Abstract
Background
Adequate hydration remains the mainstay of contrast‐induced nephropathy prevention, and nitrates could reduce cardiac preload.
Hypothesis
This study aimed to explore the adequate hydration with nitrates for patients with chronic kidney disease (CKD) and congestive heart failure (CHF) to reduce the risk of contrast‐induced nephropathy (CIN) and at the same time avoid the acute heart failure.
Methods
Three hundred and ninty‐four consecutive patients with CKD and CHF undergoing coronary procedures were randomized to either adequate hydration with nitrates (n = 196) or to routine hydration (control group; n = 198). The adequate hydration group received continuous intravenous infusion of isosorbide dinitrate combined with intravenous infusion of isotonic saline at a rate of 1.5 mL/kg/h during perioperative period. The definition of CIN was a 25% or 0.5 mg/dL rise in serum creatinine over baseline. This trial is registered with www.clinicaltrials.gov, number NCT02718521.
Results
Baseline characteristics were well‐matched between the two groups. CIN occurred less frequently in adequate hydration group than the control group (12.8% vs 21.2%; P = 0.018). The incidence of acute heart failure did not differ between the two groups (8 [4.08%] vs 6[3.03%]; P = 0.599). Cumulative major adverse events (death, myocardial infarction, stoke, hospitalization for acute heart failure) during the 90‐day follow‐up were lower in the adequate hydration with nitrates group (P = 0.002).
Conclusions
Adequate hydration with nitrates can safely and effectively reduce the risk of CIN in patients with CKD and CHF.
Keywords: congestive heart failure, contrast‐induced nephropathy, nitrates
1. INTRODUCTION
Contrast‐induced nephropathy (CIN) is one of the complications in patients who undergo coronary angiography and percutaneous coronary intervention (PCI).1 Previous studies have suggested that CIN may be caused by either renal vasoconstriction resulting in medullary hypoxemia or direct cytotoxic injury caused by the contrast agents.2 Chronic kidney disease (CKD), hypovolemia, diabetes mellitus, and congestive heart failure (CHF) are well‐recognized risk factors for CIN,3, 4, 5 and the needs for transient hemodialysis are significantly increased in this high‐risk patients.6 Hydration is the cornerstone for prevention of CIN,7 and the guidelines recommend that intravenous hydration with isotonic saline should be started at 4 to 12 hours before contrast exposure to prevent CIN.8, 9 Doctors often do not give the adequate hydration in clinical practice in fear that hydration may increase the risk of perioperative pulmonary edema; nitrates drugs could decrease cardiac pre‐load and prevent pulmonary edema. This study prospectively explored nitrates with adequate hydration for CIN prevention in high‐risk patients.
2. METHODS
2.1. Study population
The study was designed as a randomized, prospective and double‐blind clinical study. We enrolled patients from October 2016 to October 2017. The principal inclusion criteria included: (1) CHF: clinical symptoms of chronic heart failure in the past 1 year, which present with exertional dyspnea or nocturnal paroxysmal dyspnea or orthopnea and with objective laboratory findings, such as left ventricular ejection fraction <40% showed by echocardiography and blood biochemical results showed brain natriuretic peptide (BNP) > 500 pg/mL; (2) CKD: estimated glomerular filtration rate (eGFR) < 60 mL/min, according to Modification of Diet in Renal Disease study equation10; (3) patients were scheduled for coronary angiography; (4) signed informed consent. Exclusion criteria included: (1) patients received hemodialysis or eGFR < 15 mL/min; (2) patients required emergency coronary intervention (eg, primary PCI for ST‐ segment elevation myocardial infarction); (3) suffering from malignant tumors, severe liver failure, respiratory failure or other short‐term progressive disease; (4) allergy to contrast agent; (5) nitroglyme drugs contraindications; (6) severe hypotension, systolic blood pressure < 90 mmHg.
2.2. Study protocol
Patients were randomly assigned to isosorbide dinitrate with adequate hydration or standard hydration protocol with sealed envelopes that contained a computer‐generated randomization sequence. The control group received a continuous intravenous infusion of isotonic saline at a rate of 0.5 mL/kg/h 6 hours before and 12 hours after the operation. The experiment group received continuous intravenous infusion of isosorbide dinitrate at a rate of 2 mg/h combined with intravenous infusion of isotonic saline at a rate of 1 mL/kg/h 6 hours before and 12 hours after the operation. Blood urea nitrogen, serum creatinine (Scr), serum electrolytes and cardiac enzymes were evaluated at baseline, the day of coronary angiography, each day for the following 3 days and at hospital discharge. All patients had an electrocardiogram on baseline, immediately after procedure. Acute heart failure (AHF) was observed during perioperative period, and we followed up these high‐risk patients by routine clinical visit and recorded any main adverse cardiac events from hospitalization to 90 days after the procedure, medications were changed as required by the clinical situation.
2.3. Study end points
The primary end point was incidence of CIN, which was defined as a 25% or 0.5 mg/dL rise in SCr over baseline during the first 72 hours post‐procedure.1 Secondary end points were major post‐procedure adverse clinical events including AHF, myocardial infarction, all‐cause death and CIN requiring renal replacement therapy. AHF was defined as a symptom of heart failure (paroxysmal nocturnal dyspnea or orthopnea with rales in both lungs) with objective examination (hypoxemia in blood gas analysis and pulmonary edema in X‐ray). Non‐Q‐wave myocardial infarction was defined as a creatine kinase‐myocardial band enzyme elevation three times the upper normal value without new Q‐waves on the electrocardiogram. Q‐wave myocardial infarction was defined as presence of new pathologic Q‐waves on an electrocardiogram in conjunction with an elevation in creatine kinase‐myocardial band enzyme elevation three times the upper normal value. All adverse clinical events, as well as study end points, were monitored and adjudicated by the independent event committee.
2.4. Statistical analysis
Continuous variables are presented as mean ± SD and were compared using the t test for independent samples. Variables not normally distributed are presented as median and interquartile range, and compared with the Wilcoxon rank sum test. Categorical data are presented as percentages and were compared using the χ 2 test or the Fisher exact test when there <5 values in a given cell. Relative risks (RR) are reported with 95% confidence intervals (CIs). All statistical analyses were performed using SPSS statistical software (version 18.0, SPSS, Chicago, Illinois).
3. RESULTS
3.1. Baseline characteristics and procedures
Of 508 eligible patients, a total of 400 consecutive patients were enrolled and randomly allocated to the nitrates group (n = 200) or the control group (n = 200), and 394 enrollments patients was included in primary analysis (Figure 1). All randomly assigned patients received their allocated treatment. Baseline clinical characteristics for study population are shown in Table 1. The mean age of the cohort was 66 years, and 34% were female. The two groups were comparable regarding gender, type of procedure (diagnostic angiography or PCI) and baseline SCr, while the ejection fraction was 38% ± 8% in the adequate hydration group and 37% ± 8% in the control group (P = 0.271). Prevalence of diabetes was also no significant difference between the two groups (adequate hydration group, 49.5%; control group, 53.0%; P = 0.545). Mean contrast volume delivered during the entire procedure was not significantly different between groups (adequate hydration group, 172 ± 88 mL; control group, 179 ± 82 mL; P = 0.400).
Figure 1.
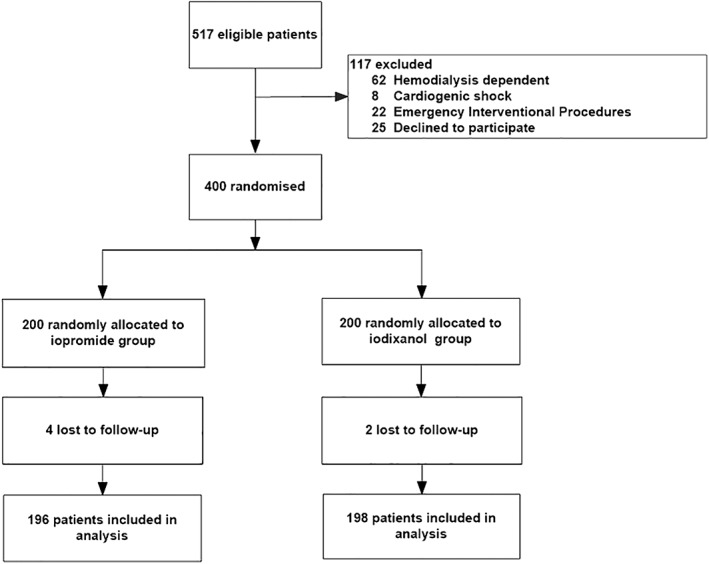
Flow diagram
Table 1.
Baseline characteristics of study patients
| Adequate hydration group (n = 196) | Control group (n = 198) | P value | |
|---|---|---|---|
| Age (years) | 66 ±10 | 66 ±9 | 0.878 |
| Gender, male/female | 144/52 | 50/148 | 0.818 |
| Weight (kg) | 71 ± 12 | 70 ± 12 | 0.509 |
| Hypercholesterolemia (%) | 41(20.9%) | 37(18.7%) | 0.614 |
| Hypertension (%) | 154(78.6%) | 161(81.3%) | 0.531 |
| Diabetes mellitus (%) | 97(49.5%) | 105(53.0%) | 0.545 |
| History of smoking (%) | 95(48.5%) | 98 (49.5%) | 0.841 |
| NSTEMI (%) | 90(45.9%) | 102(51.5%) | 0.270 |
| SBP (mmHg) | 116 ± 24 | 117 ± 26 | 0.668 |
| HR (beat/min) | 79 ± 14 | 80 ± 14 | 0.821 |
| LDL‐c (mmol/L) | 2.43 ± 0.99 | 2.41 ± 0.86 | 0.812 |
| TC (mmol/L) | 4.01 ± 1.09 | 3.99 ± 1.17 | 0.873 |
| Hemoglobin (g/L) | 120 ± 24 | 118 ± 24 | 0.321 |
| White blood cell count (109/L) | 7.93 ± 4.54 | 8.07 ± 3.70 | 0.741 |
| Ejection fraction (%) | 38 ± 8 | 37 ±8 | 0.271 |
| Serum creatinine (mmol/L) | 144(114,214) | 151(112,227) | 0.627a |
| NT‐pro BNP (pg/mL) | 2368(1343,3816) | 2234(1318,4444) | 0.855a |
| Loop diuretics (%) | 83(42.3%) | 91(45.9%) | 0.479 |
| Beta‐blockers (%) | 151(77.0%) | 158(79.8%) | 0.541 |
| ACEI/ARB (%) | 104(53.1%) | 111(56.1%) | 0.613 |
| PCI (%) | 156(79.6%) | 155(78.3%) | 0.805 |
| Contrast media volume(%) | 172 ± 88 | 179 ± 82 | 0.400 |
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitors or angiotensin receptor blockers; DBP, diastolic blood pressure; eGFR, evaluated glomerular filtration rate; HR, heart rate; LDL‐c, low density lipoprotein cholesterol; NSTEMI, non‐ST elevated myocardial infarction; NT‐proBNP, N terminal‐pro brain natriuretic peptide; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TC, total cholesterol.
Wilcoxon rank sum tests.
3.2. Incidence of CIAKI
At least two SCr values were available for all participants after procedure. The mean Scr (median [interquartile range]) was similar between the two groups (144 [114 to 214] mmol/l vs 151 [112 to 227] mmol/l; P = 0.627). The total mean volume saline administered in the adequate hydration group was twice than the control group, so patients in the adequate hydration group had a higher volume of urine output than the control group (1558 ± 514 mL vs 1051 ± 312 mL; P < 0.001, Figure 2). Patients who received larger volumes of normal saline had a lower rate of contrast‐induced acute kidney injury, and CIN was developed in 67 patients (17.0%), of whom 25 (12.8%) were in the adequate hydration group and 42 (21.2%) in the control group. The proportion of patients with >0.3 mg/dL increase of SCr from the baseline was noticeably less in the adequate hydration group than in the control group within 7 days of the CM administration (15.8% vs 27.3%, P = 0.004). The proportion of patients with SCr increasing >0.5 mg/dL and >25% from baseline were both significantly lower in the adequate group than in the control group (10.2% vs 17.8% in SCr > 0.5 mg/dL; 10.7% vs 18.7% in SCr > 25%), which was showed in Table 2.
Figure 2.
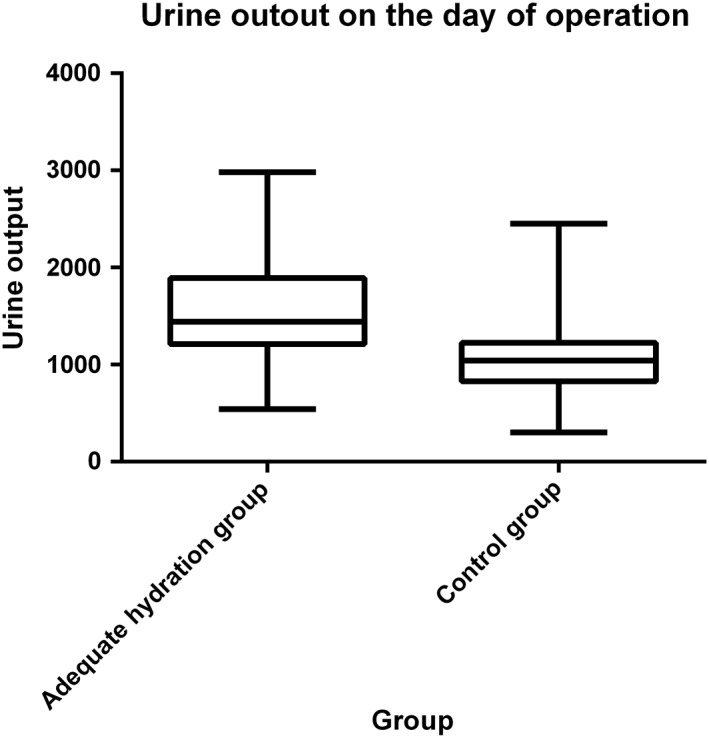
Urine output in study patients during the operation day
Table 2.
Incidence of CIAKI and AHF
| Definition of CIAKI | Adequate hydration group (n = 196) | Control group (n = 198) | P value |
|---|---|---|---|
| SCr >50% ↑ n (%) | 9(4.6%) | 15(4.4%) | 0.152 |
| SCr >25% ↑ n (%) | 21(10.7%) | 37(18.7%) | 0.018 |
| SCr >0.5 mg/dL ↑ n (%) | 20(10.2%) | 35(17.8%) | 0.023 |
| SCr >0.3 mg/dL ↑ n (%) | 31 (15.8%) | 54(27.3%) | 0.004 |
| Incidence of CIAKI n (%) | 25(12.8%) | 42(21.2%) | 0.018 |
| Incidence of AHF n (%) | 8(4.08%) | 6(3.03%) | 0.599 |
Abbreviations: AHF, acute heart failure; CIAKI, contrast‐induced acute kidney injury; SCr, serum creatinine.
Fisher's Exact.
3.3. Incidence of AHF and major adverse cardiovascular events
The incidence of AHF after procedure did not differ between the two groups (8 [4.08%] vs 6[3.03%]; P = 0.599), and the dose of needed to treat with diuretic during the perioperative period is similar between the two groups. Figure 3 showed the occurrence of hospitalization for AHF and other major adverse cardiovascular events in the both groups during the 90‐day follow‐up. A total of four patients died during follow‐up, and two in adequate hydration group, two in control group. Less occurrences of cumulative major adverse events were observed in the adequate hydration group compared with the control group (P for log‐rank = 0.002). Myocardial infarction and stroke developed in 18 patients and was more frequently observed in the control group than in the adequate group (11 vs 7 events, P = 0.346).
Figure 3.
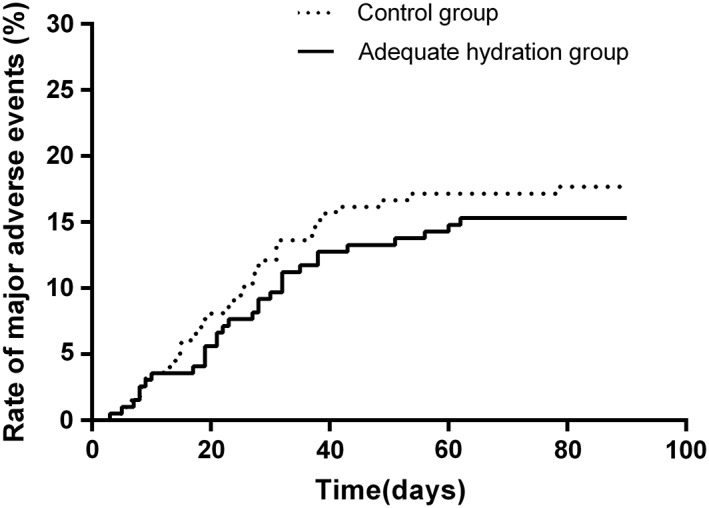
Cumulative major adverse events at 90 days for the adequate hydration groups and control group
4. DISCUSSION
The main finding of our study is that in patients with CKD and CHF undergoing coronary angiography, a prophylactic nitrates with matched adequate hydration is an effective and safe strategy for the prevention of CIN in this patients.
Hydration of high‐risk patients for CIN before contrast administration is a universally accepted measure to prevent CIN. It expands the volume, suppresses the renin‐angiotensin‐aldosterone system, reduces tubuloglomerular feedback and dilutes contrast media.1, 11, 12 However, hydration could further increase cardiac pre‐load and induce pulmonary edema. Patients with CKD or CHF did not receive routine adequate hydration because of fear of pulmonary edema.13, 14 CHF or CKD itself is independent risk factor for CIN.3, 4, 5 First, patients with CHF often have a decreased effective circulatory volume and increased release of vasoconstrictive hormones, which may contribute to medullary hypoxia. Second, approximately 90% of patients hospitalized with AHF in the United Stated receive intravenous (IV) loop diuretics during the hospitalization,15 which can induce reduction of the circulating volume, prostaglandin‐mediated venodilation, and dehydration,15, 16 and they are always susceptible to CIN. Nitrates administration may have some positive effects when combined with hydration. It is universally accepted that nitrates decrease cardiac pre‐load and reduce risk of pulmonary edema. Nitrates increased safety and effectiveness of hydration by decreased cardiac pre‐load and increased levels of nitric oxide in the renal vasculature.
There is no direct evidence on the optimal hydration protocol on patients with CKD and CHF. We tried to develop a noninvasive and feasible strategy of hydration, which can benefit patients with high risk of CIN. The POSEIDON (Prevention of Contrast Renal Injury With Different Hydration Strategies) trial have revealed that left ventricular end‐diastolic pressure‐guided fluid administration is safe and effective in preventing CIN in patients undergoing cardiac catheterization.17 The MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast‐induced Nephropathy Prevention) trial found that furosemide with hydration induced high urine output significantly reduces the risk of CIN and may be associated with improved in‐hospital outcome.18 Our previous study also reported that CVP‐guided fluid administration could safely and effectively reduce the risk of CIN in patients with CKD and CHF.19 There has been a retrospective study demonstrated that the use of nitrates, particularly intravenous nitroglycerin prior to and during PCI may be associated with a decreased incidence of CIN.20 Previous studies have shown that intravenous isosorbide dinitrate treatment of CHF patients was significantly more effective than nitroglycerin group, and had less adverse effects.21 In our study, we applied isosorbide dinitrate instead of nitroglycerin to prevent CIN, and made similar conclusions.
It should be emphasized that although hydration could prevent CIN,22 adequate hydration also could increase the incidence of pulmonary edema for CHF patients. In our study, we applied nitrates to ensure safety of adequate hydration, and we monitored the blood pressure, oxygen saturation and other signs of AHF during perioperative period. Incidence of AHF didn't differ between two groups. The incidence of CIN was significantly lower in the nitrates group than in the control group (12.8% vs 21.2%; P = 0.018). We hypothesized nitrates could formed of nitric oxide, which could act to protect renal medulla from regional hypoxia and oxidative stress.23, 24
4.1. Limitation
First, this study is a single‐center study. Incidence of AHF in two groups had no significant difference, but we cannot ensure the safety of adequate hydration because of the limited cases. Therefore, our conclusion needs further validation in large‐scale prospective multicenter studies. Finally, the physicians in the hydration procedure are not blind, which may affect our results.
ACKNOWLEDGMENTS
This study was funded by Chinese PLA General Hospital Support Foundation (2016‐FC‐TSYS‐1001), Chinese Sailing Foundation (LHJJ201610713), and Chinese Medical Association Exploration Foundation.
Conflict of interest
There is no any potential conflict of interest.
Qian G, Liu C‐F, Guo J, Dong W, Wang J, Chen Y. Prevention of contrast‐induced nephropathy by adequate hydration combined with isosorbide dinitrate for patients with renal insufficiency and congestive heart failure. Clin Cardiol. 2019;42:21–25. 10.1002/clc.23023
Funding information Chinese Medical Association Exploration Foundation; Chinese Sailing Foundation, Grant/Award Number: LHJJ201610713; Chinese PLA General Hospital Support Foundation, Grant/Award Number: 2016‐FC‐TSYS‐1001
REFERENCES
- 1. Barrett BJ. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(17):1853‐1855. [DOI] [PubMed] [Google Scholar]
- 2. Feldkamp T, Kribben A. Contrast media induced nephropathy: definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99(2):177‐196. [PubMed] [Google Scholar]
- 3. McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368‐375. [DOI] [PubMed] [Google Scholar]
- 4. Ezekowitz J, Mcalister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44(8):1587‐1592. [DOI] [PubMed] [Google Scholar]
- 5. Hannan EL, Wu C, Bennett EV, et al. Risk stratification of in‐hospital mortality for coronary artery bypass graft surgery. J Am Coll Cardiol. 2006;47(3):661‐668. [DOI] [PubMed] [Google Scholar]
- 6. Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high‐risk patients undergoing angiography[J]. N Engl J Med. 2003;348(6):491‐499. [DOI] [PubMed] [Google Scholar]
- 7. Han Y. Reply: intravenous hydration (with or without rosuvastatin) should remain the cornerstone of the prevention of contrast‐induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;64(3):332. [DOI] [PubMed] [Google Scholar]
- 8. Thomsen HS. Guidelines for contrast media from the European Society of Urogenital Radiology. Am J Roentgenol. 2003;181(6):1463. [DOI] [PubMed] [Google Scholar]
- 9. Mccullough PA, Stacul F, Becker CR, et al. Contrast‐induced nephropathy (CIN) consensus working panel: executive summary. Rev Cardiovasc Med. 2006;7(4):177‐197. [PubMed] [Google Scholar]
- 10. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929‐937. [DOI] [PubMed] [Google Scholar]
- 11. Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 12. Persson PB, Tepel M. Contrast medium‐induced nephropathy: the pathophysiology. Kidney Int Suppl. 2006;69(100):S8‐S10. [DOI] [PubMed] [Google Scholar]
- 13. Rosenstock JL, Gilles E, Geller AB, et al. Impact of heart failure on the incidence of contrast‐induced nephropathy in patients with chronic kidney disease. Int Urol Nephrol. 2010;42(4):1049‐1054. [DOI] [PubMed] [Google Scholar]
- 14. Schilp J, de Blok C, Langelaan M, Spreeuwenberg P, Wagner C. Guideline adherence for identification and hydration of high‐risk hospital patients for contrast‐induced nephropathy. BMC Nephrol. 2014;15(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duan N, Zhao J, Li Z, et al. Furosemide with saline hydration for prevention of contrast‐induced nephropathy in patients undergoing coronary angiography: a meta‐analysis of randomized controlled trials. Med Sci Monit. 2015;21:292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic‐guided fluid administration for the prevention of contrast‐induced acute kidney injury: the POSEIDON randomised controlled trial. Journal of Vascular Surgery. 2014;383(9931):1814. [DOI] [PubMed] [Google Scholar]
- 18. Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial[J]. JACC Cardiovasc Interv. 2012;5(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 19. Qian G, Fu Z, Guo J, Cao F, Chen Y. Prevention of contrast‐induced nephropathy by central venous pressure‐guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Interv. 2015;9(1):89. [DOI] [PubMed] [Google Scholar]
- 20. Peguero JG, Cornielle V, Gomez SI, et al. The use of nitrates in the prevention of contrast‐induced nephropathy in patients hospitalized after undergoing percutaneous coronary intervention. J Cardiovasc Pharmacol Ther. 2014;19(3):310‐314. [DOI] [PubMed] [Google Scholar]
- 21. Curfman GD, Heinsimer JA, Lozner EC, Fung HL. Intravenous nitroglycerin in the treatment of spontaneous angina pectoris: a prospective, randomized trial. Circulation. 1983;67(2):276‐282. [DOI] [PubMed] [Google Scholar]
- 22. Dorval JF, Dixon SR, Zelman RB, Davidson CJ, Rudko R, Resnic FS. Feasibility study of the RenalGuard™ balanced hydration system: a novel strategy for the prevention of contrast‐induced nephropathy in high risk patients. Int J Cardiol. 2013;166(2):482‐486. [DOI] [PubMed] [Google Scholar]
- 23. Wakai A, Mccabe A, Kidney R, et al. Nitrates for acute heart failure syndromes. Cochrane Database Syst Rev. 2013;8(8):CD005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat[J]. J Clin Investig. 1994;94(3):1069‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]


