Abstract
Objectives:
The cognitive functions, motor coordination, and social behavior were studied in rodents after adding different doses of caffeine in their drinking water.
Methodology:
BLC57 mice were divided into four groups: Control (n = 8), chronic moderate dose (n = 8, Ch] MD), Ch high dose (n = 8, Ch HD), and withdrawal (n = 8, WD). Caffeine was administered for 1 month to all groups. Spatial memory was tested by Morris water maze, motor coordination by rotarod (RR), social behavior by (Crawley’s test), and anxiety by elevated plus maze (EPM) test.
Results:
In water maze, the latency to reach the platform was significantly shorter in Ch MD group compared to the control and the Ch HD groups. WD group showed the worst performance. RR results showed that the groups treated with caffeine performed poor in comparison to the control group where their latency to fall was significantly less. In the three-chamber test, the Ch MD group showed enhanced sociability (session 1) and social novelty behavior (session 2). On the other hand, both Ch HD and WD showed a lack in sociability and a deficit in social novelty. In the EPM, results showed that all caffeine administrated mice where more anxious than the control group.
Conclusion:
We concluded that chronic administration of caffeine in MD resulted in enhancement of spatial memory, motor functions, sociability, and social novelty. The anxiety in these animals was, however, increased. On the other hand, Ch HD caffeine had opposite effects on all the parameters except for anxiety.
Keywords: Caffeine, motor function, cognitive functions, anxiety, social behavior
Introduction
The most widely consumed psychostimulant substance is caffeine which is found in many drinks and products. Caffeine is regularly consumed all over the world.[1] Interestingly, caffeine has been reported to enhance memory in animal models as well as humans.[2,3] However, heavy caffeine consumption has been associated with many side effects.[4]
Memory consolidation occurs mainly during sleep. Sleep disorders were shown to affect memory storage through its effects on neuronal circuits.[5]
When administrated acutely, caffeine improved learning and memory. Furthermore, caffeine was used to prevent memory defects and treat other cognitive malfunctions.[6] On the other hand, one of the caffeine side effects is sleep disturbances which were shown to be associated with deterioration of memory.[7]
Caffeine has the potency to modulate behavioral activity including locomotor activity by affecting both central and peripheral nervous systems’ neuronal pathways. Caffeine is recognized as a non-selective adenosine receptor antagonist, especially for A1 and A2A receptors, which is reflected by an increase in locomotor behavior in rodents.[7] It exerts a stimulating effect on locomotor activity at low-to-moderate doses (MD), but weaker stimulatory or even depressive effects at higher doses. In addition, chronic caffeine administration has been shown to impair locomotor activity in rats.[8]
Anxiety disorder is an exaggerated fear and anxiety feelings compared to normal response. This mental disorder affects 18.1% of adults in the United States.[9] Interestingly, low caffeine dose can be anxiolytic, while high dose (HDs) may cause anxiety.[10] The suggested mechanisms for the anxiogenic effect of caffeine may be through adenosine and/or GABAA receptors antagonism.[11]
Social behavior defined as the collection of mannerisms and actions made by two or more organisms from the same species.[12] There is a popular belief that some people’s behavior or mood is not normal or positive without the first sip of caffeine-containing beverages. In fact, the negative mood is increased following the withdrawal (WD) of caffeine, but this effect could not be recorded properly due to the failure to conduct “blind” studies and the expectancies of the volunteers.[13]
In this study, we conducted several tests to evaluate the brain cognitive function as learning and memory, motor coordination, anxiety levels, and sociability and social novelty parameters. To achieve this goal, the following tests were used: Morris water maze (MWM), rotarod (RR) test, elevated plus maze (EPM), and the three-chamber social apparatus, respectively.
Materials and Methods
Animals
Caffeine was mixed with water (the MD is 0.1 g/L/day (20 mg/kg/day), and the HD is 1 g/L/day, i.e., 200 mg/kg/day) and administered to BLC57 mice.[14] To be sure that animals consume the given dose of caffeine, the daily water intake of each animal was calculated, and the caffeine dose was dissolved in half of the measured water intake and given to the animals at the beginning of the day. When they drank that amount, they were provided with water ad libitum afterword and during the rest of the day. In this case, all the animals were given the desired dose of caffeine in the first half of the day. The animals were obtained from Arabian Gulf University Animal Facility Department. The mice (average weight 20–30 gm) were divided into 4 groups with 8 animals per group: Control group (n=8), chronic (Ch) MD of caffeine (n = 8, administered for 4 weeks duration, Ch MD), Ch high caffeine dose (n = 8, administered for 4 weeks duration, Ch HD), and WD (n = 8, HD caffeine was administered for 4 weeks, and it was stopped 3 days before testing, WD). The animals were tested when they reached adulthood (8 weeks of age). Animal care and experimental ethics of the Arabian Gulf University/Bahrain were followed during this study.
Tests
RR
This test is aiming to estimate the motor power and motor coordination of the animals. Caffeine as a CNS stimulant may modulate these parameters. The accelerating RR assesses motor coordination and balance. The time the animals can stay on a rotating rod with a fixed velocity (4.5 m/min) before falling was measured. The average latency before falling was calculated from three trials (each of 2 min) per animal. All the animals of the six groups were tested in the same day.
EPM test
The EPM tests anxiety-like behavior.[15] The maze is composed of a central area (6 cm × 6 cm) where the mice were placed at the beginning of each experiment. The central area has access to two open and two enclosed arms (surrounded by 17 cm high walls) of same length and size. In each experiment, the number of entries to and the total time spent in each arm was measured during 10 min. The setup was cleaned by 70% alcohol before the start of any new experiment with a new mouse. Anxiety is indicated if the mouse spent little time in the open arms.
MWM test
Spatial learning and memory were tested by MWM.[16] The maze is a circular swimming pool of 140 cm diameter and 60 cm height, filled with water which was maintained at 26°C–28°C. The set up was placed in a dark room illuminated by sparse red light and provided with visual cues. The maze program divided the pool by imaginary diagonal lines into four quadrants.
The test composed of two sessions: The first session (training day) trained the mouse to locate a hidden platform placed underwater level by 2 cm so that to escape swimming. Each animal had five training trials, each one composed of four tests with different predetermined departure points along the pools boundary. Each test consisted of 120 s to find the hidden platform and reside on it. Mice that fail to find the platform within the 120 s were placed on it and left there for 20 s.
The test was performed using a video camera to capture and analyze the animal position every 0.2 s (ANY - maze-video-tacking; Stoelting Co., Wood Dale, IL, USA). The parameters measured included the latency needed and the distance swum by the animal to reach the platform. The speed of swimming of the animals was also measured and used as a control between the groups. Data from the four tests in each trial were averaged to give one data point.
Social behavior: Sociability and social novelty[17]
This test has two sessions. In session 1, it tests the sociability of the animal or its preference to stay in a chamber containing other animal than in an empty chamber. The three-chamber test is composed of a test box (20 cm × 40 cm × 20 cm) made up of three Plexiglas interconnected chambers. Within each chamber was a circular wire cage of 11 cm height and 9 cm diameter. Each test started by 5 min habituation followed by two sessions, each one lasted 10 min. In session 1, the subject mouse was placed in the middle chamber and left to move freely between the chambers. The time spent by this mouse to stay in the side chamber containing unfamiliar mouse (stranger 1) in the wire cage as well as the number of entries to this chamber were measured and compared to the time spent and number of entries to the other empty chamber. Session 1 determines the level of sociability of the subject (tested) mouse.
Session 2 measures the social novelty of the animal which is the preference of the animal to spend more time in a chamber containing new animals than in a chamber containing an already explored animal. In this session, another unfamiliar mouse (Stranger 2) was placed in the wire cage of the previously empty chamber in session 1. The subject mouse was left to move freely between the chamber that contained the mouse of session 1 (stranger 1) and the chamber that contained the new, unfamiliar mouse (stranger 2). Stranger 1 and 2 mice were of same species, gender, and age. The time spent in each chamber, as well as number of entries, was recorded. In the beginning of every experiment, the apparatus was cleaned with 70% alcohol. When a mouse spent more times and entered more frequently the chamber that housed (stranger 2), it was considered as having a preference for social novelty.
Statistical analysis
Each point in the data was expressed as the average ± standard error of means. Statistical significance between the groups in the water maze, RR, EPM, and the three-chamber tests was measured by analysis of variance post hoc test (ANOVA). Statistical analysis between individual groups was done using the t-test. All tests were done using Microsoft Excel ITM 2010.
Results
RR
Generalized decrease in the latency to fall in caffeine-treated groups
The groups treated with caffeine performed poorly in comparison to the control group where their latency to fall was significantly less [Cont = 33.2 ± 3.4 s, Ch MD = 20.5 ± 1.4 s, Ch HD = 23.4 ± 1.9 s, WD = 23.5 ± 2.7 s, ANOVA test, P < 0.005, F = 5.069079, F crit = 2.636391, Figure 1].
Figure 1.

Latency to fall (Mean ± Standard error of means seconds) in the rotarod test for the control, moderate dose (MD), and HD group. The time spent by the caffeine-treated groups was significantly less than the control group (ANOVA test, P < 0.005, F = 5.069079, F crit = 2.636391). Significant differences were recorded when comparing the control group to chronic (Ch) HD (P < 0.01), Ch MD (P < 0.001), WD (P < 0.01) groups. No significant difference was obtained between Ch MD, Ch HD, and WD groups (ANOVA, P = 0.49, F = 0.7069, Fcrit = 3.038)
MWM
Latency to reach the platform of Ch MD group (41.6 ± 8.1 s) was significantly better [ANOVA test, P < 0.0005, F = 7.37491, F crit = 2.67218, Figure 2a] compared to the other groups (Cont = 54.3 ± 9.1 s, Ch HD = 74.6 ± 8.4 s). WD group showed the worst performance when compared to the treated groups and control (92.5 ± 7.3 s). This significant difference in the latency to reach the hidden platform was not due to a difference in the animal’s speed of swimming in the pool [Figure 2c]. There were no significant outcomes between the groups in regard to velocity (P = 0.3348; F = 1.1538, Fcrit = 2.9466; ANOVA test). Similar to the latency results, measurement of the distance swum by the animals to reach the platform confirms that the Ch MD were performing significantly better than the other groups. The control (9.1 ± 1.5 dm) and Ch MD (10.7 ± 2.7 dm) had a significantly less crossing distance when compared to both Ch HD (18.6 ± 1.5 dm) and WD (18.2 ± 1.9 dm) [Figure 2b]. In the probe experiment, the platform was removed and the animals were allowed to swim for 2 min. The percentage of time the animal spent in each quadrant of the swimming pool was calculated. Spending >25% of the time in the quadrant contained the hidden platform is considered significant, and the mouse is considered as learned the test. The Ch MD (50.5 ± 7.3 s) spent significantly higher percentage of time in the disk zone when compared to the other groups [Cont = 42.9 ± 3.0 s, Ch HD = 18.8 ± 3.5 s, WD= 17.1 ± 3.1 s, ANOVA test, P < 0.0001, F = 14.91209, F crit = 2.682809; Figure 2d].
Figure 2.

(a-d) Effect of the administration of caffeine on a mouse model in Morris water maze test to assess spatial memory and learning. The results displayed different levels of cognitive effects based on the chronic doses and withdrawal (WD) of caffeine. Statistical analysis for the latency required by the animals to reach the platform (a) revealed that a significant difference was recorded between the groups (ANOVA P < 0.0005). Two trial t-test calculated a significant difference between the control group and Ch HD (P < 0.05) and WD (P < 0.001) groups. No significant difference was recorded between the Cont group and the Ch MD group (P = 0.15). Figure 2c shows that there are no significant differences between the groups concerning their speed of swimming the pool during testing (ANOVA = 0.3448). In the probe trial (D) ANOVA test between the groups showed a significant difference (P < 0.005). Two trial t-test showed that the control group stayed significantly more time in the platform quadrant than the Ch HD (P < 0.0001) and WD (P < 0.0005) groups. No significant differences between the Cont and the Ch MD groups (P = 0.169). The Ch MD group of animals stayed significantly more time in the platform quadrant than the Ch HD (P < 0.0001) and WD (P < 0.005) groups
EPM
The results showed that the Ch HD and WD groups (time in the open arms of the test was 18.9 ± 18.8 and 59 ± 15.24 s, respectively) were showing significantly more anxiety than the control group (time spend in the open arm was 118 ± 18.1, P < 0.05, t-test). The Ch HD treated mice were the most anxious group, represented by spending the last time in the open arm. There was a slight difference between the Ch MD (74.3 ± 24.3 s) and WD groups. However, the Ch MD group showed less anxious behavior than the other caffeinated groups. Although the Ch MD animals stayed less time in the open arms, they were not significantly different from the control group [t-test P > 0.05, Figure 3].
Figure 3.
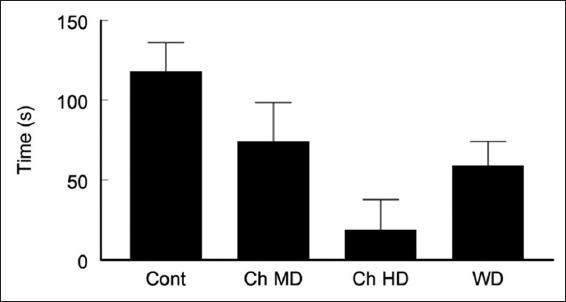
The effect of different doses and withdrawal of caffeine on anxiety, assessed by elevated plus Maze test. ANOVA test showed a significant difference between the groups (P < 0.05) in the total time the animals spent in the open arm of the maze. Two trials t-test showed that the control animals stayed significantly more time in the open arm of the elevated plus maze (less anxiety) when compared to the chronic high dose (Ch HD) (P < 0.005) and WD (P < 0.05) groups. No significant difference was recorded between the control and the Ch MD groups (P = 0.279)
Three-chamber social apparatus (Crawley’s sociability and preference for social novelty test)
In the three-chamber test, Cont group stayed significantly more time (315.4 ± 18 s) in the chamber with mouse than in the empty chamber (217 ± 22.4 s). This session represents sociability of the animal (S1) (t-test, P < 0.05, t critical two-tail = 2.364). Furthermore, this group showed a preference for social novelty behavior (stayed 184.8 ± 29.5 s in the chamber with the old mouse and 347 ± 78.1 s in the chamber with the new mouse) in session 2 (S2) (P < 0.05, paired t-test, t critical two-tail = 2.364) [Figure 4].
Figure 4.
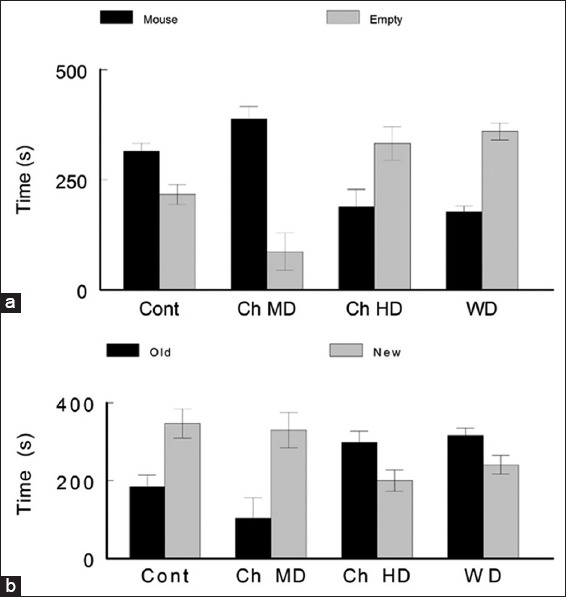
Three-chamber test to assess sociability and preference for social novelty. (a) Sociability (Session 1: Time spent in chamber with a mouse vs. chamber without mouse). (b) Preference for social novelty (Session 2: Time spent in chamber with the old mouse vs. chamber with new mouse). Control animals showed normal sociability and preference for social novelty. Within the caffeine-treated groups, only the chronic moderate dose group performed similarly to the control by showing a significant increase in sociability and preference for social novelty (see the text for statistical significances and P values)
Ch MD group showed an increase in sociability (session 1) by preferring to be in the chamber containing another mouse (387.9 ± 28.8 s) rather than staying in the empty chamber (86.5 ± 42.4 s) S1 (t-test, P < 0.005, t critical two-tail = 2.364). They also showed a normal preference for social novelty (session 2) by spending more time in the chamber which contained a new mouse (330.3 ± 45.5 s) than the chamber containing the old (102.7 ± 52.8 s). ChMD S2 (t-test, P < 0.05, t critical two-tail=2.364).
On the other hand, Ch HD (chamber with mouse: 189 ± 39.2 s, chamber without mouse: 332.5 ± 38.3 s) (t-test, P < 0.05, t critical two-tail = 2.364) and WD (chamber with mouse: 176.7 ± 13.1 s, chamber without mouse: 360.1 ± 19.2 s) (t-test, P < 0.0005, t critical two-tail = 2.364) displayed a lack in sociability (session 1). Furthermore, in session 2, Ch HD (chamber with old mouse: 298.4 ± 29.6 s, chamber with new mouse: 200.3 ± 26.8 s) (t-test, P > 0.05, t critical two-tail = 2.364) and WD (chamber with old mouse: 316.6 ± 19.2 s, chamber with new mouse: 240.8 ± 24.4 s) illustrated a deficit in preference for social novelty with no significant differences (t-test, P < 0.05, t critical two-tail = 2.364).
Discussion and Conclusion
This study demonstrates the long-term stimulating effects of caffeine given in two different doses to different groups of mice in regard to four parameters. The parameters that were investigated are motor function, cognitive function, anxiety, and social behavior. There is a lack of researches investigating all of the aforementioned parameters under the effect of different doses for long durations as well as the WD of caffeine in one study. There was a variation in the response of mice to different doses of caffeine which was illustrated by Ch MD showing the best performance in both cognitive function and sociability. Overall, the treated groups displayed better performance than the WD group.
Most of the biological effects of caffeine are secondary to its antagonizing effects on all types of adenosine receptors.[11] Some evidence suggests that adenosine inhibits the release of excitatory neurotransmitters more strongly than that of inhibitory neurotransmitters.[18] It has been shown that adenosine acts on A1 receptors and it decreases the central neurons firing rate.[19,20] This is due to an activation of potassium channels through adenosine A1 receptors.[21]
In addition, it has been shown that adenosine decrease calcium entry through N-type channels in hippocampal CA1 and CA3 neurons.[19,20] A1 receptors influence the binding of dopamine D1 agonists.[22] Interestingly, a study[23] presented strong evidence on that the release of adenosine is increased by combined D1 and NMDA receptor stimulation.
Data from different researches stated that caffeine has a dose-dependent effect on cognitive function,[24-26] delivering significantly different effects when taken at low doses than at high ones. The beneficial effect of caffeine on cognitive function is seen with low doses, while HDs of caffeine intake show deterioration in function. This study presented similar results in terms of cognitive function, as the latency of the group treated with moderated doses of caffeine in MWM test (Ch MD = 41.6 ± 8.1 s) was significantly better (ANOVA test, P < 0.0005) than the group treated with HDs (Cont = 54.3 ± 9.1 s, Ch HD = 74.6 ± 8.4 s, WD = 92.5 ± 7.3 s).
It has been illustrated that caffeine increases the firing rate of mesocortical cholinergic neurons by inhibiting its tonic inhibitor, adenosine.[27] This effect is what causes arousal following caffeine ingestion. Furthermore, the effect of benzodiazepines on behavior is modified by administration of caffeine in animals as well as humans.[28] The proposed mechanism for this process is by blocking the benzodiazepine receptors which requires very high concentrations of caffeine.[29]
Caffeine having an anxiogenic effect is a well-known fact according to many studies.[30,31] The outcomes of our study were in favor of the aforementioned thesis in that a significant increase in anxiety was detected in the treated groups. In regard to the anxiogenic effect, not many researches addressed the correlation between the dose and effect. In our study, we were able to assort the anxiogenic effect according to the dosing profile. The results conveyed a directly proportional relation between the caffeine dose and the anxiogenic effect. Ch MD (74.3 ± 24.5 s) were the least anxious among the treated groups whereas Ch HD (18.9 ± 18.8 s) showed the highest levels of anxiety compared to the other groups.
Although no significant difference was observed between the Ch HD and WD groups, the data show some tendency of the Ch HD group for anxiety more than the WD group [Figure 3].
Based on many conducted researches, it showed that caffeine’s effect on motor and cognitive functions are dose-dependent. In fact a biphasic effect, hence on low to moderate it causes an increase in motor and cognitive functions and vice versa.[12,17,24,25] The early mechanism proposes mobilization of intracellular calcium. High concentrations (1–10 mM) of caffeine were found to interfere with the uptake and storage of calcium in the sarcoplasmic reticulum of striated muscle and to increase the translocation of Ca++ through the plasma membrane.[32] Furthermore, its binding to ryanodine receptors in calcium channels of muscle and brain; caffeine increases myofilament sensitivity to Ca++.[33]
Caffeine selectively targets the striatopallidal neurons in the striatum and their counterparts in the nucleus accumbens causing a decrease in its activity when given in low doses. The basal expression of mRNA for NGFI-A[34] and NGFI-B[35] is relatively high in the striatum. A number of recent studies which tested the expression of NGFI-A and NGFI-B mRNA.[36] Showed that lower doses of caffeine (7.5–25 mg/kg) decrease the expression of NGFI-A and NGFI-B mRNA levels in the striatum, suggesting that caffeine in low doses works as a stimulant. The blockade of adenosine receptors present in the striatum is the reason behind it. This is also supported by the fact that caffeine-induced changes are located in the striatopallidal neuron, which expresses adenosine A2 receptor.
According to different studies, there is a variation in the outcomes of locomotor performance in chronically caffeine-treated mice. Some researches support that chronic administration of caffeine enhances locomotor[13,30,37] activity, while other studies presented the opposite.[26,30] The results of our study demonstrated a significant deterioration in locomotor function in all treated groups with the Ch MD (20.5 ± 1.4 s) showing the highest reduction in performance.
Our study supports the conclusion obtained by other studies regarding sociability and preference to social novelty. Studies suggest that Ch MD of caffeine improve both behaviors while HD administration showed a regression.[30,38] In this study, the chronically treated groups of mice with MD (chamber with mouse = 387.9 ± 28.8 s, empty chamber = 86.5 ± 42.4 s) were found to have significantly higher levels of sociability in comparison to the control, Ch HD, and WD groups. The results obtained for WD and Ch HD groups were quite similar to each other. Both aforementioned groups displayed a drastically decreased sociability and social novelty in comparison to the other groups.
In the previous study, we demonstrated the acute effects of caffeine on mice.[39] In comparison to the present study, mice treated with MD of caffeine displayed similar results in terms of cognitive function as well as anxiety levels, whereas both high and MDs of caffeine displayed lack of sociability and social novelty.
Overall this study documents the diverse effects of caffeine on different parameters. Ch MD group showed better results in sociability, memory, and cognitive function; however, Ch MD group was more anxious than the control group and surprisingly exhibited the lowest results in motor function. The Ch HD group was the most anxious group and presented deterioration in all the other parameters. The most striking result was that sociability increased in Ch MD group exceeding the results obtained from the control group. However, further investigations and more experiments are needed to clarify a link between caffeine dose and level of sociability.
References
- 1.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 2.Ardais AP, Borges MF, Rocha AS, Sallaberry C, Cunha RA, Porciúncula LO. Caffeine triggers behavioral and neurochemical alterations in adolescent rats. [[Last cited on 2017 Aug 17]];Neuroscience. 2014 270:27–39. doi: 10.1016/j.neuroscience.2014.04.003. Available from: http://www.linkinghub.elsevier.com/retrieve/pii/S0306452214002917 . [DOI] [PubMed] [Google Scholar]
- 3.Bolton S, Null G. Caffeine psychological effects, use and abuse. J Orthomol Psychiatry. 1981;10:202–11. [Google Scholar]
- 4.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging:Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 5.Angelucci ME, Vital MA, Cesário C, Zadusky CR, Rosalen PL, Da Cunha C, et al. The effect of caffeine in animal models of learning and memory. Eur J Pharmacol. 1999;373:135–40. doi: 10.1016/s0014-2999(99)00225-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Yu YP, Ye YL, Zhang JT, Zhang WP, Wei EQ, et al. Spatiotemporal properties of locomotor activity after administration of central nervous stimulants and sedatives in mice. Pharmacol Biochem Behav. 2011;97:577–85. doi: 10.1016/j.pbb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Bădescu SV, Tătaru CP, Kobylinska L, Georgescu EL, Zahiu DM, Zăgrean AM, et al. Effects of caffeine on locomotor activity in streptozotocin-induced diabetic rats. J Med Life. 2016;9:275–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Loke WH. Effects of caffeine on mood and memory. Physiol Behav. 1988;44:367–72. doi: 10.1016/0031-9384(88)90039-x. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-air-land. Psychopharmacology (Berl) 2002;164:250–61. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 12.Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20(Suppl 1):S239–48. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- 13.Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the morris water maze. Neurobiol Learn Mem. 1997;68:203–20. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- 14.Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G. Caffeine/sleep-deprivation interaction in mice produces complex memory effects. Ann Neurosci. 2015;22:139–49. doi: 10.5214/ans.0972.7531.220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. Vis Exp. 2008;22:1–4. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelucci ME, Cesário C, Hiroi RH, Rosalen PL, Da Cunha C. Effects of caffeine on learning and memory in rats tested in the morris water maze. Braz J Med Biol Res. 2002;35:1201–8. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- 17.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011;48:6–10. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988;9:130–4. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson SM, Haas HL, Gähwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–63. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogul DJ, Adams ME, Fox AP. Differential activation of adenosine receptors decreases N-type but potentiates P-type ca2+current in hippocampal CA3 neurons. Neuron. 1993;10:327–34. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- 21.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 22.Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, et al. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–8. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- 23.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–80. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains:An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 25.Waldeck B. Effect of caffeine on locomotor activity and central catecholamine mechanisms:A study with special reference to drug interaction. Acta Pharmacol Toxicol (Copenh) 1975;36:1–23. doi: 10.1111/j.1600-0773.1975.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 26.Nikodijević O, Jacobson KA, Daly JW. Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav. 1993;44:199–216. doi: 10.1016/0091-3057(93)90299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons:Implications for EEG arousal. Science. 1994;263:689–92. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattila ME, Mattila MJ, Nuotto E. Caffeine moderately antagonizes the effects of triazolam and zopiclone on the psychomotor performance of healthy subjects. Pharmacol Toxicol. 1992;70:286–9. doi: 10.1111/j.1600-0773.1992.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 29.Nehlig A, Daval JL, Pereira de Vasconcelos A, Boyet S. Caffeine-diazepam interaction and local cerebral glucose utilization in the conscious rat. Brain Res. 1987;419:272–8. doi: 10.1016/0006-8993(87)90593-2. [DOI] [PubMed] [Google Scholar]
- 30.López-Cruz L, San-Miguel N, Bayarri P, Baqi Y, Müller CE, Salamone JD, et al. Ethanol and caffeine effects on social interaction and recognition in mice:Involvement of adenosine A2Aand A1receptors. Front Behav Neurosci. 2016;10:206. doi: 10.3389/fnbeh.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148:153–63. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- 32.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system:Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 33.McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C, et al. The brain ryanodine receptor:A caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- 34.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–9. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 35.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–8. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 36.Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB, et al. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–85. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 37.Acevedo J, Santana-Almansa A, Matos-Vergara N, Marrero-Cordero LR, Cabezas-Bou E, Díaz-Ríos M, et al. Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms. Neuropharmacology. 2016;101:490–505. doi: 10.1016/j.neuropharm.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–64. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Almosawi S, Baksh H, Qareeballa A, Falamarzi F, Alsaleh B, Alrabaani M, et al. Acute administration of caffeine:The effect on motor coordination, higher brain cognitive functions, and the social behavior of BLC57 mice. Behav Sci (Basel) 2018;8:E65. doi: 10.3390/bs8080065. [DOI] [PMC free article] [PubMed] [Google Scholar]


