Abstract
Objectives:
This study was performed to determine the prevalence of uropathogens causing urinary tract infections (UTIs) and to determine their pattern of antimicrobial resistance.
Methods:
This study was conducted on 273 urine samples collected from outpatient departments (OPDs) of Qassim University affiliated hospitals. Fully automated VITEK 2 compact system was used in the identification and antimicrobial susceptibility testing of causative microorganisms.
Results:
Of 273 urine samples, only 89 (32.6%) were found to show significant growth for UTI, and overall, drug resistance was found in 92% (n = 82/89) of samples, with most (80%) being resistant to at least two drugs. Antibiotic resistance was commonly observed in ampicillin (88.3%), piperacillin (72.7%), clindamycin (66.7%), amoxicillin/clavulanic acid (66.2%), and trimethoprim/sulfamethoxazole (50%). The commonly isolated microorganisms were Escherichia coli 24 (27%), Klebsiella pneumoniae 11 (12.4%), Proteus mirabilis 4 (4.5%), Pseudomonas aeruginosa 4 (4.5%), Enterobacter cloacae 5 (5.6%), Enterococcus faecalis 5 (5.6%), and Staphylococcus saprophyticus 3 (3.4%).
Conclusions:
This research work has shown that patients with UTI in Qassim are at high risk of antibiotic resistance. The work also showed that multidrug-resistant bacteria can lead to momentous therapeutic problems in OPD patients.
Keywords: Antimicrobial resistance, Escherichia coli, Klebsiella, multidrug resistance, Pseudomonas aeruginosa, qassim, uropathogens, VITEK system
Introduction
Urinary tract infection (UTI) is a common health problem in both community and nosocomial settings. UTI is among one of the most common infections occurring particularly in women. As reported by the National Ambulatory Medical Care Survey, UTI alone is responsible for nearly seven million patient visits in outpatient department (OPD) as well as up to one million visits in hospital emergency department, resulting in about 100,000 hospitalizations.[1] Nearly 50–60% of all women suffer from an episode of UTI at least once in their lifetime.[2] If the predisposing factors which are responsible for the occurrence of UTI are not timely diagnosed and treated, then it is also common for UTI episodes to reoccur.[3] Untreated UTI can result in serious complications such as kidney damage, renal scarring, and renal failure. UTI is commonly caused by bacteria mostly by Gram-negative bacteria such as Escherichia coli, Proteus species, Pseudomonas aeruginosa, Acinetobacter species, Klebsiella species, Enterobacter species, and Citrobacter species. Among Gram-positive bacteria, Staphylococcus saprophyticus, Enterococcus species, and Coagulase-negative Staphylococcus are common predictable spectrum of bacteria which are responsible for causing UTIs.[4,5]
As compared to non-pathogenic bacteria, the bacteria which are responsible for causing UTIs have more aggressive virulence factors which enhance their host cell attachment, colonization as well as invasion abilities. These bacteria avoid evasion of the immune system of host by the help of certain virulence factors which may be comprised of various cellular components such as pili, capsule, lipopolysaccharides, and various other cell surface structures.[4] Certain human anatomical as well as physiological factors are also responsible for increasing the incidence of UTI, for example, length of the urethra is shorter in females as compared to males which leads to an increased chance of acquiring UTI. Similarly, incomplete emptying of bladder particularly in old age results in accumulation of residual urine remaining in the bladder and vesicoureteral reflux which frequently occurs among pregnant women which is the vital factors that can predispose host to UTIs.[6]
UTI is treated often by broad-spectrum antibiotics, and treatment is started empirically without performing culture and sensitivity. This inappropriate and non-judicious usage of antibiotics has resulted in the development of worldwide antibiotic resistance in bacteria, leading to the emergence of multiresistant strains of bacterial pathogens.[7] According to a survey conducted by the European Survey of Antibiotic Consumption, multidrug-resistant (MDR) bacterial strains were accountable for a mortality rate nearly about 25,000 Europeans/year usually due to complications of UTIs.[8] Hence, it is necessary to circumvent non-judicious use of antibiotics that lead to the emergence of antimicrobial resistance and most appropriate antibiotics should be opted for first-choice empiric treatment of UTI. The antimicrobial susceptibility pattern among bacteria varies from country to country.[9] The Infectious Diseases Society of America recommends that regional surveillance should be conducted to monitor changes in susceptibility of uropathogens in specific regions.[10]
The main aim of this study was to determine the prevalence of UTI causing pathogens according to age and sex at a medical college hospital among OPD and their antibiotic susceptibility pattern to provide a database for reference. Keeping this study in view, insufficient research work has been published on this particular topic.
In the current scenario, where the antimicrobial resistance pattern is changing very alarmingly and new MDR bacteria are emerging frequently leading to enhance morbidity and mortality. This study focuses on highlighting the guidelines for the usage of appropriate antibiotics which can be used in treating UTI. In addition, our study will assist concerned authorities in preparing antibiotic prescription policies and evaluating their antibiotic formulary guidelines. Increase awareness and annual reporting for these findings will help in preventing the immerged strains from spread within the community.
Methods
Study design and setting
This is a cross-sectional study, and the retrospective analysis was carried out at the College of Medicine OPD in Buraydah, Qassim area, Saudi Arabia. We included all urine sample results submitted for urine culture and sensitivity in this study. We enrolled a total of 273 urine samples. The identification and susceptibility of causative microorganisms were performed by VITEK 2 compact system. Ethical approval was taken from the ethical committee of the Ministry of Health, KSA (Ethical approval number #20180314).
Measurements
Colony count method is a technique by which the number of viable bacterial colonies can be counted numerically in milliliter of urine sample; it is a quantitative measurement by which we can differentiate true bacteriuria from bacterial contamination that often occurs during improper collection of mid-stream or “clean-catch” urine.[11] UTI occurs when there is a presence of >105 colony-forming units (CFUs)/ml of mid-stream urine, which is diagnostic. UTI was defined as urine culture plates growing bacterial colonies of ≥105 CFU/mL in a mid-stream urine sample.[11,12]
Collection and process of urine samples
Mid-stream urine samples were collected in a sterile container and were processed within 2 h of collection time. Urine sample was inoculated on a standard culture media Cystine–Lactose–Electrolyte-Deficient (CLED) agar, using a calibrated (1 μL) loop. Culture plates were incubated at 35–37°C ambient air incubator for 18 h. After the allocated time period, the culture plates were visualized for the presence of bacterial colonies. They were reported as significant or non-significant growth on the basis of colony count method. These urine samples were also centrifuged, and urine sediment was used for direct microscopic examination of red blood cells (RBCs), leukocytes, epithelial cell, casts, crystals, and parasites. In the normal urine sediment, a few count of RBCs, pus cells (0–5/high power field), and epithelial cells may present. Epithelial cell count reported as “few,” “moderate,” or “many” per low-power field.
Bacterial identification and susceptibility testing
The identification and antimicrobial susceptibility of bacteria isolated from urine samples were performed by VITEK 2 Compact System. Initially, the urine samples are cultured on CLED agar and were incubated at ambient temperature 35–37°C in 5% CO2, after 18 h of incubation; the bacterial growth was preliminary identified by their colony morphology as well as gram staining. The culture plates showing growth of significant bacterial colonies were used to formulate a standardized saline inoculum recommended for VITEK identification. For the identification of bacteria through VITEK, we used special ID: Gram-negative ID card (GN Reference 21 341) and Gram-positive ID card (GP Reference 21 342).
The antimicrobial susceptibility tests (AST) and the minimum inhibitory concentrations were determined by special sensitivity (AST) cards. Clinical and Laboratory Standards Institute criteria were used for the interpretation of AST results as per manufacturer’s instructions (BioMérieux, France) and the Advanced Expert System.
Isolated Gram-negative uropathogens were tested against different AST-N panels including ampicillin, cefepime, norfloxacin, cefoxitin, amoxicillin/clavulanic acid, piperacillin, piperacillin/tazobactam, cefalotin, amikacin, tobramycin, cefuroxime, cefuroxime, cefpodoxime, cefotaxime, meropenem, ceftazidime, nitrofurantoin, trimethoprim/sulfamethoxazole, ciprofloxacin, and gentamicin [Figure 1].
Figure 1.
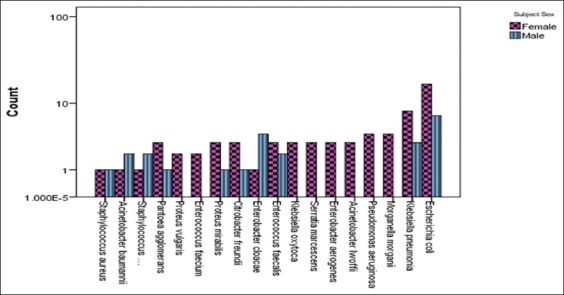
Frequency distribution of isolated microorganisms from patients with catheter-associated urinary tract infection
Whereas, Gram-positive uropathogens were tested against AST-P which include benzylpenicillin, erythromycin, tetracycline, oxacillin, moxifloxacin, trimethoprim/sulfamethoxazole, cefalotin screen, gentamicin, tobramycin, levofloxacin, clindamycin, inducible clindamycin resistance, linezolid, teicoplanin, vancomycin, fosfomycin, and nitrofurantoin [Figure 1].
Data collection and statistical analysis
A structured questionnaire was designed to collect the demographic and clinical data of the patients. On arrival of a patient in the OPD, gender, age, medical history as well as vital signs were noted. We performed descriptive statistical methods for the data analysis. The antimicrobial resistance prevalence was calculated as the proportion of positive results over the entire study sample. Those bacteria which showed resistance to two antimicrobial agents of different class of antibiotics were termed as MDR.
Data analysis was performed using SPSS software. Statistical tests of significance were performed using the Student’s t-test and Chi-square (χ2) test, and variables were compared using cross-tabulation statistical methods. P < 0.05 was considered statistically significant.
Results
A total of 273 urine samples have been received in the Microbiology Laboratory, College of Medicine OPD in Qassim University, Saudi Arabia. Only 89 (32.6%) urine samples were showing significant growth for UTI, among whom, the females were 65 (73%) episodes. The average age (±SD) was 35.82±15.3 years. Frequency of gender distribution of UTI cases admitted to OPD of Qassim University medical clinics, Buraydah, Qassim, Saudi Arabia are summarized in Table 1.
Table 1.
Frequency of gender distribution of UTI cases admitted to OPD of Qassim University medical clinics, Buraydah, Qassim, Saudi Arabia, 2014

The most common Gram-negative urinary pathogens isolated were E. coli, Klebsiella pneumoniae, Proteus mirabilis, P. aeruginosa, Enterobacter cloacae, Enterobacter aerogenes, and Morganella morganii [Table 2]. The frequency of Acinetobacter, Citrobacter, and Pantoea agglomerans is mentioned in Table 4 due to their clinical relevance.
Table 2.
Number (%) of common Gram-negative urinary pathogens resistant (R) to antimicrobial agents
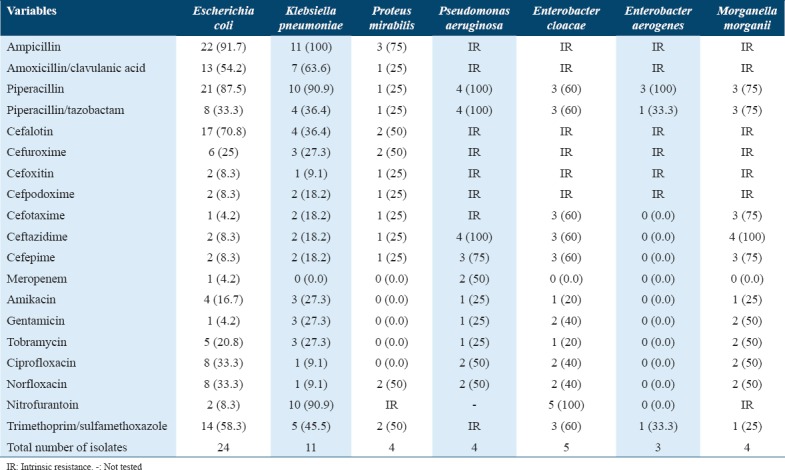
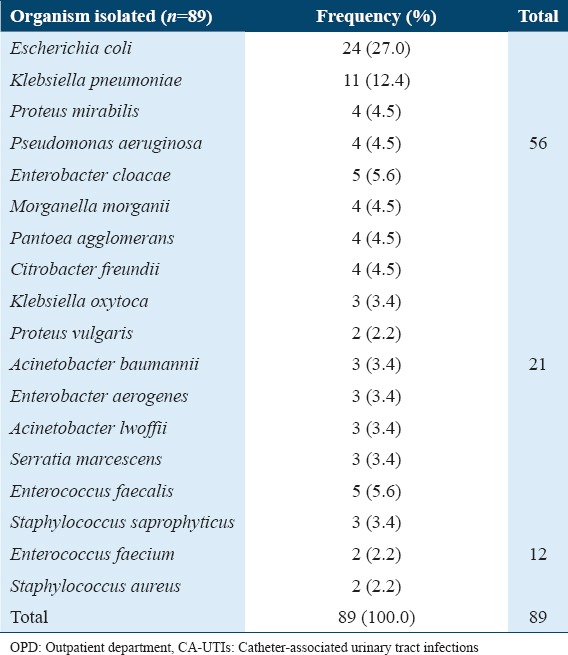
Table 4.
Frequency distribution of microorganisms isolated from patients with CA-UTIs admitted to OPD of Qassim University medical clinics, Buraydah, Qassim, Saudi Arabia, 2014
The number (percentage) of common Gram-negative urinary pathogens resistant (R) to antimicrobial agents is shown in Table 2. The common urinary pathogens such as E. coli, K. pneumoniae, and P. mirabilis showed high resistance when they were tested against amoxicillin/clavulanic acid (66.2) and piperacillin (72.7). In comparison, low resistance rates were found against cefoxitin, cefpodoxime, ceftazidime, meropenem, and amikacin [Table 2]. E. coli showed a relatively low rate of resistance to nitrofurantoin (8.3% of the isolates) when compared with K. pneumoniae isolates (90.9%), and only 4.2% of E. coli isolates were resistant to gentamicin. In contrast, K. pneumoniae showed an 27% resistance rates.
Multiple resistances
Antimicrobial resistance was seen both in Gram-positive and Gram-negative bacteria. Multiple resistances were high among the isolated urinary pathogens. Particularly, E. coli had a <50% resistance rate to at least five of 20 antimicrobial agents [Table 3], while K. pneumoniae and P. mirabilis had <50% and <30% resistance rates, respectively.
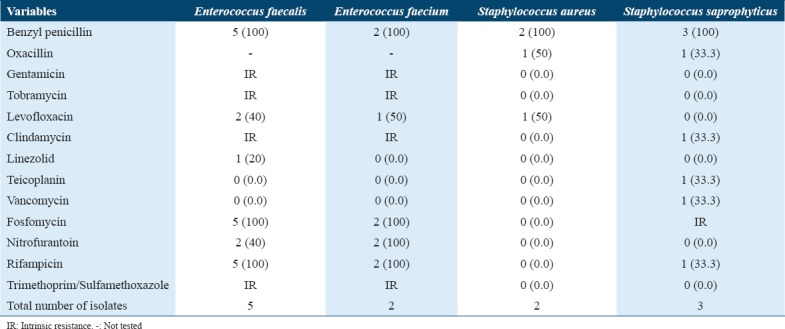
Table 3.
Number (%) of common Gram-positive urinary pathogens resistant (R) to antimicrobial agents*
Discussion
UTI is listed among one of the most common infections caused by various Gram-positive and Gram-negative bacteria.[13] To successfully treat the patients who are suffering from UTI, it is crucial to accurately identify the causative pathogen. Failure to do so will not only prolong the disease and will render the patient to complications but will also promote negative consequences of bacterial resistance due to a non-judicious use of inappropriate antibiotics. It is onerous to precisely measure the incidence and prevalence of UTIs because it is not a reportable disease within Saudi Arabia. In addition, the diagnosis of UTI is dependent on both the presence of clinical signs and symptoms along with a positive urine culture; however, in majority of health-care settings on the scene, this diagnosis is accomplished and treatment is started without performing the culture and antimicrobial sensitivity.
The emergence of antimicrobial resistance is a global problem as it affects people all over the globe. Based on the results of the urinary samples at the College of Medicine OPD in Buraidah, Qassim area, Saudi Arabia, we have shown that approximately 92% (n = 82/89) of samples were resistant to antibiotic drugs and ~80% (n = 72) were MDR.
In our research study, the ratio of female patients with UTI was more than the males. This was inconsistent with the study by Kattel et al.[14] Higher prevalence of UTI among females is due to various factors that predispose women to UTI.[15] The most prevalent urinary tract pathogen in both the genders in our study was E. coli and K. pneumoniae in concordance with other studies from Saudi Arabia conducted by Ahmed et al. and Al-Tawfiq and Anani.[16,17]
In comparison to previous studies related to UTI since 1995, the prevalence rate of UTIs and the antibiotic resistance pattern in the Qassim area have enhanced over the past 20 years.[18] For example, in our study, the prevalence rate of UTI by urinary pathogens is concluded to be 32%, and these results are slightly higher than a study by Ahmad S, 1995 conducted in King Fahad Specialist Hospital in Buraidah, Qassim area, Saudi Arabia, who concluded a prevalence of 20.54%.[18] Furthermore, we found that the distribution of the common pathogens was slightly different from previous studies in the same area. As shown in Table 4, the common isolated pathogens were E. coli which was the most common organism (50.11%), followed by Klebsiella spp. (28.33%), Pseudomonas spp. (7.84%), and Proteus spp. (4.91%). Other bacterial pathogens were Enterococcus spp. (3.98%), Acinetobacter spp. (1.84%), Staphylococcus aureus (1.63%), and Enterobacter spp. (0.35). However, in our study, the most common pathogen was E. coli (26.9 %), K. pneumoniae (12.4%), E. cloacae (5.6%), P. mirabilis (4.5%), P. aeruginosa (4.5%), E. aerogenes (3.3%), M. morganii (4.5%), Citrobacter freundii (4.5%), Pantoea agglomerans (4.5%), Enterococcus faecalis (5.6%), S. saprophyticus (3.3%), and Staphylococcus aureus (2.2%). These data pointed out that there were decreased in the prevalence of E. coli and increased of Enterococcus sp.
An alarming finding from this study showed a high degree of drug resistance among pathogens. Our study showed a very high rate of resistance (>70%) among E. coli isolates to piperacillin. Among Klebsiella isolates, no resistance was found for meropenem and low resistance was found for ciprofloxacin (9.1), norfloxacin (9.1), and cefotaxime (9.1) but high for nitrofurantoin and trimethoprim/sulfamethoxazole. Resistance to piperacillin, piperacillin/tazobactam, and ceftazidime among P. aeruginosa, E. cloacae, and M. morganii goes in similar pattern consistently over 75%.[19] This resistance is most likely due to the massive use of third-generation cephalosporins and fluoroquinolone antibiotics in UTIs patients. Therefore, they were increasingly recognized as important causes of UTIs and our study findings highlight the significance of this species as a leading cause of MDR infection in patients with UTIs.
The etiology of bacteria causing UTI as well as their susceptibility to antimicrobials continue to vary over time period and it is different among different countries.[10,20,21] Sensitivity to cotrimoxazole is a crucial feature in choosing the empirical treatment for UTIs, as recommended by the European Urology Association Guidelines,[22,23] which suggest cotrimoxazole as the first-line antibiotic choice for empirical therapy in uncomplicated community-acquired UTIs when the local rates of trimethoprim/sulfamethoxazole resistance in uropathogens are <10–20%. However, our study revealed an overall resistance of trimethoprim/sulfamethoxazole of 49.4% and E. coli-specific resistance of 58.3%. Similar results were obtained in a recent study of this nature conducted in Sultanate Oman by Sharef et al.,[24] which revealed an overall resistance of 47% and E. coli specific resistance of 50%, to this antibiotic. This is in contrast to various studies conducted in European countries which revealed low resistance ranging from 28% to 30%[25,26] and other African countries where resistance is comparatively high ranging from 88.3% to 98.6% [27,28] The high resistance in trimethoprim/sulfamethoxazole susceptibility pattern may be due to non-judicious use and over-the-counter selling of this antibiotic; however, recently, steps have been taken by the Ministry of Health, KSA, to restrict over-the-counter selling of antibiotics without prescription of doctor; implantation of this rule will have a significant impact in controlling antibiotic resistance.
Only few researches have been conducted to reveal the latest trends in etiological agents causing UTI among outpatients and community. In contrast, significant changes in the causes of hospital-acquired UTIs have been reported since 1980.[29] The laboratory tests, especially urine culture and sensitivity, are mandatory to establish an accurate diagnosis and antibiotic susceptibility pattern of pathogens causing UTI.
As part of infection control, we use the VITEK System to detect and report uropathogenic etiology and to detect the antibiotic susceptibility to limit the therapeutic failures that may inherently be caused by the conventional methods used. The phenotyping techniques which have been used to identify uropathogenic bacteria and to confirm the antimicrobial resistance data profile find concurrently agreements to the previously used molecular genotyping methods.
We use a fully automated machine. The use of the VITEK 2 system enables the identity of broad spectrum of bacteria and their susceptibility up to 20 different antimicrobials.
In the current study, the urinary pathogens of a relatively low prevalence or newly emerged uropathogens are described in Table 5. These included Citrobacter freundii, Klebsiella oxytoca, Proteus vulgaris, Acinetobacter baumannii, Acinetobacter lwoffii, Serratia marcescens, and Pantoea agglomerans. Information on Acinetobacter, Citrobacter, and Pantoea agglomerans are included in Table 5 die to their clinical importance. Mostly, Acinetobacter species are acquired from nosocomial infections, particularly, in patients who are catheterized or having debilitating disease, but, in our research, we isolated it from it in community-acquired UTI.
Table 5.
Number (%) of less common Gram-negative urinary pathogens resistant (R) to antimicrobial agents*
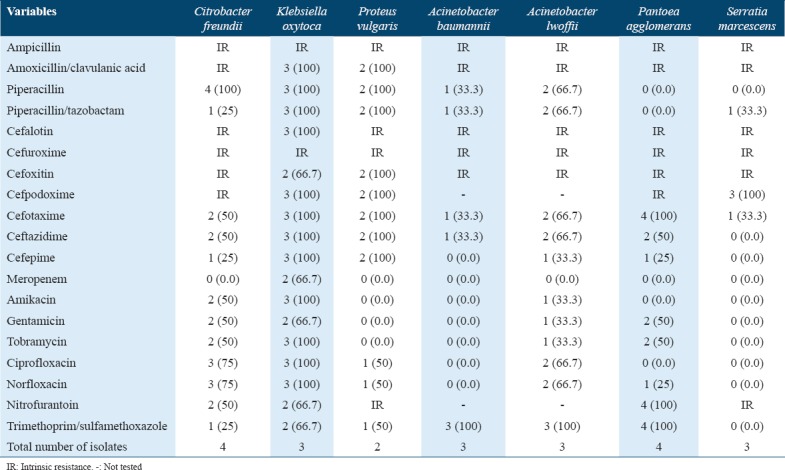
The antibiotic susceptibility of uropathogenic bacteria is known to change with time and is inconsistent in different regions.[20] Here, we have described the impact of the best antimicrobials with low resistance rate (overall resistance %) against the uropathogens in this study. The best antimicrobials for Gram-negative organisms (n = 77) were meropenem (6.5%), amikacin (20.8%), gentamicin (20.8%), tobramycin (27.3%), and cefepime (28.6%) and moderate resistance rate were ciprofloxacin (31.2%), cefotaxime (32.5%), cefoxitin (35.1%), norfloxacin (35.1%), ceftazidime (36.4%), cefpodoxime (41.6%), piperacillin/tazobactam (44.2%), and cefuroxime (46.8%); however, the high resistance rate was found to be against cefuroxime axetil (49.4%), trimethoprim/sulfamethoxazole (49.4%), nitrofurantoin (50.6%), amoxicillin/clavulanic acid (66.2%), piperacillin (72.7%), and ampicillin (88.3%).
In contrast, the sensitivity pattern of antimicrobials for Gram-positive organisms (n = 12) was linezolid (8.3%), teicoplanin (8.3%), vancomycin (8.3%), cefalotin screen (16.7%), moxifloxacin (25%), nitrofurantoin (33.3%), and levofloxacin (33.3%); however, the high resistance rate was found to be against erythromycin (50%), trimethoprim/sulfamethoxazole (50%), gentamicin (58.3%), tobramycin (58.3%), fosfomycin (58.3%), clindamycin (66.7%), oxacillin (75%), tetracycline (75%)m and benzylpenicillin (100%) resistance rate. Among our Gram-positive isolated organisms, there was no inducible clindamycin resistance (0.0%).
This research work will have an important impact in the treatment and management of patients with catheter-associated (CA)-UTI, particularly those patients who are infected with MDR uropathogens. It should be noted that MDR is gradually increasing throughout the world and it is an alarming sign as with time we are losing our therapeutic options for treating simple infections caused by bacteria. Efforts should be done to make clinicians realize the fact that there is a high possibility of multidrug resistance. Second, the occurrence of MDR which we observed in this study is a serious threat in the management of patients with CA-UTI. It highlights the relevance of a more serious systematic approach to decrease the antibiotic resistance rates. In this particular era, when antibiotic resistance is growing with an alarming rate, more research work is highly needed on a priority basis for the development of rapid diagnostic test (point of care testing) for prompt targeted therapy. The execution of a drug monitoring system that augments drug administration and assists a more personalized methodology to recommended treatment is also needed.
Moreover, education programs should be conducted to reduce the prevalence of disease in the community as well as enlighten the quality of life for patients living in low- and middle-income regions. In the near future, we aim to implement and weigh the efficiency of such programming in improving health literacy as well as decreasing infection rates.
Conclusions
This research work has shown that patients who presented with UTI in Qassim are at high risk of antibiotic resistance. The research also has shown that MDR bacteria can lead to momentous therapeutic problems in OPD patients.
Declarations
Ethics approval and consent to participate
Taken.
Availability of data and materials
All the data which are supporting our research findings are present in this work.
Competing Interests
Authors mention that they do not have any competing interests in this research.
Funding
There has been no funding for this research study.
References
- 1.Foxman B. Epidemiology of urinary tract infections:Incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. ACOG practice bulletin no 91:Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785–94. doi: 10.1097/AOG.0b013e318169f6ef. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen HT. Smith's General Urology. 16th ed. New York (USA): Mcgraw-Hill Medical; 2004. Bacterial Infections of the Genitourinary Tract; p. 220. [Google Scholar]
- 5.Momoh A, Orhue P, Idonije O, Oaikhena A, Nwoke E, Momoh A. The antibiogram types of Escherichia coli isolated from suspected urinary tract infection samples. J Microbiol Biotech Res. 2011;1:57–65. [Google Scholar]
- 6.Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008;38(Suppl 2):50–7. doi: 10.1111/j.1365-2362.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 7.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuiston Haslund J, Rosborg Dinesen M, Sternhagen Nielsen AB, Llor C, Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in europe. Scand J Prim Health Care. 2013;31:235–40. doi: 10.3109/02813432.2013.844410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance:A cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 10.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious diseases society of America (IDSA) Clin Infect Dis. 1999;29:745–58. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 11.Hamdan HZ, Ziad AH, Ali SK, Adam I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum north hospital. Ann Clin Microbiol Antimicrob. 2011;10:2. doi: 10.1186/1476-0711-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdan HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO, Adam I, et al. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14:26. doi: 10.1186/s12941-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–60. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 14.Kattel HP, Acharya J, Mishra SK, Rijal BP, Pokhrel BM. Bacteriology of urinary tract infection among patients attending Tribhuvan university teaching hospital Kathmandu, Nepal. J Nepal Assoc Med Lab Sci. 2008;25:29. [Google Scholar]
- 15.August SL, De Rosa MJ. Evaluation of the prevalence of urinary tract infection in rural panamanian women. PLoS One. 2012;7:e47752. doi: 10.1371/journal.pone.0047752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad S, Al-Juaid NF, Alenzi FQ, Mattar EH, Bakheet Oel-S. Prevalence, antibiotic susceptibility pattern and production of extended-spectrum beta-lactamases amongst clinical isolates of Klebsiella pneumoniae at armed forces hospital in Saudi Arabia. J Coll Physicians Surg Pak. 2009;19:264–5. [PubMed] [Google Scholar]
- 17.Al-Tawfiq JA, Anani AA. Antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infections in a Saudi Arabian hospital. Chemotherapy. 2009;55:127–31. doi: 10.1159/000198698. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S, Ahmad F. Urinary tract infection at a specialist hospital in Saudi Arabia. Bangladesh Med Res Counc Bull. 1995;21:95–8. [PubMed] [Google Scholar]
- 19.Gupta K, Stamm WE. Outcomes associated with trimethoprim/sulphamethoxazole (TMP/SMX) therapy in TMP/SMX resistant community-acquired UTI. Int J Antimicrob Agents. 2002;19:554–6. doi: 10.1016/s0924-8579(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 20.Livermore DM, Pearson A. Antibiotic resistance:Location, location, location. Clin Microbiol Infect. 2007;13(Suppl 2):7–16. doi: 10.1111/j.1469-0691.2007.01724.x. [DOI] [PubMed] [Google Scholar]
- 21.Magliano E, Grazioli V, Deflorio L, Leuci AI, Mattina R, Romano P, et al. Gender and age-dependent etiology of community-acquired urinary tract infections. ScientificWorldJournal. 2012;2012:349597. doi: 10.1100/2012/349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidelines on Urological Infections. Netherlands: European Association of Urology; 2014. European Association of Urology. [Google Scholar]
- 23.Grabe M, Bartoletti R, Bjerklund-Johansen T. Guidelines on Urological Infections. Netherlands: European Association of Urology; 2015. [Google Scholar]
- 24.Sharef SW, El-Naggari M, Al-Nabhani D, Al Sawai A, Al Muharrmi Z, Elnour I, et al. Incidence of antibiotics resistance among uropathogens in Omani children presenting with a single episode of urinary tract infection. J Infect Public Health. 2015;8:458–65. doi: 10.1016/j.jiph.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 25.De Francesco MA, Ravizzola G, Peroni L, Negrini R, Manca N. Urinary tract infections in Brescia, Italy:Etiology of uropathogens and antimicrobial resistance of common uropathogens. Med Sci Monit. 2007;13:BR136–44. [PubMed] [Google Scholar]
- 26.Miragliotta G, Di Pierro MN, Miragliotta L, Mosca A. Antimicrobial resistance among uropathogens responsible for community-acquired urinary tract infections in an Italian community. J Chemother. 2008;20:721–7. doi: 10.1179/joc.2008.20.6.721. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim ME, Bilal NE, Hamid ME. Increased multi-drug resistant Escherichia coli from hospitals in Khartoum state, Sudan. Afr Health Sci. 2012;12:368–75. doi: 10.4314/ahs.v12i3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim ME, Bilal NE, Magzoub MA, Hamid ME. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from hospitals in Khartoum state, Sudan. Oman Med J. 2013;28:116–20. doi: 10.5001/omj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronsema DA, Adams JR, Pallares R, Wenzel RP. Secular trends in rates and etiology of nosocomial urinary tract infections at a university hospital. J Urol. 1993;150:414–6. doi: 10.1016/s0022-5347(17)35497-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data which are supporting our research findings are present in this work.


