Abstract
In this study, we investigated the diversity of drug‐resistant Mycobacterium tuberculosis isolates from families who own cattle in the Eastern Cape Province of South Africa using spoligotyping and mycobacterial interspersed repetitive‐unit‐variable number tandem repeat (MIRU‐VNTR) typing. The Mycobacterium tuberculosis was investigated using MIRU‐VNTR and the Mycobacterium tuberculosis families were evaluated using spoligotyping. Spoligotyping grouped 91% of the isolates into seven clusters, while 9% of the deoxyribonucleic acid (DNA) from TB isolates were unclustered from a total of 154 DNA used. Previously described shared types were observed in 89.6% of the isolates, with the Beijing family, SIT1, the principal genotype in the province, while the families T, SIT53 and X1, SIT1329 were the least detected genotypes. MIRU‐VNTR grouped 81% of the isolates in 23 clusters while 19% were unclustered. A combination of the VNTR and spoligotyping grouped 79% of the isolates into 23 clusters with 21% unclustered. The low level of diversity and the clonal spread of drug‐resistant Mycobacterium tuberculosis isolates advocate that the spread of TB in this study may be instigated by the clonal spread of Beijing genotype. The results from this study provide vital information about the lack of TB control and distribution of Mycobacterium tuberculosis complex strain types in the Eastern Cape Province of South Africa.
Keywords: Beijing, M. tuberculosis, MIRU‐VNTR, Multidrug resistance, Spoligotyping
1. INTRODUCTION
Mycobacterium tuberculosis complex (MTBC) grounds eight million novel cases of tuberculosis (TB) and two million deaths every year. However, TB still remains a global epidemic with 8 million new‐fangled cases and 2 million deaths every year (Villemagne et al., 2012; WHO 2014). Although the World Health Organization has made strides in achieving the Millenium Development Goal of halting and reversing the incidence of TB, South Africa still has high incidences of TB, between 410, 000 and 520,000 (WHO 2014). The introduction of modern TB drugs in South Africa has decreased mortality and morbidity; however, the introduction of new and faster detection methods such as GeneXpert detected more cases because of its accuracy (Muller, 2001). The compounding factor for TB in South Africa is the advent and spread of drug‐resistant TB, especially multidrug‐resistant TB (MDR‐TB) [resistant to at least isoniazid (INH) and rifampicin (RIF)] and extensively drug‐resistant strains (XDR) TB [MDR‐TB with further resistance to any fluoroquinolone (FLQ) and to a minimum of one of the three injectable second‐line drugs, kanamycin (KAN), amikacin (AMK), and/or capreomycin (CAP)] (CDC 2006; WHO 2010). In 2006, the Eastern Cape Province of South Africa had a TB incidence of 705 of 100,000. In 2008, it had the solitary utmost MDR‐TB caseload in South Africa (WHO 2012). Deprived of adequate chemotherapy, TB could become increasingly mortal.
The World Health Organization reported that 274 patients were detected with XDR‐TB in October 2006 from the Eastern Cape Province, of which 23% died before treatment was initiated (WHO 2012; WHO/IUATLD, 2008). It further explains that, of the 206 patients who started treatment, 58.4% detected with XDR‐TB died. According to the National Health Laboratory Services, Eastern Cape had a total of 808 XDR‐TB between 2006 and 2010 (NDH 2011). Management of MDR and XDR‐TB strategy design hinge on the drivers of the epidemic, which are the prevalence and banquet of drug‐resistant strains as well as the understanding of the population structure (Said et al., 2012). TB drug‐resistant strains have been documented from previous studies in all the provinces in South Africa (Mlambo et al., 2008; Streicher et al., 2004; Warren et al., 1996). However, insufficient data have been reported from these studies. To the best of our knowledge, a comprehensive genotypic diversity of circulating MTBC strains is reported for the first time in the Eastern Cape Province of South Africa. Consequently, studies on the characterization of drug‐resistant strains are necessary for accurate assessments of population structure and to determine the common families of mingling drug‐resistant M. tuberculosis strains. Different genotypes occurring at different frequencies cause the global TB epidemiology (Bifani, Mathema, Kurepina, & Kreiswirth, 2000; van Soolingen et al., 1999).
The source and transmission patterns of drug‐resistant isolates can be traced using genotyping. Several methods such as IS6110 restriction fragment length polymorphism (RFLP), spoligotyping, and mycobacterial interspersed repetitive‐unit‐variable numbers of tandem repeat (MIRU‐VNTR), have been used for molecular typing of M. tuberculosis (Cavusoglu, Turhan, Akinci, & Soyler, 2006; Cerezo et al., 2012; Mokrousov et al., 2004; Sola et al., 2003). IS6110 RFLP typing is arduous, requires enormous quantities of DNA and has deprived discriminatory power on M. tuberculosis isolates with little or no IS61 l0 copy number (Chaoui, Zozio, & Lahlou, 2014), although it is known as the orientation technique for genotyping of M. tuberculosis strains (Mathema, Kurepina, Bifani, & Kreiswirth, 2006; van Soolingen, 2001). These limitations of IS6110 have been compensated by the development of PCR‐based methods such as spoligotyping and MIRU‐VNTR (Supply, Magdalena, Himpens, & Locht, 1997; Supply et al., 2006). Spoligotyping utilizes polymorphism in the direct repeat region, an organization belonging to a family of repeats called clustered repetitive interspersed palindromic repeats (CRISPRs) located in the genomes of bacteria and archaea (Kamerbeek et al., 1997; Pourcel, Salvignol, & Vergnaud, 2005). The method is practical and rapid in both clinical and molecular epidemiology and the results are uttered in an undemanding digital pattern, named and data based (Kamerbeek et al., 1997). However, when used unaided, spoligotyping tends to overrate the proportion of clustered strains. It is therefore required that spoligotype‐defined clusters be further defined using other methods such as MIRU‐VNTR (Sola et al., 2003). MIRU‐VNTR genotyping is a PCR‐based technique used to distinguish the number of tandem repeats at a specified genetic locus (Bifani et al., 1996). Most genotyping studies in South Africa were conducted in provinces with high multidrug‐resistant strains (MDRs) such as Western Cape (Warren et al., 1996), Gauteng (Hove, Molepo, Dube, & Nchabeleng, 2012), and KwaZulu‐Natal (Gandhi et al., 2014) and samples were collected from hospitals. However, little data are available from most of the provinces of South Africa, especially the cattle farmers’ in the Eastern Cape region. In this paper, we report on the clonality and genetic profiles of MTBC among drug‐resistant isolates from the Eastern Cape Province of South Africa as part of our bigger studies on reservoirs of antibiotic resistance determinants in South Africa.
2. EXPERIMENTAL PROCEDURES
2.1. Study population, Sample collection, and Ethical approval
A total of 6,000 suspected TB cases from households who own cattle in the Eastern Cape Province were considered in the study. This study is part of a larger research that investigated the prevalence of MTBC from cattle lymph nodes. The samples from this study were collected from people who own cattle and not from TB patients. The study was conducted at the Molecular Pathogenicity and Molecular Epidemiology Research Laboratory, University of Fort Hare, Alice. This project was approved by the University of Fort Hare Research Ethics Committee (UREC) and an ethical clearance certificate was issued; REC‐270710‐028‐RA Level 01. All individual identifiers have been detached or disguised, so the person(s) described are not identifiable and cannot be known through the details provided.
2.2. Sputum smear microscopy and Culture
Sputum smears were Ziehl–Neelsen (ZN)‐stained and acid fast bacilli (AFB) were examined under a bright field microscope. Similarly, all the collected sputum samples were also processed for in vitro drug susceptibility testing. Sputum samples were decontaminated using N‐acetyl L‐cysteine and neutralized with sodium hydroxide (Stroup et al., 2014). MTBC isolates were cultured on a Lowenstein–Jensen (LJ) slant followed by incubation at 37°C for at least 6–8 weeks.
2.3. Confirmation of Mycobacterium tuberculosis complex
DNA was extracted following the method by Yates, Drobniewski, & Wilson, 2002 and amplified using the Polymerase Chain Reaction (PCR) targeting the 240‐bp region of the mpb64 gene using the outlined primer sequences F(460‐479) 5′‐TCCGCTGCCAGTCGTCTTCC‐3′ and R (700‐681) 5′‐GTCCTCGCGAGTCTAGGCCA‐3′ (Madhavan et al., 2000). The amplification was done in a 25‐μl reaction mixture, which consisted of 2.5 μl of 5× buffer (500 mmol/L potassium chloride, 100 mmol/L Tris chloride, 15 mmol/L magnesium chloride, gelatin 0.1%, pH 8.3), 100 ng each of primers, 200 mmol/L of each deoxyribonucleotide triphosphate, 1 U Taq DNA polymerase (Kapa Biosystems, South Africa), and 5 μl of DNA template. Nuclease‐free water was added to make the total volume to 25 μl. Amplification was executed using thermal cycler MyCycler™ (BioRad, Cape Town, South Africa). The protocol entailed of one cycle at 94°C for 5 min, 35 cycles of denaturation at 94°C for 1 min, annealing for 1 min at 55°C, and extension for 1 min at 72°C followed by one cycle of final extension at 72°C for 10 min (Madhavan et al., 2000).
2.4. Drug susceptibility testing (DST)
Confirmed MTBC isolates were tested for in vitro drug sensitivity testing for RIF, INH, and ethambutol (EMB) using the standard LJ proportion method as described earlier (Stroup et al., 2014; WHO 2009). Isolates were tested for resistance to RIF, INH, and ethambutol using concentrations of 40 μg/ml for RIF, 0.2 μg/ml for INH and 2.0 μg/ml for ethambutol. Briefly, the diluted suspension of isolates were inoculated onto LJ medium with and without drugs and incubated at 37°C. Results were read up to 42 days of incubation. An isolate was considered resistant to a given drug when growth of 1% or more as compared with the control was observed in drug‐containing medium. MTB H37Rv wild‐type strain (ATCC 27294) was used as a control for drug susceptibility testing (WHO 2009; Ali et al., 2015).
2.5. Genotyping and phylogenetic analyses
A spoligotyping commercial kit (Isogen Life Science B. V., Utrecht, The Netherlands) was used to perform spoligotyping (Kamerbeek et al., 1997) of the isolates. Spoligotypes in this study were assigned shared international types (SIT) numbers and genotypic cluster designations by comparing with the SITVIT4 database. MIRU‐VNTR typing was performed using 12 MIRU‐VNTR loci described elsewhere (Kamerbeek et al., 1997). NJ‐tree was constructed for combined spoligotypes and MIRU‐VNTR observed in our study using the MIRU‐VNTRplus web application (http://www.miru-vntrplus.org/MIRU/index.faces) (Weniger, Krawczyk, Supply, Niemann, & Harmsen, 2010).
2.6. Data analysis
The spoligotyping results were entered in an Excel sheet as an octal format and binary code representing a positive or negative hybridization result. All spoligotype binary formats were submitted to the SITVIT2 (http://www.pasteur-guadeloupe.fr:8081/SITVITDemo) (Lillebaek, Andersen, Dirksen, Glynn, & Kremer, 2003). Major spoligotyping‐based phylogenetic subtypes were allocated according to signatures provided in SITVIT2, defining M. tuberculosis major lineages based on spoligotyping data (Demay et al., 2012). These include specific signatures for M. tuberculosis complex members, as well as rules defining major lineages/sublineages for M. tuberculosis sensu stricto as described elsewhere (Demay et al., 2012). MIRU‐VNTRplus Database (http://www.miru-vntrplus.org) was used to compare the 12 MIRU‐VNTR patterns using the Levenshtein algorithm (also called Edit‐Distance). In our case, the distance was calculated between each of our 12‐loci pattern with all the patterns available online (n = 141) in the MIRU‐VNTRplus database. Genotyping data was analyzed phylogenetically by constructing an NJ‐tree (Figure 1).
Figure 1.
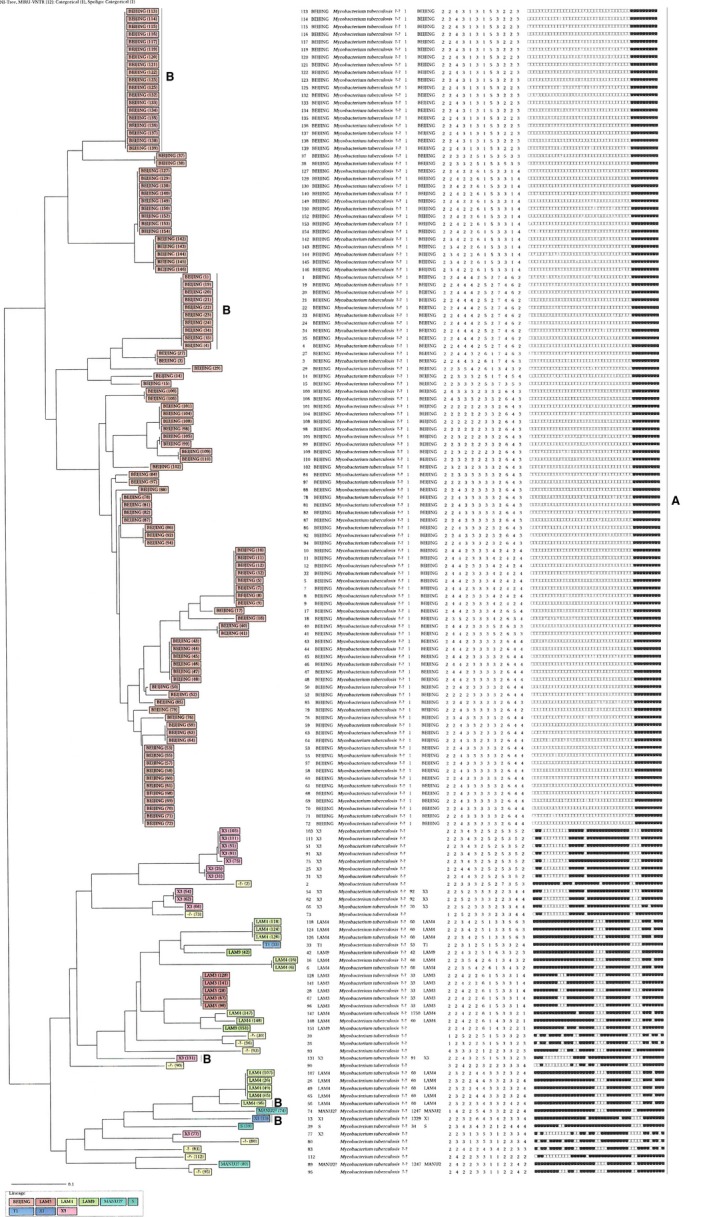
Relationship of spoligotypes of human DNA isolates from the Eastern Cape. The presented patterns were generated using the MIRU‐VNTR plus web application (http://www.miru-vntrplus.org/MIRU/index.faces. A = INR + RIF resistance; B = INH + RIF + EMB resistance
2.7. Statistical analysis
The Hunter–Gaston Index (HGI) was calculated as described previously (Hunter & Gaston, 1998), which was used in the determination of the diversity of each MIRU‐VNTR locus of the isolates, and described by the following equation, where N is the total number of isolates in the sample population for a given locus, S is the total number of distinct repeat unit values identified for the locus, and nj is the number of isolates having the jth value:
Dendrogram was generated with the program available online at www.miru-vntrplus.org (Allix‐Béguec, Harmsen, Weniger, Supply, & Niemann, 2008; Weniger et al., 2010) with the Neighbor‐joining tree generated. Fully identical patterns of M. tuberculosis isolates from different patients were assigned to the same cluster. The clustering rate was defined as (nc–c)/n, where nc is the total number of clustered cases, c is the number of clusters, and n is the total number of cases in the sample.
3. RESULTS
3.1. Sputum smear microscopy, culture, confirmation, and DST
Out of 6,000 suspected TB cases investigated in this study, 200 were positive when examined under the microscopy. As compared to microscopic examination, the numbers of positive specimens when using culture method were 156, which is lower than the initial screening. The entire specimen that were positive on culture, were also confirmed positive by PCR. Hundred and fifty‐four (98.7%) confirmed isolates were resistant to both INH and RIF, 2 (1.3%) resistant to INH alone, and 32 (20.4%) of the MDR detected were resistant to EMB.
3.2. Genotyping profiles of the M. tuberculosis DNA
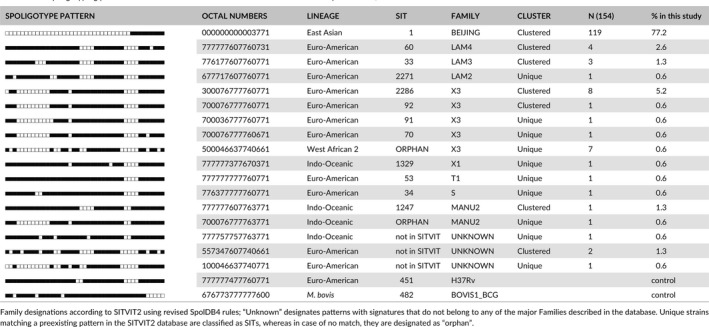
We observed 11 SITs containing (n = 138, or 88.5% of the isolates) isolates summarized in Table 1. Among the 11 SITs recorded, seven SITs (containing 121 isolates, 87.6%) matched a pre‐existing SIT in the SITVIT2 database, whereas four (2.3%) isolates did not match any SIT in the SITVIT2 database (Table 1). The largest cluster generated consisted of 119 strains, (77% of all isolates), corresponded to SIT1, a prototype of the family Beijing genotypic lineage in the SpolDB4 and SITVITWEB databases commonly found in diverse parts of the world including Southern Africa. The SIT1 cluster was followed by Orphan/X3 (n = 7 or 4.5% isolates). A total of 29 Beijing strains were resistant to all three tested anti‐TB drugs, together with 0.6% of LAM2, X3, and X1 families (Figure 1). Two isolates were excluded due to DNA shortage and nogrowth when subcultured to isolate DNA.
Table 1.
Spoligotyping patterns of 154 M. tuberculosis strains isolated in the Eastern Cape Province, South Africa
3.3. Discriminatory power of genotyping methods
The allelic variety among 12 MIRU‐VNTR loci (Table 2) differed from 0.022 to 0.787 in this study. Highly diverse (h > 0.6) (Hunter & Gaston, 1998), VNTR loci along with both Beijing and non‐Beijing isolates were found to be MIRU10, MIRU16, MIRU20, MIRU23, MIRU24, MIRU27, MIRU31, MIRU39. Nevertheless, centered on the value of allelic variety (h > 0.6) MIRU31 (h = 0.814), was more varied among non‐Beijing isolates. The biased power of each genotyping method is shown in Table 3. Spoligotyping showed a moderately biased power (HGI = 0.520) among 25 dissimilar types; spoligotyping recognized 10 distinctive isolates (6.5%) and 144 (93.5%) isolates grouped in seven clusters. The biggest cluster including 124 isolates was assigned to the Beijing family (ST1). Associated with spoligotyping, 12‐MIRUs alone recognized 25 distinctive isolates and 129 isolates in 23 clusters with a moderately biased power (HGI = 0.86). When used in combination with spoligotyping, 12‐MIRU achieved an HGI value of 0.951 (Figure 1).
Table 2.
Number of occurrences of MIRU and allelic diversity for each locus
| Allele locus | MIRU No | Allelic diversity (HGI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of alleles | Beijing | Conclusion | No. of alleles | Non‐Beijing | Conclusion | No. of alleles | All | ||
| 154 | 2 | 4 | 0.349 | MD | 3 | 0.462 | MD | 4 | 0.381 |
| 580 | 4 | 7 | 0.381 | MD | 4 | 0.729 | HD | 7 | 0.532 |
| 960 | 10 | 7 | 0.640 | HD | 5 | 0.613 | HD | 8 | 0.652 |
| 1644 | 16 | 6 | 0.682 | HD | 5 | 0.661 | HD | 8 | 0.678 |
| 2059 | 20 | 6 | 0.654 | HD | 6 | 0.822 | HD | 8 | 0.721 |
| 2531 | 23 | 7 | 0.642 | HD | 5 | 0.705 | HD | 7 | 0.658 |
| 2687 | 24 | 7 | 0.659 | HD | 6 | 0.802 | HD | 8 | 0.725 |
| 2996 | 26 | 6 | 0.605 | HD | 4 | 0.641 | HD | 6 | 0.617 |
| 3007 | 27 | 7 | 0.731 | HD | 5 | 0.527 | MD | 7 | 0.725 |
| 3192 | 31 | 8 | 0.756 | HD | 6 | 0.814 | HD | 8 | 0.787 |
| 4348 | 39 | 5 | 0.576 | MD | 4 | 0.691 | HD | 6 | 0.617 |
| 802 | 40 | 3 | 0.029 | PD | 2 | 0.041 | PD | 3 | 0.022 |
Table 3.
Discriminatory power of spoligotyping and VNTR used alone and in association
| Typing methods | Number of patterns | Number of clusters | Number of clustered isolates | Number of unique isolates | Size of clusters | Clustering Rate | HGIa |
|---|---|---|---|---|---|---|---|
| Spoligotyping | 40 | 7 | 144 | 10 | 2–114 | 89 | 0.822 |
| VNTR | 51 | 23 | 129 | 25 | 2–124 | 69 | 0.940 |
| Spoligotyping + VNTR | 55 | 24 | 123 | 31 | 2–27 | 78 | 0.951 |
HGI, Hunter–Gaston Index.
4. DISCUSSION
Previous studies (Marais et al., 2013; Said et al., 2012) described the clonal spread of genotypes of M. tuberculosis from different provinces of South Africa. However, an updated thorough analysis of the population structure of resistant M. tuberculosis isolates from the Eastern Cape Province, South Africa, was not available. As a result, this study is the first in the Eastern Cape Province to report about the population structure of resistant M. tuberculosis. This study, did not only attempt to map the population structure of drug‐resistant M. tuberculosis isolates from the Eastern Cape, but also differentiated the drug‐resistant isolates using 12‐locus MIRU‐VNTR. In this study, we collected sputum specimens from cattle farmers to trace the circulating MTBC strains that could be transmittable between humans and animals and socioeconomic activities practiced in this province. The Eastern Cape Province is one of the poorest provinces in South Africa, largely dependent on livestock farming (Katiyatiya, Muchenje, & Mushunje, 2014). As a result, there is a possibility of MTBC strains’ transmission between humans and animals; and hence, an investigation of genotypic strains in this province is highly imperative. We observed 99.4% MDR from MTBC isolates and a significant proportion (20.5%) of MDR isolates also resistant to EMB. Therefore, to minimize subsequent development of MDR strains, proper management of patients with TB is imperative. Early detection of TB is important in arresting further transmission of MDR‐TB clones, which is more expensive to manage owing to extended medication and high risk of death.
The predominant (69%) Beijing family represented by its isolated prototype SIT1 is abundant in our study. Both its elevated quantity of clonal spread and its majority among the novel patterns are appearances of the existing adaptive evolution of the South African genotype in this scenery (Streicher et al., 2004). In the Western Cape region, the Beijing genotype was vastly prevalent, and represented 36.5% of the drug‐resistant cases (Johnson et al., 2010). The Eastern Cape and Western Cape regions share a boarder on the western side of the Eastern Cape, and many residents of both Western Cape and Eastern Cape region have relatives in both regions. Strains might be carried from one region to another. Beijing family represented a high proportion of M. tuberculosis isolates causing ongoing TB transmission in Russia, Central Asia, and East Asia (Niemann et al., 2009; Wada, Iwamoto, & Maeda, 2009). Having 13% of the isolates globally and 50% of the isolates in Asia (Kim et al., 2001; Park, Bai, & Kim, 2000; Parwati, van Crevel, & van Soolingen, 2010), this family still has higher incidences (up to 92.59%) in Beijing and its immediate areas in China (Dong et al., 2010). Said et al. (2012) did not find any Beijing family in their study. The difference in the clonal spread of Beijing family in our study could be due to the difference in sampling size, time, and locations.
The LAM lineage (10.7%) was the subsequent most predominant in our study and was present in six out of 12 reported sublineages throughout the world (Demay et al., 2012). In contrast to other studies, the LAM family was reported at 22% of a total of 147 isolates in a study reported in Dar es Salaam, Tanzania (Eldholm, Matee, Mfinanga, Heun, & Dahle, 2006), while in Kenya, 11% of the families were LAM (Githui et al., 2004). Among the six sublineages present in South Africa, LAM4 was the most predominant (SIT60, 5.8% of strains). Different LAM subfamilies dominate in diverse coastal regions of Sub‐Saharan Africa (Pillay & Sturm, 2007; Viegas et al., 2010). LAM lineage is vastly prevalent in Latin American and the Caribbean's (Brudey et al., 2006); however, the clonal spread of LAM lineage (10.7%) found in the Eastern Cape is different; the spoligotype of LAM4 in our study (5.7%) with its prototype SIT60 and its variant 1750 (0.51%) were different from that of LAM9 (0.51%) with its prototype SIT42; altogether different from that obtained in Bogotá, Colombia (27.6%). We also found one strain in the LAM9 lineage that did not match any in the SITVIT2 and was considered as orphan. A study by Asiimwe, Ghebremichael, Kallenius, Koivula, and Joloba (2008) from Kampala showed proportions of LAM9 to be 2.6%. Out of the 12 sublineages that have been described globally for the LAM family (Brudey et al., 2006; Demay et al., 2012) a total of six sublineages were detected in our study. LAM3 in our study, represented by its prototype SIT33 was 2.6% which is higher than that obtained by Asiimwe et al. (2008) (1.7%). We also found LAM2 with its prototype SIT2271 (0.51%) in minority to all the LAM isolates. Of noteworthy, LAM6 was absent in our study. This was in contrast to the report elsewhere (Martins et al., 2013).
The X family of strains is defined by two associated features, a small number of IS6110 copies and the lack of spacer 18 in the spoligotype (Sebban, Mokrousov, Rastogi, & Sola, 2002). The latter trait universal to at least three spoligotype shared types: ST119, ST137, and ST92 (Sebban et al., 2002). In our study, the X family with its variants was detected in 7.64%. This is not surprising as variants of this genotype family have been described in South Africa (Kim et al., 2001). Other noteworthy families identified in this study included the X family which was fairly distributed, T (mainly T1) and S families, which were among the minor families.
We also found the existence of a few ancestral Manu lineage strains (n = 4/193 or 2.07%) as clustered strains within our study sample. This lineage was first described as a new family in India in 2004 (Brudey et al., 2006) and later related strains in minute quantities were reported in a study from Madagascar (Ferdinand, Sola, Chanteau, & Sola, 2005). Rapidly later, it was cautiously subdivided into Manu‐1 (deletion of spacer 34), Manu‐2 (deletion of spacers 33‐34), and Manu‐3 (deletion of spacers 34‐36) sublineages, and suggested that it might denote an ancestral replica of main genetic group 1 strains (Brudey et al., 2006). Manu lineage strains were reported from Saudi Arabia (Al‐Hajoj et al., 2007), Tunisia (Namouchi et al., 2008), and in Egypt (Helal et al., 2009). In this study, 17 (8.8%) of the spoligo patterns could not be typed based on the current SpolDB4 database. This suggests the existing absence of information on the genetic diversity of M. tuberculosis strains from this region; therefore, appeals for additional clinical epidemiological studies in different provinces of South Africa to comprehend the genetic diversity of the TB epidemic in the country.
A high diversity among M. tuberculosis in this study was observed when MIRU‐VNTR genotyping technique was used. On its own, spoligotyping was the least discriminatory method when compared to MIRU‐VNTR typing and combined typing with spoligotyping and MIRU‐VNTR using Hunter–Gaston index (HGI). The peak allelic variety was witnessed for MIRU loci 10, 16, 20, 24, 27, 31. Loci 10, 16, 23, 26, and 40 were announced as the loci with the greatest allele polymorphisms, while loci 4, 20, 24, and 27 were the most poorly discriminated loci (Sola et al., 2003). However, in this study, MIRU loci 20, 24, and 27 were highly discriminative. This study was not on a population of TB patients, but it is based on cattle owners who had their animals investigated for TB. Hence, some degree of selection bias cannot be excluded. We could not conclude on aspects relating to drug resistance versus lineage in this study since the isolates used were not from a population study, but concentrated only on cattle owners, therefore introducing bias.
The evaluation of the stretch of M. tuberculosis families in this study might be difficult because of the shorter sampling period (2 months); however, it is still adequate in understanding the transmission patterns. Generally, clustering is indicative of continuing or current spread, while unique patterns indicate recrudescence events (Zozio et al., 2005). The spoligotyping and cluster analysis of 154 clinically isolated strains of MTBC showed that these 154 isolated strains had 60 genotypes. When spoligotyping is utilized together with MIRU‐VNTRs, the two methods are very discriminatory, supplying information for both the epidemiological and phylogenic characteristics of tubercle bacilli (Supply et al., 2006). In our study, the discriminating power of spoligotyping and MIRU‐VNTR separate were lower than the discriminating powers of both methods combined. Using the combined methods, the clustering rates were at 67%, whereas spoligotyping alone identified 87% clustering and MIRU alone identified 58.5% clustering. This suggests that each method alone can overestimate or underestimate clustering. Therefore, spoligotyping combined with MIRU‐VNTR can easily discriminate more isolates. The low level of diversity and the clonal spread of drug‐resistant M. tuberculosis isolates suggest that the spread of TB in this study may be instigated by the spread of Beijing genotype. A clearer picture of the spread and persistency of Beijing genotype in the Eastern Cape will be obtained once all isolates, susceptible and resistant, are investigated. However, this research still represents an important contribution in the lack of TB control and distribution of MTB strain types in the Eastern Cape Province. We conclude that spoligotyping and MIRU‐VNTR typing technique assists in widespread access and plays an imperative role in the epidemiological research of MTBC.
CONFLICT OF INTEREST
No conflict of interest declared by authors.
ACKNOWLEDGMENTS
We are grateful to the Nelson Mandela Metropolitan University in Port Elizabeth, South Africa for providing us with samples. We also acknowledge the National Research Foundation (NRF) of South Africa and the South Africa Medical Research Council (SAMRC) for financial support.
Bhembe NL, Nwodo UU, Okoh AI, Obi CL, Mabinya LV, Green E. Clonality and genetic profiles of drug‐resistant Mycobacterium tuberculosis in the Eastern Cape Province, South Africa. MicrobiologyOpen. 2019;8:e449 doi: 10.1002/mbo3.449
REFERENCES
- Al‐Hajoj, S. A. , Zozio, T. , Al‐Rabiah, F. , Mohammad, V. , Al‐Nasser, M. , Sola, C. , & Rastogi, N. (2007). First insight into the population structure of Mycobacterium tuberculosis in Saudi Arabia. Journal of Clinical Microbiology, 45, 2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A. , Hasan, Z. , McNerney, R. , Mallard, K. , Hill‐Cawthorne, G. , Coll, F. , … Hasan, R. (2015). Whole genome sequencing based characterization of Extensively Drug‐Resistant Mycobacterium tuberculosis isolates from Parkistan. PLoS ONE, 10(2), e0117771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allix‐Béguec, C. , Harmsen, D. , Weniger, T. , Supply, P. , & Niemann, S . (2008). Evaluation and strategy for use of MIRU‐VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. Journal of Clinical Microbiology, 46, 2692–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiimwe, B. B. , Ghebremichael, S. , Kallenius, G. , Koivula, T. , & Joloba, M. L. (2008). Mycobacterium tuberculosis spoligotypes and drug susceptibility pattern of isolates from tuberculosis patients in peri‐urban Kampala, Uganda. BMC Infectious Diseases, 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifani, P. J. , Mathema, B. , Kurepina, N. E. , & Kreiswirth, B. N. (2000). Global dissemination of the Mycobacterium tuberculosis W‐Beijing family strains. Trends in Microbiology, 432(10), 45–52. [DOI] [PubMed] [Google Scholar]
- Bifani, P. J. , Plikaytis, B. B. , Kapur, V. , Stockbauer, K. , Pan, X. , Lutfey, M. L. , … Kreiswirth, B. N. (1996). Origin and interstate spread of a New York City multidrug‐resistant Mycobacterium tuberculosis clone family. JAMA, 275, 452–457. [PubMed] [Google Scholar]
- Brudey, K. , Driscoll, J. R. , Rigouts, L. , Prodinger, W. M. , Gori, A. , Al‐Hajoj, S. A. , … Sola, C. (2006). Mycobacterium tuberculosis complex genetic diversity: Mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiology, 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavusoglu, C. , Turhan, A. , Akinci, P. , & Soyler, I. (2006). Evaluation of the Genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis isolates. Journal of Clinical Microbiology, 44, 2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2006). Notice to readers: Revised definition of extensively drug‐resistant tuberculosis. Centers for Disease Control and Prevention MMWR, 55, 1176. [Google Scholar]
- Cerezo, I. , Jiménez, Y. , Hernandez, J. , Zozio, T. , Murcia, M. I. , & Rastogi, N. (2012). A first insight on the population structure of Mycobacterium tuberculosis complex as studied by spoligotyping and MIRU‐VNTRs in Bogotá, Colombia. Infection, Genetics and Evolution, 12, 657–663. [DOI] [PubMed] [Google Scholar]
- Chaoui, I. , Zozio, T. , & Lahlou, O. (2014). Contribution of spoligotyping and MIRU‐VNTRs to characterize prevalent Mycobacterium tuberculosis genotypes infecting tuberculosis patients in Morocco. Infection, Genetics and Evolution, 21, 463–471. [DOI] [PubMed] [Google Scholar]
- Demay, C. , Liens, B. , Burguière, T. , Hill, V. , Couvin, D. , Millet, J. , … Rastogi, A. (2012). SSITVITWEB, a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infection, Genetics and Evolution, 12, 755–766. [DOI] [PubMed] [Google Scholar]
- Dong, H. Y. , Liu, Z. G. , Lv, B. , Zhang, Y. Y. , Liu, J. , & Zhao, X. Q. (2010). Spoligotypes of Mycobacterium tuberculosis from different provinces of China. Journal of Clinical Microbiology, 48, 4102–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldholm, V. , Matee, M. , Mfinanga, S. G. , Heun, M. , & Dahle, U. R. (2006). A first insight into the genetic diversity of Mycobacterium tuberculosis in Dar es Salaam, Tanzania, assessed by spoligotyping. BMC Microbiology, 6, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand, S. , Sola, C. , Chanteau, S. , & Sola, C. (2005). A study of spoligotyping‐defined Mycobacterium tuberculosis clades in relation to the origin of peopling and the demographic history in Madagascar. Infection, Genetics and Evolution, 5(4), 340–348. [DOI] [PubMed] [Google Scholar]
- Gandhi, N. R. , Brust, J. C. M. , Moodley, P. , Weissman, D. , Heo, M. , Ning, Y. , … Shah, N. S. (2014). Minimal diversity of drug‐resistant Mycobacterium tuberculosis strains, South Africa. Emerg. Infect. Diseases., 20(3), 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githui, W. A. , Jordaan, A. M. , Juma, E. S. , Kinyanjui, P. , Karimi, F. G. , Kimwomi, J. , … Victor, T. C. (2004). Identification of MDR‐TB Beijing/W and other Mycobacterium tuberculosis genotypes in Nairobi, Kenya. The International Journal of Tuberculosis and Lung Disease, 8(3), 352–360. [PubMed] [Google Scholar]
- Helal, Z. H. , Ashour, M. S. , Eissa, S. A. , Abd‐Elatef, G. , Zozio, T. , Babapoor, S. , … Khan, M. I. (2009). Unexpectedly high proportion of ancestral Manu genotype Mycobacterium tuberculosis strains cultured from tuberculosis patients in Egypt. Journal of Clinical Microbiology, 47, 2794–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove, P. , Molepo, J. , Dube, S. , & Nchabeleng, M. (2012). Genotypic diversity of Mycobacterium tuberculosis in Pretoria. Southern African Journal of Epidemiology and Infection, 27(2), 77–83. [Google Scholar]
- Hunter, P. R. , & Gaston, M. A. (1998). Numerical index of the discriminatory ability of typing systems: An application of Simpson's index of diversity. Journal of Clinical Microbiology, 26(11), 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. , Warren, R. M. , van der Spuy, G. D. , Gey van Pittius, N. C. , Theron, D. , Streicher, E. M. , … Victor, C. (2010). Drug‐resistant tuberculosis epidemic in the Western Cape driven by a virulent Beijing genotype strain. The International Journal of Tuberculosis and Lung Disease, 14, 119–121. [PubMed] [Google Scholar]
- Kamerbeek, J. L. , Schouls, A. , van Kolk, M. , van Agterveld, D. , Soolingen, S. , Kuijper, A. , … van Embden, J. (1997). Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. Journal of Clinical Microbiology, 35, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyatiya, C. L. F. , Muchenje, V. , & Mushunje, A. (2014). Farmers’ perceptions and knowledge of cattle adaptation to heat stress and tick resistance in the Eastern Cape, South Africa. Asian‐Australasian Journal of Animal Sciences, 27, 1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. J. , Bai, G. H. , Lee, H. , Kim, H. J. , Lew, W. J. , & Park, Y. K. (2001). Transmission of Mycobacterium tuberculosis among high school students in Korea. The International Journal of Tuberculosis and Lung Disease, 5, 824–830. [PubMed] [Google Scholar]
- Lillebaek, T. , Andersen, A. B. , Dirksen, A. , Glynn, J. R. , & Kremer, K. (2003). Mycobacterium tuberculosis Beijing genotype. Emerging Infectious Diseases, 9, 1553–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan, K. , Hajib, N. , Therese, L. , Gunisha, P. , Jayanthi, U. , & Biswas, J. (2000). Polymerase Chain Reaction for Detection of Mycobacterium tuberculosis in Epiretinal Membrane in Eales’ Disease. Investigative Ophthalmology & Visual Science, 41, 822–825. [PubMed] [Google Scholar]
- Marais, B. J. , Mlambo, C. K. , Rastogi, N. , Zozio, T. , Duse, A. G. , Victor, T. C. , … Warren, R. M. (2013). Epidemic spread of multidrug‐resistant tuberculosis in Johannesburg, South Africa. Journal of Clinical Microbiology, 51(6), 1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, M. C. , Giampaglia, C. M. , Oliveira, R. S. , Simonsen, V. , Latrilha, F. O. , Moniz, L. L. , … Rastogi, N. (2013). Population structure and circulating genotypes of drug‐sensitive and drug‐resistant Mycobacterium tuberculosis clinical isolates in São Paulo state, Brazil. Infection, Genetics and Evolution, 14, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathema, B. , Kurepina, N. E. , Bifani, P. J. , & Kreiswirth, B. N. (2006). Molecular epidemiology of tuberculosis: Current insights. Clinical Microbiology Reviews, 19(4), 658–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlambo, C. K. , Warren, R. M. , Poswa, X. , Victor, T. C. , Duse, A. G. , & Marais, E. (2008). Genotypic diversity of extensively drug‐resistant tuberculosis (XDR‐TB) in South Africa. The International Journal of Tuberculosis and Lung Disease, 12, 99–104. [PubMed] [Google Scholar]
- Mokrousov, I. , Narvskaya, O. , Limeschenko, E. , Vyazovaya, A. , Otten, T. , & Vyshnevsiy, B. (2004). Analysis of the allelic diversity of the mycobacterial interspersed repetitive units in Mycobacterium tuberculosis strains of the Beijing family: Practical implications and evolutionary considerations. Journal of Clinical Microbiology, 42, 2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, A . (2001). Genotype MTBDRplus assay and INNO‐LiPA Rif.TB assay. Available from: http://www.tbonline.info/posts/2011/10/15/genotype-mtbdrplus-assay-and-inno-lipa-riftb-assay/
- Namouchi, A. , Karboul, A. , Mhenni, B. , Khabouchi, N. , Haltiti, R. , & Ben Hassine, R. (2008). Genetic profiling of Mycobacterium tuberculosis in Tunisia: Predominance and evidence for the establishment of a few genotypes. Journal of Medical Microbiology, 57, 864–872. [DOI] [PubMed] [Google Scholar]
- NDH . (2011). Multi‐drug resistant tuberculosis: A policy framework on decentralized and institutionalized management for South Africa, National Department of Health.
- Niemann, S. , Köser, C. U. , Gagneux, S. , Plinke, C. , Homolka, S. , Bignell, H. , … Rigatti, R. (2009). Genomic diversity among drug sensitive and multidrug resistant isolates of Mycobacterium tuberculosis with identical DNA fingerprints. Plosone., 4, e7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y. K. , Bai, G. H. , & Kim, S. J. (2000). Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from countries in the Western Pacific region. Journal of Clinical Microbiology, 38, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwati, I. , van Crevel, R. , & van Soolingen, D. (2010). Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. The Lancet Infectious Diseases, 10, 103–111. [DOI] [PubMed] [Google Scholar]
- Pillay, M. , & Sturm, A. W. (2007). Evolution of extensively drug‐resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu‐Natal, South Africa. Clinical Infectious Diseases, 45, 1409–1414. [DOI] [PubMed] [Google Scholar]
- Pourcel, C. , Salvignol, G. , & Vergnaud, G. (2005). CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology, 15, 653–663. [DOI] [PubMed] [Google Scholar]
- Said, H. M. , Kock, M. M. , Ismail, N. A. , Mphahlele, M. , Baba, K. , Omar, S. V. , … Ehlers, M. M. (2012). Molecular characterization and second‐line anti‐TB drug‐resistance patterns of multidrug resistant tuberculosis isolates from the northern region of South Africa. Journal of Clinical Microbiology, 50(9), 2857–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban, M. , Mokrousov, I. , Rastogi, N. , & Sola, C. (2002). A data‐mining approach to spacer oligonucleotide typing of Mycobacterium tuberculosis . Bioinformatics, 18, 235–243. [DOI] [PubMed] [Google Scholar]
- Sola, C. , Filliol, I. , Legrand, E. , Lesjean, S. , Locht, C. , Supply, P. , & Rastogi, N. (2003). Genotyping of the Mycobacterium tuberculosis complex using MIRUs: Association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infection, Genetics and Evolution, 3, 125–133. [DOI] [PubMed] [Google Scholar]
- Streicher, E. M. , Warren, R. M. , Kewley, C. , Simpson, J. , Rastogi, N. , der Sola, C. , … Victor, T. C. (2004). Genotypic and phenotypic characterization of drug‐resistant Mycobacterium tuberculosis isolates from rural districts of the Western Cape Province of South Africa. Journal of Clinical Microbiology, 42, 891–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup, S. , Pholwat, S. , Heysell, S. K. , Khan, E. , Ferdous, S. S. , & Ahmed, S. (2014). Discordance across several methods for drug susceptibility testing of drug‐resistant Mycobacterium tuberculosis isolates in a single laboratory. Journal of Clinical Microbiology, 52(1), 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply, P. , Allix, C. , Lesjean, S. , Cardoso‐Oelemann, M. , Rüsch‐Gerdes, S. , Willery, E. , … van Soolingen, D. (2006). Proposal for standardization of optimized mycobacterial interspersed repetitive unit variable‐number tandem repeat typing of Mycobacterium tuberculosis . Journal of Clinical Microbiology, 44, 4498–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply, P. , Magdalena, J. , Himpens, S. , & Locht, C. (1997). Identification of novel intergenic repetitive units in a mycobacterial two‐component system operon. Molecular Microbiology, 26, 991–1003. [DOI] [PubMed] [Google Scholar]
- van Soolingen, D. (2001). Molecular epidemiology of tuberculosis and other mycobacterial infections: Main methodologies and achievements. Journal of Internal Medicine, 249, 1–26. [DOI] [PubMed] [Google Scholar]
- van Soolingen, D. , Borgdorff, M. W. , de Haas, P. E. , Sebek, M. M. , Veen, J. , Dessens, M. , … van Embden, J. D. (1999). Molecular epidemiology of tuberculosis in the Netherlands: A nationwide study from 1993 through 1997. Journal of Infectious Diseases, 180, 726–736. [DOI] [PubMed] [Google Scholar]
- Viegas, S. O. , Machado, A. , Groenheit, R. , Ghebremichael, S. , Pennhag, A. , Guso, P. S. , … Koivula, T. (2010). Molecular diversity of Mycobacterium tuberculosis isolates from patients with pulmonary tuberculosis in Mozambique. BMC Microbiology, 10, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne, B. , Crauste, C. , Flipo, M. , Baulard, A. R. , Déprez, B. , & Willand, N. (2012). Tuberculosis: The drug development pipeline at a glance. European Journal of Medicinal Chemistry, 51, 1–16. [DOI] [PubMed] [Google Scholar]
- Wada, T. , Iwamoto, T. , & Maeda, S. (2009). Genetic diversity of the Mycobacterium tuberculosis Beijing family in East Asia revealed through refined population structure analysis. FEMS Microbiology Letters, 291, 35–43. [DOI] [PubMed] [Google Scholar]
- Warren, R. , Hauman, J. , Beyers, N. , Richardson, M. , Schaaf, H. S. , Donald, P. , & van Helden, P. (1996). Unexpectedly high strain diversity of Mycobacterium tuberculosis in a high incidence community. South African Medical Journal= Suid‐Afrikaanse Tydskrif vir Geneeskunde, 86(1), 45–49. [PubMed] [Google Scholar]
- Weniger, T. , Krawczyk, J. , Supply, P. , Niemann, S. , & Harmsen, D . (2010). MIRU‐VNTRplus: A web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Research, 38(web server issue), W326–W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2009). Guidelines for surveillance of drug resistance in tuberculosis, 4th ed. Document WHO/HTM/TB/, 2009, 422. [Google Scholar]
- WHO . (2010). Multidrug and extensively drug‐resistant TB (M/XDR‐TB) Global report on surveillance and response, Available from: http://whqlibdoc.who.int/publications/2010/9789241599191_ eng. pdf
- WHO . (2012). Implementation and rollout of Xpert MTB/RIF, Geneva, Switzerland. Available from http://www.who.int/tb/laboratory/mtbrifrollout/en/
- WHO . (2014). Global Tuberculosis Report, ISBN 978 92 4 156480 992:4 156438 0. WHO/HTM/TB/2014.08
- WHO/IUATLD . 2008. Global Project on Anti‐Tuberculosis Drug Resistance Surveillance. Anti‐Tuberculosis Drug Resistance in the World: Fourth Global Report. Geneva, Switzerland: Report no. WHO/HTM/TB/2008.394.
- Yates, M. , Drobniewski, F. , & Wilson, S. (2002). Evaluation of a rapid PCR‐based epidemiological typing for routine studies of Mycobacterium tuberculosis . Journal of Clinical Microbiology, 40, 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Prakash, C. S. , & He, G. (2012). Characterization and compilation of polymorphic simple sequence repeat (SSR) markers of peanut from public database. BMC Research Notes, 5, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozio, T. , Allix, C. , Gunal, S. , Saribas, Z. , Alp, A. , Durmaz, R. , … Sola, C. (2005). Genotyping of Mycobacterium tuberculosis clinical isolates in two cities of Turkey: Description of a new family of genotypes that is phylogeographically specific for Asia Minor. BMC Microbiology, 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]


