Abstract
Objective:
Cluster of differentiation (CD) 74, CD44, and macrophage migration inhibitory factor (MIF) are well known for their immunological functions; however, it has been shown that recently, CD74, CD44, and MIF have a role in tumor and tumor progression. This study was undertaken to investigate the expression of CD74, MIF, and CD44 in breast cancer cells.
Materials and Methods:
The expression of CD74, MIF, and CD44 molecules on the breast cancer-derived cell lines CAMA-1, 3,4-methylenedioxyamphetamine (MDA)-MB-231, and MDA-MB-43 was determined by flow cytometry, western immunoblotting, and confocal microscope. To validate the study the studying expression of CD74, MIF, and CD44 on the normal breast cell line 266LDM, whole cell lysate obtained from adult normal breast tissue and normal breast tissue.
Results:
The results show that all breast cancer cells overexpress CD74 isoforms, MIF, and CD44, in contrast to the normal cell lines and normal breast tissues, which express only CD44 and MIF in low levels. The expression of CD74, MIF, and CD44 was studied in the immortalized normal breast luminal cell line 226LDM, normal breast tissues, and lysate to validate the study.
Conclusion:
The data show, in this study, the evidence that breast cancer cell lines expressing three different isoforms of CD74. The results of the present study indicate a crucial role of CD74 in breast cancer cells along with MIF and CD44. The results also suggest that CAMA-1, MDA-MB-231, and MDA-MB-435 cells are poorly immunogenic, expressing low levels of HLA-A, B, and C and HLA-DR.
Keywords: Breast cancer cells, cluster of differentiation 44, cluster of differentiation 74, immunophenotyping, migration inhibitory factor
Introduction
A number of molecules play a key role to play a crucial role in tumor progression. These include cluster of differentiation (CD) 74, macrophage migration inhibitory factor (MIF), and CD44.[1] These molecules have been recognized in several types of cancers and are supposed to be playing a crucial role in immunotolerance.[2] CD74 expression has been observed in several types of cancer including multiple myeloma and in various solid tumor cancers such as gastric cancer, kidney, and small-cell lung cancer.[3-6] CD74, along with CD44, has been suggested to mediate MIF signaling pathway in bladder and prostate cancer cell lines.[1,7] MIF has a critical role in tumorigenesis due to its overexpression by cancer cells; it is widely documented that MIF is overexpressed in breast cancer, prostate cancer, and colon carcinoma tumors.[8] CD44 is able to promote tumor cell invasiveness, self-renewal and sustained survival, and has the ability to prompt many types of cancer.[9,10] Till date, CD74 coexpression with MIF or with CD44 was not fully defined in breast cancer; however, few studies have shown their expression in prostate cancer and also in lung cancer.[7,11] Recently, Richard et al.[12] studied the involvement of MIF and its receptor CD74 in human breast cancer. However, they did not consider the relationship between CD74 and MIF and the expression of CD44. It is proposed herein that coexpression of CD74/MIF and CD44 might play a key role in progression and survival of breast tumor, in addition to the role that each molecule individually has in breast tumor. The primarily purpose of this study was to study the expression profile of CD74, MIF, and CD44 molecules on the breast cancer-derived cell lines CAMA-1, 3,4-methylenedioxyamphetamine (MDA)-MB-231, and MDA-MB-435. To validate this study the expression of CD74, MIF, and CD44 on the normal breast cell line 266LDM, whole cell lysate obtained from adult normal breast tissue and normal breast tissue section was investigated. To test this, flow cytometry, western blotting, and microscopy were employed.
Materials and Methods
Cell culture
Human mammary gland cell lines CAMA-1, MDA-MB-231, MDA-MB-435, and immortalized normal breast luminal cells (226LDM) were commercially obtained from American Type Culture Collection (ATCC, Middlesex, UK) and were cultured as described by manufacturer’s instructions (ATCC), whereas HeLA cells, Jurkat cells, and Raji cell were also purchased from ATCC (Middlesex, UK) and were used as positive control.
Flow cytometry
All cell lines used in this study were subjected to analyze the cell surface or intracellular expression of CD74, MIF, and CD44 by flow cytometry (FlowJo 8.8.6) using monoclonal antibodies By2 (anti-CD74), ab55445 (anti-MIF), 156-3C11 (anti-CD44), W6/32 (anti-HLA-A, B, and C), and LN3 (anti-HLA-DR) as described previously.[13,14]
Western blotting
Protein expression of the desired proteins was analyzed by western immunoblotting using anti-CD74 (clone: By2), anti-MIF (clone: ab55445), and anti-CD44 (clone: 156-3C11) antibodies as described previously.[14]
Immunostaining
CAMA-1, MDA-MB-231, and Raji cell lines were grown individually in 8 well chambers (LabTek, Thermo Fisher Scientific) for 48 h and staining was conducted when reached at appropriate density. All staining steps were performed at 25°C, cells were fixed with paraformaldehyde (4%, Sigma-Aldrich, St. Louis, MO, USA) and were blocked with bovine serum albumin (2%, Sigma-Aldrich). Using anti-CD74-monoclonal, anti-MIF-monoclonal, or anti-CD44-monoclonal antibodies, immunostaining was performed as described previously.[13,14]
Statistical analysis
All statistical determinations were carried out by the Statistical Package for the Social Sciences (IBM Statistics, USA). P < 0.05 was considered statistically significant.
Results
Cell surface expression of HLA-A, B, and C and HLA-DR
Cell surface of CAMA-1, MDA-MB-231, and MDA-MB-435 cells revealed that the expression of HLA-A, B, and C and HLA-DR molecules and normal breast cells was tested to examine the immunogenicity of these cell lines as models of malignant and non-malignant cells. Figure 1 shows the results obtained from a flow cytometer. The results showed that MDA-MB-231 and MDA-MB-435 cells express the same level of HLA-A, B, and C and HLD-DR, respectively. In contrast, CAMA-1 and 266LDM cells did not express HLA-A, B, and C and HLA-DR or the expression was very feeble.
Figure 1.
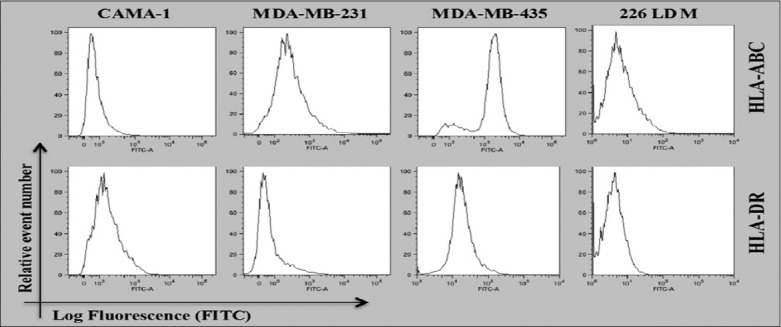
Flow cytometric analysis for cell surface expression of HLA-A, B, and C and HLA-DR in the cells displayed. The data shown are the representative of three independent expressions
CD74, MIF, and CD44 detection and quantification
The intracellular and cell surface expression of CD74, CD74, and MIF were analyzed in CAMA-1, MDA-MB-231, and MDA-MB-435 cells. Non-permeabilized and permeabilized cells with 0.1% Triton X-100 were stained with an appropriate concentration of By2 (anti-CD74), 156-3C11 (anti-CD44), and ab55445 (anti-MIF) antibodies followed by 1 μl Rabbit anti Mouse albumin, conjugated with FITC (RAM-FITC) secondary antibody. Cells without staining and isotype cells, stained with only secondary antibody, were used as a negative control. CD74, CD44, and MIF expression was detected on the cell surface and cytoplasmic of CAMA-1, MDA-MB-231, and MDA-MB-435 cells [Figure 2a-c]. Monocytes, Raji cells, cervical cancer HeLa cells and lymphocytes, and Jurkat cells, were used as a positive control as they express high levels of CD74, CD44, and MIF, respectively. Results are shown as histograms where mean fluorescence intensity is along the horizontal axis (X-axis) versus total cell count on vertical axis (Y-axis) [Figure 2a-c].
Figure 2.
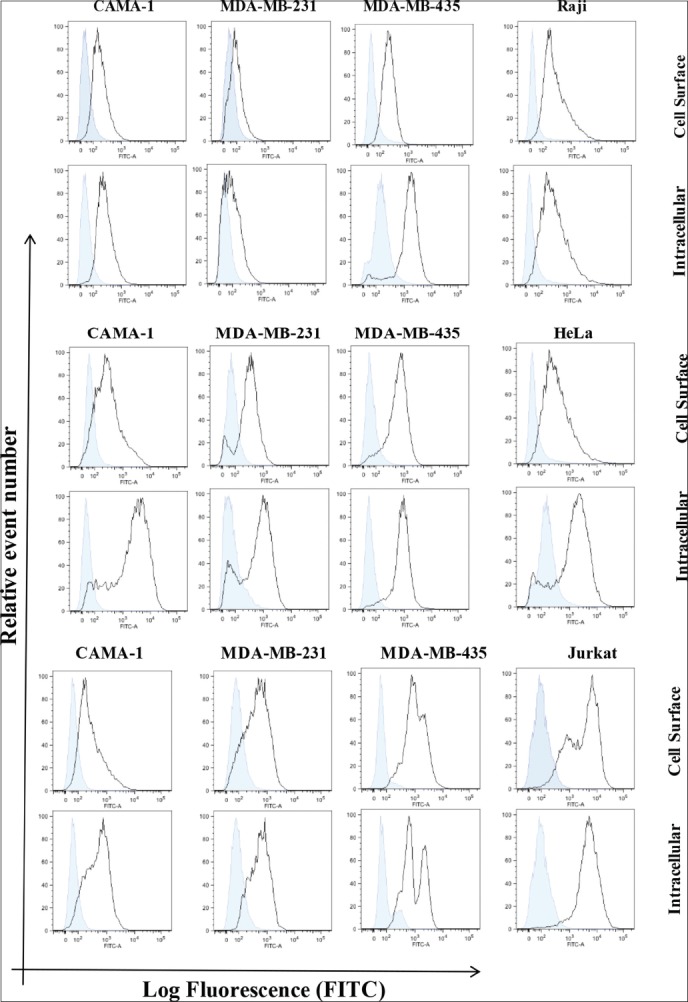
Flow cytometric analysis for cell surface and intracellular expression of the cluster of differentiation (CD) 74, macrophage migration inhibitory factor (MIF), and CD44 in the breast cancer cells displayed. Empty histograms represented the expression of CD74, MIF, and CD44. Expression in Raji, Jurkat, and HeLa cells is used positive controls, whereas blue-filled histograms were shown as a negative control obtained from isotype matched with control antibody. The data are representative of three independent assays
Immunoblot analysis of CD74, MIF, and CD44
Western blot analysis was used to detect CD74, CD44, and MIF protein expression in MDA-MB-231, CAMA-1, and MDA-MB-435 cells By2 (anti-CD74), D-2 (anti-MIF), 156-3C11 (anti-CD44), and TU-02 (anti-α-tubulin) and Poly6221 (anti-β-actin). By2 (anti-CD74) is specific for CD74 isoforms 31–45 kDa and 156-3C11 (anti-CD44) is a mouse mAb that detects endogenous levels of total CD44 protein and is specific for most isoforms (80–90 kDa). The MIF-specific antibody, D-2, is a mouse monoclonal antibody mapping an epitope between amino acids 7–39 at the N-terminus of the MIF protein. THP-1 monocytic cells, Jurkat cells, and cervical cancer HeLa cells were used as a positive control, expressing high levels of CD74, MIF, and CD44.
The results obtained show that the molecular weight of CD44, α-tubulin, β-actin, CD74, and MIF is 80–90 kDa, 50–55 kDa, 42 kDa, 33–41 kDa, and 12 kDa, respectively. β-actin and α-tubulin were used as a loading control since their expression not affected by any treatments such as interferon-γ or lipopolysaccharide. MDA-MB-231, CAMA-1, and MDA-MB-435 cell lines expressed CD74 isoforms, MIF, and CD44; however, CAMA-1 cells expressed two isoforms of CD44 (CD44s and CD44v) [Figure 3a]. To assess the variance in the loading of protein from the cell lysates on the polyacrylamide gel during performing of western immunoblotting, the band intensities of CD44, CD74, and MIF were measured by Image Studio Lite (LI-COR Biosciences Software) and were normalized with the band intensities of β-actin and were presented in Figure 3b.
Figure 3.
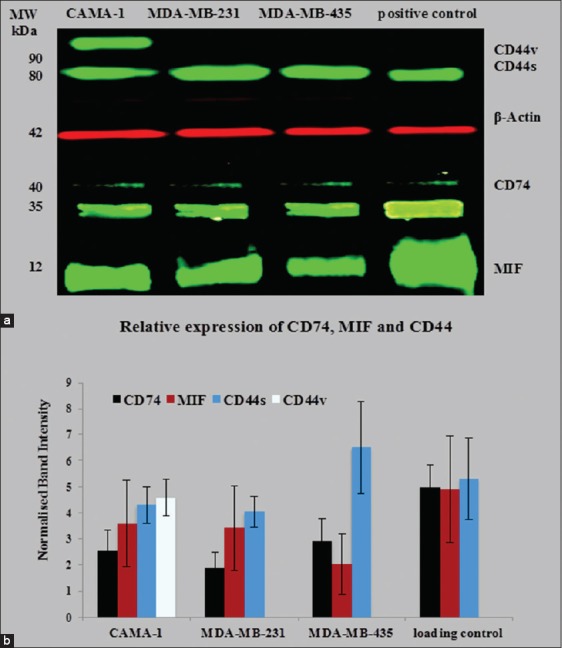
Semi-quantitative western blot for cluster of differentiation (CD) 74, macrophage migration inhibitory factor (MIF), and CD44 in CAMA-1, 3,4-methylenedioxyamphetamine (MDA)-MB-231, and MDA-MB-435 cells (a); normalized band intensities of CD74, CD44, and MIF with β-actin were plotted. Each bar represents protein expression in mean ± standard deviation of five independent scanned (b). The data are the representative of three independent assays
Confocal microscopy
Laser scanning microscopy was performed to see the cell surface and intracellular expression of CD44, CD74, and MIF proteins using different wavelengths. The CAMA-1, MDA-MB-435, and MDA-MB-231 cell lines all exhibited expression of CD74, CD44, and MIF on the surface and intracellular membranes of cells [Figure 4a and b]. CD74 or MIF was labeled with Alexa Fluor® 488 (green) and CD44 Alexa Fluor® 555 (red), and cell nuclei were stained with 4’, 6-diamidino-2-phenylindole (blue).
Figure 4.
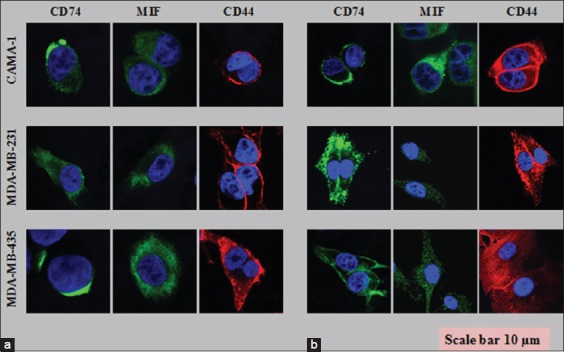
Cell surface (a) and intracellular staining (b) of the cluster of differentiation (CD) 74, CD44, and macrophage migration inhibitory factor on CAMA-1, 3,4-methylenedioxyamphetamine (MDA)-MB-231, and MDA-MB-435 cells visualized by confocal laser scanning microscopy. Each photomicrograph derived from three independent experiments
Validation study of tumor antigens
To authenticate this study, the CD74, MIF, and CD44 expressions were determined in 226LDM cells, a normal immortalized breast luminal cells. Flow cytometric analysis was conducted to determine cell surface and intercellular expression of CD44, CD74, and MIF and the results are shown in Figure 5a. To further validate the role of CD74, MIF, and CD44 in these cancer cells, western immunoblotting was performed in which α-tubulin was used instead of β-actin as a loading control and the results of western immunoblotting are shown in Figure 5b. These results were further revalidated again by confocal laser scanning microscopy using an intracellular staining of CD74, MIF, and CD44 in 226LDM cells. The laser scanning showed no expression of CD74, but the expression of MIF and CD44 was noticed in these 226LDM cells [Figure 5c]. Flow cytometry data show that MIF was not detectable and CD44 was very weak on the cell surface but was higher intracellularly.
Figure 5.
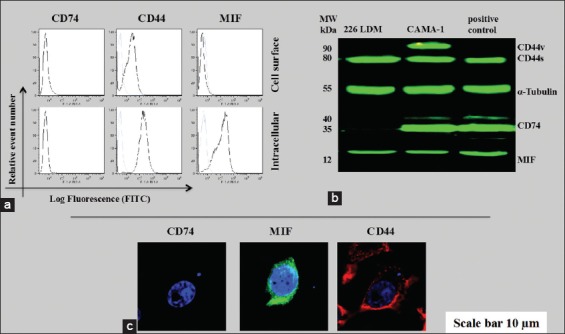
The cluster of differentiation (CD) 74, macrophage migration inhibitory factor (MIF), and CD44 protein expression in 226LDM cells. (a) Cell surface and intracellular expression of CD74, MIF, and CD44. Empty histograms are shown for 226LDM cells stained with indicated antibody. Blue-filled histograms are shown for negative controls obtained from an isotype matched with control antibody. (b) Western immunoblot for CD74, MIF, and CD44. (c) Confocal laser scanning for intracellular staining of CD74, MIF, and CD44 in 226LDM cells. The data are the representative of three independent assays
In the same manner, the expression of CD74, MIF, and CD44 on normal breast lysate and tissues (available commercially) was examined. The results revealed that CD74 was not expressed on normal breast lysate and tissues in contrast to MIF and CD44 [Figure 6a and b]. Although normal breast lysate and tissues expressed MIF and CD44, the level was low compared to that in CAMA-1, MDA-MB-231, and MDA-MB-435 cells.
Figure 6.
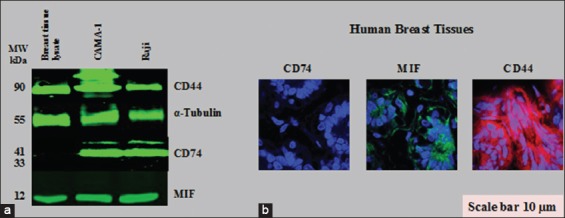
Cluster of differentiation (CD) 74, macrophage migration inhibitory factor (MIF), and CD44 proteins expression in normal human breast cell lysate and tissues. (a) Western blot for CD74, MIF, and CD44 expression. (b) Histological immunostaining of CD74, MIF, and CD44 in normal breast tissue cells, as visualized by confocal laser scanning microscopy
Discussion
To further understand the immunogenicity and tumorigenicity of breast cancer cells, the expression of HLA-A, B, and C and HLA-DR molecules in MDA-MB-231, CAMA-1, 226LDM, and MDA-MB-435 cells was investigated. HLA-A, B, and C expression was detected in MDA-MB231 and MDA-MB435 cells, but not in CAMA-1 and 226LDM cells. Conversely, HLA-DR was not detectable in any of the cell lines except MDA-MB-435 cells, which showed weak expression, as shown in Figure 1. The expression of major histocompatibility (MHC) Class I and II in cancer cells, including lung, liver, melanoma, colon, and breast cancer, has been previously observed.[15-18] Notably, Jabrane-Ferrat et al.[19,20] reported that four of five different breast adenocarcinoma cell lines expressed cell-surface MHC Class I proteins. On the other hand, MHC Class I was either not expressed or downregulated in primary breast cancer tissue.[21]
In this respect, it has been revealed that the expression of CD74, HLA-DR, and HLA-DM by tumor cells is a marker of better prognosis in breast cancer patients. Number of investigators noticed that HLA molecular expression improves cancer cells immunogenicity, and for this reason, they emphasized on antitumor T-cell response.[22-24] Thus, the expression of HLA-A, B, and C and HLA-DR molecules in breast cancer cell lines could be of considerable value for tumor immunity, potentially leading to efficient tumor antigen processing and presentation to T cells.[25] In the tumor immunotherapy design field, MHC Class II presentation has received much recent attention.[26,27] Recently published data indicate that CD74, MIF, and CD44 have a role in the pathogenesis of solid tumors.[1,7,28] The data obtained have confirmed that cell surface and intracellular MIF, CD74, and CD44 molecules are obvious by flow cytometry and confocal microscopy. The western blotting results revealed that breast cancer cells express total protein of CD74, MIF, and CD44. Significantly, western blot analysis showed that the studied breast cancer cells express three different isoforms of CD74, the P33, P35, and P41 isoforms, which were detected at the expected molecular weights, the major form being the 33 kDa isoform [Figure 6a].[29] These findings differ from those obtained by Verjans et al.[30] who found that MDA-MB-231 and MDA-MB-468 cells express one CD74 isoform; however, it was not specified which isoform. Similarly, reports also showed that MDA-MB-435 also expresses P35 isoform of CD74.[31] Not only have these, THP-1 cells show expression of P33 and P43 isoforms of CD74. The cell surface and the total protein expression of CD74 were also studied in the MCF-7 cell line.[32] Data obtained from flow cytometry showed that MCF-7 cells express a moderate level of CD74 compared to the positive control Raji cell lines. Western blot results confirmed that MCF-7 cells express the total protein of CD74. However, MCF-7 cells expressed the P33 and P41 isoforms of CD74, in contrast to breast cancer cells. Theoretically, an additional 43 kDa form of CD74, the longest isoform, could be expected, but it has not yet been unequivocally detected in immunoprecipitates from human cells. Regulation of Class II MHC antigen presentation is regulated by the P33 and P35 isoforms while the T-cell selection in the thymus is monitored by P41 isoform.[29] CD74 aids in the export of MHC II/CD74 complexes from the endoplasmic reticulum as well as blocking untimely loading of peptides onto the MHC II molecules.[33-37] However, it has been newly suggested that elevated levels of CD74 expression may avert tumor antigen presentation by closing peptide cleft of MHC Class II molecule, thus preventing binding of antigenic peptides for T-cells presentation.[38,39] In Humans, nearly 80% of the CD74 protein pool consists of the P33 isoform. However, P35 is considered the most enigmatic isoform.[36] Metodieva et al.[37] have suggested that P33 and P35, together, assist in antigen presentation; moreover, this process does not require the coexpression of further more CD74 isoform.[37,38]
Western blotting also demonstrated that CAMA-1 cells express two different isoforms of CD44; CD44 variant (CD44v) and CD44 standard (CD44s) [Figure 6a]. Few breast cancer cell lines, such as MDA-MB-468 and SUM149, express a number of isoforms of CD44s and CD44v.[40] It is likely that CD44 expression in breast cancer may be related with highly aggressive breast tumor subtypes or extremely invasive breast cancer cells.[41]
To validate the present study, immortalized normal breast luminal cells (226LDM cells), breast (human) whole cell lysate obtained from adult normal tissue, and normal breast tissue slides were used as a model. The expression of CD74, MIF, and CD44 was investigated by flow cytometry, western blot, and microscopy. The findings confirmed that 226LDM cells did not express CD74 on the cell surface or intracellularly but express MIF and CD44, although in low levels compared to breast cancer cells. In the same manner, the results obtained from western blotting of normal breast lysate and histological immunostaining of breast tissue slides showed only positive detection of MIF and CD44 but not CD74. This result was expected because it has been established that the expression of CD74 is restricted to antigen-presenting cells including B cells, monocytes, macrophages, and dendritic cells (e.g., Langerhans cells).[42]
Conclusion
The study shows evidence that breast cancer cells expressing three different isoforms of CD74. The CD74 plays a crucial role in breast cancer cells along with MIF and CD44. The results clearly indicate that CAMA-1, MDA-MB-231, and MDA-MB-435 cells are poorly immunogenic, expressing low levels of HLA-A, B, and C and HLA-DR. Longitudinal studies in breast cancer animal model and also in breast cancer patients are necessary to invent novel agents that can antagonize the effects of CD74, CD44, and MIF, thereby, we can better treat, prevent, or control the onset and/or progression of breast cancer.
References
- 1.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–9. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 2.Butrym A, Majewski M, Dzietczenia J, Kuliczkowski K, Mazur G. High CD74 expression correlates with ZAP70 expression in B cell chronic lymphocytic leukemia patients. Med Oncol. 2013;30:560. doi: 10.1007/s12032-013-0560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell. 2005;16:5061–9. doi: 10.1091/mbc.E05-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, et al. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87–91. doi: 10.1016/s0304-3835(01)00503-1. [DOI] [PubMed] [Google Scholar]
- 6.Young AN, Amin MB, Moreno CS, Lim SD, Cohen C, Petros JA, et al. Expression profiling of renal epithelial neoplasms:A method for tumor classification and discovery of diagnostic molecular markers. Am J Pathol. 2001;158:1639–51. doi: 10.1016/S0002-9440(10)64120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, et al. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res. 2004;10:6606–11. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 8.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer:Macrophage migration inhibitory factor (MIF) –the potential missing link. QJM. 2010;103:831–6. doi: 10.1093/qjmed/hcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toole BP, Slomiany MG. Hyaluronan, CD44 and emmprin:Partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorchenko O, Stiefelhagen M, Peer-Zada AA, Barthel R, Mayer P, Eckei L, et al. CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood. 2013;121:4126–36. doi: 10.1182/blood-2012-11-466250. [DOI] [PubMed] [Google Scholar]
- 11.McClelland M, Zhao L, Carskadon S, Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol. 2009;174:638–46. doi: 10.2353/ajpath.2009.080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard V, Kindt N, Decaestecker C, Gabius HJ, Laurent G, Noël JC, et al. Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol Rep. 2014;32:523–9. doi: 10.3892/or.2014.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ssadh HA, Spencer PS, Alabdulmenaim W, Alghamdi R, Madar IH, Miranda-Sayago JM, et al. Measurements of heterotypic associations between cluster of differentiation CD74 and CD44 in human breast cancer-derived cells. Oncotarget. 2017;8:92143–56. doi: 10.18632/oncotarget.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alabdulmonem W, Alhomaidan HT, Rasheed Z, Madar IH, Alasmael N, Alkhatib S, et al. CD74 a potential therapeutic target for breast cancer therapy:Interferon gamma up-regulates its expression in CAMA-1 and MDA-MB-231 cancer cells. Int J Cancer Res. 2018;14:58–69. [Google Scholar]
- 15.Moldenhauer G, Henne C, Karhausen J, Möller P. Surface-expressed invariant chain (CD74) is required for internalization of human leucocyte antigen-DR molecules to early endosomal compartments. Immunology. 1999;96:473–84. doi: 10.1046/j.1365-2567.1999.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczmarek M, Frydrychowicz M, Rubis B, Mizera-Nyczak E, Nieruchalska E, Sikora J, et al. Analysis of expression of MHC Class I molecules and TAP genes in malignant human cell lines. Folia Histochem Cytobiol. 2007;45:205–14. [PubMed] [Google Scholar]
- 17.Huang X, van den Berg A, Gao Z, Visser L, Nolte I, Vos H, et al. Expression of HLA class I and HLA class II by tumor cells in Chinese classical Hodgkin lymphoma patients. PLoS One. 2010;5:e10865. doi: 10.1371/journal.pone.0010865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the MHC class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology. 2012;1:908–16. doi: 10.4161/onci.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabrane-Ferrat N, Calvo F, Faille A, Lagabrielle JF, Boisson N, Quillet A, et al. Recombinant gamma interferon provokes resistance of human breast cancer cells to spontaneous and IL-2 activated non-MHC restricted cytotoxicity. Br J Cancer. 1990;61:558–62. doi: 10.1038/bjc.1990.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabrane-Ferrat N, Faille A, Loiseau P, Poirier O, Charron D, Calvo F, et al. Effect of gamma interferon on HLA class-I and -II transcription and protein expression in human breast adenocarcinoma cell lines. Int J Cancer. 1990;45:1169–76. doi: 10.1002/ijc.2910450630. [DOI] [PubMed] [Google Scholar]
- 21.Pistillo MP, Nicolò G, Salvi S, Capanni P, Perdelli L, Pasciucco G, et al. Biochemical analysis of HLA class I subunits expression in breast cancer tissues. Hum Immunol. 2000;61:397–407. doi: 10.1016/s0198-8859(99)00179-2. [DOI] [PubMed] [Google Scholar]
- 22.Meazza R, Comes A, Orengo AM, Ferrini S, Accolla RS. Tumor rejection by gene transfer of the MHC class II transactivator in murine mammary adenocarcinoma cells. Eur J Immunol. 2003;33:1183–92. doi: 10.1002/eji.200323712. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys RE, Hillman GG, von Hofe E, Xu M. Forcing tumor cells to present their own tumor antigens to the immune system:A necessary design for an efficient tumor immunotherapy. Cell Mol Immunol. 2004;1:180–5. [PubMed] [Google Scholar]
- 24.Oldford SA, Robb JD, Codner D, Gadag V, Watson PH, Drover S, et al. Tumor cell expression of HLA-DM associates with a th1 profile and predicts improved survival in breast carcinoma patients. Int Immunol. 2006;18:1591–602. doi: 10.1093/intimm/dxl092. [DOI] [PubMed] [Google Scholar]
- 25.Feinmesser M, Sulkes A, Morgenstern S, Sulkes J, Stern S, Okon E, et al. HLA-DR and beta 2 microglobulin expression in medullary and atypical medullary carcinoma of the breast:Histopathologically similar but biologically distinct entities. J Clin Pathol. 2000;53:286–91. doi: 10.1136/jcp.53.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajnavölgyi É, Lanyi A. Role of CD4+T lymphocytes in antitumour immunity. Adv Cancer Res. 2003;87:195–249. doi: 10.1016/s0065-230x(03)87298-6. [DOI] [PubMed] [Google Scholar]
- 27.Velders MP, Markiewicz MA, Eiben GL, Kast WM. CD4+T cell matters in tumour immunity. Int Rev Immunol. 2003;22:113–40. doi: 10.1080/08830180305220. [DOI] [PubMed] [Google Scholar]
- 28.Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–92. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 29.Liu YH, Lin JY. Recent advances of cluster of differentiation 74 in cancer. World J Immunol. 2014;4:174–84. [Google Scholar]
- 30.Verjans E, Noetzel E, Bektas N, Schütz AK, Lue H, Lennartz B, et al. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer. 2009;9:230. doi: 10.1186/1471-2407-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metodieva G, Nogueira-de-Souza NC, Greenwood C, Al-Janabi K, Leng L, Bucala R, et al. CD74-dependent deregulation of the tumor suppressor scribble in human epithelial and breast cancer cells. Neoplasia. 2013;15:660–8. doi: 10.1593/neo.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín-Ventura JL, Madrigal-Matute J, Muñoz-Garcia B, Blanco-Colio LM, Van Oostrom M, Zalba G, et al. Increased CD74 expression in human atherosclerotic plaques:Contribution to inflammatory responses in vascular cells. Cardiovasc Res. 2009;83:586–94. doi: 10.1093/cvr/cvp141. [DOI] [PubMed] [Google Scholar]
- 33.Weenink SM, Gautam AM. Antigen presentation by MHC class II molecules. Immunol Cell Biol. 1997;75:69–81. doi: 10.1038/icb.1997.11. [DOI] [PubMed] [Google Scholar]
- 34.Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:210–5. doi: 10.1097/00129039-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–36. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 36.Bergmann H. Business as usual:The p35 isoform of human CD74 retains function in antigen presentation. Immunol Cell Biol. 2012;90:839–40. doi: 10.1038/icb.2012.51. [DOI] [PubMed] [Google Scholar]
- 37.Laetitia G, Chemali M, Desjardins M, Labrecque N, Thibodeau J. Human invariant chain isoform p35 restores thymic selection and antigen presentation in CD74-deficient mice. Immunol Cell Biol. 2012;90:896–902. doi: 10.1038/icb.2012.27. [DOI] [PubMed] [Google Scholar]
- 38.Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol. 2009;15:2855–61. doi: 10.3748/wjg.15.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng YX, Yang M, Rong TT, Yuan XL, Ma YH, Wang ZH, et al. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J Gastroenterol. 2012;18:2253–61. doi: 10.3748/wjg.v18.i18.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery N, Hill A, McFarlane S, Neisen J, O'Grady A, Conlon S, et al. CD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activity. Breast Cancer Res. 2012;14:R84. doi: 10.1186/bcr3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold DV, Stein R, Burton J, Goldenberg DM. Enhanced expression of CD74 in gastrointestinal cancers and benign tissues. Int J Clin Exp Pathol. 2010;4:1–2. [PMC free article] [PubMed] [Google Scholar]


