), all hormone responsive tumors. Epidemiologic studies have also reported increased risks of colon (26,27) and non-Hodgkin lymphoma (28) associated with shift work
The unexpectedly broad pattern of associations across multiple tumor types that we observed accords with the evidence in animal models, studies of shift workers, and mechanistic studies that indicate circadian rhythm alterations have manifold biological consequences. Increased cancer risks in animal models have been documented in a broad group of tumors including blood, liver, ovary, intestine, colon and skin (29–31). Mechanistically, many non-hormone related pathways involved in carcinogenesis are under circadian control (32), such as cell proliferation, DNA repair (33), apoptosis (34), and immune response (35). Therefore, it is possible that circadian disruption has effects across a wider group of tumors than previously thought.
Alternatively, the findings may be due to confounding, or other bias. Geographical regions could subsume many cancer-related factors, such as the degree of rural/urban, tax policies affecting smoking, poverty levels, cancer screening and hospitalization, as well as behavior and lifestyles. Although we adjusted for many of these community level factors, given the limitations of ecological studies, study of individual-level subjects is needed to confirm these findings.
We note that our strongest and most consistent effect was observed for chronic lymphocytic leukemia, a tumor that lacks strong extrinsic environmental risk factors and which has recently been a focus of studies of dysregulation, altered expression (36) (37) and methylation (38) of specific circadian genes. Further investigation of this association along with other categories of lymphoproliferative malignancies is warranted.
The hypothesis that exposure to light at night contributes to circadian disruption, previously associated with breast (39), and prostate (40) cancers, is generally concordant with our findings. There is evidence that exposure to light at night suppresses melatonin production, which has anti-oncotic properties (41). Consistent with this is the finding of reduced breast cancer in blind women, in whom light does not suppress melatonin since the pathway for light-induced suppression is via the eye (42).
Our study has several strengths. Using position in a timezone to study circadian misalignment is a unique design. The SEER registry is closely monitored and controlled, and therefore has high data quality (10). The opportunity to link the SEER registry with substantial numbers of cancer cases (2,095,394 in men, 1,972,514 in women) with geographic information allowed us to explore multiple cancer sites. Other independent variables are derived from high quality government data allowing us to consider several important potential confounders.
As mentioned above, the ecological study is limited by the inability to adjust for person-level risk factors, although we were able to adjust for county–level data on cigarette smoking, latitude, poverty, and state to partially mitigate these limitations. Separately, we examined obesity, Hispanic make-up of counties and urbanicity in sensitivity analyses. None of these variables had a noteworthy effect on the findings.
The inability to track migration and the impact of temporal lag between exposure and outcome are limitations. The United States is a relatively mobile society, and residence may have changed one or more times, but such changes would tend to bias data towards the null, making real differences harder to detect. It has been hypothesized that the natural seasonal adaptation is disrupted by Daylight Savings Time (43) but since all the states included in our study are equally affected, this would not influence our findings.
If these findings are verified, what measures should be considered to reduce their potential public health impact? It is worth noting that the magnitudes of risks are in a very small range (generally RR <1.1 per 5 degrees longitude). Measures to reduce social jetlag such as improved sleep hygiene (reducing light at night, earlier bedtimes) and promoting later and more flexible school and work schedules have been advocated by the United States Centers for Disease Control and Prevention (19) and are reasonable steps. The social jet lag effect itself, i.e., the tendency for chronotypes to move later with westward movement across the timezone is well documented based on questionnaire and personal device data from Europe, Russia, China, South Africa, and the United States (44–46). Further studies to investigate the relationship of timezone position to diabetes, obesity, cardiovascular endpoints, and overall mortality as well as follow-up of the cancer findings reported here are needed. Our findings suggest that circadian disruption is not a rare phenomenon affecting only shift workers or international travelers but is common in the general population and therefore has broader implications for public health than generally appreciated.
In summary, this ecological study shows residency in the western part of a timezone is associated with increased rates of total cancer and many specific cancers, in accord with the circadian disruption hypothesis. Replication in analytic epidemiology studies is warranted.
Supplementary Material
Acknowledgements
We thank David Check of the Biostatistics Branch, DCEG, for figure development.
Funding: This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring, Md) 2009;17(11):2100–2 doi 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. International journal of obesity (2005) 2013;37(4):604–11 doi 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Current biology : CB 2012;22(10):939–43 doi 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Rosenwasser AM. Chronobiology of ethanol: animal models. Alcohol 2015;49(4):311–9 doi 10.1016/j.alcohol.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol 2009;38(4):963–70 doi 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology 2007;8(12):1065–6. [DOI] [PubMed] [Google Scholar]
- 7.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiology international 2006;23(1-2):497–509 doi 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 8.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Med Rev 2007;11(6):429–38 doi 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Borisenkov MF. Latitude of residence and position in time zone are predictors of cancer incidence, cancer mortality, and life expectancy at birth. Chronobiology international 2011;28(2):155–62 doi 10.3109/07420528.2010.541312. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973-2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. [Google Scholar]
- 11.Howlader N NA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2013, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 12.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking−-50 years of progress”: a paradigm shift in cancer care. Cancer 2014;120(13):1914–6 doi 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. The Lancet Oncology 2009;10(11):1033–4. [DOI] [PubMed] [Google Scholar]
- 14.IARC. IARC Monograph on the Evaluation of Carcinogenic Risks in Human, Report of the Advisory Group to Recommend Priorities for IARC Monographs 2015-2019. 2014;4.47(Internal Reprot 14/002) France. [Google Scholar]
- 15.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 2012;104(14):1094–101 doi 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egevad L H M, Berney D, Fleming K, Ferley J Cancer Incidence in Five Continents. IARC Scientific Publication No 160,. Volume IX p 61–6. [Google Scholar]
- 17.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer 2014;120(18):2883–92 doi 10.1002/cncr.28749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, Flaxman AD, Hansen GM, Murray CJ. Cigarette smoking prevalence in US counties: 1996-2012. Population health metrics 2014;12(1):5 doi 10.1186/1478-7954-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roenneberg T, Merrow M. The Circadian Clock and Human Health. Current biology : CB 2016;26(10):R432–43 doi 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Brockmann PE, Gozal D, Villarroel L, Damiani F, Nunez F, Cajochen C. Geographic latitude and sleep duration: A population-based survey from the Tropic of Capricorn to the Antarctic Circle. Chronobiology international 2017:1–9 doi 10.1080/07420528.2016.1277735. [DOI] [PubMed] [Google Scholar]
- 21.Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet (London, England) 2015;386(9991):369–75 doi 10.1016/s0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwyer-Lindgren L, Freedman G, Engell RE, Fleming TD, Lim SS, Murray CJ, et al. Prevalence of physical activity and obesity in US counties, 2001-2011: a road map for action. Population health metrics 2013;11:7 doi 10.1186/1478-7954-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolstad HA. Nightshift work and risk of breast cancer and other cancers--a critical review of the epidemiologic evidence. Scand J Work Environ Health 2008;34(1):5–22. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. American journal of epidemiology 2006;164(6):549–55 doi 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer research 2007;67(21):10618–22 doi 10.1158/0008-5472.can-07-2485. [DOI] [PubMed] [Google Scholar]
- 26.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst 2003;95(11):825–8. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Ji A, Zhu Y, Liang Z, Wu J, Li S, et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget 2015;6(28):25046–60 doi 10.18632/oncotarget.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. International journal of cancer 2008;123(9):2148–51 doi 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 29.Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proceedings of the National Academy of Sciences of the United States of America 2012;109(29):11758–63 doi 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PloS one 2010;5(6):e10995 doi 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Molecular cancer research : MCR 2008;6(11):1786–93 doi 10.1158/1541-7786.mcr-08-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 2003;3(5):350–61 doi 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman AE, Zheng T, Ba Y, Zhu Y. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Molecular cancer research : MCR 2008;6(9):1461–8 doi 10.1158/1541-7786.mcr-07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JY, Joh HM, Park JM, Kim MJ, Chung TH, Kang TH. Non-thermal plasma-induced apoptosis is modulated by ATR- and PARP1-mediated DNA damage responses and circadian clock. Oncotarget 2016;7(22):32980–9 doi 10.18632/oncotarget.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsoumtsa LL, Torre C, Ghigo E. Circadian Control of Antibacterial Immunity: Findings from Animal Models. Frontiers in cellular and infection microbiology 2016;6:54 doi 10.3389/fcimb.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisele L, Prinz R, Klein-Hitpass L, Nuckel H, Lowinski K, Thomale J, et al. Combined PER2 and CRY1 expression predicts outcome in chronic lymphocytic leukemia. European journal of haematology 2009;83(4):320–7 doi 10.1111/j.1600-0609.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 37.Rana S, Munawar M, Shahid A, Malik M, Ullah H, Fatima W, et al. Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Molecular biology reports 2014;41(1):95–103 doi 10.1007/s11033-013-2841-7. [DOI] [PubMed] [Google Scholar]
- 38.Hanoun M, Eisele L, Suzuki M, Greally JM, Huttmann A, Aydin S, et al. Epigenetic silencing of the circadian clock gene CRY1 is associated with an indolent clinical course in chronic lymphocytic leukemia. PloS one 2012;7(3):e34347 doi 10.1371/journal.pone.0034347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Park MS, Lee E, Choi JW. High Incidence of Breast Cancer in Light-Polluted Areas with Spatial Effects in Korea. Asian Pac J Cancer Prev 2016;17(1):361–7. [DOI] [PubMed] [Google Scholar]
- 40.Rybnikova NA, Haim A, Portnov BA. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch Environ Occup Health 2016:1–12 doi 10.1080/19338244.2016.1169980. [DOI] [PubMed] [Google Scholar]
- 41.Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog 2007;13(4):303–28. [DOI] [PubMed] [Google Scholar]
- 42.Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. Total visual blindness is protective against breast cancer. Cancer Causes Control 2009;20(9):1753–6 doi 10.1007/s10552-009-9405-0. [DOI] [PubMed] [Google Scholar]
- 43.Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Current biology : CB 2007;17(22):1996–2000 doi 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Current biology : CB 2007;17(2):R44–5 doi 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Shawa N, Roden LC. Chronotype of South African adults is affected by solar entrainment. Chronobiology international 2016;33(3):315–23 doi 10.3109/07420528.2016.1144608. [DOI] [PubMed] [Google Scholar]
- 46.Randler C Differences in sleep and circadian preference between Eastern and Western German adolescents. Chronobiology international 2008;25(4):565–75 doi 10.1080/07420520802257794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


