Abstract
Purpose
This case series details the clinical progression of patients with primary progressive apraxia of speech (PPAOS) to illustrate, using several methods and supplemental material examples, the changes that occur in speech and language functioning in this patient population.
Method
Four patients who presented with PPAOS were followed between 5 and 6 years. Two patients had predominant articulatory abnormalities (termed phonetic PPAOS), 1 had predominant prosodic abnormalities (prosodic PPAOS), and 1 had relatively equal articulatory and prosodic abnormalities (mixed PPAOS). Detailed speech (including acoustics), language, neurologic, and neuropsychological data were collected.
Results
At initial exam, the patients ranged from 60 to 77 years old, with presenting disease duration of 1.5–10 years. Although all patients presented with an isolated apraxia of speech, all developed varying degrees of aphasia and dysarthria. Patients with phonetic PPAOS developed relatively more severe aphasia than the other 2 patients. All patients eventually had severe functional communication limitations and required alternative or augmentative means of communication, although at varying times postonset of their initial speech problem. Two patients developed dysphagia, 3 showed mild–moderate Parkinsonism, and 2 developed depression. For all patients, simple temporal acoustic measurements documented slowed speech rate over time.
Conclusions
This case series demonstrates that patients who initially present with PPAOS may develop aphasia and dysarthria, cognitive and behavioral changes, and other neurologic signs. Whether these changes can be predicted by the perceptual characteristics of the apraxia of speech is yet to be determined. The detailed longitudinal profiles provide valuable clinical insight into the progression of disease in people with PPAOS.
Supplemental Material
In 1967, Darley described a motor speech disorder (MSD) characterized by varying combinations of slow speaking rate, syllable segmentation, abnormal prosody, distorted sound substitutions, additions, and prolongations, sometimes accompanied by groping and trial-and-error articulatory movements (Darley, 1967, 1969). The disorder was termed apraxia of speech (AOS), an MSD reflecting a problem with the planning and/or programming of speech. AOS is well recognized in the context of stroke, where onset is acute and the condition improves or becomes stable and chronic (McNeil, Robin, & Schmidt, 2009). AOS that is insidious in onset and progresses over time because of neurodegeneration is less well recognized and understood.
For the past decade, patients with primary progressive apraxia of speech (PPAOS) have been the focus of a body of research. It has been demonstrated that AOS can be the earliest and sole manifestation of an underlying neurodegenerative disease (Duffy, 2006; Josephs et al., 2012). Although these patients may technically meet criteria for the nonfluent/agrammatic variant of primary progressive aphasia (PPA), they do not meet the requisite root criteria for PPA, as outlined in the consensus criteria (Gorno-Tempini et al., 2011), as they are not initially aphasic. Prior studies, focusing primarily on longitudinal change of broad neurologic symptoms and neuroimaging of these patients over a single time interval, have shown that disease progression is variable among these patients. Some patients develop features of progressive supranuclear palsy (PSP; Josephs et al., 2006, 2014) or corticobasal syndrome (CBS; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004; Josephs et al., 2006), whereas others may develop agrammatic aphasia (Josephs et al., 2014; Whitwell et al., 2017). Overall, counseling about the prognosis is challenging because we do not yet have a complete picture of the clinical disease course. There is a need for speech-language pathologists (SLPs) who serve individuals with neurodegenerative diseases to better understand the clinical presentation of PPAOS; how it changes over time; whether and when other speech, language, cognitive, and other neurologic signs and symptoms emerge; and how best to stage efforts to manage and treat problems with communication that, by definition, are core clinical features of the syndrome.
Notably, research has demonstrated that the profile of initial AOS characteristics can differ among affected patients. In some cases, the speech pattern is dominated by distorted sound substitutions and additions and other features attributable to articulatory difficulty, whereas, in other cases, the pattern is dominated by slow, prosodically segmented speech (Utianski et al., 2018). The first profile has been designated as phonetic (articulatory) PPAOS and the second as prosodic PPAOS (previously referred to as Types 1 and 2, respectively; Josephs et al., 2013). If there is no clear predominance of characteristics or speech is too mildly or severely affected to permit judgments, the profile is referred to as mixed PPAOS.
Importantly, it appears that the PPAOS pattern subtype may have prognostic implications. In a recent longitudinal study of the evolution of PPAOS in 13 patients, evaluated at two time points, it was observed that, although not broken down by subtype, in some patients with PPAOS, AOS remained the most salient feature for an average of 7 years (Josephs et al., 2014). Other patients developed a severe extrapyramidal syndrome resembling PSP within 5 years, causing significant morbidity. Retrospectively, this more aggressive course was associated with the prosodic subtype (Josephs et al., 2014), which provides support for considering the PPAOS subtypes as a prognostically important variable in the studies of disease progression. Toward that end, a more recent study examined imaging and clinical characteristics of patients with progressive AOS, with and without aphasia, over four visits, spanning 5 years (Whitwell et al., 2017). That study provided converging evidence that patients with phonetic PPAOS showed the development of more severe aphasia and faster rates of progression in motor speech impairments, whereas patients with prosodic PPAOS showed earlier and more rapid rates of decline in Parkinsonism.
The purpose of this study was to carefully illustrate the clinical presentation, the evolution, and the kinds of measures that can describe and measure change in this uncommon patient population. Toward that end, we present a thorough description of four patients who were seen for detailed speech, language, neurologic, and neuropsychological assessment on five or six occasions over a half-decade period, including supplemental materials. This case series documents, in detail, the clinical presentation of representative patients who present with each subtype of PPAOS and lays the groundwork to identify patterns of speech, language, motor, and cognitive changes over time. The data further illustrate means for quantifying the severity and salient and predominant perceptual characteristics of AOS. A better understanding of the evolution of PPAOS will facilitate more accurate prognoses and assist patient counseling and care.
Method
Participants
The study was approved by the Mayo Clinic Institutional Review Board, and all patients consented for enrollment into the study. All participants gave permission to disseminate the video recordings for educational purposes. Starting in 2011, patients with PPAOS and other neurodegenerative speech and language disorders were recruited for enrollment in a longitudinal research study. Patients over 18 years of age, with an informant to provide an independent evaluation of functioning and who spoke English as their primary language, were included. All patients underwent a detailed speech and language examination, neurologic evaluation, neuropsychological testing, and neuroimaging analysis. Imaging will not be discussed here. As of October 2017, all patients who presented with PPAOS (without evidence of aphasia or dysarthria) and who were seen for five or six annual visits were selected for this case series. Four patients met these criteria, out of 38 total patients enrolled with PPAOS; the rest of whom were seen for four or fewer visits. The speech patterns of these four patients are fairly representative of those in the larger cohort, but the length of their follow-up allows for a more detailed illustration of disease progression. Patients were diagnosed with PPAOS at baseline if the dominant presenting sign was that of AOS, without evidence of concomitant aphasia or dysarthria. They did not meet clinical criteria for any other neurologic diagnosis at initial testing, including, but not limited to, PSP (Hoglinger et al., 2017; Litvan et al., 1996), CBS (Armstrong et al., 2013; Boeve, Lang, & Litvan, 2003), or Alzheimer's disease dementia (Albert et al., 2011; Dubois et al., 2014; McKhann et al., 1984). Although these patients were included in prior group imaging studies (Josephs et al., 2014; Josephs et al., 2013; Josephs et al., 2012), their clinical profiles have not previously been detailed. The demographics and details of the presenting clinical signs and symptoms of these patients are described below.
Patients travel from across the United States to participate in this research; as such, the professionals involved in this research do not provide ongoing treatment. Counseling and recommendations regarding treatment are provided at each research session.
Patient 1: Initial Presentation
At initial presentation, Patient 1 (P1) was a 60-year-old Caucasian right-handed woman and a retired 6th-grade math teacher. Ten years prior, she gradually began having trouble expressing herself. Her husband reported that “sounds did not come out right” and that she would “drop sounds.” She further described her speech as slow and halting. Her difficulty had been gradually progressing. At another clinic, she received a diagnosis for her speech difficulty of “conversion disorder” for which she underwent psychiatric intervention with no improvement in her speech symptoms. Later, at another clinic, she received a diagnosis of “laryngeal dysphonia” and was given laryngeal Botox injections with standard side effects (temporary weak voice and dysphagia) but no benefits. At the time of the initial evaluation at the Mayo Clinic, she denied difficulty with spoken or written language comprehension or changes in memory. She denied word retrieval difficulties. She denied change or difficulty with chewing or swallowing, gait, balance, or coordination. She had a lisp as a child for which she received speech therapy, with resolution of the problem. She reported normal language development. Four years prior to the presentation at the Mayo Clinic, she retired because her speech difficulties were interfering with teaching.
Patient 2: Initial Presentation
At initial presentation, Patient 2 (P2) was a 75-year-old Caucasian right-handed woman and a retired registered nurse. She had a 1.5-year history of difficulties in speaking. She reported slow, minimal progression of her difficulties. She described her speech as “slow” and admitted to having difficulty pronouncing words with several syllables and a need to concentrate to maintain accuracy. She denied difficulties with spoken or written language comprehension, memory, changes in personality, or depression. She denied difficulty with word retrieval and reported being able to write or type words that she had difficulty saying. She denied changes in gait, balance, or coordination. She denied changes or difficulty with chewing or swallowing, although she reported occasional problems swallowing liquids mixed with a more solid substance (e.g., grapes). She denied developmental speech or language difficulty. She initiated on-time retirement 10 years prior to the onset of symptoms. At the time of initial presentation, she spent her time volunteering but reported talking less during her volunteer work because of her speech difficulty.
Patient 3: Initial Presentation
At initial presentation, Patient 3 (P3) was a 76-year-old Caucasian right-handed man and a semiretired lawyer. Four years prior, he noted difficulty in making sounds precisely and accurately, particularly during lengthier utterances. At first, these difficulties were only noticeable to him, but they gradually became apparent to family members. Imaging and other assessment with neurology and ear, nose, and throat at outside clinics were unremarkable and did not yield a diagnosis. He denied difficulty with any aspect of language, except for an occasional word retrieval error, not felt to fall outside the normal range either by him or the evaluating SLP (JRD). Reading comprehension and written communication were unaffected. Memory was unaffected. He denied changes in gait, balance, or coordination. He denied changes or difficulty with chewing or swallowing. He denied developmental speech or language difficulty. At the time of the initial evaluation, he was working 20% of the time as a lawyer, but his speech problem was making work increasingly difficult.
Patient 4: Initial Presentation
At initial presentation, Patient 4 (P4) was a 65-year-old Caucasian left-handed man and a retired emergency department physician. Two years prior, he noticed difficulty in pronouncing multisyllabic words and that he was speaking at a slower-than-usual rate. He reported that his speech difficulties gradually worsened over time; friends and family reportedly agreed. He denied difficulty with spoken language or reading comprehension or difficulty with written language expression. He denied memory loss, confusion, hallucinations, or changes in behavior, mood, or sleep. He denied changes in gait, balance, coordination, or penmanship. He denied changes or difficulty with chewing or swallowing. He denied any childhood history of language difficulty but reported mild persistent developmental stuttering, which had not worsened since the onset of his new speech difficulties. Largely because of his speech difficulty, he retired from work as an emergency room physician.
Speech and Language Examinations
Several speech and language measures were administered as part of the standard research protocol, as previously reported (Duffy et al., 2017; Josephs et al., 2012, 2013, 2014). Perceptual judgments of speech included (a) a 0–4 rating of AOS severity (1 = mild; 4 = severe), as an index of AOS severity regardless of its specific features; (b) a 1–10 rating (10 = normal) of MSD severity (adapted from Yorkston, Strand, Miller, & Hillel, 1993), which indexed the degree of functional impairment associated with the speech difficulty; (c) the Apraxia of Speech Rating Scale–Third Edition (ASRS-3), which quantified the severity and prominence of several AOS features, detailed below; and (d) an articulation error score (AES), also described below (Strand, Duffy, Clark, & Josephs, 2014; Utianski et al., 2018). Language measures included the Western Aphasia Battery (WAB; Kertesz, 2007), from which the Aphasia Quotients (AQs) served as a composite measure of global language ability; the WAB includes measures of repetition, naming, spontaneous speech fluency, word finding, grammatical competence, verbal and reading comprehension, and writing. A WAB-AQ score of 93.8 or above was considered normal, consistent with standard test guidelines. Additional supplementary reading and writing tasks from the WAB were also administered. A 22-item version of the Token Test, Part V (De Renzi & Vignolo, 1962), was used to assess verbal comprehension of complex instructions. Age-related norms were utilized to determine an abnormal score on the Token Test (Wertz, Keith, & Custer, 1971). In addition to the animal fluency task (FAS) included in the WAB, both letter (Loonstra, Tarlow, & Sellers, 2001) and action/verb (Woods et al., 2005) word fluency were assessed. Grammar was assessed by review of conversational speech and verbal and written picture description tasks. Agrammatism was assessed in both speech and writing, after which a consensus determination was made as to whether a patient qualified as agrammatic. The patients included in this study showed no evidence of agrammatism. Specifically, they demonstrated no more than a single instance of omission of articles/function words or syntax errors in conversational speech, narrative picture description, and writing at initial presentation. The 15-item Boston Naming Test (BNT; Lansing, Ivnik, Cullum, & Randolph, 1999) served as a sensitive measure of a confrontation naming ability; a score of 13 or above was considered normal. The Pyramids and Palm Trees Test was also administered as a measure of semantic access (Howard & Patterson, 1992); a score of 49 or above was considered normal. As some of the WAB subtests can be influenced by nonaphasic deficits, including, but not limited to, AOS, participants were required to perform below normal on at least two measures of language, including a demonstration of agrammatism in spoken language or writing, poor scores on the BNT, or reduced WAB-AQ. The composite of the aforementioned tests was utilized in the judgment of aphasia severity (1 = mild; 4 = severe). No patients in this study met criteria for aphasia at the initial visit.
Two SLPs (authors JRD, EAS, or HMC) made independent judgments regarding the presence, nature, and severity of AOS (including subtype designation), dysarthria, and aphasia. All judgments were made following the administration of standardized testing but prior to scoring the AES and the Apraxia of Speech Rating Scale (ASRS; Strand et al., 2014). Raters were blinded to previous scores and consensus diagnoses. In the event of a disagreement, the SLPs discussed until an agreement was reached; no such disagreements occurred in the patients described here.
PPAOS Subtype Assessment
A previous study of reliability for the PPAOS subtype designation demonstrated 95% independent interrater agreement (Josephs et al., 2012); a second independent consensus classification was conducted 1 to 5 years following initial consensus diagnosis. A designation of phonetic PPAOS was made if distorted sound substitutions or additions (often increasing in prominence with increased utterance length or syllable or word complexity) were judged to clearly dominate the speech pattern. A designation of prosodic PPAOS was made if syllable segmentation or lengthened intersegment durations between syllables, words, or phrases were judged to clearly dominate the speech pattern. Importantly, the subtype designation is reflective of the pattern that predominates; it does not imply that it is the sole disruption of speech output. If there was no clear predominance of characteristics for phonetic PPAOS or prosodic PPAOS, the patient received a diagnosis of mixed PPAOS.
Judgments of the PPAOS and its subtype were based on spoken responses to the Spontaneous Speech subtests of the WAB and several supplementary motor speech tasks that included vowel prolongation, speech-alternating motion rates (e.g., rapid repetitions of /pΛ/, /tΛ/, and /kΛ/), speech sequential motion rates (e.g., rapid repetitions of /pΛtΛkΛ/), a word and sentence repetition task (see Appendix of Duffy et al., 2015, for the full list of stimuli), and a conversational speech sample. The combination of these sources of speech responses allows for the reliable judgments of the PPAOS subtype (Josephs et al., 2013). The consensus classification of the PPAOS subtype was made by the perceptual judgment of speech characteristics only, independent of the ASRS scores (see below), acoustic data, results of clinical neurology and neuropsychology assessments, and neuroimaging.
ASRS-3
The ASRS-3 (see Appendix of Utianski et al., 2018) was used to rate the presence or absence, relative prominence, and severity of several characteristics associated with AOS. An earlier version of the ASRS was used in previous studies of progressive AOS (Duffy et al., 2015, 2017; Josephs et al., 2013, 2012; Strand, Duffy, Clark, & Josephs, 2014). The current 13-item version has been reorganized to highlight the different features of apraxia speech in the domains of articulation and prosody. The ASRS-3 has fewer items than the original version, and the scoring guidelines have been made more explicit, both intended to improve reliability. The total ASRS-3 score can range from 0 to 52 (where 0 = no abnormal speech characteristics); the total score captures the severity of AOS but does not capture the presence and prominence of specific perceptual features. For this study, those items that capture the salient features associated with each subtype and their change over time were of particular interest. Toward that end, the sum of scores for the four items that best capture phonetic (articulatory) problems, Items 1–4, is the phonetic subscore. The sum of scores for the four items that best capture prosodic abnormalities, Items 5–8, is the prosodic subscore. The items were chosen to capture the articulatory and prosodic processes that relate to the features described in recent papers (Ballard et al., 2015; McNeil et al., 2009). In its current form, the ASRS can be used as a list of features whose presence may help point to the diagnosis, characterize the nature of the problem, and index the severity of the problem.
AES
An AES was derived from the proportion of incorrectly produced words on the supplementary speech tasks. This included three repetitions of 13 words plus one repetition of three sentences for a total of 56 words. Productions were scored as incorrect if any of the following characteristics were noted: distorted or undistorted sound substitutions, additions, or repetitions, sound omissions, sound prolongations, beyond those consistent with overall speech rate, false starts, and successful or unsuccessful attempts to correct sound errors. This error score may underestimate total abnormalities within and across words because a word scored as incorrect might contain more than one error and because distortions that did not cross phonemic boundaries were not scored as incorrect. If we scored distortions that did not cross phonemic boundaries as errors, many, if not most, patients would be at 100% error rate. In addition, we wanted to minimize the overlap with dysarthria; distortions associated with dysarthria would artificially inflate the error score.
Acoustic Measurements
Imitation of words of increasing length and multisyllabic words are known to be sensitive to AOS and its distinction from aphasia (Ballard et al., 2014; Duffy et al., 2017), AOS subtype (Duffy et al., 2015), and AOS progression (Laganaro, Croisier, Bagou, & Assal, 2012). Note that the patients presented here are unique from those previously assessed longitudinally using temporal acoustic measurements (Duffy et al., 2015). Toward that end, temporal measures were completed for three consecutive repetitions for the word catastrophe, selected for illustrative purposes and previously documented diagnostic sensitivity and specificity (Duffy et al., 2017). Durations were measured from the initial stop release or the initial onset of noise energy or voicing for each response to cessation or a marked reduction of the acoustic energy at the end of the response. Syllables per second rates were derived by dividing the number of syllables in the target response by the average duration. In some instances, the production could not be measured validly secondary to the severity of distortion, including loss of syllables, interference from pseudobulbar affect, and multiple restarts, revisions, and repetitions. In the final visits, some of the patients were not asked to complete the task secondary to fatigue and frustration, so those data are missing.
Dysarthria
Dysarthria was diagnosed when the features consistent with dysarthria or a given dysarthria type were noted (Duffy, 2005). For instance, the presence of flutter, reduced loudness, and breathy vocal quality were considered consistent with a diagnosis of hypokinetic dysarthria, whereas strained voice quality and hypernasality, in the presence of confirmatory signs (e.g., pathologic reflexes, pseudobulbar affect), were consistent with a diagnosis of spastic dysarthria.
Neurologic Evaluation
Clinical and demographic information were obtained. Neurologic testing was conducted by a behavioral neurologist (KAJ) and included assessments to characterize general cognitive ability (Montréal Cognitive Assessment [MoCA]; Nasreddine et al., 2005, with a cutoff score of 26 or lower to indicate impairment), frontal lobe function (Frontal Assessment Battery [FAB]; Dubois, Slachevsky, Litvan, & Pillon, 2000, where the maximum score is 18 and higher scores indicate better performance and a measure of behavioral dysfunction, and the Frontal Behavioural Inventory [FBI]; Kertesz, Davidson, & Fox, 1997, where the maximum score is 72 and lower scores indicate better performance), neuropsychiatric features (Neuropsychiatric Inventory Questionnaire [NPI-Q]; Kaufer et al., 2000, where the maximum score is 36 and lower scores indicate better performance), and motor impairments (the Movement Disorders Society-sponsored version of the Unified Parkinson's Disease Rating Scale motor subsection [UPDRS III]; Goetz et al., 2008, where the maximum score is 120 and lower scores indicate better performance). Nonverbal oral apraxia (NVOA) was assessed with an 8-item measure consisting of four gestures (“cough,” “click your tongue,” “blow,” “smack your lips”), each repeated twice. Each item received 0 to 4 points, with a score of 4 awarded for an immediate, accurate response; a score of 32 indicates no errors. A cutoff of 29 was used to establish the presence of NVOA (Botha et al., 2014).
Neuropsychological Testing
A clinical neuropsychologist (MMM) oversaw the test administration, scoring accuracy, and quality control of the neuropsychological assessment. Tests were selected to assess different domains of cognitive function and to complement the speech and language battery. The domains tested include memory (Auditory Verbal Learning Test; Rey, 1964), motor speed (Trail-Making Test A; Spreen & Strauss, 1998), divided attention/cognitive flexibility (Trail-Making Test B), visuospatial and visuoperceptual function (Cube Analysis and Incomplete Letters of the Visual Object and Space Perception Battery, respectively; Warrington & James, 1991), and visual constructional skills (Rey–Osterrieth Complex Figure Test; Osterrieth, 1944; Rey, 1964).
Results
This case series followed four patients (two women, two men) who presented with PPAOS. Three of the four patients were right-handed. At the initial exam, the patients ranged from 60 to 77 years old, with presenting disease duration of 1.5–10 years. They had between 15–20 years of education. The demographic information is reported in Table 1. Results of the speech, language, neurologic, and neuropsychological assessments are detailed below and in Tables 2–5.
Table 1.
Demographic information for all patients for each visit.
| Patient ID | Visit number | Age at exam (years) | Education | Sex | Handedness | Disease duration (years) | Consensus diagnosis | Mode of communication |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 60 | 18 | F | Right | 10 | Phonetic | Verbal |
| 1 | 2 | 62 | 12 | Phonetic + aphasia | Verbal | |||
| 1 | 3 | 63 | 13 | Phonetic + aphasia + dysarthria | Writing/ASL | |||
| 1 | 4 | 64 | 14 | Mixed + aphasia + dysarthria | ASL/writing tablet | |||
| 1 | 5 | 65 | 15 | Mixed + aphasia | iPad speaking app | |||
| 1 | 6 | 66 | 16 | Mixed + aphasia | Alphabet board | |||
| 2 | 1 | 76 | 15 | F | Right | 1.5 | Prosodic | Verbal |
| 2 | 2 | 77 | 3.5 | Prosodic + dysarthria | Verbal | |||
| 2 | 3 | 78 | 4.5 | Prosodic + dysarthria | Verbal | |||
| 2 | 4 | 79 | 5.5 | Prosodic + dysarthria | Verbal | |||
| 2 | 5 | 80 | 6.5 | Prosodic + dysarthria + aphasia | Verbal | |||
| 2 | 6 | 81 | 7.5 | Mixed + dysarthria + aphasia | Writing/writing tablet | |||
| 3 | 1 | 77 | 20 | M | Right | 4 | Mixed | Verbal |
| 3 | 2 | 79 | 5 | Mixed + dysarthria | Verbal | |||
| 3 | 3 | 80 | 6 | Prosodic + dysarthria | Verbal | |||
| 3 | 4 | 81 | 7 | Mixed + dysarthria | Writing | |||
| 3 | 5 | 82 | 8 | Mixed + dysarthria | Writing tablet | |||
| 3 | 6 | 83 | 9 | Mixed + dysarthria + aphasia | Writing tablet | |||
| 4 | 1 | 65 | 20 | M | Left | 2 | Phonetic | Verbal |
| 4 | 2 | 67 | 4 | Phonetic + aphasia | Verbal | |||
| 4 | 3 | 68 | 5 | Phonetic + aphasia | Verbal | |||
| 4 | 4 | 69 | 6 | Phonetic + aphasia + dysarthria | Verbal | |||
| 4 | 5 | 70 | 7 | Mixed + aphasia + dysarthria | Writing |
Note. Disease duration is time from reported symptom onset to exam. F = female; ASL = American Sign Language; M = male.
Speech Findings
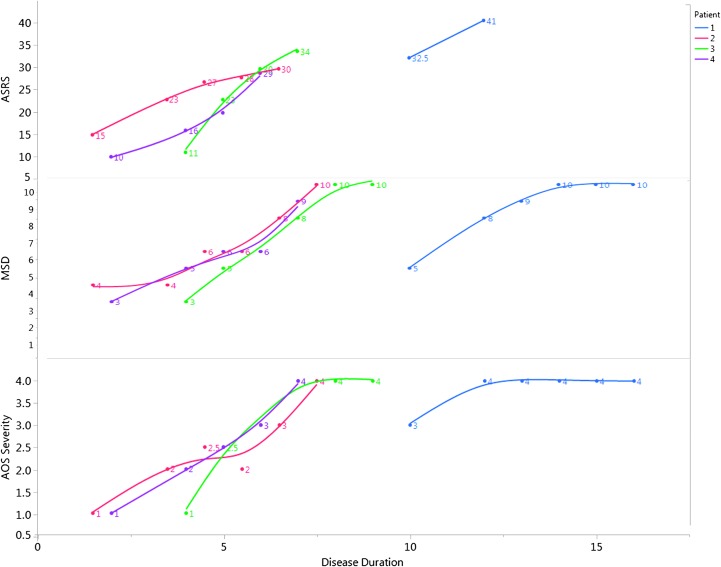
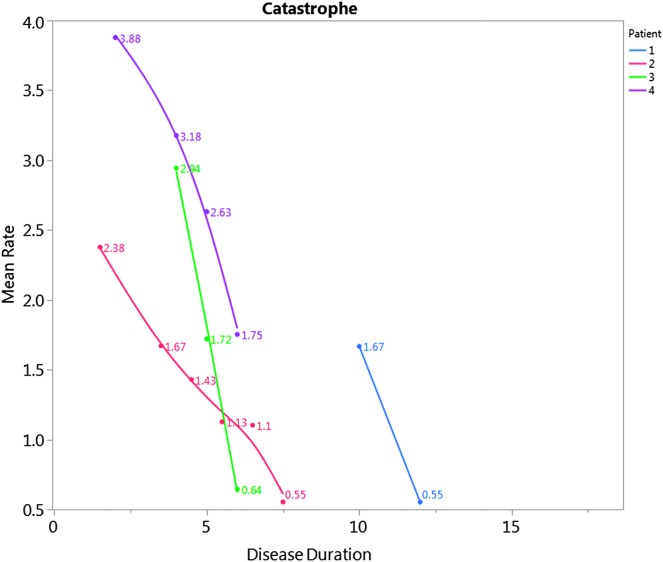
Speech findings are reported in Table 2. Two patients presented with phonetic PPAOS (P1 and P4), in which articulatory difficulties predominated; one presented with a prosodic PPAOS (P2), in which slow, segmented speech predominated; and one patient presented with a mixed PPAOS (P3), in which the AOS was too mild to reliably determine if there was a predominant phonetic or prosodic pattern. The patient who was reported to have mixed PPAOS at initial presentation (P3) was deemed to have a prosodic PPAOS at initial review, but when the videos were reviewed several years later, he was reclassified as having mixed PPAOS; as he progressed, he was diagnosed with prosodic PPAOS. Interestingly, all four patients were classified as mixed in the final evaluation, associated with increased severity of AOS (see Table 1 and Figure 1), concomitant aphasia and dysarthria, and, ultimately, significantly reduced verbal output. The MSD severity score, which reflects functional impairment secondary to MSDs, increased for all four patients (see Figure 1). The ASRS score increased over time (see Figure 1), reflecting increased severity, and the phonetic and prosodic subscores matched the overall perceptual consensus diagnosis of the subtype. The AES increased (worsened) over time for all patients. During the later follow-up visits, verbal output was reduced to a degree that the AES and ASRS could not be validly scored. The average word syllable rate decreased for the production of the word catastrophe over time in all patients (see Figure 2). Overall, all four patients reported here took longer to say the word than healthy controls (Duffy et al., 2017), even at initial presentation (3+ SDs beyond the mean).
Table 2.
Speech information for all patients for each visit.
| Patient ID | Visit number | AOS severity (/4) | Aphasia severity (/4) | Dysarthria severity (/4) | Dysarthria type | Dysphagia (present?) | MSD severity (/10) | ASRS 3 total | ASRS 3 phonetic | ASRS 3 prosodic | AES % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 3 | 0 | 0 | None | No | 6 | 33 | 14 | 11 | 67.9 |
| 1 | 2 | 4 | 1 | 0 | None | No | 3 | 41 | 13 | 13 | 86 |
| 1 | 3 | 4 | 1 | 0.5 | Spastic | Yes | 2 | 48 | 12 | 16 | CND |
| 1 | 4 | 4 | 2 | 0.5 | Spastic | Yes | 1 | CND | CND | CND | CND |
| 1 | 5 | 4 | 2 | CND | CND | Yes | 1 | CND | CND | CND | DNT |
| 1 | 6 | 4 | 3 | CND | CND | Yes | 1 | CND | CND | CND | DNT |
| 2 | 1 | 1 | 0 | 0 | None | No | 7 | 15 | 4 | 8 | 12.5 |
| 2 | 2 | 2 | 0 | 0.5 | Hypokinetic | No | 7 | 23 | 6 | 11 | 7.1 |
| 2 | 3 | 2.5 | 0 | 1 | Hypokinetic | No | 5 | 27 | 6 | 12 | 16.4 |
| 2 | 4 | 2 | 0 | 1 | Hypokinetic | No | 5 | 28 | 7 | 12 | 30.4 |
| 2 | 5 | 3 | 1 | 2 | Hypokinetic | No | 3 | 30 | 10 | 11 | 40.8 |
| 2 | 6 | 4 | 1 | 1 | Hypokinetic | No | 1 | CND | CND | CND | DNT |
| 3 | 1 | 1 | 0 | 0 | None | No | 8 | 11 | 4 | 4 | 5.4 |
| 3 | 2 | 2.5 | 0.5 | 1.5 | Spastic | Yes | 6 | 23 | 7 | 9 | 32.1 |
| 3 | 3 | 3 | 0.5 | 1.5 | Spastic | Yes | 5 | 30 | 8 | 11 | 48.2 |
| 3 | 4 | 4 | 0.5 | 2 | Spastic | Yes | 3 | 34 | 9 | 12 | 46.7 |
| 3 | 5 | 4 | 0.5 | 2 | Spastic | Yes | 1 | CND | CND | CND | CND |
| 3 | 6 | 4 | 2 | CND | Spastic | Yes | 1 | CND | CND | CND | CND |
| 4 | 1 | 1 | 0 | 0 | None | No | 8 | 10 | 4 | 3 | 10.7 |
| 4 | 2 | 2 | 1 | 0 | None | No | 6 | 16 | 6 | 4 | 33.9 |
| 4 | 3 | 2.5 | 1 | 0 | None | No | 5 | 20 | 8 | 4 | 39.3 |
| 4 | 4 | 3 | 1.5 | 1.5 | Hypokinetic | No | 5 | 29 | 12 | 7 | 78.2 |
| 4 | 5 | 4 | 3 | CND | Hypokinetic | No | 2 | CND | CND | CND | DNT |
Note. Maximum score noted in the column header. For the ASRS and AES, this indicates that a patient did not produce enough speech to validly and reliably score these measures. AOS = Apraxia of Speech; MSD = Motor Speech Disorders Severity Rating; ASRS-3 = Apraxia of Speech Rating Scale–Third Edition; AES = articulation error score; CND = could not determine, although the test was attempted; DNT = did not test.
Figure 1.
Total score on the Apraxia of Speech Rating Scale (ASRS; top), motor speech disorders (MSDs) severity rating score (middle), and apraxia of speech (AOS) severity (where 1 = mild and 4 = severe; bottom), against relative reported disease duration for each patient. The MSD severity rating scores are reported in inverse to facilitate ease of comparison; here, 1 = not impaired and 10 = severely impaired.
Figure 2.
Mean rate (syllables/second) for productions of the word catastrophe against relative reported disease duration for each patient. In healthy controls, the average syllable rate for production of catastrophe was reported as 4.84 syllables/second (SD = 0.41; Duffy et al., 2017).
Although all patients presented with an isolated AOS, all of them developed dysarthria at some point during the course of their follow-up visits. Two developed a hypokinetic dysarthria (P2 and P4; characterized by reduced loudness and a hoarse-breathy voice quality at 3.5 and 6 years postsymptom onset, respectively) and, two, a spastic dysarthria (P1 and P3; characterized predominantly by a strained voice quality at 13 and 5 years postsymptom onset, respectively). The two patients with spastic dysarthria reported concomitant dysphagia. The two patients with hypokinetic dysarthria denied any difficulty with chewing or swallowing during all follow-up visits. At initial examination, all four patients were using speech to communicate, but all of them eventually used augmentative and alternative communication (AAC; e.g., writing tablet, iPad with speaking app). Patients were between 7 and 10 years postonset before AAC was needed and/or before muteness emerged.
A brief video of each patient repeating words of increasing length and complexity at each visit are provided in Supplemental Materials S1–S4. Among the videos, increased and increasing difficulty with lengthier and more complex words, advancing severity of AOS, development of dysarthria, and relative frustration and reactive emotional responses are illustrated. During the final visits, some patients were no longer able to complete the tasks.
Language Findings
Language findings are reported in Table 3. Although all patients presented with an isolated AOS at initial presentation, all of them developed aphasia during the course of the follow-up visits. Scores on the WAB were overall consistent with clinical judgments about the presence and severity of aphasia. The scores on each WAB subtest, for each visit for all patients, are provided in Supplemental Material S6. Writing samples for each patient, over time, are shown in Figures 3 –6. The data document that WAB Confrontation Naming was largely preserved until the final visits, although the BNT scores suggest reduced naming in P1 and P4. Scores on the Pyramids and Palm Trees Test were normal in all patients over time, suggesting preserved vocabulary. Verbal fluency was initially reduced for all patients, possibly associated with AOS at presentation but also declined over time in all patients. The severity of aphasia at final visits appears greater in P1 and P4 (aphasia severity = 3, or marked; see Table 2), who presented with phonetic PPAOS. All patients turned to AAC when the severity of motor speech impairment hindered effective verbal communication. Writing was largely preserved for most patients, who preferred to use the writing tablet or iPad over pen-to-paper writing for efficiency. This was a successful form of communication, but its effectiveness was impeded when aphasia became more prominent (as in P1) and/or when limb motor impairments became more prominent (as in P3).
Table 3.
Language data for all patients for each visit.
| Patient ID | Visit number | Token Test (/22) | WAB-AQ (/100) | BNT total (/15) | Action fluency | Letter fluency | PPTT (/52) | Agrammatism |
|
|---|---|---|---|---|---|---|---|---|---|
| Speaking | Writing | ||||||||
| 1 | 1 | 22 | 94.6 | 14 | 12 | 28 | 51 | No | No |
| 1 | 2 | 21 | 84 | 15 | 11 | 22 | 52 | No | No |
| 1 | 3 | 22 | CND | 15 | 11 | 25 | 52 | Yes | Yes |
| 1 | 4 | 16 | 74.6 | 14 | DNT | DNT | 51 | Presumed | Yes |
| 1 | 5 | 11 | CND | 9 | DNT | DNT | 50 | Presumed | Yes |
| 1 | 6 | DNT | CND | 8 | DNT | DNT | DNT | Presumed | Presumed |
| 2 | 1 | 22 | 98.7 | 14 | 11 | 15 | 50 | No | No |
| 2 | 2 | 22 | 96.4 | 15 | 9 | 11 | 50 | No | No |
| 2 | 3 | 21 | 96 | 15 | 7 | 6 | 50 | No | No |
| 2 | 4 | 20 | 95.4 | 15 | 8 | 9 | 50 | No | No |
| 2 | 5 | 20 | 85.6 | 15 | 3 | 7 | 50 | Yes | Yes |
| 2 | 6 | 18 | CND | DNT | DNT | DNT | 49 | Presumed | Yes |
| 3 | 1 | 19 | 100 | 13 | 16 | 18 | 51 | No | No |
| 3 | 2 | 20 | 96.6 | 12 | 12 | 15 | 51 | No | No |
| 3 | 3 | 19 | 96.6 | 14 | 11 | 22 | 52 | No | No |
| 3 | 4 | 21 | 97 | 13 | DNT | DNT | 52 | No | Yes |
| 3 | 5 | 19 | CND | 13 | 6 | 17 | 50 | No | Yes |
| 3 | 6 | DNT | CND | DNT | DNT | DNT | 51 | Yes | Yes |
| 4 | 1 | 20 | 97.4 | 15 | 15 | 24 | 50 | No | No |
| 4 | 2 | 15 | 96 | 15 | 11 | 27 | 51 | No | No |
| 4 | 3 | 15 | 87.6 | 12 | 13 | 23 | 51 | No | No |
| 4 | 4 | 7 | 79.9 | 14 | 12 | 20 | 51 | Yes | Yes |
| 4 | 5 | 4 | CND | 10 | 0 | 13 | 51 | Presumed | Yes |
Note. Maximum score noted in each column header. If patients were unable to complete any portion of the WAB testing, whether that was secondary to motoric limitations or, more often, fatigue, a WAB-AQ could not be calculated. For the ASRS and AES, this indicates that a patient did not produce enough speech to validly and reliably score these measures. WAB-AQ = Western Aphasia Battery–Aphasia Quotient; BNT = Boston Naming Test–Short Form; PPTT = Pyramids and Palm Trees Test; CND = could not determine, although the test was attempted; DNT = did not test; Presumed = not enough produced to judge but previously demonstrated.
Figure 3.
Writing samples for P1. In Visit 5, the patient used an iPad with predictive text. In Visit 6, the patient was not asked to attempt the written picture description secondary to the severity of difficulties and associated fatigue. The spelling sample (irregular words to dictation) was obtained as the patient pointed to letters on an alphabet board. Circles and values (e.g., .5) were added by the examiner during scoring.
Figure 4.
Writing samples for Patient 2. Articles and words circled or in brackets (e.g., [A]) were added by the examiner during scoring.
Figure 5.
Writing samples for Patient 3. Articles and words circled or in brackets (e.g., [A]) were added by the examiner during scoring.
Figure 6.
Writing samples for Patient 4. Articles and words circled or in brackets (e.g., [A]), along with values (e.g., 8) were added by the examiner during scoring.
Neurologic Findings
Neurologic findings are reported in Table 4. Scores on the MoCA, a test of general cognitive functioning, generally declined over time, with fluctuations in scores over time noted in P2 and P3. All patients demonstrated scores below the normal range on the MoCA between Visits 3 and 4, suggesting the emergence of cognitive impairment. Not surprisingly, the scores consistently declined in the patients for whom aphasia was more severe (P1 and P4). On the FAB, there was a general decline in scores over time in all patients, suggesting impaired frontal lobe function. Scores on the FBI fluctuated for P1 and P2 but reliably increased (worsened) in P3 and P4, further supporting the impaired frontal lobe function. Scores on the NPI-Q were mildly elevated in P1 and P4 but did not meet the threshold for diagnostic psychiatric concerns. One patient developed a mild facial dystonia (P1). All patients demonstrated reduced motor function, as reflected by increased scores on the UPDRS III over time. This was mainly due to bradykinesia (slowness of movement) and, in some instances, postural instability; tremor was rarely observed. Three of the four patients (P1, P2, and P3) developed mild–moderate Parkinsonism. One patient ultimately met diagnostic criteria for CBS (Armstrong et al., 2013; Boeve et al., 2003) at 15 years disease duration (P1). Two patients developed symptoms of depression (P2 and P4). All patients presented with or developed an NVOA.
Table 4.
Neurologic data for all patients for each visit.
| Patient ID | Visit number | MoCA (/30) | FAB (/18) | FBI (/72) | UPDRS III (/120) | NPI-Q (/36) | NVOA (/32) | Additional symptoms |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 27 | 18 | 12 | 5 | 5 | 14 | Pseudobulbar affect |
| 1 | 2 | 27 | 16 | 6 | 16 | 4 | 0 | Mild Parkinsonism |
| 1 | 3 | 25 | 16 | 6 | 13 | 2 | 3 | |
| 1 | 4 | 23 | 14 | 7 | 41 | 4 | 0 | Facial dystonia |
| 1 | 5 | 21 | 8 | 16 | 49 | 5 | 0 | CBS |
| 1 | 6 | 12 | DNT | DNT | 69 | DNT | 0 | Myoclonus/mod. Parkinsonism |
| 2 | 1 | 28 | 17 | 3 | 6 | 1 | 32 | |
| 2 | 2 | 21 | 15 | 1 | 13 | 0 | 31 | Mild Parkinsonism |
| 2 | 3 | 25 | 15 | 8 | 21 | 0 | 20 | Yes/no reversal |
| 2 | 4 | 27 | 15 | 2 | 34 | 2 | 21 | |
| 2 | 5 | 24 | 15 | 5 | 34 | 2 | 6 | Depression |
| 2 | 6 | 23 | 1 | 13 | 61 | 0 | 0 | Mod. Parkinsonism |
| 3 | 1 | 25 | 17 | 3 | 15 | 0 | 31 | Postural tremor |
| 3 | 2 | 27 | 15 | 3 | 24 | 0 | 30 | Yes/no reversal |
| 3 | 3 | 29 | 16 | 9 | 32 | 1 | 20 | Mild Parkinsonism |
| 3 | 4 | 24 | 13 | 20 | 30 | 2 | 19 | Mild rigidity |
| 3 | 5 | 26 | 14 | 13 | 55 | 1 | 11 | Mod. Parkinsonism |
| 3 | 6 | 24 | 12 | 28 | 58 | 6 | 2 | |
| 4 | 1 | 27 | 18 | 2 | 8 | 0 | 32 | |
| 4 | 2 | 26 | 17 | 4 | 6 | 2 | 32 | Pronoun reversal |
| 4 | 3 | 25 | 16 | 14 | 6 | 2 | 26 | Hearing loss |
| 4 | 4 | 21 | 17 | 17 | 7 | 6 | 8 | Stereotype (“ok”)/depression |
| 4 | 5 | 21 | 14 | 26 | 15 | 6 | 1 |
Note. Maximum score noted in each column header. MoCA = Montréal Cognitive Assessment; FAB = Frontal Assessment Battery; FBI = Frontal Behavioural Inventory; UPDRS III = the Movement Disorders Society-sponsored version of the Unified Parkinson's Disease Rating Scale motor subsection; NPI-Q = Neuropsychiatric Inventory Questionnaire; NVOA = nonverbal oral apraxia; CBS = corticobasal syndrome; Mod. = moderate; DNT = did not test.
Neuropsychological Findings
Neuropsychological findings are reported in Table 5. Scores on the Auditory Verbal Learning Test suggest overall average to above average short- and long-term memory in all patients. Declining scores on Trail A for P3 and P4 suggest declining motor speed, whereas declining scores on Trail B for all patients suggest reduced cognitive flexibility/divided attention. The scores on the Rey–Osterrieth Complex Figure Test are difficult to interpret, given fluctuations across visits; this most likely reflects nonsystematic variability in performance between visits rather than “true” sporadic improvement and subsequent decline. Visuospatial skills appear to decline in P3 and P4, whereas the visuoperceptual function remained normal across all visits during which it was tested.
Table 5.
Neuropsychological testing for all patients for each visit.
| Patient ID | Visit number | AVLT ST % retention | AVLT LT % retention | Trails A | Trails B | Rey-O | VOSP letters (/20) | VOSP cube (/10) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 14 | 13 | 11 | 11 | 16 | 20 | 10 |
| 1 | 2 | 14 | 13 | 10 | 9 | 16 | 20 | 10 |
| 1 | 3 | 14 | 14 | 11 | 11 | 13 | 20 | 10 |
| 1 | 4 | 14 | 14 | 8 | 7 | 16 | 20 | 10 |
| 1 | 5 | 14 | 13 | DNT | DNT | DNT | 19 | DNT |
| 1 | 6 | DNT | DNT | DNT | DNT | DNT | DNT | DNT |
| 2 | 1 | 14 | 15 | 9 | 10 | 11 | 20 | 10 |
| 2 | 2 | 12 | 13 | 8 | 10 | 17 | 20 | 9 |
| 2 | 3 | 9 | 10 | 8 | 10 | 12 | 19 | 9 |
| 2 | 4 | 12 | 15 | 9 | 7 | 12 | 19 | 10 |
| 2 | 5 | 11 | 15 | DNT | DNT | DNT | 19 | DNT |
| 2 | 6 | 12 | 15 | DNT | DNT | DNT | 19 | DNT |
| 3 | 1 | 10 | 11 | 6 | 8 | 7 | 20 | 8 |
| 3 | 2 | 12 | 10 | 6 | 7 | 6 | 20 | 9 |
| 3 | 3 | 14 | 15 | 6 | 7 | 11 | 20 | 8 |
| 3 | 4 | 14 | 15 | 4 | 4 | 11 | 20 | 10 |
| 3 | 5 | 9 | 15 | DNT | DNT | DNT | 20 | DNT |
| 3 | 6 | 12 | 15 | DNT | DNT | DNT | 20 | DNT |
| 4 | 1 | 11 | 8 | 15 | 13 | 10 | 20 | 10 |
| 4 | 2 | 11 | 14 | 10 | 10 | 14 | 20 | 9 |
| 4 | 3 | 10 | 14 | 9 | 8 | 14 | 20 | 8 |
| 4 | 4 | 14 | 11 | 6 | 7 | 14 | 20 | 7 |
| 4 | 5 | 10 | 11 | DNT | DNT | DNT | 20 | DNT |
Note. Scores for Auditory Verbal Learning Test (AVLT) short term (ST) and long term (LT), Trails A, Trails B, and Rey-O are Mayo's Older Americans Normative Studies scores (Machulda et al., 2007; Steinberg, Bieliauskas, Smith, & Ivnik, 2005). Cube Analysis and Incomplete Letters of the Visual Object and Space Perception Battery (VOSP) are presented in raw scores (maximum scored noted in column header). Rey-O = Rey–Osterrieth Complex Figure Test; DNT = did not test (too severe).
Discussion
In this report, we detail the profiles of speech, language, neurologic, and neuropsychological functioning in four patients who presented with PPAOS and were comprehensively assessed over a 5- to 6-year period. The progression of their communication difficulties was documented on a number of speech and language measures that included standardized tests, clinical tasks administered and scored in a standard manner, reliably scored rating scales, and an acoustic measure of syllable rate. Several of those measures documented the baseline features of their AOS and how those features changed over time as indexed by a perceptual rating scale of the presence and prominence of its possible features, a measure of articulatory impairment, an acoustic measure of rate, and rating scales of intelligibility and global severity. An additional assessment also documented the emergence and progression of aphasia and dysarthria and the neurological and neuropsychological symptoms and signs that also emerged over time.
Problems in addition to AOS (e.g., aphasia, dysarthria, Parkinsonism) emerged anywhere between 3.5 years, the earliest onset of dysarthria, and 6.5 years, the earliest onset of aphasia, to 12 years postonset. Overall, it appears that the rate of progression of AOS and the emergence and progression of additional problems varies among individuals with PPAOS. This is consistent with recent clinical neuroimaging findings that assessed changes in 34 patients with progressive AOS with and without aphasia (Whitwell et al., 2017). Similarly, in this study, patients with phonetic PPAOS (P1 and P4) showed more severe aphasia and a pronounced decline in motor speech, whereas the patient with prosodic PPAOS (P2) showed more prominent emergence of Parkinsonism, as indicated by higher UPDRS III scores (Whitwell et al., 2017).
The notion that there are perceptually distinct presentations of PPAOS is one that is still under scrutiny and exploration (Utianski et al., 2018). Three of the four patients were reliably rated as having either predominant phonetic or prosodic disturbances associated with AOS. However, an important point is made with the difficulty of categorizing P3, whose subtype classification was not consistent between consensus meetings. The mild nature of his AOS, consistent with a low total ASRS and AES, was not clearly predominated by prosodic disturbances at first visit but rather there was an equal prominence of each perceptual characteristic. As P3 became more moderately affected, the predominance of prosodic features became evident. On the other end of the spectrum, all patients were deemed as mixed by their final visit, largely reflecting pervasive and substantial impairment of articulatory and prosodic aspects of speech production and possibly a blurring of distinctions by the dysarthria that emerged in each case. This suggests that the distinction among separable features of AOS diminishes over time as AOS becomes more severe, dysarthria develops, and there is a reduction in verbal output. Supplemental Material S5, the commentary on Supplemental Materials S1–S4, further describes the illustrated speech patterns. Increased severity of AOS and the consensus diagnosis regarding the predominance of phonetic and prosodic disturbances is further corroborated by the ASRS. Crucially, inherent in its design, the ASRS is vulnerable to the effects of aphasia and dysarthria as the AOS features assessed in the ASRS overlap with the features of aphasia and dysarthria; a systematic investigation of these influences is warranted.
The simple temporal acoustic measures of productions of the word catastrophe have been demonstrated as sensitive and specific to the presence of the PPAOS (Duffy et al., 2017). In healthy controls, the average syllable rate for production of catastrophe was reported as 4.84 syllables/second (SD = 0.41; Duffy et al., 2017). All four patients reported here took longer to say the word than healthy controls, even at the initial presentation. It took each patient progressively longer to produce the word over time until they were so impaired that measurements could no longer be made. This supports the use of simple temporal acoustics to documenting change over time, at least in the earlier stages of the condition, in patients with PPAOS. These findings are consistent with past studies of stroke-induced AOS (Ballard et al., 2016; McNeil, Liss, Tseng, & Kent, 1990), nonfluent/agrammatic variant of PPA when AOS is present (Ballard et al., 2014; Knibb, Woollams, Hodges, & Patterson, 2009), and other studies of PPAOS (Laganaro et al., 2012), which also quantified the slowed rate in AOS and changes over time. Of course, the patients in the current study also developed dysarthria, which is expected to influence acoustic measures. The combined effects of AOS and dysarthria on acoustic measures have not yet been examined and warrant further investigation in this population.
While the initial presentation was that of an MSD, all four patients developed aphasia. The severity of aphasia at the final visit was greater in P1 and P4, the two patients who presented with phonetic PPAOS. Again, this is consistent with past research, which has associated more cortically mediated symptoms (e.g., language, behavioral impairments) with this presentation of AOS (Josephs et al., 2014; Whitwell et al., 2017). Nonetheless, the association between the presentation of AOS and the subsequent severity of language impairment is not yet entirely understood. In examining the writing of these patients (Figures 3–6), it appears that agrammatism may occur prior to the detection of difficulties with grammar and syntax on formal, nonwritten testing (e.g., Northwestern Anagram Test [Weintraub et al., 2009], Token Test, Boston Diagnostic Aphasia Examination subtest of syntactic processing, not reported here). Although agrammatism includes many variables, it is the case that the omission of articles and function words, over frank syntax errors, are among the initial and primary complaints from many patients.
Two patients developed a spastic dysarthria and concomitant dysphagia (P1 and P3), whereas two patients developed hypokinetic dysarthria (P2 and P4). Given the possible relationship between PPAOS and both CBS (Assal, Laganaro, Remund, & Ragno Paquier, 2012; Josephs & Duffy, 2008; Sánchez-Valle, Forman, Miller, & Gorno-Tempini, 2006; Wadia & Lang, 2007) and PSP (Josephs et al., 2005, 2014; Spaccavento, Del Prete, Craca, & Loverre, 2014), the emergence of dysarthria is a potential harbinger for the evolving neurologic disease. The disease evolution in these cases also supports monitoring for signs and symptoms of Parkinsonism (per increasing UPDRS III scores). Clinicians should be wary of the development of frontal lobe impairment, such as dysexecutive and behavioral symptoms (per increasing FBI, FAB, and NPI-Q scores). In these four patients, memory and visuoperceptual function were preserved when tested and can be viewed as relative persisting strengths. It is recognized that final data for these exams are missing, so it is unclear if they remain preserved as disease end stages are reached. Consistent with past studies, all patients presented with, or developed, an NVOA (Botha et al., 2014). Despite the aforementioned developments, it is the case that the AOS remained the most pronounced impairment throughout the time these cases were followed.
Our understanding of PPAOS continues to grow as we follow these patients over a longer period. For instance, our past research, based on evaluations at two to three time points, suggested that patients with prosodic PPAOS might not develop aphasia (Josephs et al., 2014), but the prosodic PPAOS patient presented here did indeed eventually develop aphasia. As we follow these patients for longer periods, our understanding becomes more complete. Whether and how subtyping relates to the underlying disease and anticipated disease progression remain empirical questions. Similarly, this may offer insights into whether the initial clinical presentation of AOS, as seen in PPAOS, versus aphasia, as seen in PPA, has implications for an underlying disease or disease progression.
This study is a case series and, as such, carries inherent limitations. Continuing to follow a larger cohort of patients will allow for the identification and verification of trends in clinical changes over time. It is possible that the patients presented here are not reflective of the general population of patients with PPAOS, although based on our experience, their presentation at the first assessment seems quite representative. Given the higher-than-average educational attainment for each of the presented individuals, selection bias needs to be considered, although we have no reason to suspect that educational achievement would alter the patterns of impairment demonstrated here. In addition, there were some fluctuations in scores on standardized testing that are difficult to explain in the context of a degenerative disease. This is not necessarily a flaw of the well-validated measures but warrants consideration of variability of an individual's performance between visits and recognition that an evaluation on any given day may not always capture his or her average level of functioning. It also argues for the use of self- or proxy ratings to supplement formal testing. Finally, we have examined the scores relative to the reported disease duration, which carries the limitations inherent in the patient-reported history of symptom onset. Despite these limitations, the detailed clinical profiles outlined here, covering an extended course of time, afford previously undocumented insight into the progression of patients with PPAOS.
Given the current data, the measures that may prove most helpful in monitoring PPAOS over time include the AES and ASRS to quantify the nature and severity of the AOS, a spontaneous writing sample to monitor for agrammatism, and the MSD's severity rating scale to monitor functional impairment. The aforementioned global severity rating, on a scale of 1–4, does not appear to fully capture the degree of change seen in these other measures. Although this rating is useful for the descriptive, ordinal classification of severity (mild, moderate, marked, severe), it may prove less useful in monitoring the spectrum of change over time. Continuing to follow patients longitudinally will allow us to assess whether the aforementioned patterns hold true in a larger cohort of patients with PPAOS. In doing this and collecting corresponding neuroimaging it is necessary to assess clinical–anatomical correlates of changes seen in patients who initially present with PPAOS to identify possible biomarkers of disease progression.
Conclusions
Following four patients with PPAOS for over half of a decade, coupled with findings of other cross-sectional studies, allows us to draw some tentative conclusions and outline hypotheses that require prospective investigations. First, it appears that the rate of progression varies among individuals with PPAOS. Second, people with PPAOS may develop dysarthria, NVOA, and dysphagia, the latter of which is possibly associated with the presence of a spastic dysarthria. Third, people with PPAOS are likely to develop aphasia, with greater severity for those with the phonetic predominant presentation of PPAOS. Fourth, people with PPAOS typically will develop additional neuromotor and neurocognitive signs and symptoms, although the MSD remains the predominant functionally significant deficit. Finally, the functional limitations of PPAOS are not inconsequential; here, each patient reported that their speech difficulty led to early retirement or altered daily activities, reduced interactions and, ultimately, loss of verbal means of communication. Patients with PPAOS will ultimately require alternative and augmentative means of communication, the nature of which will be influenced by the presence and severity of aphasia and additional neuromotor and neurocognitive symptoms.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Deafness and Communication Disorders Grants R01-DC010367 and R01-DC014942, awarded to Keith A. Josephs, and Grant R01-DC012519, awarded to Jennifer L. Whitwell.
Funding Statement
This work was supported by National Institute on Deafness and Communication Disorders Grants R01-DC010367 and R01-DC014942, awarded to Keith A. Josephs, and Grant R01-DC012519, awarded to Jennifer L. Whitwell.
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., … Phelps C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia, 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. J., Litvan I., Lang A. E., Bak T. H., Bhatia K. P., Borroni B., … Weiner W. J. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assal F., Laganaro M., Remund C. D., & Ragno Paquier C. (2012). Progressive crossed-apraxia of speech as a first manifestation of a probable corticobasal degeneration. Behavioural Neurology, 25(4), 285–289. https://doi.org/10.3233/BEN-2012-110219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K. J., Azizi L., Duffy J. R., McNeil M. R., Halaki M., O'Dwyer N., … Robin D. A. (2016). A predictive model for diagnosing stroke-related apraxia of speech. Neuropsychologia, 81, 129–139. https://doi.org/10.1016/j.neuropsychologia.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Ballard K. J., Savage S., Leyton C. E., Vogel A. P., Hornberger M., & Hodges J. R. (2014). Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS ONE, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K. J., Wambaugh J. L., Duffy J. R., Layfield C., Maas E., Mauszycki S., & McNeil M. R. (2015). Treatment for acquired apraxia of speech: A systematic review of intervention research between 2004 and 2012. American Journal of Speech-Language Pathology, 24(2), 316–337. https://doi.org/10.1044/2015_AJSLP-14-0118 [DOI] [PubMed] [Google Scholar]
- Boeve B. F., Lang A. E., & Litvan I. (2003). Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology, 54(Suppl. 5), S15–S19. [DOI] [PubMed] [Google Scholar]
- Botha H. A., Duffy J. R., Strand E. A., Machulda M. M., Whitwell J. L., & Josephs K. J. (2014). Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology, 82(19), 1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley F. L. (1967). Lacunae and research approaches to them. In Milliken C. & Darley F. L. (Eds.), Brain mechanisms underlying (pp. 236–240). New York, NY: Grune & Stratton. [Google Scholar]
- Darley F. L. (1969). Aphasia: Input and output disturbances in speech and language processing. Paper presented to the annual convention of the American Speech and Hearing Association, Chicago [unpublished]. [Google Scholar]
- De Renzi E., & Vignolo L. (1962). The Token Test: A sensitive test to detect receptive disturbances in aphasics. Brain, 85, 665–678. [DOI] [PubMed] [Google Scholar]
- Dubois B., Feldman H. H., Jacova C., Hampel H., Molinuevo J. L., Blennow K., … Cummings J. L. (2014). Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. Lancet Neurology, 13, 614–629. [DOI] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., & Pillon B. (2000). The FAB: A Frontal Assessment Battery at bedside. Neurology, 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- Duffy J. R. (2005). Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis, MO: Elsevier Mosby. [Google Scholar]
- Duffy J. R. (2006). Apraxia of speech in degenerative neurologic disease. Aphasiology, 20, 511–527. [Google Scholar]
- Duffy J. R., Hanley H., Utianski R. L., Clark H. M., Strand E. A., Josephs K. A., & Whitwell J. L. (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. R., Strand E. A., Clark H. M., Machulda M. M., Whitwell J. L., & Josephs K. A. (2015). Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American Journal of Speech-Language Pathology, 24(2), 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C., Tilley B., Shaftman S., Stebbins G., Fahn S., Martinex-Martin P., … LaPelle N. (2008). Movement disorder society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders, 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. E., & Manes F. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M., Murray R., Rankin K., Weiner M., & Miller B. (2004). Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: A case report. Neurocase, 10, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger G. U., Respondek G., Stamelou M., Kurz C., Josephs K. A., Lang A. E., … Litvan I. (2017). Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders, 32(6), 853–864. https://doi.org/10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D., & Patterson K. (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and picture. Bury St. Edmunds, United Kingdom: Thames Valley Test Company. [Google Scholar]
- Josephs K. A., Boeve B. F., Duffy J. R., Smith G. E., Knopman D. S., Parisi J. E., … Dickson D. W. (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 11(4), 283–296. [DOI] [PubMed] [Google Scholar]
- Josephs K. A., & Duffy J. (2008). Apraxia of speech and nonfluent aphasia: A new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Current Opinion in Neurology, 21(6), 688–692. https://doi.org/10.1097/WCO.0b013e3283168ddd [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Whitwell J. L., Layton K. F., Parisi J. E., … Petersen R. C. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Gunter J. L., … Whitwell J. L. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Lowe V. J., … Whitwell J. L. (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Master A. V., … Whitwell J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(5), 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D., Cummings J., Ketchel P., Smith V., MacMillan A., Shelley T., … DeKosky S. (2000). Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12, 233–239. [DOI] [PubMed] [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery (Revised). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Kertesz A., Davidson W., & Fox H. (1997). Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Knibb J. A., Woollams A. M., Hodges J. R., & Patterson K. (2009). Making sense of progressive non-fluent aphasia: An analysis of conversational speech. Brain, 132(Pt. 10), 2734–2746. https://doi.org/10.1093/brain/awp207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganaro M., Croisier M., Bagou O., & Assal F. (2012). Progressive apraxia of speech as a window into the study of speech planning processes. Cortex, 48, 963–971. [DOI] [PubMed] [Google Scholar]
- Lansing A. E., Ivnik R. J., Cullum C. M., & Randolph C. (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14, 481–487. [PubMed] [Google Scholar]
- Litvan I., Agin Y., Calne D., Campbell G., Dubois B., Duvoisin R. C., … Zee D. S. (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology, 47, 1–9. [DOI] [PubMed] [Google Scholar]
- Loonstra A., Tarlow A., & Sellers A. (2001). COWAT metanorms across age, education, and gender. Applied Neuropsychology, 8, 161–166. [DOI] [PubMed] [Google Scholar]
- Machulda M. M., Ivnik R. J., Smith G. E., Ferman T. J., Boeve B. F., Knopman D., … Tangalos E. G. (2007). Mayo's Older Americans Normative Studies: Visual form discrimination and copy trial of the Rey–Osterrieth Complex Figure. Journal of Clinical and Experimental Neuropsychology, 29(4), 377–384. https://doi.org/10.1080/13803390600726803 [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., & Stadlan E. M. (1984). Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Liss J. M., Tseng C. H., & Kent R. D. (1990). Effects of speech rate on the absolute and relative timing of apraxic and conduction aphasic sentence production. Brain and Language, 38(1), 135–158. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Robin D. A., & Schmidt R. A. (2009). Apraxia of speech: Definition and differential diagnosis. Clinical management of sensorimotor speech disorders (pp. 249–268). New York, NY: Thieme. [Google Scholar]
- Nasreddine Z., Phillips N., Bedirian V., Charbonneau S., Whitehead V., Collin I., … Chertkow H. (2005). The Montréal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. (1944). Test of copying a complex figure: Contribution to the study of perception and memory. Archives de Psychologie, 30, 286–356. [Google Scholar]
- Rey A. (1964). The Clinical Psychological Examination. Paris, France: Presses Universitaires de France. [Google Scholar]
- Sánchez-Valle R., Forman M., Miller B., & Gorno-Tempini M. (2006). From progressive nonfluent aphasia to corticobasal syndrome: A case report of corticobasal degeneration. Neurocase, 12(6), 355–359. [DOI] [PubMed] [Google Scholar]
- Spaccavento S., Del Prete M., Craca A., & Loverre A. (2014). A case of atypical progressive supranuclear palsy. Clinical Interventions in Aging, 9, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O., & Strauss E. (1998). Compendium of neuropsychological tests: Administration, norms and commentary (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Steinberg B. A., Bieliauskas L. A., Smith G. E., & Ivnik R. J. (2005). Mayo's Older Americans Normative Studies: Age- and IQ-adjusted norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clinical Neuropsychology, 19(3–4), 329–377. https://doi.org/10.1080/13854040590945210 [DOI] [PubMed] [Google Scholar]
- Strand E. A., Duffy J. R., Clark H. M., & Josephs K. (2014). The Apraxia of Speech Rating Scale: A new tool for diagnosis and description of AOS. Journal of Communication Disorders, 51, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski R. L., Duffy J. R., Clark H. M., Strand E. A., Botha H., Schwarz C. G., … Josephs K. A. (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. https://doi.org/10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia P., & Lang A. (2007). The many faces of corticobasal degeneration. Parkinsonism and Related Disorders, 13(Suppl. 3), S336–S340. https://doi.org/10.1016/S1353-8020(08)70027-0 [DOI] [PubMed] [Google Scholar]
- Warrington E., & James M. (1991). The Visual Object and Space Perception Battery. Bury St. Edmonds, United Kingdom: Thames Valley Test Company. [Google Scholar]
- Weintraub S., Mesulam M.-M., Wieneke C., Rademaker A., Rogalski E. J., & Thompson C. K. (2009). The Northwestern Anagram Test: Measuring Sentence Production in Primary Progressive Aphasia. American Journal of Alzheimer’s Disease and Other Dementias, 24(5), 408–416. https://doi.org/10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz R., Keith R., & Custer D. (1971). Normal and aphasic behavior on a measure of auditory input and a measure of verbal output. Paper presented at the annual convention of the American Speech and Hearing Association, Chicago. [Google Scholar]
- Whitwell J., Weigand S., Duffy J., Clark H., Strand E., Machulda M., … Josephs K. (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89, 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S., Scott J., Sires D., Grant I., Heaton R., & Troster A. (2005). Action (verb) fluency: Test–retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11, 408–415. [PubMed] [Google Scholar]
- Yorkston K., Strand E. A., Miller R., & Hillel A. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for timing of intervention. Journal of Medical Speech-Language Pathology, 1, 35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.