Abstract
Background
In ST‐segment elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention (PPCI), current oral P2Y12 platelet inhibitors do not provide maximal platelet inhibition at the time of reperfusion. Furthermore, administration of cangrelor prior to reperfusion has been shown in pre‐clinical studies to reduce myocardial infarct (MI) size. Therefore, we hypothesize that cangrelor administered prior to reperfusion in STEMI patients will reduce the incidence of microvascular obstruction (MVO) and limit MI size in STEMI patients treated with PPCI.
Methods
The platelet inhibition to target reperfusion injury (PITRI) trial, is a phase 2A, multi‐center, double‐blinded, randomized controlled trial, in which 210 STEMI patients will be randomized to receive either an intravenous (IV) bolus of cangrelor (30 μg/kg) followed by a 120‐minute infusion (4 μg/kg/min) or matching saline placebo, initiated prior to reperfusion (NCT03102723).
Results
The study started in October 2017 and the anticipated end date would be July 2020. The primary end‐point will be MI size quantified by cardiovascular magnetic resonance (CMR) on day 3 post‐PPCI. Secondary endpoints will include markers of reperfusion, incidence of MVO, MI size, and adverse left ventricular remodeling at 6 months, and major adverse cardiac and cerebrovascular events.
Summary
The aim of the PITRI trial is to assess whether cangrelor administered prior to reperfusion would reduce acute MI size and MVO, as assessed by CMR.
Keywords: cangrelor, cardiovascular magnetic resonance imaging, microvascular obstruction, myocardial infarct size, primary percutaneous coronary intervention, reperfusion injury, ST‐segment elevation myocardial infarction
1. INTRODUCTION
Although timely reperfusion by primary percutaneous coronary intervention (PPCI) and secondary preventative therapies have resulted in a substantial decline in mortality following ST‐segment myocardial infarction (STEMI), significant morbidity and mortality remain, with 7% death and 22% heart failure at 1 year.1 This is because, in part, to the existence of “myocardial reperfusion injury,” a phenomenon in which the restoration of coronary blood flow to acutely ischemic myocardium, results, paradoxically, in further myocardial injury and cardiomyocyte death.2 The main irreversible components of reperfusion injury include1: microvascular obstruction (MVO), which is defined as an inability to reperfuse previously ischemic myocardium at the microvascular level despite a patent epicardial coronary artery, occurs in up to 50% of reperfused STEMI patients, and its presence portends to a worse prognosis3, 4, 5; and2 lethal myocardial reperfusion injury, which is a consequence of oxidative stress, calcium overload, adenosine triphosphate depletion, and mitochondrial dysfunction, and which contributes up to 50% of the final MI size, thereby mitigating the full benefits of timely reperfusion in terms of MI size reduction.2
Despite numerous cardioprotective agents showing promising results in the experimental setting for preventing reperfusion injury, their translation into the clinical setting for patient benefit has been challenging, and there is still no effective therapy for preventing MVO and reducing MI size following STEMI.6 It has been shown that current oral P2Y12 platelet inhibitors, such as prasugrel and ticagrelor, administered to STEMI patients prior to PPCI, can sometimes take up to 4 to 6 hours to achieve maximal platelet inhibition,7 particularly in situations of delayed gastric emptying, meaning that at the time of reperfusion, the platelets are not maximally inhibited, resulting in an increased risk of MVO and worse clinical outcomes. In contrast, the intravenous (IV) P2Y12 platelet inhibitor, cangrelor, has a rapid onset of action and induces maximal platelet inhibition within 1 to 2 minutes of administration. As such, administration of cangrelor prior to PPCI would be expected to offer maximal platelet inhibition at the time of reperfusion, thereby reducing the risk of developing MVO. Moreover, a number of experimental small and large animal studies have demonstrated that administering cangrelor prior to reperfusion has the ability to reduce MI size through a cardioprotective effect on the cardiomyocyte involving the activation of established pro‐survival signaling cascades such as Akt and Erk1/2.8, 9, 10, 11, 12, 13
These findings suggest that administering cangrelor prior to reperfusion in STEMI patients, has the therapeutic potential to target myocardial reperfusion injury through two distinct mechanisms maximal platelet inhibition at time of PPCI thereby preventing MVO, and a cardioprotective effect on the cardiomyocyte, thereby reducing MI size.
Cangrelor at dose of a single IV bolus (30 μg/kg) followed by an IV infusion (4 μg/kg/min) for at least 120 minutes has been used in the CHAMPION trials14, 15, 16, 17 and this dose was also found to be cardioprotective in a pre‐clinical model using rabbits.18 Furthermore, a recent proof‐of‐concept study showed that cangrelor was safe to be administered in ticagrelor‐loading STEMI patients, and cangrelor provided faster and stronger peri‐procedural platelet inhibition than ticagrelor alone.19
Cardiovascular magnetic resonance (CMR) is a robust tool to assess MI size as a surrogate marker in clinical cardioprotection studies.20, 21 Therefore, in the platelet inhibition to target reperfusion injury (PITRI) trial, we aim to investigate whether administering cangrelor prior to reperfusion, can prevent MVO and reduce acute MI size, assessed by CMR, in STEMI patients treated with PPCI.
2. METHODS
2.1. Trial design
The PITRI trial is a Phase 2A, double‐blind, randomized, placebo‐controlled clinical trial (https://clinicaltrials.gov; NCT03102723). It will recruit 210 STEMI patients treated with PPCI from cardiac centers within Singapore. The study will be conducted in accordance with the Declaration of Helsinki and has been approved by the SingHealth Centralized Institutional Review Board. All patients will provide written informed consent. The patient recruitment pathway is shown in Figure 1.
Figure 1.
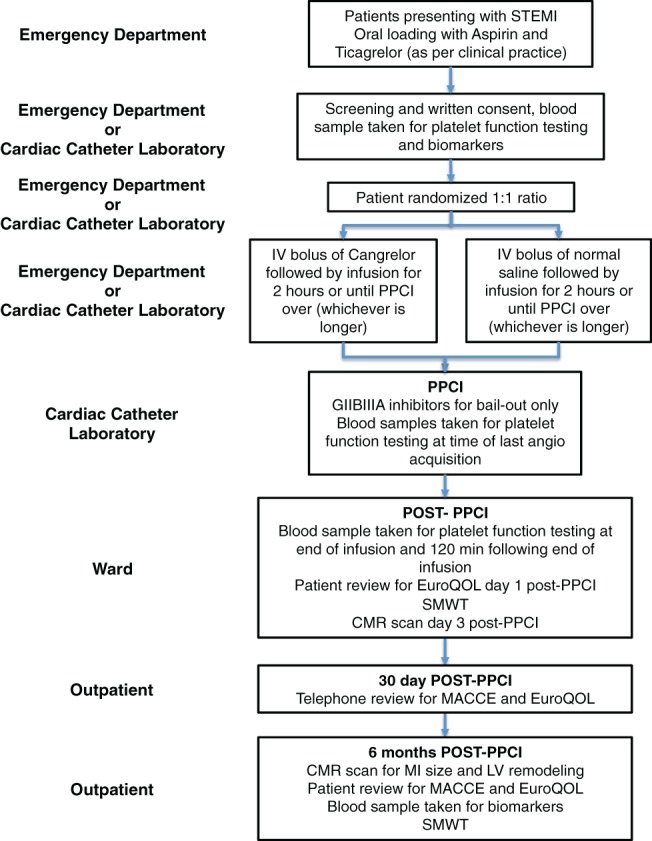
Patient pathway in platelet inhibition to target reperfusion injury trial (for information see main text). CMR, cardiovascular magnetic resonance; GIIAIIB, glycoprotein 2B3A; IV, intravenous; LV, left ventricular; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; PPCI, percutaneous coronary intervention; SMWT, 6‐minute walk test; STEMI ST‐segment elevation myocardial infarction
2.2. Overall objective
The overall objective of the PITRI trial will be to investigate whether an IV infusion of cangrelor initiated prior to reperfusion will prevent MVO and reduce MI size in STEMI patients treated with PPCI.
2.3. Patient inclusion and exclusion criteria
On arrival at the hospital, patients presenting with a suspected STEMI will be screened for study eligibility and written informed consent will be obtained. Oral loading with 180 mg of ticagrelor and 300 mg of aspirin will be performed as per current standard of care. Patient inclusion and exclusion criteria are listed in Table 1. In brief, the patient inclusion criteria include those patients presenting within 6 hours of symptom onset with >20 minutes chest pain and ST‐segment elevation on electrocardiography (ECG).
Table 1.
Patient inclusion and exclusion criteria for PITRI trial
| Inclusion criteria |
| 1. Age ≥ 21 and < 80 years of age |
2. STEMI as defined by:
|
| 3. ≤6 hour of onset of chest pain |
| Exclusion criteria |
| 1. History of previous myocardial infarction, stroke, transient ischemic attack or prior coronary artery bypass graft surgery |
| 2. Known contraindications to CMR (eg, CMR contraindicated implanted devices, allergy to contrast, severe renal insufficiency (estimated glomerular filtration rate < 40 mL/min/1.73 m2 and claustrophobia) |
| 3. Prior therapy before admission within 7 days of anticoagulant (warfarin, phenindione, dabigatran, apixaban, and rivaroxaban), glycoprotein IIb/IIIa inhibitor, oral P2Y12 inhibitor, or thrombolytic therapy |
4. Significant co‐morbidities
|
5. Contraindications to heparin or antiplatelet therapy
|
| 6. Pregnancy |
| 7. Patients on strong CYP3A inhibitors or inducers (eg, atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole, rifampicin, dexamethasone, phenytoin, carbamazepine, and phenobarbital) |
2.4. Patient randomization and treatment allocation
Consented patients will be randomized to receive either cangrelor or placebo using web‐based randomization through the Singapore Clinical Research Institute. Randomization will be stratified according to each recruiting center. Once randomized, the patient will be started on either cangrelor or placebo as soon as possible, prior to reperfusion of the culprit artery, but without delaying the procedure. The treatment allocation will be concealed from the patient, the interventional cardiologist, the blinded research staff collecting the data and clinical endpoints, and the core lab staff analyzing the CMR and platelet aggregation data.
The eligible consented patient will then be randomized to receive either:
1. Cangrelor treatment: Cangrelor will be administered as a single IV bolus (30 μg/kg) followed by an IV infusion (4 μg/kg/min) for at least 120 minutes or until PPCI procedure has ended (whichever is longer)—this will be initiated prior to reperfusion and will not delay the onset of reperfusion. This dosing regimen is identical to that used in the CHAMPION trials.14, 15, 16, 17 Furthermore, this dose was also found to be cardioprotective in a pre‐clinical model using rabbits.18
2. Placebo control: IV normal saline as a single IV bolus followed by an infusion of at least 120 minutes or until PPCI procedure has ended (whichever is longer)—this will be initiated prior to reperfusion, and will not delay the onset of reperfusion.
PPCI will be performed according to local practice with the use of thrombectomy, bivalirudin or heparin at the discretion of the interventional cardiologist. The use of glycoprotein IIb/IIIa inhibitors will be discouraged except for bail‐out procedures (to treat new or persistent thrombus formation, slow or no reflow, or distal embolization). Each site will be encouraged to continue oral ticagrelor (90 mg BD) as oral P2Y12 agent of choice together with aspirin for at least 6 months post‐PPCI.
2.5. Outcomes
2.5.1. Efficacy outcomes
The primary endpoint will be MI size at 3 days post‐PPCI, as quantified on CMR and expressed as the mass of late gadolinium enhancement (LGE) as a percentage of the LV mass.
The secondary endpoints will include the incidence and extent of MVO, myocardial salvage index, markers of successful myocardial reperfusion (ST‐segment resolution at 90 minutes, thrombolysis in myocardial infarction [TIMI] flow grade, TIMI frame count and TIMI blush grade), MI size by CMR at 6 months, post‐MI LV remodeling at 6 months, major adverse cardiac and cerebrovascular event (MACCE all‐cause death, hospitalization for heart failure [HHF], stent thrombosis, ischemia‐induced coronary revascularization, re‐infarction, and stroke), incidence of definite stent thrombosis at 48 hours (as defined by the Academic Research Consortium), platelet function tests (subset of 70 patients), blood biomarkers, 6‐minute walk test (SMWT) and quality of life (using the EuroQol EQ‐5D health‐related quality of Life questionnaire) will be collected at 30 days and 6 months (these data will be collected by telephone and review of medical notes at 30 days and at the time of the outpatient CMR scan at 6 months).
2.5.2. Safety outcomes
The primary safety endpoint will be bleeding not related to coronary artery bypass grafting (CABG), and classified according to the Bleeding Academic Research Consortium (BARC),22 at 48 hours.
2.6. CMR protocol
Each patient will undergo two CMR scans: the first scan will be performed on day 3 post‐PPCI and the second scan will be performed at 6‐month post‐PPCI (see Table 2 for CMR protocols). Before each scan, a blood sample will be taken prior to the scan to measure the hematocrit, a parameter that is required to calculate extracellular volume fraction by T1‐mapping CMR. The CMR scans use standard published protocols.23
Table 2.
CMR protocols for PITRI trial
| First CMR scan at day 3 post‐PPCI |
|
| Second CMR scan at 6 months post‐PPCI |
|
Abbreviations: CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; MI, myocardial infarction; MVO, microvascular obstruction; PITRI, platelet inhibition to target reperfusion injury; PPCI, percutaneous coronary intervention.
2.7. Platelet function testing
This will be assessed with the point‐of‐care platelet function test (VerifyNow System) according to manufacturer instructions to ensure maximum platelet inhibition at the time of PPCI in a subset of 70 STEMI patients. The VerifyNow System (Accumetrics Inc., San Diego, California) is a point‐of‐care turbidimetry‐based optical detection system that measures platelet‐induced aggregation.24, 25 It has received clearance from the FDA for measurement of the level of P2Y12 receptor blockade and uses a proprietary algorithm to report values in P2Y12 reaction units (PRU).26 Blood samples will be taken at 4 time‐points: prior to cangrelor or placebo, at time of final coronary angiography acquisition post‐PPCI, end of cangrelor or placebo infusion, and 120 minutes following the termination of the cangrelor or placebo infusion. Patients subsequently receiving glycoprotein IIb/IIIa inhibitors for bail‐out will be excluded from the platelet function sub‐study.
2.8. Blood biomarkers
Blood samples will be taken to measure blood biomarkers for myocardial fibrosis and inflammation, and other factors related to cangrelor treatment or adverse post‐STEMI LV remodeling. Blood markers for myocardial fibrosis will include matrix metalloproteinases (MMPs) 1, 2 3, 8, 9, Tissue inhibitor of metalloproteinases (TIMP)‐1 and − 4, galectin‐3 and osteopontin. Blood biomarkers for inflammation will include C‐reactive protein (CRP), interleukin (IL)‐6, IL‐1, IL‐10, sCD40 ligand, and TNF‐α. Blood samples will be taken at 2 time‐points in total: prior to cangrelor or placebo treatment and at 6 months. At each of the time‐points, 30 mL of blood will be obtained.
2.9. 6‐minute walk test
Functional capacity of patients will be measured using the SMWT. The test will be carried out twice, the first measurement before discharge from the index admission, and the second measurement will be done at 6 months follow‐up.
2.10. Statistical analysis
2.10.1. Sample size
The primary endpoint will be MI size quantified by LGE expressed as a percentage of LV mass measured on day 3 by CMR. In recently published studies in STEMI patients, presenting within 6 hours of chest pain onset, treated with PPCI, the weighted mean MI size (as a percentage of the LV) in the control group was 22% (SD of 12%).21 Experimental animal studies have reported that cangrelor administered prior to reperfusion reduce MI size by 50% in the in situ rabbit heart.8 We estimate a more conservative effect size with cangrelor in STEMI patients of 25% given patient variability in terms of ischemic time, and presence of co‐morbidities (age, diabetes) and co‐medications (nitrates, statins, and morphine) which may impact on cardioprotective efficacy. In order to demonstrate a 25% reduction in MI size from 22% to 16.5%, 76 STEMI patients per treatment group or 152 in total would be required (80% power, 2‐sided test at 5% significance level). However, the patients most likely to benefit from cardioprotection against reperfusion injury are those presenting with pre‐PPCI TIMI grade flow ≤1.21 Because of the intervention or placebo would be initiated prior to knowing the TIMI flow grade, then the sample size is inflated to a total of 190 patients (given that 25% of the STEMI patients may be expected to have a pre‐PPCI TIMI coronary flow 2‐3). To allow for a 10% to 12% dropout rate,27, 28, 29, 30, 31, 32 we intend to recruit 210 patients in total. After 100 PPCI patients have been recruited (about 50% of target), a pre‐specified interim analysis of acute MI size data on the first scan will be undertaken by the study statistician to investigate the effect of cangrelor on MI size. If there is <5% difference in MI size with patients randomized to receive cangrelor then the PITRI study may be terminated on the basis of futility. The data and safety monitoring committee (DSMC) charter will provide details on how the study would be terminated on grounds of futility.
2.10.2. Primary outcome analysis
The primary endpoint is MI size as a percentage of LV mass measured by CMR at day 3. The statistical analysis will be performed by analysis of covariance with the MI size as the response variable with the treatment group included as a binary covariate in the model. If the data are non‐normally distributed, a transformation may be applied to the primary endpoint or a non‐parametric analysis performed, such as Mann‐Whitney‐Wilcoxon test or permutation test. The primary analyses will be by intention to treat analysis but there will also be a per protocol analysis. If important differences in the baseline and angiography characteristics are noted between the two groups, an adjusted comparison of the primary analysis will also be performed.
2.10.3. Secondary outcome analysis
In the case of secondary endpoint, Fishers exact test will be used to compare categorical variables. Continuous variables will be compared with a mixed effects model to account for repeated measures. Time‐to‐event analyses (secondary clinical endpoints), based on all available follow‐up data, will be performed with the use of Kaplan‐Meier estimates and will be compared between groups with the use of the log‐rank test. A generalized linear model will be used to calculate risk ratios in the subgroup analyses and to test for interactions.
2.10.4. Sensitivity and other planned analyses
A sensitivity analysis will be performed to understand the implications of missing data. In addition, the following subgroup analyses will be performed: patients with pre‐PPCI TIMI flow ≤1 vs >1; LAD STEMI vs non‐LAD STEMI; below and above the median age; diabetes vs non‐diabetes. Subgroups will be analyzed using an interaction test by fitting an interaction term of treatment and the relevant subgroups in the appropriate regression model.
2.11. Trial governance, data management and funding
The trial management group will meet regularly to discuss the execution of the trial. The SingHealth Clinical Trials Compliance Unit will ensure full safety monitoring of the research study is performed in compliance with Singapore Guidelines for Good Clinical Practice and local regulatory requirements.
Data will be collected by a paper case report form and entered onto the Web‐based electronic RedCap database. An independent DSMC has been installed to monitor the progress of the study as well as to raise any safety concerns. All expected or unexpected adverse events will be reviewed continuously by the investigators, the sponsor and the DSMC according to the sponsor's regulations.
3. RESULTS
The study started in November 2017 and it is anticipated that it would be completed in July 2020. To date (October 2018), 47 patients have been recruited from the 3 sites.
4. DISCUSSION
Cangrelor, a nonthienopyridine adenosine triphosphate analog, is an IV antagonist of the adenosine diphosphate receptor P2Y12. It offers rapid‐onset reversible and predictable platelet inhibition. This promises an ideal pharmacokinetic profile for emergency patients presenting with an acute coronary syndrome (reviewed in Reference 33). It has a plasma half‐life of 3 to 5 minutes with platelet function rapidly returning to baseline levels, within 60 minutes, respectively, upon completion of an IV infusion.34, 35
The first experimental study to provide evidence of a direct cardioprotective effect with P2Y12 receptor antagonists was by Yang et al.18 who showed that administering Cangrelor prior to reperfusion reduced MI size by 30% in rabbits. Crucially, cangrelor was only effective at limiting MI size if it was administered prior to reperfusion, confirming that cangrelor targeted myocardial reperfusion injury.18 Interestingly, the MI limiting effects of cangrelor but not its platelet inhibitory effects were abrogated by pharmacological inhibitors of PI3K and MEK1/2, known signaling mediators of cardioprotection, suggesting a dissociation between the MI‐limiting effect of cangrelor and its antiplatelet effects. Pre‐treatment of rabbits with clopidogrel was also shown to reduce MI size and again this cardioprotective effect was abrogated by signal pathway inhibitors. The interpretation of these findings was that the platelet P2Y12 inhibitors might reduce MI size independent of their antiplatelet effect. The MI limiting effects of platelet P2Y12 inhibition have been confirmed using pre‐treatment with oral ticagrelor in rats,10, 36 and cangrelor in primates.9 More recently, it has been shown that intraperitoneal ticagrelor administered 5 minutes prior to reperfusion reduced MI size in an in vivo rat model of acute reperfusion injury, confirming that cardioprotective strategy can target myocardial reperfusion injury.37 Furthermore, pre‐treatment with ticagrelor was also shown to reduce MI size in a porcine MI model.38 The mechanism underlying cardioprotection by P2Y12 inhibition by cangrelor or ticagrelor is unclear but may be because of the activation of protective signaling of postconditioning through sphingosine‐1 phosphate.18, 39
However, because of reduced bioavailability of the oral P2Y12 platelet inhibitors that are given to STEMI patients, platelet inhibition is not maximal during PPCI. Up to 90% of STEMI patients have been shown to have negligible levels of platelet inhibition at the beginning of the procedure after pre‐hospital administration of 600 mg of clopidogrel.40, 41 These findings have been attributed to impaired bioavailability of clopidogrel which characterizes STEMI patients because of delayed intestinal absorption, systemic vasoconstriction, and hemodynamic disturbances. More surprisingly, in a randomized study42 comparing ticagrelor and prasugrel in STEMI patients undergoing PPCI, both agents showed an important delay of action with high on‐treatment platelet reactivity (OPR) rates (46.2% and 34.6%, respectively) 2 hours after the loading dose. These findings were confirmed by Parodi et al.7 where prasugrel and ticagrelor provided similar platelet inhibition and this was effective in only half of the patients after 2 hours of the loading dose. At least 4 hours was required to achieve effective platelet inhibition in the majority of patients in that study. A similar finding was recently confirmed, where >2 hours were required to achieve adequate platelet inhibition with ticagrelor in STEMI patients treated with PPCI.43 Although IV glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban) would have a faster onset of action than oral P2Y12 inhibitors, they also increase the bleeding risk, and their offset of action is much longer than that of cangrelor.44 In the PITRI trial, we intend to explore whether the fast‐acting IV P2Y12 inhibitor, cangrelor, can reduce MI size and MVO because to its combination of potent antiplatelet (to mitigate against distal embolization of platelet aggregation) and cardioprotective effects (to mitigate against reperfusion injury), when initiated prior to PPCI in STEMI patients.
In the setting of PCI for acute coronary syndrome (ACS) and stable angina, none of the initial phase 3 trials (CHAMPION‐PCI14 and CHAMPION‐PLATFORM15) showed any benefit of cangrelor over clopidogrel in reducing the primary endpoint but it was associated with reductions in secondary endpoints, including the rate of stent thrombosis, and with no excess in severe bleeding. A pooled analysis of these two CHAMPION trials using the universal definition of MI was subsequently carried out and showed that cangrelor was associated with a significant reduction in early ischemic events when compared with clopidogrel in patients with non‐ST‐elevation ACS undergoing PCI, and a lower rate of stent thrombosis was observed.45 This led to the design of CHAMPION‐PHOENIX trial46 which randomized 11 145 patients undergoing urgent or elective PCI to receive cangrelor or a 600 or 300 mg loading dose of clopidogrel. Cangrelor significantly reduced the rate of ischemic events, including stent thrombosis, during PCI, with no significant increase in severe bleeding at 48 hours. However, many of the patients received 300 mg of clopidogrel instead of the 600 mg dose and 37% of patients received the loading dose during or after PCI rather than before the procedure.47 So far, no study has assessed the benefit of IV cangrelor over oral ticagrelor in reducing MI size and MVO, in the setting of STEMI.
Cangrelor administered in combination with ticagrelor has recently been shown to provide consistent and potent P2Y12 inhibition during PPCI in real‐world patients.48 Furthermore, a recent post‐hoc analysis of the three CHAMPION studies showed that cangrelor's efficacy in reducing ischemic complications in patients undergoing PCI was maintained irrespective of whether there was concomitant use of glycoprotein IIb/IIIa inhibitors or not.49 However, use of glycoprotein IIb/IIIa inhibitors was associated with higher bleeding rates, regardless of the randomization to cangrelor or clopidogrel.49 In a study of South Korean patients with ACS, those treated with ticagrelor had the lowest OPR with 87.5% of the patients having OPR values in the range for increased risk of bleeding.50 Therefore, to minimize the potential for harm, use of glycoprotein IIb/IIIa inhibitors will be discouraged in the PITRI trial except for bail‐out and the platelet function sub‐study will provide additional insights into the response of this Asian cohort (consisting predominantly of ethnic Chinese patients) to ticagrelor and cangrelor.
5. CONCLUSION
The aim of the PITRI trial is to assess whether cangrelor administered prior to reperfusion and concomitantly with ticagrelor, will not only provide effective platelet inhibition during the PPCI procedure but would also potentially reduce reperfusion injury, both factors that may lead to a reduction in MVO and MI size, as assessed by CMR.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The PITRI trial is funded by a Singapore Ministry of Health's National Medical Research Council under its Clinical Trials Grant (NMRC/CTGIITE/0005/2016). DJH was supported by the British Heart Foundation (FS/10/039/28270), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke‐National University Singapore Medical School, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist‐Senior Investigator scheme (NMRC/CSA‐SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016‐T2‐2‐021). This article is based upon work from COST Action EU‐CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
Bulluck H, Chan MHH, Bryant JA, et al. Platelet inhibition to target reperfusion injury trial: Rationale and study design. Clin Cardiol. 2019;42:5–12. 10.1002/clc.23110
Funding information COST Action EU‐CARDIOPROTECTION, Grant/Award Number: CA16225; Singapore Ministry of Health's National Medical Research Council, Grant/Award Number: NMRC/CTGIITE/0005/2016; Duke‐National University Singapore Medical School; National Institute for Health Research University College London Hospitals Biomedical Research Centre; British Heart Foundation, Grant/Award Number: FS/10/039/28270; National Medical Research Council
Heerajnarain Bulluck and Mervyn Chan made an equal contribution, and should be considered Joint First Authors.
REFERENCES
- 1. Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373(11):1021‐1031. [DOI] [PubMed] [Google Scholar]
- 2. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121‐1135. [DOI] [PubMed] [Google Scholar]
- 3. Krug A, de Rochmonte WDM, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ Res. 1966;19(1):57‐62. [DOI] [PubMed] [Google Scholar]
- 4. White SK, Sado DM, Flett AS, Moon JC. Characterising the myocardial interstitial space: the clinical relevance of non‐invasive imaging. Heart. 2012;98(10):773‐779. [DOI] [PubMed] [Google Scholar]
- 5. Bulluck H, Hausenloy DJ. Microvascular obstruction: the bane of myocardial reperfusion. Rev Espanola de cardiologia. 2015;68(11):919‐920. [DOI] [PubMed] [Google Scholar]
- 6. Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart. 2016;102(5):341‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST‐segment elevation myocardial infarction patients: RAPID (Rapid activity of platelet inhibitor drugs) primary PCI study. J Am Coll Cardiol. 2013;61(15):1601‐1606. [DOI] [PubMed] [Google Scholar]
- 8. Yang X‐M, Liu Y, Cui L, et al. Platelet P2Y12 blockers confer direct postconditioning‐like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther. 2012;18(3):251‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X‐M, Liu Y, Cui L, et al. Two classes of anti‐platelet drugs reduce anatomical infarct size in monkey hearts. Cardiovasc Drugs Ther. 2013;27(2):109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang XM, Cui L, Alhammouri A, Downey JM, Cohen MV. Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther. 2013;27(5):403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bell RM, Sivaraman V, Kunuthur SP, Cohen MV, Downey JM, Yellon DM. Cardioprotective properties of the platelet P2Y(12) receptor inhibitor, Cangrelor: protective in diabetics and reliant upon the presence of blood. Cardiovasc Drugs Ther. 2015;29(5):415‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang XM, White J, WKuck J, Ruchko MV, Wilson GL, et al. Mitochondrially targeted endonuclease III has a powerful anti‐infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2015;110(2):1435‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen MV, Yang XM, White J, Yellon DM, Bell RM, Downey JM. Cangrelor‐mediated Cardioprotection requires platelets and Sphingosine phosphorylation. Cardiovasc Drugs Ther. 2016;30(2):229‐232. [DOI] [PubMed] [Google Scholar]
- 14. Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318‐2329. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330‐2341. [DOI] [PubMed] [Google Scholar]
- 16. Genereux P, Stone GW, Harrington RA, Gibson CM, Steg PG, et al. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX trial (clinical trial comparing Cangrelor to Clopidogrel standard of care therapy in subjects who require percutaneous coronary intervention). J Am Coll Cardiol. 2014;63(7):619‐629. [DOI] [PubMed] [Google Scholar]
- 17. White HD, Bhatt DL, Gibson CM, Hamm CW, Mahaffey KW, et al. Outcomes with cangrelor versus clopidogrel on a background of bivalirudin: insights from the CHAMPION PHOENIX (a clinical trial comparing Cangrelor to Clopidogrel standard therapy in subjects who require percutaneous coronary intervention [PCI]). JACC Cardiovascular . interventions. 2015;8(3):424‐433. [DOI] [PubMed] [Google Scholar]
- 18. Yang XM, Liu Y, Cui L, et al. Platelet P2Y12blockers confer direct postconditioning‐like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther. 2013;18(3):251‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexopoulos D, Pappas C, Sfantou D, et al. Cangrelor in ticagrelor‐loaded STEMI patients undergoing primary percutaneous coronary intervention. J Am Coll Cardiol. 2018;72(14):1750‐1751. [DOI] [PubMed] [Google Scholar]
- 20. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST‐segment‐elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137(18):1949‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bulluck H, Hammond‐Haley M, Weinmann S, Martinez‐Macias R, Hausenloy DJ. Myocardial infarct size by CMR in clinical cardioprotection studies: Insights from randomized controlled trials. JACC Cardiovasc. Imaging. 2017;10(3):230‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736‐2747. [DOI] [PubMed] [Google Scholar]
- 23. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a VerifyNow‐P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol. 2006;28(5):315‐322. [DOI] [PubMed] [Google Scholar]
- 25. Malinin A, Pokov A, Spergling M, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow‐P2Y12(R) rapid analyzer: the VERIfy thrombosis risk ASsessment (VERITAS) study. Thromb Res. 2007;119(3):277‐284. [DOI] [PubMed] [Google Scholar]
- 26. Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119(19):2625‐2632. [DOI] [PubMed] [Google Scholar]
- 27. Freixa X, Bellera N, Ortiz‐Perez JT, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 28. Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 29. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, et al. Exenatide reduces reperfusion injury in patients with ST‐segment elevation myocardial infarction. Eur Heart J. 2012;33(12):1491‐1499. [DOI] [PubMed] [Google Scholar]
- 30. Tarantini G, Favaretto E, Marra MP, et al. Postconditioning during coronary angioplasty in acute myocardial infarction: the POST‐AMI trial. Int J Cardiol. 2012;162(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 31. Woo JS, Kim W, Ha SJ, et al. Cardioprotective effects of exenatide in patients with ST‐segment‐elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33(9):2252‐2260. [DOI] [PubMed] [Google Scholar]
- 32. Siddiqi N, Neil C, Bruce M, et al. Intravenous sodium nitrite in acute ST‐elevation myocardial infarction: a randomized controlled trial (NIAMI). Eur Heart J. 2014;35(19):1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marino M, Rizzotti D, Leonardi S. Cangrelor: review of the drug and the CHAMPION programme (including PHOENIX). Curr Cardiol Rep. 2014;16(6):493. [DOI] [PubMed] [Google Scholar]
- 34. Ueno M, Ferreiro JL, Angiolillo DJ. Update on the clinical development of cangrelor. Expert Rev Cardiovasc Ther. 2010;8(8):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 35. Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 36. Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase‐2‐dependent effect. Arterioscler Thromb Vasc Biol. 2014;34(9):2078‐2085. [DOI] [PubMed] [Google Scholar]
- 37. Ye Y, Birnbaum GD, Perez‐Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(8):1805‐1814. [DOI] [PubMed] [Google Scholar]
- 38. Vilahur G, Gutierrez M, Casani L, Varela L, Capdevila A, et al. Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2016;134(22):1708‐1719. [DOI] [PubMed] [Google Scholar]
- 39. Downey JM, Cohen MV. Letter by Downey and Cohen Regarding Article,Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2017;135(17):e1000‐e1001. [DOI] [PubMed] [Google Scholar]
- 40. Ferreiro JL, Homs S, Berdejo J, Roura G, Gomez‐Lara J, et al. Clopidogrel pretreatment in primary percutaneous coronary intervention: prevalence of high on‐treatment platelet reactivity and impact on preprocedural patency of the infarct‐related artery. Thromb Haemost. 2013;110(1):110‐117. [DOI] [PubMed] [Google Scholar]
- 41. Biscaglia S, Tebaldi M, Vranckx P, Campo G, Valgimigli M. Effects of pre‐hospital clopidogrel administration on early and late residual platelet reactivity in ST‐segment elevation myocardial infarction patients undergoing primary intervention. J Thromb Haemost. 2013;11(1):192‐194. [DOI] [PubMed] [Google Scholar]
- 42. Alexopoulos D, Xanthopoulou I, Gkizas V, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5(6):797‐804. [DOI] [PubMed] [Google Scholar]
- 43. Bergmeij TO, Godschalk TC, Janssen PWA, Berge KVD, Breet NJ, et al. How long does it take for clopidogrel and ticagrelor to inhibit platelets in patients undergoing primary percutaneous coronary intervention? A detailed pharmacodynamic analysis: time course of platelet reactivity in STEMI (TOPS). Semin Thromb Hemost. 2017;43(4):439‐446. [DOI] [PubMed] [Google Scholar]
- 44. Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA. 2000;284(12):1549‐1558. [DOI] [PubMed] [Google Scholar]
- 45. White HD, Chew DP, Dauerman HL, et al. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163(2):182‐190.e4. [DOI] [PubMed] [Google Scholar]
- 46. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303‐1313. [DOI] [PubMed] [Google Scholar]
- 47. Siller‐Matula JM, Huber K, Christ G, et al. Impact of clopidogrel loading dose on clinical outcome in patients undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. Heart. 2011;97(2):98‐105. [DOI] [PubMed] [Google Scholar]
- 48. Mohammad MA, Andell P, Koul S, James S, Schersten F, et al. Cangrelor in combination with ticagrelor provides consistent and potent P2Y12‐inhibition during and after primary percutaneous coronary intervention in real‐world patients with ST‐segment‐elevation myocardial infarction. Platelets. 2017;28(4):414‐416. [DOI] [PubMed] [Google Scholar]
- 49. Vaduganathan M, Harrington RA, Stone GW, et al. Cangrelor with and without glycoprotein IIb/IIIa inhibitors in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2017;69(2):176‐185. [DOI] [PubMed] [Google Scholar]
- 50. Lee JH, Ahn SG, Park B, et al. A pharmacodynamic study of the optimal P2Y12 inhibitor regimen for east Asian patients with acute coronary syndrome. Korean J Intern Med. 2015;30(5):620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


