Abstract
Background
Epidemiological studies suggest that a diet high in marine fatty acids (fish oil) may have beneficial effects on inflammatory conditions such as rheumatoid arthritis and possibly asthma.
Objectives
(1) To determine the effect of marine n‐3 fatty acid (fish oil) supplementation in asthma. (2) To determine the effect of a diet high in fish oil in asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register. We also searched bibliographies of retrieved trials and contacted fish oil manufacturers. Searches were current as of May 2010.
Selection criteria
We included randomised controlled trials in patients with asthma more than two years of age. The study duration had to be in excess of four weeks. Double blind trials were preferred, but we also reviewed single‐blind and open trials for possible inclusion.
All four reviewers read each paper, blind to its identity. Decisions concerning inclusion were made by simple majority. We all performed quality assessment independently.
Data collection and analysis
The only comparison possible was between marine n‐3 fatty acid supplementation and placebo. There were insufficient trials to examine dietary manipulation alone.
Main results
Nine randomised controlled trials conducted between 1986 and 2001 satisfied the inclusion criteria. Seven were of parallel design and two were cross‐over studies. Eight compared fish oil with placebo whilst one compared high dose versus low dose marine n‐3 fatty acid supplementation. Two studies were conducted in children, whilst the remaining seven studies were conducted in adults. None of the included studies reported asthma exacerbations, health status or hospital admissions.
There was no consistent effect on any of the analysable outcomes: FEV1, peak flow rate, asthma symptoms, asthma medication use or bronchial hyper reactivity. One of the studies performed in children which combined dietary manipulation with fish oil supplementation showed improved peak flow and reduced asthma medication use. There were no adverse events associated with fish oil supplements.
Authors' conclusions
There is little evidence to recommend that people with asthma supplement or modify their dietary intake of marine n‐3 fatty acids (fish oil) in order to improve their asthma control. Equally, there is no evidence that they are at risk if they do so.
Plain language summary
Dietary marine fatty acids (fish oil) for asthma in adults and children
Eating more fish has been recommended as one way of possibly reducing asthma. Populations (such as Eskimo communities) with diets high in fish also have low rates of asthma. As diets in other communities have become higher in saturated fats, asthma has also increased. The theory has been that an ingredient in fish oil may reduce inflammation. Inflammation causes the swelling in the airways of the lungs that leads to asthma attacks. However, this review of trials found that people with asthma changing their diets to include more fish oil did not improve their asthma.
Background
Interest in the possible health benefits of dietary marine n‐3 fatty acids (fish oil) developed following observations that those populations, such as Eskimos, with a high dietary intake of fish also have a low incidence of atherosclerotic and thrombotic disorders, as well as inflammatory conditions such as rheumatoid arthritis. The recent understanding of asthma being a chronic inflammatory disease of the airways and that our dietary patterns have changed over the past 30 years has led to speculation that a diet deficient in fish may also lead to worsening of asthma. The potential anti inflammatory effect of fish oil is that its active ingredient, eicosapentaenoic acid (EPA), is a competitive substrate with arachidonic acid in generating metabolites. EPA is a substrate for the generation of less active prostenoids and leukotrienes than arachidonic acid, thereby potentially acting to reduce airway inflammation and bronchoconstriction.
To date, the evidence for beneficial effects of a dietary intake which is high in marine n‐3 fatty acids is controversial. Whilst some support for this hypothesis has occurred in epidemiological studies, the data from intervention studies has been conflicting. Black 1997 hypothesised that dietary fatty acid intake may influence the development of allergic sensitisation by increasing the formation of prostaglandin E2; which in turn promotes T helper lymphocyte Th2 responses and stimulates the formulation of immunoglobulin E. As dietary fat intake patterns have changed over the past few decades, with an increase in saturated fat consumption and a reduction in polyunsaturated fat intake, there is some suggestion that this may be an important factor in the increasing prevalence of asthma and other allergic diseases. However, a review of the clinical trials of asthma and fish oils (Monteleone 1997), concluded that there was no evidence of clinical improvement in people with asthma using fish oil supplementation, despite some changes seen in inflammatory cell functions.
Objectives
To identify published randomised controlled trials of marine n‐3 fatty acid modification in adults and children (> two years of age) with asthma.
To assess the methodological quality of these trials.
To determine the effect of fish oil supplementation in asthma.
To determine the effect of a diet high in marine n‐3 fatty acids in asthma.
Methods
Criteria for considering studies for this review
Types of studies
Studies had to be randomised controlled trials.
Types of participants
We only included studies which focused upon asthma, however we considered the results of studies in related conditions if the results for the subjects with asthma could be identified separately. We excluded studies in infants less than two years of age.
Types of interventions
Any supplementation of marine n‐3 fatty acids to the diet and/or any manipulation of dietary intake of marine n‐3 fatty acids. Intervention time must have exceeded four weeks and some compliance assessment must have been evident. We only considered the oral route of administration. Double blind trials were preferred, but we also reviewed single blind and open studies for possible inclusion.
Types of outcome measures
Lung function measurements such as Forced Expiratory Volume in one second (FEV1) and/or Peak Expiratory Flow (PEF).
Asthma medication usage.
Asthma symptom scores.
Number of asthma exacerbations.
Health status (quality of life) scores.
Bronchial hyper responsiveness (BHR).
Hospital admissions.
It was anticipated that not all studies would have results pertaining to all of the outcomes listed above. However we only included studies which had at least one of the above outcome measures. We analysed data on an intention to treat basis wherever possible.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
"fatty acid*" or fatty‐acid* or (fish and oil*) or "eicosapentaenoic acid*" or "icosapentaenoic acid*" or EPA or diet* or nutrition* or omega*
Subsequent search updates have been run on the Specialised Register on an annual basis. The most recent search was conducted in May 2010.
Searching other resources
In addition, we reviewed reference lists of all available primary studies and review articles to identify other potentially relevant citations. We also completed a search of the Cochrane Central Register of Controlled Trials (CENTRAL) using the search strategies listed above. A further search was performed using Current Contents which included studies published up to August 2001. We made inquiries regarding other published or unpublished studies known and/or supported by the authors of the included studies and/or companies who manufacture fish oil supplements and fish industry resources, so that these results could be included in this review. Finally, we made personal contact with colleagues, collaborators and other trialists working in the field of marine fatty acids and/or asthma to identify potentially relevant studies.
Data collection and analysis
Selection of studies
From the title, abstract or descriptors, one reviewer (RW) reviewed the literature searches to identify potentially relevant trials for full review. Searches of bibliographies and texts were conducted to identify additional studies. All three reviewers independently read the abstract and methods sections of the papers to select the trials for inclusion in this review, using specific criteria. All three reviewers were blinded to the identity of each paper. Agreement was by a simple majority of all three reviewers and we resolved disagreement by consensus.
Data extraction and management
We (RW, MA) extracted data for the trials independently. We obained confirmation of methodology in two studies (Dry 1991; Nagakura 2000) and obtained additional data in three studies (Hodge 1998; Thien 1993; McDonald 1990). In some cases, expansions of graphic reproductions and estimations from other data presented in the paper were performed.
Assessment of risk of bias in included studies
All three reviewers independently performed methodological quality assessment. All used two different methods of assessment. First, using the Cochrane approach to assessment of allocation concealment, all trials were scored and entered using the following scheme: Grade A: Adequate concealment Grade B: Unclear concealment Grade C: Obviously not adequate concealment.
We measured inter‐rater reliability using simple agreement. For seven of the nine studies there was 67% agreement and 100% agreement for the other two studies in the first instance. After discussion with all reviewers we achieved 100% agreement.
We assessed concealment of allocation as clearly adequate (Grade A) in only 1 study (McDonald 1990). The adequacy or otherwise of the seven other studies could not be determined from the details published in the papers (Grade B). One study was deemed to have obviously inadequate allocation concealment (Stenius‐Aarniala1989).
In addition, we assessed each study using a 0 to 5 scale described by Jadad 1996 and summarised as follows:
Was the study described as randomised? (1 = Yes, 0 = No)
Was the study described as being double blind? (1 = Yes, 0 = No)
Was there a description of withdrawals and drop puts? (1 = Yes, 0 = No)
Was the method of randomisation well described and appropriate? (1 = Yes, 0 = No)
Was the method of double blinding well described and appropriate? (1 = Yes, 0 = No)
Deduct one point if methods for randomisation or blinding were inappropriate.
Measures of treatment effect
The planned analyses included: Comparison 1: marine n‐3 fatty acid supplementation versus placebo Comparison 2: marine n‐3 fatty acid supplementation vs untreated control Comparison 3: dietary marine n‐3 fatty acid manipulation vs untreated control Comparison 4: dietary marine n‐3 fatty acid manipulation vs placebo Comparison 5: where different doses of marine n‐3 fatty acid supplements/manipulation were used, dose‐response analysis was attempted , categorising studies by mean plasma/red cell fatty acid levels where available.
These comparisons were performed separately for each outcome, namely lung function measurements (FEV1 and PEF), asthma medication usage, asthma symptom scores, number of asthma exacerbations, health status (quality of life) scores, bronchial hyper responsiveness (BHR) and hospital admissions, whenever the results were reported.
To compare studies which used different doses of marine fatty acids, we converted doses to daily equivalents of eicosapentaenoic acid (EPA) in grams. Where appropriate, we entered continuous data as negative values to conform to the Cochrane convention whereby effects that favour the treatment under review move to the left of the line of no effect.
Data synthesis
We calculated the weighted mean difference (WMD) for continuous outcomes to provide pooled effect sizes and 95% confidence intervals (95% CI) where the outcome of interest was expressed as the same scale for all included studies. If we used different scales to measure the same outcome e.g. Peak Expiratory Flow (PEF) measured in absolute units and as a percentage of predicted normal we used the standardised mean difference (SMD). For both methods, random and fixed effect models were compared. Where there was a significant difference in the pooled effect sizes and their 95% confidence intervals between the random and fixed effects models, the random effects model has been used. We performed Chi square tests to assess heterogeneity between studies.
For dichotomous variables, we calculated individual and pooled statistics as odds ratios (OR) with 95% confidence intervals (95% CI): both fixed and random effects models were used.
Subgroup analysis and investigation of heterogeneity
We planed three specific subgroups a priori:
Comparison of adults with children
Comparison of dietary n‐3 manipulation with n‐3 supplementation
Comparison of the different dosage schedules based on the dose equivalent of eicosapentaenoic acid (EPA) previously described.
Sensitivity analysis
We planned five separate sensitivity analyses.
Inclusion criteria of patients in individual studies. Excluding studies which did not have supportive evidence of diagnosed asthma.
Severity of asthma. Excluding studies with subjects whose baseline FEV1 or peak expiratory flow on admission to the study was greater than 80% predicted.
Intervention compliance. Excluding studies which did not report, or report poor compliance measures for the intervention.
Methodological quality. Excluding studies of lower methodological quality (i.e. Jadad methodological quality score of two or less out of five).
Methods of meta‐analysis. Random effects models were compared with fixed effect models.
Results
Description of studies
We identified a total of 265 citations from the Cochrane Airways Group trials register. We obtained a further 89 citations from the Current Contents database and two citations were found through inquiries to fish oil manufacturers and personal contact with colleagues. Of these, a total of 22 articles, which related to 21 studies, were identified for possible inclusion. After reviewing the methods section of each of these studies, we excluded twelve from the review.
Of the nine studies which met the inclusion criteria, seven were of parallel design (Arm 1988; Arm 1989; Dry 1991; Hodge 1998; Kirsch 1988, Nagakura 2000; Thien 1993) whilst two were cross‐over trials (McDonald 1990; Stenius‐Aarniala1989). Both cross‐over studies compared marine n‐3 fatty acid supplementation with placebo. The study by Stenius‐Aarniala1989 was a three arm study, comparing evening primrose oil, fish oil and olive oil (as placebo). However, only the fish oil and olive oil (placebo) arms were considered in this review. We performed separate analyses for each of these study designs.
Of the seven parallel studies, five compared marine n‐3 fatty acid supplementation with placebo (Arm 1988; Arm 1989; Dry 1991; Nagakura 2000; Thien 1993); one compared dietary marine n‐3 fatty acid manipulation with dietary n‐6 fatty acid manipulation (Hodge 1998) and one compared high dose marine n‐3 fatty acid supplementation with low dose marine n‐3 fatty acid supplementation (Kirsch 1988).
Seven of the studies were in adults, and two were in children (Hodge 1998; Nagakura 2000).
A total of 187 people with asthma were included in these nine studies.
Reasons for excluding studies were: not randomised controlled trial (5), not marine fatty acids in asthma (3), no outcome measurements (3) and inadequate intervention period (3). One abstract contained insufficient information for further assessment and we therefore excluded it. A further abstract was excluded as the published article which related to the abstract was included in the review.
Risk of bias in included studies
Four studies obtained quality scores of four out of five (Arm 1988; Arm 1989; Nagakura 2000; Thien 1993). However the study by Nagakura 2000 initially received a score of two, but clarification from the authors as to the methods of randomisation and double‐blinding increased the score to four. Two studies received quality scores of three (Hodge 1998; Kirsch 1988). The remaining three studies obtained quality scores of two (Dry 1991; McDonald 1990; Stenius‐Aarniala1989).
Marine n‐3 fatty acids have a distinctive taste and texture so oily placebos are needed, preferably contained in a capsule. The constituents of the placebos used in these studies were: Arm 1988 Olive oil capsule Arm 1989 Olive oil capsule Dry 1991 Unknown placebo, unknown formulation Hodge 1998 Capsule containing n‐6 fatty acids: palm oil, olive oil, safflower oil (plus usual dietary fat replaced by sunflower oil) Kirsch 1988 Comparator was low dose marine n‐3 fatty acid (capsule) McDonald 1990 Olive oil capsule Stenius‐Aarniala1989 Olive oil liquid Thien 1993 Olive oil capsule Nagakura 2000 Olive oil capsule
It should be noted that in all studies, the placebo was an n‐6 fatty acid (usually olive oil), except Kirsch 1988 and Dry 1991(where the identity of the placebo was unknown).
Effects of interventions
None of the studies reported asthma exacerbations, health status (quality of life) or hospital admissions. One adverse event was reported in a participant receiving placebo by Arm 1989.
The study by Kirsch 1988 met our inclusion criteria, but compared high dose marine n‐3 fatty acid supplementation (4 gm EPA/day) with low dose marine n‐3 fatty acid supplementation (0.1 gm EPA/day) so we did not combine it with the studies that used placebos. In this study, there was no difference in FEV1 or symptom scores between the two comparison groups.
Results of lung function measurements, asthma medication usage, asthma symptom scores and bronchial hyper‐reactivity (BHR) from the eight placebo controlled studies were combined.
Lung function
FEV1 measurements were performed in two of the parallel studies (Dry 1991; Hodge 1998). There was no significant effect of marine n‐3 fatty acids. The combined WMD (fixed effect model) for FEV1 following marine n‐3 fatty acid supplementation was 2.08 % predicted (95% CI ‐4.77 to 8.93, Analysis 1.4).
1.4. Analysis.
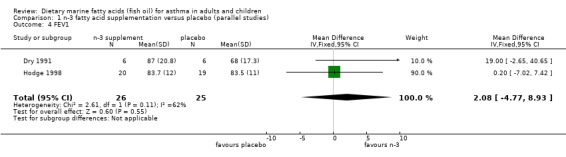
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 4 FEV1.
The PEF was reported in four parallel studies (Arm 1988; Arm 1989; Hodge 1998; Thien 1993) and the two cross‐over studies (McDonald 1990; Stenius‐Aarniala1989). In the parallel studies, the pooled SMD for the PEF at the end of the studies was 1.05 (95% CI 0.02 to 2.08), using a random effects model. This suggests a significant improvement following marine n‐3 fatty acid supplementation. However there was significant heterogeneity between the studies (Chi‐squared = 15.34, p < 0.01). The two studies by Arm et al (Arm 1988; Arm 1989), showed clear differences in PEF at the end of the studies that favoured marine n‐3 fatty acids. This may, however, have been due to differences in PEF at baseline. These values were lower in the placebo group than in participants who were allocated to receive marine n‐3 fatty supplementation. When the results were expressed as the change in PEF from baseline, there was no significant effect of marine n‐3 fatty acids. The SMD using a random effects model was 0.51 (95% CI ‐0.46 to 1.47), Chi squared = 15.53, p < 0.001. Heterogeneity was attributable to the significant effect of the dietary supplement on change in PEF in one study (Hodge 1998).
In the cross‐over studies, the WMD for PEF using a fixed effect model was 5.1 l/min (95% CI ‐34.29, 44.38).
Asthma symptoms
Seven studies reported asthma symptom scores. The scales differed between studies, but five of the six reported symptom scores as a numerical figure, with a higher score representing worse asthma symptoms. One study (Stenius‐Aarniala1989) reported symptoms in terms of "same, better or worse".
Five of the six parallel studies reported asthma symptoms (Arm 1988, Arm 1989, Hodge 1998; Nagakura 2000; Thien 1993). The pooled SMD using a fixed effect model was ‐0.09 (95% CI ‐0.45 to 0.27). No significant heterogeneity was found between these studies (Chi‐squared = 5.44, p = 0.24).
Both of the cross‐over studies reported asthma symptom scores, but the study by Stenius‐Aarniala1989 reported symptoms in terms of "same, better or worse" whilst that by McDonald 1990 gave actual scores. These scoring systems could not be combined in a meta analysis. The study by Stenius‐Aarniala1989 reported an improvement in symptoms, whilst that by McDonald 1990 found no improvement.
Asthma medication
Five studies reported asthma medication usage. These scores all differed, but all used the same basic approach: the more asthma medication was required, the higher the score. In the studies by Arm 1988, Arm 1989 and Thien 1993, the medication score was based on the total number of doses of bronchodilator medication used throughout the study period. The studies by Hodge 1998 and McDonald 1990 also gave total asthma medication usage scores throughout the study period, but the score combined bronchodilator, preventive and other asthma medication usage.
Four of the five parallel studies reported asthma medication usage (Arm 1988; Arm 1989; Hodge 1998, Thien 1993). The pooled SMD using a random effects model was ‐0.84 (95% CI ‐2.11 to 0.44). Significant heterogeneity between these studies existed for asthma medication usage (Chi‐squared = 24.78, p < 0.001). This was attributable to the study of Hodge 1998 which showed a significant reduction in medication use, in contrast to the other studies.
Of the two cross‐over studies, only that by McDonald 1990, reported asthma medication usage. No effect was found.
Bronchial Hyper‐responsiveness (BHR)
Five studies (Arm 1988; Arm 1989; Hodge 1998; Nagakura 2000; Thien 1993), all of parallel design, reported BHR. Three of these (Arm 1988; Arm 1989; Thien 1993) reported BHR in terms of PD35sGaw measurements (the provocation dose of histamine required to produce a 35% fall in specific conductance), whilst Hodge 1998 reported it as the dose‐response slope for each subject and Nagakura 2000 reported it as the PC20 for each subject. The results from all studies were log transformed for this analysis. The pooled SMD using a fixed effect model for the studies was ‐0.14 (95% CI ‐0.52 to 0.24), showing no overall change. No significant heterogeneity between these studies was apparent (Chi‐squared = 2.37, p = 0.67).
Subgroup analysis
Only one of the three planned sub‐group analyses could be conducted due to the small number of studies included in this review. The two studies conducted in children (Hodge 1998, Nagakura 2000) were compared to the four studies of parallel design in adults (Arm 1988; Arm 1989; Dry 1991; Thien 1993) for BHR and asthma symptoms outcomes. Neither BHR nor asthma symptom scores were significantly different between children and adults.
A comparison of the different dosage levels of EPA could not be performed because of the small number of studies and the limited range of EPA doses (1 to 4 gm EPA/day) used in the studies.
Discussion
We found nine studies which met the inclusion criteria. A further fourteen were excluded from this review because they were not randomised controlled trials, did not study marine fatty acids in asthma, did not report relevant outcome measures and/or had inadequate intervention periods. The methodological quality of most of the included studies, particularly the concealment of allocation following randomisation, could not be determined clearly from the information presented in the papers. We would encourage authors of future papers to follow the CONSORT (1996) guidelines and fully report such details. The two studies by Mickleborough (well performed RCTs) deserve special attention. The first (2003) was excluded because authors studied the effects of fish oil supplementation on exercise induced bronchoconstriction in elite athletes, which may have different pathogenetic mechanisms from chronic persistent asthma. Moreover, the duration of intervention in this study was less than 4 weeks. The more recent sudy (2006) conducted in asthmatic subjects similarly had a short duration (less than 4 weeks), with the main outcome studied also being the effect on exercise‐induced bronchoconstriction. However, it is noted that data on lung function measurements, medication use and compliance assessment were also collected.
The review has found no convincing evidence that supplementation of diet with marine n‐3 fatty acids leads to an improvement in asthma symptoms, asthma medication usage, bronchial hyper‐reactivity or FEV1. Unfortunately none of the studies reported asthma exacerbations, health status (quality of life) or hospital admissions.
There was no consistent effect of n‐3 supplementation on PEF as a measure of lung function and there was significant heterogeneity between studies. The apparent improvement in PEF following marine n‐3 fatty acid supplementation is probably due to imbalance in the baseline values of PEF in the studies by Arm 1988 and Arm 1989.
The study by Stenius‐Aarniala1989 showed an improvement in symptoms although the remaining five studies found no improvement. Interestingly the original paper by Stenius‐Aarniala1989 did not report this improvement in asthma symptoms, however this was probably because the analysis in the original paper was a comparison of all three arms of the study, whilst in this review analysis was restricted to only the fish oil and placebo arms of the study.
Whilst the review has found insufficient evidence to promote the addition of marine n‐3 fatty acid supplements to the diets of asthmatics, it also found no evidence of adverse events either. This suggests that whilst there may be no benefit in marine n‐3 fatty acid supplementation, there may be no harm in doing so either.
Much of the interest in n‐3 fatty acid supplementation for asthma began in the era when the neutrophil was considered to possibly have an important role in the pathogenesis of asthma. Certainly, the most profound anti‐inflammatory effects of n‐3 fatty acids are on neutrophil function and mediator generation. Hence, this may explain the clinical benefit in diseases where there is neutrophilic inflammation such as rheumatoid arthritis, psoriasis, cystic fibrosis and inflammatory bowel disease. However, in asthma, eosinophils and mast cells are now thought to be more important effector cells. N‐3 fatty acids do not have a significant anti‐inflammatory effect on eosinophils and mast cells in‐vitro. Hence, this may explain their relative lack of efficacy in asthma compared with these other diseases. Nevertheless, with current emergent understanding of asthma phenotypes, there may be subtypes of asthma with predominant neutrophilic inflammation, such as exercise‐induced bronchoconstriction or non‐eosinophilic asthma, where dietary n‐3 fatty acid supplementation may have a therapeutic role.
It should be noted that, only in one study (Hodge 1998), was dietary manipulation of marine n‐3 fatty acids carried out as part of the treatment phase. It is noteworthy that this trial showed a significant improvement in peak expiratory flow and asthma medication use. It remains to be seen whether this finding would be replicated in other studies in children, or studies in which dietary manipulation was carried out as well as supplementation.
Most of the epidemiological evidence which gave rise to the hypothesis that marine n‐3 fatty acids may have a protective effect, came from studies that found that the regular consumption of fish has a protective effect. It has been assumed from these epidemiological studies that as fish is the major source of n‐3 fatty acids in the human diet, then n‐3 fatty acids have been the component responsible for this protective effect. This review has not addressed the issue of whether the regular consumption of fish per se, or whether there is another active component in fish that may result in improved asthma control.
Authors' conclusions
Implications for practice.
The results from this review suggest that there is little evidence to recommend that people with asthma supplement or modify their dietary intake of marine n‐3 fatty acids (fish oil) in order to improve their asthma control. Equally, there is no evidence that they are at risk if they do so.
Implications for research.
Given the small number of studies, which have been conducted to date, and the limited range of clinically important outcomes that have been reported there is a need for further research in this area. Specifically, since the total number of people with asthma who have been involved in the studies has only been 187, future studies should include larger numbers of subjects. Similarly the outcome measures of asthma exacerbations, hospital admissions and quality of life scores should be included in future studies. Only two studies have been conducted to date (Hodge 1998; Nagakura 2000) in children so it is difficult to make any firm conclusions as to the benefits, or otherwise, of marine n‐3 fatty acid supplementation in this age group.
This review has not been able to answer the question whether increasing the dietary intake of marine n‐3 fatty acids by increased fish intake (but not n‐3 supplementation) result in improved asthma control. Further studies would be required to address this issue. The study of Hodge 1998 provides the most encouragement for this further work.
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2019 | Amended | Potentially eligible studies added to Studies awaiting classification. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 13 May 2010 | New search has been performed | Literature searches conducted. No new studies identified. |
| 17 May 2008 | New search has been performed | A new search was run in May 2008; no new studies met the eligibility criteria of the review |
| 15 May 2008 | Amended | Converted to new review format. |
| 11 February 2002 | New citation required and conclusions have changed | A new study (Nagakura 2000) was added to this review in February 2002. Therefore a major update of the review has been performed. However, the conclusions and recommendations arising from this review remain essentially the same. Further studies were analysed and excluded in May 2007. |
Acknowledgements
We wish to acknowledge the following in helping achieve the completion of this review: Ms Cheryl Salome, Dr Frank Thien, Assistant Professor Jonathan Arm, Dr Christine McDonald and Dr Boyd Strauss for providing additional data relating to the studies by Hodge 1998, Thien 1993, Arm 1989, and McDonald 1990 respectively. Dr Vincent and Dr. Nagakura for providing clarification of information relating to the studies by Dry 1991 and Nagakura 2000 respectively.
Associate Professor Peter Gibson for his continued patience in editing this review, Dr Chris Cates, Mr Steve Milan, Miss Anna Bara and Ms Jane Dennis of the Cochrane Collaboration Airways Review Group for providing much needed and continued support, encouragement and guidance. We also wish to thank Mr Anthony Mortimer for his assistance with the preparation of the synopsis.
Ms Cathryn Wharton for the enormous amount of effort and work performed to double‐blind all of the potential studies for this review and for providing excellent support in following up additional articles and authors.
Kirsty Olsen who has copy edited this review.
Data and analyses
Comparison 1. n‐3 fatty acid supplementation versus placebo (parallel studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PEF ‐ end | 4 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.02, 2.08] |
| 2 PEF ‐ baseline | 4 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.48, 0.56] |
| 3 PEF ‐ change | 4 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.46, 1.47] |
| 4 FEV1 | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | 2.08 [‐4.77, 8.93] |
| 5 Asthma symptom scores | 5 | 124 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.45, 0.27] |
| 6 Asthma medication usage | 4 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐2.11, 0.44] |
| 7 Bronchial hyperresponsiveness (logged) | 5 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.52, 0.24] |
1.1. Analysis.
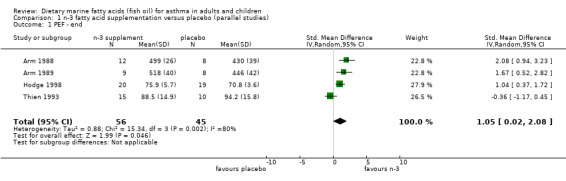
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 1 PEF ‐ end.
1.2. Analysis.
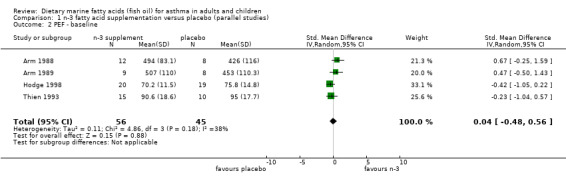
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 2 PEF ‐ baseline.
1.3. Analysis.
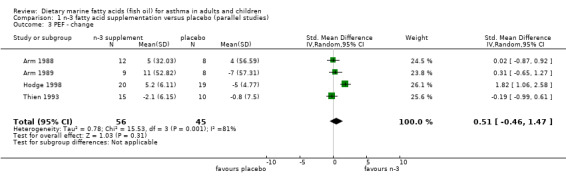
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 3 PEF ‐ change.
1.5. Analysis.
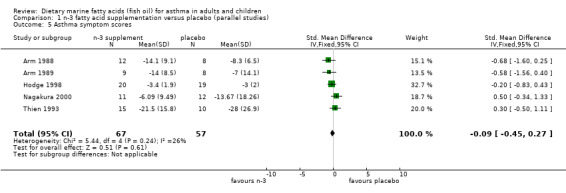
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 5 Asthma symptom scores.
1.6. Analysis.
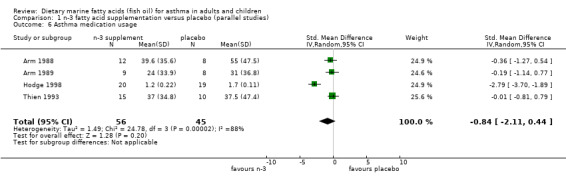
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 6 Asthma medication usage.
1.7. Analysis.
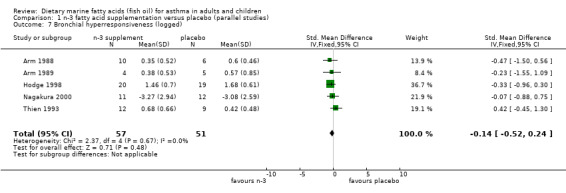
Comparison 1 n‐3 fatty acid supplementation versus placebo (parallel studies), Outcome 7 Bronchial hyperresponsiveness (logged).
Comparison 2. n‐3 supplementation versus placebo (cross‐over studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PEF ‐ end | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 5.05 [‐34.29, 44.38] |
| 3 Asthma symptom scores | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐1.75, 1.69] |
| 4 Symptomatic deterioration | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.09, 0.84] |
2.1. Analysis.
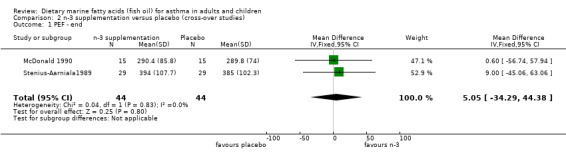
Comparison 2 n‐3 supplementation versus placebo (cross‐over studies), Outcome 1 PEF ‐ end.
2.3. Analysis.
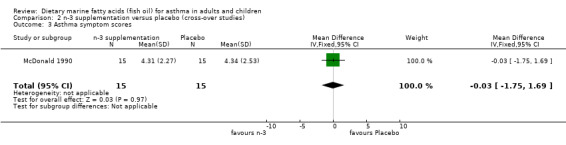
Comparison 2 n‐3 supplementation versus placebo (cross‐over studies), Outcome 3 Asthma symptom scores.
2.4. Analysis.
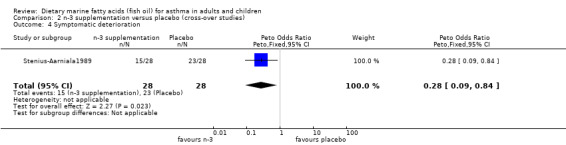
Comparison 2 n‐3 supplementation versus placebo (cross‐over studies), Outcome 4 Symptomatic deterioration.
Comparison 3. adults versus children ‐ bronchial hyperresponsiveness (logged).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adults versus Children | 5 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.52, 0.25] |
| 1.1 Adults | 3 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.61, 0.59] |
| 1.2 Children | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.72, 0.28] |
3.1. Analysis.
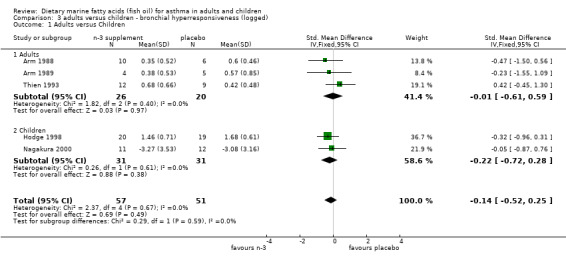
Comparison 3 adults versus children ‐ bronchial hyperresponsiveness (logged), Outcome 1 Adults versus Children.
Comparison 4. adults versus children ‐ Asthma symptom scores.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adults versus children | 5 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.41, 0.29] |
| 1.1 adults | 3 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.76, 0.27] |
| 1.2 Children | 2 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
4.1. Analysis.
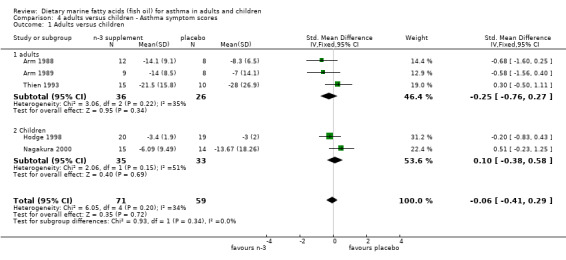
Comparison 4 adults versus children ‐ Asthma symptom scores, Outcome 1 Adults versus children.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arm 1988.
| Methods | Randomised controlled trial. Double blind. Placebo controlled. Parallel design. Ten week intervention period. Comparison of omega‐3 supplementation with placebo supplementation. Identical capsules used. | |
| Participants | 25 asthmatic subjects (20 completed). Aged 15 to 42 years. No one taking oral steroids or had history of aspirin sensitivity. Formal exclusion criteria not reported. Asthma severity determined from symptom diaries and PEF measurements. No mention of how subjects were recruited or diagnostic criteria for asthma. | |
| Interventions | Active (omega‐3) group: 18 x MaxEPA capsules/day (provided 3.2g EPA and 2.2g DHA) Placebo group: 18 x matched placebo (olive oil) capsules/day. All subjects asked not to change their usual dietary patterns (but no further information provided). Oral route of administration. Two to four week run‐in period, followed by 10 week treatment period. | |
| Outcomes | Lung function measurements ‐ PEF Asthma medication usage Asthma symptom scores Airway conductance (sGaw) | |
| Notes | 5 losses to follow‐up: 3 found capsules unmanageable, 1 'personal circumstances', 1 withdrawn due to acute asthma. Other characteristics or outcome measures of this group not reported. Quality score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Arm 1989.
| Methods | Randomised controlled trial. Double blind. Placebo controlled. Parallel design. Comparison of omega‐3 (EPA) capsules with placebo (olive oil) supplementation. 10 week intervention period. Capsules were identical for both groups. | |
| Participants | 22 atopic, non‐smoking asthmatic volunteers entered and 17 completed the trial aged 18‐42 years. Asthma severity determined from asthma symptoms and PEF measurements. No one currently using oral steroids or theophylline or gave history of aspirin sensitivity. No formal exclusion criteria reported. | |
| Interventions | Active (EPA) group: 18 MaxEPA capsules/day (provided 3.2g EPA and 2.2g DHA) Placebo group: 18 matched capsules containing olive oil (amount and composition not provided). Both groups were asked to maintain their usual diets (no compliance measures of this reported). Oral route of administration. 2 week run‐in period followed by 10 week treatment period. | |
| Outcomes | Lung function measurement ‐ PEF Asthma medication usage Asthma symptom scores Airway conductance (sGaw) Allergen responsiveness | |
| Notes | 5 losses to follow‐up: 2 found capsules unmanageable, 2 'personal circumstances', 1 withdrawn as became pregnant. No further characteristics or outcomes reported for this group. Quality score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Dry 1991.
| Methods | Randomised controlled trial. Double blind, placebo controlled. Parallel group design.12 month treatment phase. Comparison of omega‐3 supplementation ( 1g/day) versus placebo. No mention of whether placebo was matched to active or how ingested (?capsule, fluid) | |
| Participants | 12 allergic asthmatics. Age, setting, diagnostic criteria, severity and comorbidities unknown. | |
| Interventions | Active group: 1g/day of EPA and DHA Placebo group: constituents unknown, unknown whether placebo was matched/identical to active group. Duration of treatment 12 months.Oral administration however unknown whether in capsule, fluid or other format. | |
| Outcomes | Lung function measurements: ‐ FEV1 | |
| Notes | Have contacted author for additional information. Reply from author suggested compliance data recorded. No losses to follow‐up reported. Quality score = 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not available |
Hodge 1998.
| Methods | Randomised controlled trial. Double blind. Parallel design. Comparison of diet high in omega‐6 fatty acids (placebo capsules: safflower/palm/olive oil) and diet high in omega‐3 fatty acids (capsules: MaxEPA). Omega‐3 diet participants advised to eat fish at least once per month, omega‐6 group to avoid fish. Six month intervention period. | |
| Participants | 45 asthmatic children, aged 8 to 12 years. 39 completed the study (6 dropped out at baseline prior to randomisation). Asthma defined as reported episodic wheeze in past 12 months and airway hyperresponsiveness. Asthma severity scores based on PEF, day and night symptom scores and medication usage. Excluded those on oral steroids, "significant other diseases", known aspirin or salicylate sensitivity. No mention as to how subjects were recruited. | |
| Interventions | Omega‐3 (active) group: 4 x MaxEPA capsules/day (total of 1.2g omega‐3/day). Dietary control consisted of use of canola oil and canola based margarines and salad dressings which were supplied in unmarked containers and used in place of usual fats/oils. Advised to consume fish at least once a month. omega‐6 (placebo) group: 4 x matched placebo capsules containing safflower (1.8g/day), palm (1.8g/day) and olive oil (0.4g/day). Usual fat/oil intake replaced with sunflower oil and sunflower oil based margarines and salad dressings. Advised to avoid fish. Route of administration was oral. Two week run‐in period followed by 6 month intervention period. | |
| Outcomes | Paper reported:‐ FEV1 measurements,
Bronchial hyperresponsiveness, and
Asthma severity scores. Asthma severity scores comprised PEF data, symptom scores and medication usage. For the purposes of this review the authors provided additional data to allow asthma severity score data to be broken down into PEF data, symptom scores and medication scores. Hence this review is based upon: Lung function measurements ‐ PEF, FEV1 Asthma medication usage Asthma symptom scores Bronchial hyperresponsiveness |
|
| Notes | No mention of why the 6 dropped out at baseline and no information provided on this group. Obtained raw data from authors to determine medication usage, PEF changes and symptom score results as paper added all of these outcomes together to give an overall "asthma severity score". Quality score = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Kirsch 1988.
| Methods | Randomised controlled trial. Double blind. Parallel design. Comparison of high dose EPA versus low dose EPA supplementation. 8 week treatment period. Study conducted in 1984 to 1985. Identical capsules used for both groups. | |
| Participants | 12 consecutive asthma patients, aged 42 to 73 years. Asthma defined as having had symptoms of reversible airflow obstruction on at least half of the days in the preceeding year and a history of asthma for at least 3 years. Asthma severity determined from symptom index, and physical evaluation. No one had experienced (in the previous 12 months) status asthmaticus, pneumonitis, pneumothorax or major lung disease. Formal exclusion criteria not reported. No mention of where or how subjects were recruited. 10 subjects currently using oral steroids and 3 were aspirin sensitive. | |
| Interventions | High dose EPA group: 8 gelatine capsules/day (4.0g EPA/day) Low dose EPA group: 8 gelatine capsules/day (0.1g EPA/day). No mention of dietary modifications or changes. Oral route of administration. 6 week run‐in period, 8 week treatment period followed by 2 week close‐out period. Oral route of administration. 6 week run‐in period, 8 week treatment phase and 2 week close‐out phase. | |
| Outcomes | Lung function measurements ‐ FEV1 Asthma symptom scores Hospital admissions (qualitative statement only) | |
| Notes | No mention of any losses to follow up. Requested additional data from author, but no response. Quality score = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
McDonald 1990.
| Methods | Randomised controlled trial. Double blind. placebo controlled. Cross‐over design. Ten week intervention period, followed by 6 week washout and then 10 week cross‐over intervention period. Comparison of omega‐3 supplementation with placebo supplementation. | |
| Participants | 15 non‐smoking asthmatics, aged 28 to 72 years completed the study. Subjects recruited from Chest clinic at a Hospital. Diagnosis of asthma based on history of recurrent reversible symptoms. Those with peptic ulcers, cardiovascular diseases and other potential bleeding disorders were excluded. At least 7 subjects were ex‐smokers (ceased smoking 1 to 31 years ago and smoked for 3 to 50 years prior to cessation). | |
| Interventions | Active (EPA) group: 15 Lipitac capsules/day (provided 2.7g EPA & 1.8g DHA). Placebo group: 15 placebo capsules/day (15g olive oil). Oral route of administration. Subjects asked to keep their dietary fish intake unchanged throughout study period. 2 week run‐in period, followed by 10 week treatment, 6 week washout and 10 week crossover treatment period. | |
| Outcomes | Lung function measurement ‐ PEF Asthma medication usage Asthma symptom scores. | |
| Notes | Information about study obtained from abstract, which reported 15 subjects and thesis which reported on 8 subjects only. No information provided in abstract about withdrawals, however thesis reported 5 withdrawals. Reasons for withdrawal were: 2 had problems with swallowing the capsules, 3 unrelated medical problems. Quality score=2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Nagakura 2000.
| Methods | Randomised controlled trial. Double blind. Placebo controlled. Parallel design. 10 month intervention period. Comparison of omega‐3 supplementation with placebo supplementation. Visually identical capsules used. | |
| Participants | 30 asthmatic children, aged 4 to 17 years entered the study & 23 completed. Asthma defined from hospital admission for asthma. None using oral steroids, 6 were using preventor medications, some (number unknown) were using Theophylline. Exclusion criteria unknown. | |
| Interventions | Active group: 6 to 12 capsule/day based on body weight (17 to 26.8 mg/kg EPA & 7.3 to 11.5 mg/kg DHA). Placebo group: Visually identical olive oil capsules; number of capsules matched for body weight. Oral route of administration. Two month run‐in followed by 10 month intervention. Co‐interventions ‐ reduced allergen exposure, dietary intake constant (study conducted as in‐patients). | |
| Outcomes | Bronchial hyperresponsiveness. Asthma symptom scores | |
| Notes | 6 dropped out during study without reasons given. Author asked to clarify ‐ subjects were discharged from hospital. 1 subject unable to swallow capsules. Quality score = 2 from publication, 4 after clarification from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Stenius‐Aarniala1989.
| Methods | Randomised controlled trial. Cross over design. Three arm comparison of fish oil (omega‐3 group) versus evening primrose oil (omega‐6 group) versus olive oil using liquid oil supplementation. Thirty week treatment period (10 weeks per each study arm). No wash out period between treatments. arms. | |
| Participants | 40 asthmatics selected, 36 entered study and 29 completed study, aged 19‐61 years. Asthma defined as those fulfilling ACCPATS criteria. Severity of asthma determined by PEF variability of greater than 15%. Exclusion criteria: fish allergy, diabetes, coagulation disorders. 8 subjects were currently using oral steroids and 4 were aspirin sensitive. | |
| Interventions | Fish oil: 20 mL/day for 10 weeks (MaxEPA 18% EPA, 12% DHA: approx. 3.6g EPA) Evening Primrose oil: 20 mL/day for 10 weeks (72% cis linoleic, 9% gamma linoleic) Olive oil: 20 mL/day for 10 weeks (77% monoenoic mainly oleic) Oral route of administration, treatment doses taken in liquid form (not adequate concealment) Two week run‐in period followed by 30 week intervention (10 weeks per treatment arm) period. No wash out period between treatment periods. | |
| Outcomes | Lung function measurement ‐ PEF Asthma symptom scores | |
| Notes | 11 losses to follow up: 4 during run‐in period due to PEF variability < 15%, 7 withdrawn during treatment phase as either unable to tolerate taste of oil or found it troublesome to keep asthma diary. No other characteristics or outcome measures provided for this group. Quality score=2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Double blind but no attempt to conceal taste' (inadequate blinding). |
Thien 1993.
| Methods | Randomised controlled trial. Double blind, placebo controlled. Parallel design. Six month follow‐up period.. Conducted over two pollen seasons (1990, 1991). Comparison of omega‐3 supplementation with placebo supplementation. | |
| Participants | 37 non‐smoking pollen sensitive adults aged 19 to 42 years ‐ 25 completed. Recruited via Hospital Allergy Clinic. Pollen sensitive defined as having a positive skin prick test (> 5mm) to grass pollen and seasonal symptoms of hayfever. Asthma severity determined from symptom scores, medication usage, bronchial hyperresponsiveness and PEF measurements. Exclusion criteria: oral steroid dependency, aspirin sensitivity, continued use of long acting antihistamines. | |
| Interventions | Active (omega‐3) group: 18 capsules MaxEPA/day (provided 3.2g EPA and 2.2g DHA) Placebo group: 18 matched placebo capsules/day (containing olive oil, quantities or compositional analysis not provided). No dietary intervention or change mentioned. Oral route of administration. Six month treatment during pollen season. Baseline visit conducted 1 to 2 months prior to treatment period. | |
| Outcomes | Lung function measurements ‐ PEF Asthma medication usage Asthma symptom scores Airway conductance (sGaw) | |
| Notes | 12 losses to follow up: 1 nausea & vomiting, 6 found capsules unmanageable, 4 unable to record PEF/complete diary cards, 1 "personal reasons". No other characteristics or outcome measurements reported for this group. Quality score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules used throughout study |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Broughton 1997 | Not a randomised controlled trial. |
| Ebden 1989 | Not marine fatty acids in asthma. |
| Gorelova 1998 | No outcome measurements. |
| Hederos 1996 | Not marine fatty acids in asthma. Some participants < 2 years of age. |
| Machura 1996 | Not a randomised controlled trial. |
| Macyee 1997a | Intervention period < 4weeks duration. |
| Macyee 1997b | Not a randomised controlled trial. |
| Mickleborough 2006 | Intervention period < 4 weeks duration |
| Mickleborough 2003 | Duration of intervention . 4 weeks. Not all subjects had asthma |
| Palat 1988 | Abstract only. Insufficient information reported. |
| Payan 1986 | No outcome measurements. |
| Picado 1988 | Not a randomised controlled trial |
| Surette 2008 | 2 trials reported: one was not a randomised controlled trial; the other was randomised, but no validated outcomes were measured. |
| Villani 1998 | Not a randomised controlled trial and no compliance assessment. |
| vonSchacky 1993 | Not marine fatty acids in asthma and no outcome measurements. |
Contributions of authors
All reviewers contributed to this review. All were involved in selecting the studies that are included in the review, providing a synopsis of the study, assessing the methodological quality of each study and ensuring that the written review was accurate.
RW and MA were responsible for extracting the data from each study for this review.
RW co‐ordinated the process of conducting and completing this review.
SDL has taken on responsibility for the review as of September 2003.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
Garfield Weston Foundation, UK.
Declarations of interest
One of the reviewers (FT) was the Principal Investigator in one of the included trials (Thien 1993).
Edited (no change to conclusions)
References
References to studies included in this review
Arm 1988 {published data only}
- Arm JP, Horton CE, Mencia‐Huerta JM, House F, Eiser NM, Clark TJH, et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax 1988;43:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arm 1989 {published data only}
- Arm JP, Horton CE, Spur BW, Mencia‐Huerta J‐M, Lee TH. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. American Review of Respiratory Disease 1989;139:1395‐400. [DOI] [PubMed] [Google Scholar]
Dry 1991 {published and unpublished data}
- Dry J, Vincent D. Effect of a fish oil diet on asthma: results of a 1‐year double‐blind study. International Archives of Allergy and Applied Immunology 1991;95:156‐7. [DOI] [PubMed] [Google Scholar]
Hodge 1998 {published and unpublished data}
- Hodge L, Salome CM, Hughes JM, Liu‐Brennan D, Rimmer J, Allman M, et al. Effect of dietary intake of omega‐3 and omega‐6 fatty acids on severity of asthma. European Respiratory Journal 1998;11:361‐5. [DOI] [PubMed] [Google Scholar]
Kirsch 1988 {published data only}
- Kirsch CM, Payan DG, Wong MYS, Dohlman JG, Blake VA, Petri MA, et al. Effect of eicosapentaenoic acid in asthma. Clinical Allergy 1988;18:177‐87. [DOI] [PubMed] [Google Scholar]
McDonald 1990 {published and unpublished data}
- Donald CF, Vecchie L, Pierce RJ, Strauss BJG. Effect of fish‐oil derived omega‐3 fatty acid supplements on asthma control. Australian & New Zealand Journal of Medicine 1990;20:526. [Google Scholar]
- Vecchie L. The role of fish oil supplementation in the treatment of asthma [Dissertation ‐ BSc (hons)]. Melbourne, Australia: Deakin University, 1987. [Google Scholar]
Nagakura 2000 {published and unpublished data}
- Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega‐3 polyunsaturated fatty acids in children with bronchial asthma. European Respiratory Journal 2000;16:861‐5. [DOI] [PubMed] [Google Scholar]
Stenius‐Aarniala1989 {published data only}
- Stenius‐Aarniala B, Aro A, Hakulinen A, Ahola I, Seppala E, Vapaatalo H. Evening primrose oil and fish oil are ineffective as supplementary treatment of bronchial asthma. Annals of Allergy 1989;62:534‐7. [PubMed] [Google Scholar]
Thien 1993 {published and unpublished data}
- Thien FCK, Mencia Huerta JM, Lee TH. Dietary fish oil effects on seasonal hay fever and asthma in pollen‐sensitive subjects. American Review of Respiratory Disease 1993;147:1138‐43. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Broughton 1997 {published data only}
- Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n‐3 fatty acid ingestion are related to 5‐series leukotriene production. The American Journal of Clinical Nutrition 1997;65:1011‐7. [DOI] [PubMed] [Google Scholar]
Ebden 1989 {published data only}
- Ebden P, Bevan C, Banks J, Fennerty A, Walters EH. A study of evening primrose seed oil in atopic asthma. Prostaglandins Leukotrienes and Essential Fatty Acids 1989;35:69‐72. [DOI] [PubMed] [Google Scholar]
Gorelova 1998 {published data only}
- Gorelova JY, Semikina EM. The changes of lymphocyte membrane receptors in bronchial asthma and atopic dermatitis in pediatric patients receiving treatment with polyenic fatty acids. Zeitschrift für Ernährungswissenschaft 1998;37(Suppl 1):142‐3. [PubMed] [Google Scholar]
Hederos 1996 {published data only}
- Hederos CA, Berg A. Epogam evening primrose oil treatment in atopic dermatitis and asthma. Archives of Disease in Childhood 1996;75(6):494‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Machura 1996 {published data only}
- Machura E, Brus R, Kalacinski W, Lacheta M. The effect of dietary fish oil supplementation on the clinical progress of asthma in children [Wplyw diety wzbogaconej olejem rybnym na przebieg astmy u dzieci]. Pediatria Polska 1996;LXXI(2):97‐102. [PubMed] [Google Scholar]
Macyee 1997a {published data only}
- Maycee KA. The effect of omega‐3 polyunsaturated fatty acids on late phase of allergic reaction in patients with bronchial asthma. Tepanebtnyecknn apxub 1997;3:31‐3. [Google Scholar]
Macyee 1997b {published data only}
- Maycee KA. Effects of polyunsaturated fatty acids on biochemical indices in patients with bronchial asthma. Tepanebtnyecknn Apxnb 1997;3:33‐5. [Google Scholar]
Mickleborough 2003 {published data only}
- Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise‐induced bronchoconstriction in elite athletes. American Journal Respiratory and Critical Care Medicine 2003;168:1181‐9. [DOI] [PubMed] [Google Scholar]
Mickleborough 2006 {published data only}
- Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective Effect of Fish Oil Supplementation on Exercise‐Induced Bronchoconstriction in Asthma. Chest 2006;129(1):39‐49. [DOI] [PubMed] [Google Scholar]
Palat 1988 {published data only}
- Palat D, Rudolph D, Rothstein M. A trial of fish oil in asthma. American Review of Respiratory Disease 1988;137(Suppl 4 (pt 2)):329. [Google Scholar]
Payan 1986 {published data only}
- Payan DG, Wong MY, Chernov‐Rogan T, Valone FH, Pickett WC, Blake VA, et al. Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. Journal of Clinical Immunology 1986;6(5):402‐10. [DOI] [PubMed] [Google Scholar]
Picado 1988 {published data only}
- Picado C, Castillo JA, Schinca N, Pujades M, Ordinas A, Coronas A, et al. Effects of a fish‐oil enriched diet on aspirin intolerant asthmatic patients: a pilot study. Thorax 1988;43:93‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Surette 2008 {published data only}
- Surette ME, Stull D, Lindemann J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and quality of life of adult asthma patients. Current Medical Research and Opinion Feb 2008;24(2):559‐567. [PUBMED: 18194593] [DOI] [PubMed] [Google Scholar]
Villani 1998 {published data only}
- Villani F, Comazzi R, DeMaria P, Galimberti M. Effect of dietary supplementation with polyunsaturated fatty acids on bronchial hyperreactivity in subjects with seasonal asthma. Respiration 1998;65:265‐9. [DOI] [PubMed] [Google Scholar]
vonSchacky 1993 {published data only}
- Schacky C, Kiefl R, Jendraschak E, Kaminski WE. n‐3 Fatty acids and cysteinyl‐leukotriene formation in humans in vitro, ex vivo, and in vivo. Journal of Laboratory & Clinical Medicine 1993;121:302‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ade 2011 {published data only}
- Ade CJ, Rosenkranz SK, Harms CA. An airway anti‐inflammatory role for fish oil supplmentation. FASEB Journal 2011:864.5. [Google Scholar]
Brew 2015 {published data only}
- Brew BK, Toelle BG, Webb KL, Almqvist C, Marks GB. Omega‐3 supplementation during the first 5 years of life and later academic performance: A randomised controlled trial. European Journal of Clinical Nutrition 2015;69(4):419‐24. [DOI] [PubMed] [Google Scholar]
Gunaratne 2016 {published data only}
- Gunaratne AW, Makrides M, Sullivan TR, Gibson RA, Collins CT. Is allergic disease reduced at 7‐years corrected age in preterm infants who received high‐dose docosahexaenoic acid (DHA) supplementation?. Journal of Paediatrics and Child Health 2016;52:57. [Google Scholar]
Hansell 2018 {published data only}
- Hansell AL, Bakolis I, Cowie CT, Belousova EG, Ng K, Weber‐Chrysochoou C, et al. Childhood fish oil supplementation modifies associations between traffic related air pollution and allergic sensitisation. Environmental health: a global access science source 2018;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang F, Rio Navarro BE, Ramirez OJS, Mondragon MSH, Ontiveros JAP, Alcantara ST. Effect of N‐3 polyunsaturated fatty acids on adipokines and biomarkers of endothelial dysfunction in obese asthmatic adolescents with hypertriglyceridemia. Endocrine Practice 2015;21(1):8A‐9A. [Google Scholar]
Lang 2013 {published data only}
- Lang JE, Mougey EB, Allayee H, Blake KV, Lockey R, Gong Y, et al. Nutrigenetic response to omega‐3 fatty acids in obese asthmatics (NOOA): rationale and methods. Contemporary Clinical Trials 2013;34(2):326‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2018 {published data only}
- Lang J, Mougey EB, Hossain M, Lima JJ. Omega‐3 fatty acid supplementation in obese adolescents and young adults with poor asthma control. American Journal of Respiratory and Critical Care Medicine 2018;197:A1428. [Google Scholar]
Lang 2019 {published data only}
- Lang JE, Mougey EB, Hossain MJ, Livingston F, Balagopal PB, Langdon S, et al. Fish oil supplementation in overweight/obese patients with uncontrolled asthma: a randomized trial. Annals of the American Thoracic Society 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee SC, Yang YH, Chuang SY, Huang SY, Pan WH. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: a randomised controlled trial. British Journal of Nutrition 2013;110(1):145‐55. [DOI] [PubMed] [Google Scholar]
Lello 2010 {published data only}
- Lello J, Liang A, Robinson E, Leutenegger D, Wheat A. Treatment of children's asthma with a lipid extract of the new zealand green lipped mussel (perna canaliculus) (lyprinol) ‐ A double blind, randomised controlled trial in children with moderate to severe chronic obstructive asthma. Basic & Clinical Pharmacology & Toxicology 2010;107:1. [Google Scholar]
Lello 2012 {published data only}
- Lello J, Liang A, Robinson E, Leutenegger D, Wheat A. Treatment Of Children's Asthma With A Lipid Extract Of The New Zealand Green Lipped Mussel (Perna Canaliculus) (Lyprinol®)‐‐A Double Blind, Randomised Controlled Trial In Children With Moderate To Severe Chronic Obstructive Asthma. Internet Journal of Asthma, Allergy & Immunology 2012;8(1):1. [Google Scholar]
Lopez‐Frias 2018 {published data only}
- Lopez‐Frias SB, Alcantara ST, Jesus Leija Martinez J, Rio Navarro BE, Carmen Castillo Hernandez M, et al. Effect of N‐3 polyunsaturated fatty acids on oxidative stress markers and resolvin e1 in obese asthmatic adolescents with hypertriglyceridemia. Atherosclerosis. Supplements 2018;32:133. [Google Scholar]
Nsouli 2012 {published data only}
- Nsouli SM. Efficacy of fish oil oral supplementation forthe treatment of exercise induced asthma. Annals of Allergy, Asthma and Immunology. Conference: 2012 Annual Meeting of the American College of Allergy, Asthma and Immunology Anaheim, CA United States. 2012; Vol. 109 (5):A54.
Pascual 2010 {published data only}
- Pascual E, Rio Navarro B, Sienra Monge J. Effect of supplementation of omega 3, (3 gramos, 2000 mg EPA and 1000 mg DHA) vs 3 gramos of gelatin for 3 months in triglycerides and pulmonary function of asthmatic and not asthmatic obese adolescents with hypertrigly ceridemia. Annals of Allergy, Asthma and Immunology. Conference: 2010 Annual Meeting of the American College of Allergy, Asthma and Immunology Phoenix, Arizona United States. 2010; Vol. 105 (5):A44.
Shu‐Chen 2012 {published data only}
- Shu‐Chen L, Yao‐Hsu Y, Shao‐Yuan C, Wen‐Harn P. Alleviating medicine usage and improving pulmonary function with supplements of vegetable and fruit concentrate, fish oil, and probiotics in asthmatic school children. Pharmaceutical Biology. Conference: 50th Anniversary of the Phytochemical Society of North America, PSNA 2011 Hilo, HI United States. 2012; Vol. 50(5):555‐6.
Skilton 2012 {published data only}
- Skilton MR, Ayer JG, Harmer JA, Webb K, Leeder SR, Marks GB, et al. Impaired fetal growth and arterial wall thickening: a randomized trial of omega‐3 supplementation. Pediatrics 2012;129(3):e698‐703. [DOI] [PubMed] [Google Scholar]
Zeki 2018 {published data only}
- Zeki AA, Schuster GU, Rabowsky M, Cedeno M, Kivler C, Mu L, et al. Eicosapentaenoic acid (EPA)‐enriched fish oil intervention in severe asthmatics with low‐risk versus high‐risk 5‐lipoxygenase (ALOX5) gene polymorphisms. American Journal of Respiratory and Critical Care Medicine 2018;197:A2491. [Google Scholar]
Zielen 2017 {published data only}
- Zielen S, Dressler M, Boehler L, Benkner N, Reiter A, Fussbroich D, et al. Does oil supplementation with omega‐3‐fatty acids protect for exercise induced asthma? A placebo controlled trial. Allergy 2017;72:422. [Google Scholar]
Additional references
Black 1997
- Black PN, Sharpe S. Dietary fat and asthma: is there a connection?. European Respiratory Journal 1997;10:6‐12. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynalds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Monteleone 1997
- Monteleone CA, Sherman AR. Nutrition and asthma. Annals of Internal Medicine 1997;157:23‐34. [PubMed] [Google Scholar]


