Abstract
Background
Galectin‐3 has emerged as a promising novel biomarker of cardiovascular fibrosis in patients with cardiovascular diseases.
Hypothesis
We investigated whether galectin‐3 correlates with markers of vascular fibrosis, subclinical atherosclerosis, and cardiac function in patients with rheumatoid arthritis (RA), a disease accompanied by high cardiovascular risk.
Methods
RA and non‐RA individuals underwent applanation tonometry, carotid ultrasound, and impedance cardiography, to obtain markers of arterial stiffness, subclinical atherosclerosis, and myocardial function, respectively. Cardiovascular risk was estimated from the Framingham Heart Study. Serum levels of galectin‐3 were determined by enzyme‐linked immunosorbent assay.
Results
Galectin‐3 was elevated in RA patients (n = 85) compared to controls (n = 39), but this difference was no longer significant after adjustment for the presence of cardiovascular comorbidities. In the univariate analysis, galectin‐3 significantly correlated with markers of vascular stiffness (including pulse wave velocity, central blood pressure, central and peripheral pulse pressure, and total arterial compliance); atherosclerosis (carotid intima‐media thickness); myocardial blood flow (cardiac output, stroke volume) and contractibility (acceleration and velocity index); systemic vascular resistance, and estimated cardiovascular risk. Multivariate analysis models revealed an independent association between galectin‐3 and both cardiac output (β = −0.274, P = 0.039), as well as systemic vascular resistance (β = 0.266, P = 0.039).
Conclusions
In a relatively well‐controlled cohort of RA patients with low‐grade systemic inflammation and long‐standing disease, serum galectin‐3 might be useful as a marker of cardiac function and cardiovascular fibrosis.
Keywords: galectin‐3, myocardial function, rheumatoid arthritis, vascular stiffness
1. INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, autoimmune disease that activates arthritogenic immunoresponses along with several systemic inflammatory cascades.1 RA is accompanied by an aggravated cardiovascular profile, as it increases the risk of cardiovascular mortality by approximately 50% compared to the general population,2 and the incidence of heart failure (ischemic or non‐ischemic) by as much as 72%.3 Notably, the presentation of cardiac involvement in RA differs from the general population. The typical pattern of cardiac involvement is often diastolic dysfunction preceding the onset of clinical heart failure,4 while impaired myocardial perfusion has been reported even in asymptomatic individuals.5 Subsequently, these patients are less likely to exhibit typical signs and symptoms of cardiac disease and more likely to remain undiagnosed.6 Altogether, it is critical that novel markers of cardiovascular risk are implemented in patients with RA.7
Galectin‐3 has emerged as a promising novel biomarker of cardiovascular fibrosis and disease.8 Galectin‐3 is a member of a β‐galactosidase binding lectin family with a broad biological functionality, including cell proliferation, adhesion, differentiation, and apoptosis. It is expressed in fibroblasts, endothelial cells, and inflammatory cells such as activated macrophages, which are involved in most models of vascular injury, and plays an active role in both acute and chronic inflammatory response pathways.9 According to recent guidelines for the management of heart failure, it can be used to improve risk stratification in both acute and chronic heart failure.10 Its predictive value in terms of adverse cardiovascular outcomes has been demonstrated not only among high‐cardiovascular risk patients, but also in non‐heart failure population‐based cohorts.11
Despite the abundant data from high‐cardiovascular risk patients and the general population, only limited studies have evaluated galectin‐3 in patients with RA. In such studies, galectin‐3 appeared to be increased among RA patients in both peripheral blood samples and the synovial fluid and was associated with clinical disease measures.12, 13, 14 However, its potential utility as a biomarker of subclinical cardiovascular disease in patients with RA has not yet been efficiently examined. Therefore, the aim of the present study was to investigate whether serum galectin‐3 can be potentially used as marker of vascular fibrosis, subclinical atherosclerosis, and cardiac function in patients with RA.
2. METHODS
The study population consisted of consecutive patients with an established diagnosis of RA according to the 1987 American College of Rheumatology criteria,15 who were attending the Rheumatology Outpatient Unit. Non‐treated individuals without any known health problems, who attended typical check‐up appointments in the Internal Medicine Outpatient Unit, and healthy volunteers, comprised the control group. All individuals provided written informed consent before inclusion in the study. The institutional ethics committee approved the protocol of the study, which was conducted in accordance with the Helsinki declaration.
In the RA group, disease activity was assessed by the treating rheumatologist, using the 28‐joint Disease Activity Score (DAS28),16 which was calculated based on the number of tender and swollen joints, the subjective estimate (0‐100 scale) of pain/discomfort due to RA, and erythrocyte sedimentation rate (ESR) values. DAS28‐ESR thresholds of ≤3.2, 3.2‐5.1, and > 5.1 are indicative of low, moderate and high disease activity, respectively. Hypertension was defined as office systolic and/or diastolic blood pressure (SBP/DBP) ≥ 140/90 mm Hg, and/or current antihypertensive medication use.17 Previous cardiovascular events were defined as history of stroke, angina, and myocardial infarction based on self‐report and a review of medical record and current medication. Body mass index (BMI) was calculated in kg/m2. All vascular measurements were conducted within the same day between 10:00 am and 12:00 pm
2.1. Measurement of blood pressure
SBP/DBP was measured in the arm with the highest blood pressure using a validated oscillometric device (Microlife Exact BP, Microlife AG, Widnau, Switzerland) in the sitting position according to the guidelines17 Peripheral pulse pressure was estimated as an index of arterial stiffness by subtracting the DBP from the SBP.
2.2. Applanation tonometry
Applanation tonometry with the Sphygmocor device (AtCor Medical, Sydney, Australia) was applied for the assessment of a number of arterial stiffness indices. In particular, the radial pulse waveform was detected with a micromanometer‐tipped probe and was subsequently analyzed to estimate the aortic pressure waveform and calculate central aortic SBP/DBP. Central pulse pressure was obtained by subtracting the central DBP from the central SBP. Only high‐quality recordings, defined as an in‐device quality operator index of >80%, and acceptable curves on visual inspection, were included in the analysis.
Carotid‐femoral pulse wave velocity (PWV) was estimated as the gold‐standard measure of arterial stiffness, using the same device and a standard protocol. Sequential recordings of the pulse wave were obtained in the carotid and femoral artery. Wave transit time was calculated from a simultaneously recorded electrocardiogram. The distance traveled by the pulse wave was calculated from the difference between the distances from sternal notch to the recording sites, over the body surface in a direct line. PWV was calculated as the distance traveled by the pulse wave between the carotid and the femoral sampling site, divided by time (PWV = Δd/Δt). Participants were advised to abstain from caffeine, smoking, alcohol, and intense physical activity for at least 2 hours before assessment of arterial stiffness.
2.3. Carotid ultrasound
Subclinical atherosclerosis of the carotid arteries was evaluated by measurement of carotid intima‐media thickness (cIMT). Longitudinal images of the common carotid arteries were obtained with carotid ultrasound (Aloka ProSound A7 Ultrasound System, Tokyo, Japan). Mean cIMT of the left and right common carotid artery was calculated from three consecutive measurements taken in the far wall of the distal 10 mm of each artery.18
2.4. Impedance cardiography
Myocardial performance and other indices of arterial stiffness were non‐invasively assessed with impedance cardiography (Cardioscreen 1000 impedance cardiography system, Medis, Germany), which offers continuous, beat‐by‐beat measurements of central hemodynamics.19 As low‐amplitude alternating electrical current is applied through external sensors in the thorax, baseline thoracic impedance (Zo) is digitally processed along with electrocardiogram recordings, to obtain pulsatile impedance/time changes (dZ/dt). Cardiac output and stroke volume were used to evaluate myocardial blood flow. Acceleration index, which is the maximum rate of change of blood velocity in the aorta, and velocity index, which corresponds to the blood velocity in the aorta, were calculated as markers of myocardial contractibility. Systemic vascular resistance (SVR), an index of afterload, and total arterial compliance, which describes the ability of the arterial wall to distend and increase volume with increasing transmural pressure, were additionally calculated.
Cardiac output, stroke volume, SVR, and total arterial compliance were normalized for body size by indexing to each patient's body surface area to obtain cardiac index, stroke index, SVR index, and total arterial compliance index, which were used for the analyses.20
2.5. Cardiovascular risk score
As RA‐specific cardiovascular risk algorithms do not seem to predict cardiovascular risk more accurately, compared to the general population cardiovascular risk calculators, including the Framingham Risk Score,21 each patient's cardiovascular risk was calculated from the Framingham Heart Study. The algorithm uses information regarding sex, age, SBP, treatment for hypertension, smoking, diabetes, high‐density lipoprotein, and total cholesterol to estimate 10‐year risk of cardiovascular disease.22
2.6. Biochemical measurements
After completion of vascular and other non‐invasive measurements analyzed above, blood sample was drawn and fasting lipids were estimated. Samples from RA subjects were additionally analyzed for inflammatory markers (C‐reactive protein [CRP], with detection limit of 3.19 mg/L, and ESR).
Galectin‐3 was calculated in serum samples that were separated and stored at −80°C. Commercially available competitive enzyme‐linked immunosorbent assay (ELISA) kit for human galectin‐3 (Catalog No: AMS.E0497h, AMS Biotechnology [AMSBIO Europe] Ltd.) was used for the determination of serum galectin‐3 levels (detection range 2.5‐160.0 ng/dL).
2.7. Statistical analysis
Analysis was performed using the Statistical Package for Social Sciences (SPSS), version 22. Results were expressed as frequencies for qualitative variables and as mean ± SD (m ± SD) or median (interquartile range) for continuous variables, according to the normality of their distribution. Comparison of frequencies for qualitative variables was performed with the Pearson χ 2 test, while differences between mean values for continuous variables were estimated by Student t or Mann‐Whitney U test. One‐way analysis of variance (anova) with Bonferroni post‐hoc test was used to correct for multiple comparisons or the non‐parametric Kruskal‐Wallis test for non‐normally distributed variables, when more than two groups were involved. Correlation coefficients were calculated with the parametric Pearson or the non‐parametric Spearman rank tests. Multiple linear regression analysis with the “Enter” method was used to identify the statistically significant predicting factors of galectin‐3. A probability value of P ≤ 0.05 was considered statistically significant.
3. RESULTS
A total of 124 participants were included, 85 RA and 39 non‐RA individuals, with a mean age of 59.6 ± 11.6 years. RA was long‐standing (median disease duration of 10 [5‐18] years) with low inflammatory burden, based on the levels of ESR (20 [10‐30] mm/hour) and CRP (3.19 [3.19‐4.1] mg/L). The vast majority of patients (88.2%) were well‐controlled as they presented with low‐to‐moderate disease activity, with a mean DAS score of 3.6 ± 1.2. All patients were receiving treatment for RA, in particular: disease‐modifying antirheumatic drugs (69.4%), biologics (47.1%), and glucocorticoids (31.8%). Antihypertensive drugs were prescribed in 45.9% and statins in 22.4% of patients.
3.1. Comparison of galectin‐3 levels between RA patients and controls
Baseline characteristics of the study population are presented in Table 1. Serum galectin‐3 was significantly higher in RA patients, compared to the control group (17.7 [9.8‐33.5] vs 9.1 [6.0‐12.0] ng/dL, P < 0.001). In addition, RA patients exhibited higher levels of arterial stiffness and carotid atherosclerosis (Table 1).
Table 1.
Baseline characteristics of study population
| Variable | RA Group (n = 85) | Control group (n = 39) | P value |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Age (years) | 60.6 ± 11.9 | 57.2 ± 8.6 | 0.073 |
| Male sex (%) | 23.1 | 16.5 | 0.380 |
| BMI (kg/m2) | 26.5 ± 4.6 | 26.9 ± 4.2 | 0.613 |
| Office SBP (mm Hg) | 123.7 ± 14.2 | 121.5 ± 10.6 | 0.327 |
| Office DBP (mm Hg) | 73.6 ± 7.6 | 76.0 ± 8.2 | 0.140 |
| Heart rate (beats/min) | 69.9 ± 11.4 | 68.1 ± 7.7 | 0.094 |
| Smoking (%) | 22.3 | 17.9 | 0.786 |
| Hypertension (%) | 46.8 | 0.0 | <0.001 |
| Diabetes mellitus (%) | 8.2 | 0.0 | 0.081 |
| Cardiovascular events (%) | 9.4 | 0.0 | 0.059 |
| Biochemical measurements | |||
| Total cholesterol (mg/dL) | 205.9 ± 41.5 | 221.1 ± 35.1 | 0.016 |
| Triglycerides (mg/dL) | 114.0 (79.0‐150.0) | 99.5 (76.0‐142.0) | 0.150 |
| HDL‐C (mg/dL) | 54.4 ± 12.8 | 53.4 ± 12.5 | 0.677 |
| LDL‐C (mg/dL) | 128.6 ± 35.3 | 141.2 ± 27.9 | 0.073 |
| Galectin‐3 (ng/dL) | 17.7 (9.8–33.5) | 9.1 (6.0–12.0) | <0.001 |
| Markers of arteriosclerosis and atherosclerosis | |||
| Central SBP (mm Hg) | 115.7 ± 12.8 | 112.6 ± 11.6 | 0.199 |
| Central DBP (mm Hg) | 74.9 ± 7.9 | 75.8 ± 10.7 | 0.206 |
| Central pulse pressure (mm Hg) | 40.8 ± 10.8 | 36.8 ± 10.6 | 0.022 |
| Peripheral pulse pressure (mm Hg) | 50.1 ± 11.5 | 45.4 ± 7.3 | 0.007 |
| PWV (m/s) | 8.3 ± 2.2 | 7.3 ± 1.3 | 0.003 |
| cIMT (mm) | 0.68 ± 0.13 | 0.62 ± 0.09 | 0.004 |
| Systemic vascular resistance (dyn·s·cm−5/m2) | 2048 ± 620 | 1986 ± 533 | 0.813 |
| Total arterial compliance (ml/m2/mm Hg) | 1.08 ± 0.39 | 1.15 ± 0.33 | 0.273 |
| Markers of cardiac function | |||
| Cardiac output (l/min/m2) | 3.4 ± 0.8 | 3.5 ± 0.8 | 0.477 |
| Stroke volume (ml/m2) | 49.5 ± 12.4 | 52.5 ± 11.3 | 0.210 |
| Acceleration index (1/100/s2) | 86.9 ± 35.3 | 88.4 ± 35.5 | 0.835 |
| Velocity index (1/1000/s) | 56.3 ± 17.0 | 54.8 ± 15.9 | 0.662 |
Abbreviations: BMI, body mass index; cIMT, carotid intima‐media thickness; DBP: diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PWV, pulse wave velocity; RA: rheumatoid arthritis; SBP, systolic blood pressure.
Results are demonstrated as Mean ± SD / Median (IQ range).
To account for the presence of cardiovascular comorbidities (hypertension, diabetes, and established cardiovascular disease) in our RA population, we analyzed separately a subgroup of 38 RA patients free of such comorbidities, in comparison with the control group (n = 39). Both groups were comparable in terms of mean age, SBP/DBP, heart rate and sex (P > 0.05 for all comparisons). Both PWV (7.1 ± 1.4 vs 7.3 ± 1.3 m/s) and cIMT (0.62 ± 0.11 vs 0.62 ± 0.09 mm) were similar (P > 0.05 for all comparisons) between patients without cardiovascular comorbidities and controls, and the same pattern was observed with galectin‐3 [10.7 (6.4‐20.3) vs 9.1 (6.0‐12.0) ng/dL], as depicted in Figure 1.
Figure 1.
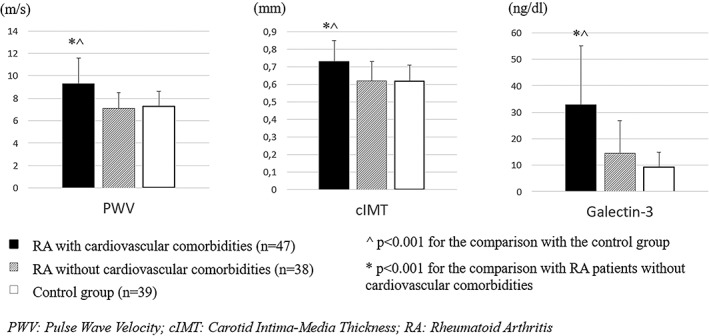
Distribution of the levels of arterial stiffness, carotid atherosclerosis, and serum galectin‐3 in RA patients, according to the presence of cardiovascular diseases, and non‐RA individuals of our study. Data are expressed as mean ± SD
To identify potential associations of galectin‐3 with markers of arteriosclerosis, atherosclerosis, and central hemodynamics, univariate analysis was performed in the RA group.
3.2. Association of galectin‐3 with anthropometric characteristics and cardiovascular risk factors
In the RA group, galectin‐3 levels were significantly associated with age (r = 0.534, P < 0.001) and BMI (r = 0.226, P = 0.037), but not with sex or lipids. A trend towards statistical significance was observed with office SBP (r = 0.210, P = 0.054). Hypertensive patients exhibited increased galectin‐3 compared to normotensives [30.5 (13.3‐44.0) vs 11.8 (6.7‐22.2) ng/dL, P < 0.001]. Galectin‐3 was non‐significantly higher in diabetics compared to non‐diabetic patients [23.6 (15.2‐61.2) vs 17.0 (9.0‐32.8) ng/dL, P = 0.225]. Patients who smoked exhibited significantly lower levels of galectin‐3 compared to non‐smokers [11.7 (6.7‐21.1) vs 20.7 (11.7‐33.7) ng/dL, P = 0.030], but smokers were significantly younger compared to non‐smokers (56.1 ± 10.1 vs 62.2 ± 12.2 years, P = 0.025).
3.3. Association of galectin‐3 with disease‐related parameters
Univariate analysis for galectin‐3 did not reveal significant associations with any of the disease‐related parameters that were studied, including ESR (r = −0.033, P = 0.768), CRP (r = 0.187, P = 0.103), DAS score (r = 0.139, P = 0.235), and disease duration (r = 0.102, P = 0.380), neither did galectin‐3 differ according to the use of biologics nor corticosteroids (P > 0.05 for the respective comparisons).
3.4. Association of galectin‐3 with central hemodynamics and markers of arteriosclerosis and atherosclerosis
A strong, positive association was observed between galectin‐3 and both PWV (r = 0.341, P = 0.002) and cIMT (r = 0.312, P = 0.004) among RA patients. In addition, increasing galectin‐3 levels were strongly associated with both increasing SVR (r = 0.364, P = 0.001) and decreasing total arterial compliance (r = −0.301, P = 0.005).
Of note, a significant association was observed between galectin‐3 and central SBP (r = 0.269, P = 0.016), in contrast with the observed association between galectin‐3 and office SBP, which only reached a trend towards statistical significance. Galectin‐3 was significantly associated with peripheral pulse pressure (r = 0.246, P = 0.023), while this association was even stronger for central pulse pressure (r = 0.345, P = 0.002).
3.5. Association of galectin‐3 with markers of cardiac function and cardiovascular risk
In the RA group, an inverse association was found between galectin‐3 and stroke volume (r = −0.279, P = 0.010), which was even stronger with cardiac output (r = −0.409, P < 0.001). In addition, galectin‐3 was strongly and inversely associated with both markers of cardiac contractibility, including acceleration index (r = −0.317, P = 0.003) and velocity index (r = −0.395, P < 0.001). Finally, galectin‐3 levels were positively associated with the estimated 10‐year cardiovascular risk from the Framingham Heart Study (r = 0.379, P = 0.001).
3.6. Multivariate analysis
Multivariate analysis revealed an independent association between galectin‐3 and age (β = 0.389, P = 0.012), as well as cardiac output (β = −0.274, P = 0.039), as depicted in Table 2. When cardiac output was replaced by SVR, which could not be entered in the same model due to multicollinearity, an independent association with SVR was likewise observed (β = 0.266, P = 0.039).
Table 2.
Multiple regression model for galectin‐3 levels among patients with rheumatoid arthritis
| Variable | Unstandardized coefficients | Standardized coefficients | 95% confidence intervals for B | P | ||
|---|---|---|---|---|---|---|
| B | SD | Β | Lower Bound | Upper Bound | ||
| Age (years) | 0.697 | 0.270 | 0.389 | 0.158 | 1.237 | 0.012 |
| Body mass index (kg/m2) | 0.810 | 0.539 | 0.177 | −0.266 | 1.886 | 0.138 |
| Hypertensiona | −5.316 | 5.222 | −0.126 | −15.742 | 5.111 | 0.312 |
| Smokinga | 2.059 | 5.106 | 0.042 | −8.135 | 12.254 | 0.688 |
| PWV (m/s) | 0.901 | 1.347 | 0.094 | −1.788 | 3.590 | 0.506 |
| Central pulse pressure (mm Hg) | 0.069 | 0.236 | 0.035 | −0.403 | 0.541 | 0.771 |
| cIMT (mm) | −42.514 | 23.732 | −0.249 | −89.896 | 4.869 | 0.078 |
| Cardiac index (l/min/m2) | −7.963 | 3.782 | −0.274 | −15.515 | −0.412 | 0.039 |
| Acceleration index (1/100/s2) | 0.072 | 0.081 | 0.122 | −0.089 | 0.233 | 0.373 |
Abbreviations: cIMT, carotid intima‐media thickness; PWV, pulse wave velocity.
Dependent variable: galectin‐3, P < 0.001, R 2 = 0.371, adjusted R 2 = 0.286.
1 = yes, 2 = no.
4. DISCUSSION
To the best of our knowledge, this is the first study to evaluate the levels of galectin‐3, a well‐established marker of vascular fibrosis and cardiovascular remodeling, in association with markers of arterial stiffness, subclinical atherosclerosis, and myocardial function in RA. In our cohort of relatively well‐controlled RA patients with long‐standing disease and low levels of systemic inflammation, serum galectin‐3 was not associated with disease‐related parameters, including inflammatory markers, disease activity, and duration. By contrast, galectin‐3 was strongly associated in the correlation analysis with essentially each and every marker of arterial stiffness that was studied, including central SBP, SVR, total arterial compliance, central and peripheral pulse pressure, and the gold‐standard PWV; carotid atherosclerosis; myocardial blood flow (cardiac output, stroke volume); cardiac contractibility (acceleration index, velocity index), and cardiovascular risk score. Of note, the association of galectin‐3 with office SBP only reached a trend towards statistical significance, in contrast with the significant association with central SBP. Decreasing cardiac output (Table 2) and increasing SVR were independently associated with elevated galectin‐3 levels in the RA population.
Another finding that merits further attention is the observed difference in galectin‐3 levels between RA and non‐RA individuals, which appears to be driven by the presence of cardiovascular comorbidities. Interestingly, the distribution of galectin‐3 levels among these groups (RA patients with and without cardiovascular comorbidities and non‐RA individuals) followed the same pattern of PWV and cIMT variance (Figure 1). Altogether, these results suggest that the increase in galectin‐3 levels in a relatively well‐controlled cohort of RA patients with low‐grade inflammation is largely the derivative of cardiovascular comorbidities and indicative of impaired cardiac output and increased SVR.
Our findings suggesting an association between galectin‐3 and both arterial stiffness and cIMT, at least in the univariate analysis, appear in accordance with previous reports. Galectin‐3 predicted PWV in community‐dwelling individuals23 and was associated with cIMT in asymptomatic individuals24 and high‐cardiovascular risk patients.25 After all, the observed independent association of galectin‐3 with both cardiac output and SVR in RA patients is consistent with a large body of evidence, mostly derived from patients with heart failure, in whom galectin‐3 correlates with cardiac function and the degree of cardiovascular remodeling.26
By contrast, available studies evaluating galectin‐3 in patients with rheumatic diseases are by far fewer. It is rather surprising that in this specific group of patients, galectin‐3 has been mainly addressed as a marker of the disease per se, whereas it has not yet been examined as a potential cardiac biomarker. Of note, galectin‐3 is markedly present in the synovium during the inflammatory flares, where it may play a causative role in the initiation and/or progression of arthritis owing to its pro‐inflammatory and pro‐catabolic actions.27 This notion is supported by studies showing elevated levels of galectin‐3 in both sera and synovial fluids,13, 14 as well as an association with CRP,14 among RA patients. According to the remarkable prospective studies by Issa et al., serum galectin‐3 is increased in patients with early undifferentiated arthritis of pre‐RA origin,12 while baseline levels of galectin‐3 in early RA correlate with anti‐CCP seropositivity and magnetic resonance imaging erosion score.13 Other than that, increased galectin‐3 has been associated with markers of disease activity and severity in other rheumatic diseases, including juvenile idiopathic arthritis,28 systemic sclerosis,29 primary Sjögren's syndrome30 and Behcet's disease.31
On the contrary, galectin‐3 was not associated with disease‐related parameters in the RA population of our study. This lack of association might be attributed to the specific characteristics of our RA population, who were relatively well‐controlled and presented with long‐standing disease and low levels of systemic inflammation. Moreover, it might be possible that circulating levels of galectin‐3 in the peripheral blood do not necessarily reflect the intra‐articular levels and activity of galectin‐3, as has been previously observed with circulating and endomyocardial galectin‐3 levels.32 Further studies are needed to examine whether galectin‐3 has a dual role in RA, either as disease biomarker in early RA and RA flares, or rather as a cardiac biomarker in well‐controlled, long‐standing disease, as is the case with other high‐cardiovascular risk populations and the general population.
The strengths of our study include the use of well‐established and validated methods for the assessment of arterial stiffness, subclinical atherosclerosis, and cardiac parameters. Our study is limited by its cross‐sectional design and a relatively small number of participants. Patients and controls were not adequately matched in terms of cardiovascular comorbidities, but we tried to overcome this limitation by performing a subgroup analysis of patients without cardiovascular comorbidities. A single measurement of inflammatory markers does not necessarily reflect their cumulative burden and fluctuation over time. In addition, RA patients of our study did not undergo cardiac ultrasound for the assessment of the cardiac structure and biometry; neither were imaging modalities implemented to evaluate joint destructive processes. Therefore, the present findings need to be regarded as preliminary, pending confirmation by larger studies.
In conclusion, in our cohort of relatively well‐controlled patients presenting with low levels of systemic inflammation and long‐standing RA, galectin‐3 levels were elevated in comparison with healthy, non‐RA individuals, but this difference appeared to be driven by the presence of hypertension and other cardiovascular comorbidities. In RA patients, serum galectin‐3 was associated with markers of arterial stiffness, subclinical atherosclerosis, cardiac function, and cardiovascular risk, but with none of the disease‐related parameters that were studied. The association between galectin‐3 and cardiac output, as well as SVR, remained significant even after adjustment for other factors. Further studies are needed to examine whether galectin‐3 can be used as a biomarker of cardiovascular fibrosis among patients with RA.
Anyfanti P, Gkaliagkousi E, Gavriilaki E, et al. Association of galectin‐3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin Cardiol. 2019;42:62–68. 10.1002/clc.23105
REFERENCES
- 1. Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376:1094‐1108. 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2. Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum. 2008;59:1690‐1697. 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 3. Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69:1275‐1285. 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 4. Davis JM III, Lin G, Oh JK, et al. Five‐year changes in cardiac structure and function in patients with rheumatoid arthritis compared with the general population. Int J Cardiol. 2017;240:379‐385. 10.1016/j.ijcard.2017.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anyfanti P, Triantafyllou A, Gkaliagkousi E, et al. Subendocardial viability ratio in patients with rheumatoid arthritis: comparison with healthy controls and identification of prognostic factors. Clin Rheumatol. 2017;36:1229‐1236. 10.1007/s10067-017-3659-9. [DOI] [PubMed] [Google Scholar]
- 6. Davis JM III, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603‐2611. 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López‐Mejías R, Castañeda S, González‐Juanatey C, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: The relevance of clinical, genetic and serological markers. Autoimmun Rev. 2016;15:1013‐1030. 10.1016/j.autrev.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 8. Hogas S, Bilha SC, Branisteanu D, et al. Potential novel biomarkers of cardiovascular dysfunction and disease: cardiotrophin‐1, adipokines and galectin‐3. Arch Med Sci. 2017;4:897‐913. 10.5114/aoms.2016.58664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can J Cardiol. 2016;32:659‐668. 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. JACC. 2013;62:e147‐e239. 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 11. Jagodzinski A, Havulinna AS, Appelbaum S, et al. Predictive value of galectin‐3 for incident cardiovascular disease and heart failure in the population‐based FINRISK 1997 cohort. Int J Cardiol. 2015;192:33‐39. 10.1016/j.ijcard.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 12. Issa SF, Duer A, Østergaard M, et al. Increased galectin‐3 may serve as a serologic signature of pre‐rheumatoid arthritis while markers of synovitis and cartilage do not differ between early undifferentiated arthritis subsets. Arthritis Res Ther. 2017;19:80 10.1186/s13075-017-1282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Issa SF, Christensen AF, Lindegaard HM, et al. Galectin‐3 is persistently increased in early rheumatoid arthritis (RA) and associates with anti‐CCP seropositivity and MRI bony lesions, while early fibrosis markers correlate with disease activity. Scand J Immunol. 2017;86:471‐478. 10.1111/sji.12619. [DOI] [PubMed] [Google Scholar]
- 14. Ohshima S, Kuchen S, Seemayer CA, et al. Galectin 3 and Its Binding Protein in Rheumatoid Arthritis. Arthritis Rheum. 2003;48:2788‐2795. 10.1002/art.11287. [DOI] [PubMed] [Google Scholar]
- 15. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315‐324. 10.2169/naika.77.742. [DOI] [PubMed] [Google Scholar]
- 16. Prevoo MLL, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LBA, van Riel PLCM. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44‐48. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 18. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima‐media thickness task force endorsed by the society for vascular. J Am Soc Echocardiogr. 2008;21:93‐111. 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 19. Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography the next vital sign technology? Chest. 2003;123:2028‐2033. 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 20. Taler SJ, Textor SC, Augustine JE. Resistant hypertension: Comparing hemodynamic management to specialist care. Hypertension. 2002;39:982‐988. 10.1161/01.HYP.0000016176.16042.2F. [DOI] [PubMed] [Google Scholar]
- 21. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis‐specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology. 2017;56:1102‐1110. 10.1093/rheumatology/kex038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743‐753. 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 23. Libhaber E, Woodiwiss AJ, Raymond A, et al. Independent associations of circulating galectin‐3 concentrations with aortic pulse wave velocity and wave reflection in a community sample. Hypertension. 2015;65:1356‐1364. 10.1161/HYPERTENSIONAHA.115.05159. [DOI] [PubMed] [Google Scholar]
- 24. Madrigal‐Matute J, Lindholt JS, Fernandez‐Garcia CE, et al. Galectin‐3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3:1‐13. 10.1161/JAHA.114.000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lisowska A, Knapp M, Tycińska A, et al. Predictive value of Galectin‐3 for the occurrence of coronary artery disease and prognosis after myocardial infarction and its association with carotid IMT values in these patients: A mid‐term prospective cohort study. Atherosclerosis. 2016;246:309‐317. 10.1016/j.atherosclerosis.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 26. Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin‐3 in Heart Failure: An Update of the Last 3 Years. Heart Fail Clin. 2018;14:75‐92. 10.1016/j.hfc.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Yéléhé‐Okouma M, Ea HK, Jouzeau JY, Reboul P. Galectin‐3: A key player in arthritis. Joint Bone Spine. 2017;84:15‐20. 10.1016/j.jbspin.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 28. Ezzat MHM, El‐Gammasy TMA, Shaheen KYA, Osman AOY. Elevated production of galectin‐3 is correlated with juvenile idiopathic arthritis disease activity, severity, and progression. Int J Rheum Dis. 2011;14:345‐352. 10.1111/j.1756-185X.2011.01632.x. [DOI] [PubMed] [Google Scholar]
- 29. Hromádka M, Seidlerová J, Suchý D, et al. Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients – Relationship with biochemical and echocardiography parameters. Int J Cardiol. 2017;249:448‐453. 10.1016/j.ijcard.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 30. Zhang R, Sun T, Song L, Zuo D, Xiao W. Increased levels of serum galectin‐3 in patients with primary Sjögren's syndrome: Associated with interstitial lung disease. Cytokine. 2014;69:289‐293. 10.1016/j.cyto.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 31. Lee YJ, Kang SW, Song JK, et al. Serum galectin‐3 and galectin‐3 binding protein levels in Behçet's disease and their association with disease activity. Clin Exp Rheumatol. 2007;25(4 Suppl 45):S41‐S45. [PubMed] [Google Scholar]
- 32. Besler C, Lang D, Urban D, et al. Plasma and cardiac galectin‐3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail. 2017;10(3). 10.1161/CIRCHEARTFAILURE.116.003804. [DOI] [PubMed] [Google Scholar]


