Abstract
Background
Patients discharged after an acute coronary syndrome (ACS) have substantial risk of recurrent ischemic events or dying.
Hypothesis
A difference may exist in risk predictors for all‐cause mortality and ischemic events between year 1 and 2 of follow‐up post‐ACS.
Methods
EPICOR (NCT01171404) was a prospective, international, real‐world cohort study of consecutive patients hospitalized for ACS within 24 hours of symptom onset and surviving to discharge. Total of 10 568 patients were enrolled (555 hospitals; 20 countries) and followed‐up for 2 years. From these, 4943 were admitted with ST‐elevation myocardial infarction (STEMI) and 5625 with non‐ST‐elevation ACS (NSTE‐ACS). Potential baseline predictors of major adverse cardiac and cerebrovascular events (MACCE; death, non‐fatal myocardial infarction [MI], non‐fatal stroke) were evaluated in year 1 and 2 post‐discharge.
Results
MACCE incidence per 100 person‐years at risk within and after 1 year was 5.3 vs 3.6, primarily death (4.1 vs 2.3), with no significant differences for MI or stroke. Older age, lack of coronary revascularization, raised creatinine, low hemoglobin, previous cardiac disease, previous chronic obstructive pulmonary disease, raised glucose, male sex, and geographic region were risk factors for MACCE in both year 1 and 2. By contrast, low ejection fraction, poorer quality of life, low body mass index (BMI) <20 kg/m2, in‐hospital cardiac complications, and Killip class lost predictive power after 1 year.
Conclusion
We observed continuous MACCE risk during 2 years of follow‐up after discharge for ACS, with greater mortality within the first year. Specific predictors at discharge for events after 1 year could not be identified.
Keywords: acute coronary syndrome, hospital discharge, mortality, prognostic model, risk predictor
1. INTRODUCTION
Prognosis of patients with acute coronary events (ACS) has improved over the past decade owing to the use of guideline‐recommended therapies, such as early revascularization, antithrombotic therapies and other secondary prevention measures.1, 2, 3 Consequently, the proportion of stable post‐ACS is growing and will represent a clinical challenge in the coming decades. Most data focus on events and predictors within the first year after an ACS,4, 5 whereas data describing events 1 year post‐ACS are scarce and limited to selected groups from clinical trials.6, 7 However, it is known that patients surviving the first year after an ACS remain at high risk for dying or having future ischemic events.3, 8, 9 Risk for further ischemic events and death is compounded by baseline characteristics and a high variability in management practice both at discharge and subsequently.3, 8 Risk stratification to guide secondary prevention therapies seems crucial for the decision‐making process.4, 10 The assessment of individual patient risk at hospital discharge provides an opportunity to potentially guide appropriate management strategies following discharge.11, 12
Little is known about the type of ischemic events occurring early and later after hospital discharge in patients post‐ACS. Similarly, there is a poor understanding of the prognostic value of baseline clinical features at different time points of the post‐ACS follow‐up. Using patient data from the EPICOR (long‐term follow‐up of antithrombotic management patterns In acute CORonary syndrome patients) registry (NCT01171404),13 we aimed to assess which predictors are associated with mortality and ischemic events within 1‐year follow‐up of an ACS event (year 1) and during a subsequent 1‐year follow‐up (year 2) in those patients who were event‐free during year 1. We tested 17 clinical predictors, which had been previously described in the EPICOR 2‐year mortality risk score.14
2. METHODS
2.1. Study design
EPICOR is a prospective, international, observational, real‐world practice, cohort study comprising consecutive patients, hospitalized for an ACS within 24 hours of symptom onset who survived to hospital discharge.13
In total, 10 568 patients were enrolled from 555 hospitals in 20 countries across Europe and Latin America (September 2010 to March 2011); 4943 were diagnosed with ST‐segment elevation myocardial infarction (STEMI) and 5625 with non‐ST‐segment elevation ACS (NSTE‐ACS, comprising non‐STEMI and unstable angina [UA]).
The study rationale and design have been described in detail elsewhere.13
2.2. Study population
The main inclusion criteria for the EPICOR registry were: hospitalization within 24 hours of symptom onset of the index event and a final diagnosis of STEMI or NSTE‐ACS at discharge, age ≥18 years, and written informed consent. Patients were excluded if they had a “secondary” ACS (precipitated by, or occurring as, a complication of surgery, trauma, gastrointestinal bleeding or percutaneous coronary intervention [PCI], or occurring during hospitalization for other reasons). Other exclusion criteria included any condition/circumstance considered likely to limit the completion of follow‐up, any serious/severe comorbidities limiting life expectancy to less than 6 months, or previous enrolment in EPICOR, or another clinical trial.
2.3. Follow‐up and event definition
Patients were followed up by centralized telephone interviews by trained native speakers of each patient's language, who were supervised by a Direct Patient Contact Manager. Patients were interviewed at 6 weeks after the index event and then every 3 months up to 24 months. Interviews included questions related to the occurrence of events, ischemic and/or bleeding; planned and unplanned hospitalizations, interventions or visits to the emergency room or other physicians (including dentists); treatment changes, including any planned/unplanned treatment interruptions; other healthcare resource utilization; and quality of life.
Events were recorded through specific questionnaires in which hospitalizations or emergency department visits were first documented. In such cases, interviewers were asked to collect all relevant clinical information (medical reports) from the patient, hospital physicians, or general practitioners. Whenever necessary, the primary study investigator was contacted to obtain confirmation or any clarification regarding the identified event.
All cardiovascular events reported by patients, relatives, or physicians were recorded together with specific information regarding each particular event, but only events in which a medical record with a specific diagnosis was available were computed. Therefore, event rates were calculated according to diagnoses reported in medical records.
In this analysis, patient follow‐up was divided into two periods: (a) first year follow‐up after hospital discharge from index ACS (year 1); and (b) second year (year 2; patients event‐free during year 1) follow‐up. Figure 1 depicts the flowchart of patients along the study.
Figure 1.
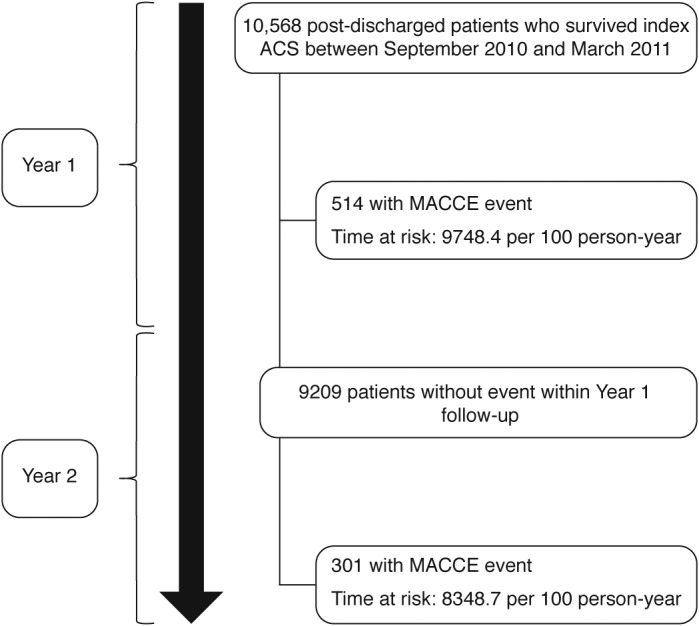
Patient flowchart. Abbreviations: ACS, acute coronary syndrome; MACCE, major adverse cardiac and cerebrovascular events
The primary endpoint during each time period was the composite of major adverse cardiac and cerebrovascular events (MACCE), defined as death, non‐fatal myocardial infarction (MI) (non‐fatal STEMI or non‐fatal non‐NSTEMI), and non‐fatal primary ischemic stroke.
2.4. Predictors of 2‐year mortality at hospital discharge after ACS
Relationships between outcomes and known baseline risk factors at discharge were also investigated. We investigated the baseline risk factors described in the 2‐year mortality risk score derived from the EPICOR and EPICOR Asia cohorts14 to describe their relationship with the outcome in our two time‐period cohorts. This risk model contains 18 predictors of 2‐year mortality: age, low ejection fraction at admission, no coronary revascularization, or thrombolysis, elevated serum creatinine at admission, poor EuroQol‐5 dimensions score (EQ‐5D, which is a patient questionnaire assessing five parameters: mobility, self‐care, ability to perform usual activities, pain/discomfort, and anxiety/depression as “no problem” for zero points, “moderate” for one point or “a severe limitation” for two points15), low hemoglobin, previous cardiac disease (MI, angina, heart failure, or atrial fibrillation), previous chronic obstructive pulmonary disease, elevated blood glucose at admission, on diuretics at discharge, male sex, lower educational level, on aldosterone inhibitor at discharge, body mass index (BMI), in‐hospital cardiac complications (MI or recurrent ischemia, cardiogenic shock, heart failure or any arrhythmia), diagnosis of STEMI, Killip class and region. The development and performance of the model have been described elsewhere.14 This risk score has been used for adjustment in previous studies.16, 17
All risk factors included in this score were used to describe patient characteristics and in‐hospital management in our study. We also used risk stratification categories to rank patients across six risk subgroups, according to 2‐year mortality risk: 1 to 4 representing the first 4 quintiles of patients, with groups 5 and 6 representing the top 2 deciles of risk.
2.5. Statistical analysis
Three groups were defined according to time of first‐event: (a) patients without events during follow‐up; (b) patients with an event during first‐year follow‐up; and (c) patients with an event during the second‐year follow‐up, who were event‐free during the first year. Patient characteristics and in‐hospital management across groups 2 and 3 were compared using χ 2 test or student t test as appropriate. Trend tests were used to compare ordinal risk factors between groups 2 and 3. Continuous parameters are presented as mean (SD) and categorical data are expressed as percentages.
Incidence rates for the primary endpoint are presented per 100 person‐years at risk. Stratified rate ratios for each time period (year 1 vs 2) were estimated and compared using the Mantel‐Haenszel test. Cumulative probabilities of the primary composite endpoint MACCE during each time period are presented as Kaplan‐Meier curves. A multivariable Cox proportional hazard regression including all variables in the 2‐year mortality risk score14 was performed to evaluate the association of these baseline risk factors with MACCE in each time period. Cumulative mortality and MACCE, stratified by six risk groups (based on EPICOR 2‐year mortality risk score)14 was calculated for each follow‐up time period, and P values were obtained using the log‐rank test for equality of survivor functions. Relationships between MACCE and known baseline risk factors are presented as hazard ratio (HR) with its 95% confidence interval (CI).
All P values were two‐sided and values of <0.05 were considered as statistically significant. All statistical analyses were performed using STATA software, version 13.1 (Stata Corp, College Station, Texas).
3. RESULTS
3.1. Descriptive MACCE events across first‐ and second‐year follow‐up
In total, 815 patients experienced MACCE during the 2‐year follow‐up, with more patients experiencing an event during year 1 (n = 514) compared with year 1 event‐free patients experiencing an event during year 2 (n = 301), P < 0.001. The difference in the outcome was driven by mortality, as death occurred more often during the first year, while non‐substantial differences in non‐fatal event rates were found between year 1 and 2. Kaplan‐Meier estimates of MACCE and incidence rate of events (by event type) for each time period are shown in Figure 2. MACCE and death incidence rates are presented in Supporting Information Table S1. Of note, 12 patients presented a subsequent event during the year 2 follow‐up.
Figure 2.
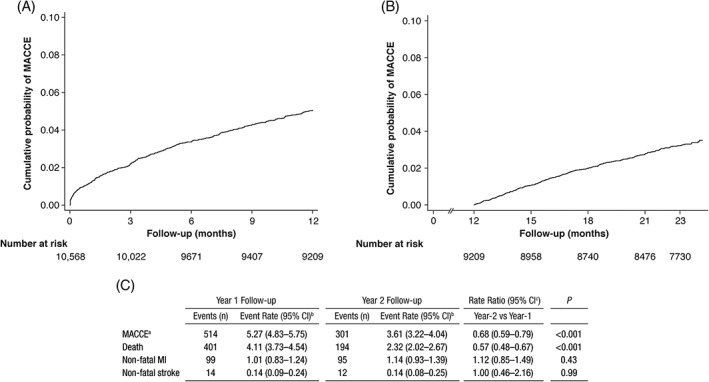
Kaplan‐Meier estimate of the risk of major adverse cardiac and cerebrovascular events (MACCE) at (A) year 1 of follow‐up after discharge, (B) year 2 of follow‐up (among year 1 MACCE‐free patients), and (C) incidence rate by event type. EPICOR had a total duration of 24 months, with the last interview conducted within ±2 weeks. Patients who completed study follow‐up were censored starting at 23.5 months. Time at risk (100 person‐years): year 1, 9748.4; year 2, 8348.7. Patients with non‐fatal event in year 1 were excluded from follow‐up in year 2. Year‐1 follow‐up, 10 568 patients at risk at start; year‐2 follow‐up, 9209 patients at risk at start. aMACCE, defined as death, non‐fatal MI and non‐fatal stroke. bPer 100 person‐years at risk. cYear 1 follow‐up as reference group. Abbreviations: CI, confidence interval; EPICOR, long‐term follow‐up of antithrombotic management patterns In acute CORonary syndrome; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction
3.2. Baseline characteristics and in‐hospital management for year 1 and 2
Baseline characteristics and in‐hospital management for populations with events (year 1 or 2) or with no events are shown in Table 1. No differences were observed in these variables between patients with an event during year 1 and those with an event during year 2 of follow‐up. Table S2 shows event rates for each variable during each time period.
Table 1.
Baseline characteristics and in‐hospital management according to time of first major adverse cardiac and cerebrovascular events (MACCE)
| Variable | Patients with no events | Patients with a year 1 event | Patients with only a year 2 event | P valuea |
|---|---|---|---|---|
| N | 9752 | 514 | 301 | — |
| Age in years, mean (SD) | 61.2 (12.1) | 68.5 (12.1) | 67.3 (12.4) | 0.201 |
| Male sex, n (%) | 7334 (75.2) | 368 (71.6) | 218 (72.4) | 0.799 |
| Education, n (%) | — | — | — | 0.949b |
| No formal | 414 (6.1) | 43 (11.7) | 20 (9.5) | — |
| Primary | 2312 (34.0) | 150 (40.8) | 97 (46.2) | — |
| Secondary | 3081 (45.3) | 130 (35.3) | 64 (30.5) | — |
| University | 997 (14.7) | 45 (12.2) | 29 (13.8) | — |
| Region, n (%) | — | — | — | 0.024 |
| Northern Europe | 3557 (36.5) | 132 (25.7) | 92 (30.6) | — |
| Southern Europe | 2167 (22.2) | 105 (20.4) | 65 (21.6) | — |
| Eastern Europe | 2174 (22.3) | 148 (28.8) | 58 (19.3) | — |
| Latin America | 1854 (19.0) | 129 (25.1) | 86 (28.6) | — |
| Ejection fraction at admission, n (%) | — | — | — | <0.001a |
| ≥40% | 8110 (90.6) | 340 (71.1) | 225 (81.6) | — |
| <40% | 670 (7.5) | 80 (16.7) | 37 (13.4) | — |
| <30% | 167 (1.9) | 58 (12.1) | 15 (5.1) | — |
| BMI <20 kg/m2, n (%) | 133 (1.6) | 16 (3.8) | 7 (2.8) | 0.517 |
| No coronary revascularization or thrombolysis, n (%) | 7136 (73.3) | 267 (52.1) | 177 (59.0) | 0.055 |
| Creatinine at admission, mg/dL, mean (SD) | 1.03 (0.48) | 1.35 (1.02) | 1.25 (0.92) | 0.164 |
| EQ‐5D, n (%)c | — | — | — | 0.094b |
| 0 | 4505 (47.3) | 158 (31.4) | 112 (37.8) | — |
| 1 | 2056 (21.6) | 98 (19.5) | 52 (17.6) | — |
| ≥2 | 2954 (31.1) | 247 (49.1) | 132 (44.6) | — |
| Hemoglobin at admission, g/dL, n (%) | — | — | — | 0.435b |
| <11 | 359 (3.9) | 49 (10.4) | 26 (9.2) | — |
| <13 | 1582 (17.3) | 132 (27.9) | 74 (26.2) | — |
| ≥13 | 7180 (78.7) | 292 (61.7) | 182 (64.5) | — |
| Prior cardiac disease, n (%) | 2484 (25.8) | 261 (51.9) | 159 (53.0) | 0.760 |
| Previous COPD, n (%) | 574 (6.0) | 66 (13.2) | 43 (14.4) | 0.617 |
| Glucose, mg/dL at admission, mean (SD) | 142.0 (78.7) | 164.0 (92.0) | 164.0 (90.7) | 0.996 |
| Diuretics at discharge, n (%) | 1665 (17.2) | 201 (39.2) | 100 (33.6) | 0.110 |
| Aldosterone inhibitor at discharge, n (%) | 751 (7.7) | 102 (19.9) | 45 (15.2) | 0.090 |
| In‐hospital cardiac complications, n (%) | 1687 (17.4) | 171 (33.4) | 85 (28.2) | 0.126 |
| Diagnosis of STEMI, n (%) | 4640 (47.6) | 193 (37.6) | 110 (36.5) | 0.775 |
| Killip class, n (%) | — | — | — | 0.002b |
| I | 7744 (88.4) | 327 (70.8) | 225 (82.4) | — |
| II | 722 (8.2) | 85 (18.4) | 27 (9.9) | — |
| III‐IV | 296 (3.4) | 50 (10.8) | 21 (7.7) | — |
Key characteristics based on the 2‐year risk score based on EPICOR and EPICOR Asia population.14 Patient characteristics and in‐hospital management across groups 2 and 3 were compared using χ2 test or student t test as appropriate.
Patients with years 1 vs 2 events.
Test for trend
The EQ‐5D is a patient‐reported Quality of life questionnaire (EQ‐5D) measured at discharge.
Abbreviations: BMI, body mass index; CABG: coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; EQ‐5D, EuroQol five‐dimension questionnaire; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Figure 3 depicts Kaplan‐Meier estimates of the risk for MACCE and MACCE incidence rates during each time period, according to the occurrence of revascularization (PCI, coronary artery bypass graft or thrombolysis) in patients with NSTE‐ACS and STEMI, respectively.
Figure 3.
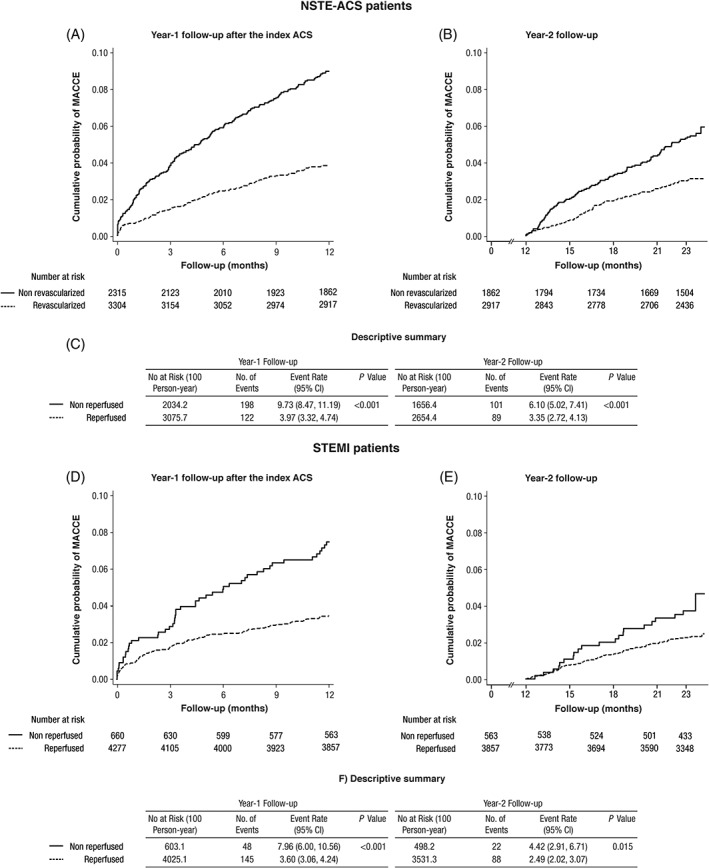
Kaplan‐Meier estimates of the risk for major adverse cardiac and cerebrovascular events (MACCE), and MACCE incidence rates according to the occurrence of revascularization (percutaneous coronary intervention [PCI], coronary artery bypass graft [CABG] or thrombolysis), in non‐ST‐segment elevation acute coronary syndrome (NSTE‐ACS) (upper panel) and ST‐elevation myocardial infarction (STEMI) patients (lower panel). Log‐rank estimates. Abbreviations: ACS, acute coronary syndrome; CI, confidence interval
3.3. Risk factors associated with MACCE during first‐year follow‐up vs second‐year follow‐up
Results of multivariable Cox proportional hazard models to elucidate those risk factors associated with MACCE in each time period are presented in Table 2. Briefly, in order of predictive strength, older age, male sex, certain geographic regions, lack of coronary revascularization or thrombolysis, higher creatinine, prior cardiac disease, prior chronic obstructive pulmonary disease (COPD), and higher glucose values were observed as risk factors for MACCE during both year 1 and 2 (Table 2). By contrast, lower ejection fraction, low BMI (<20 kg/m2), poorer quality of life, use of an aldosterone inhibitor at discharge, in‐hospital cardiac complications, and Killip class were not observed as risk factors after year 1. The use of diuretics at discharge tended to show a slightly higher degree of association with MACCE during the second year follow‐up in comparison with the first year. Neither education nor diagnosis of STEMI were related to MACCE in any of the time periods being studied.
Table 2.
Multivariable cox models for major adverse cardiac and cerebrovascular events (MACCE) separately for year 1 (all patients) and year 2 (patients MACCE‐free in year 1)
| Year 1 (n = 10 568) | Year 2 (n = 9209) | |||||
|---|---|---|---|---|---|---|
| Baseline variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (per 10 years) | 1.36 | 1.25‐1.49 | <0.001 | 1.28 | 1.15‐1.43 | <0.001 |
| Male sex | 1.32 | 1.07‐1.64 | 0.01 | 1.38 | 1.04‐1.83 | 0.02 |
| Education: No formal | 1 | — | — | 1 | — | — |
| Primary | 0.77 | 0.55‐1.08 | 0.13 | 0.95 | 0.60‐1.51 | 0.84 |
| Secondary | 0.72 | 0.51‐1.01 | 0.06 | 0.67 | 0.41‐1.09 | 0.11 |
| University | 0.72 | 0.47‐1.09 | 0.12 | 0.80 | 0.46‐1.40 | 0.44 |
| Region: Northern Europe | 1 | — | — | 1 | — | — |
| Eastern Europe | 1.87 | 1.47‐2.39 | <0.001 | 1.00 | 0.71‐1.40 | 0.99 |
| Latin America | 1.58 | 1.21‐2.04 | <0.001 | 1.49 | 1.09‐2.05 | 0.01 |
| Southern Europe | 1.17 | 0.90‐1.52 | 0.24 | 1.00 | 0.71‐1.38 | 0.99 |
| Ejection fractiona:<40% | 1.65 | 1.27‐2.13 | <0.001 | 1.34 | 0.92‐1.94 | 0.13 |
| <30% | 2.84 | 2.08‐3.90 | <0.001 | 1.45 | 0.82‐2.54 | 0.20 |
| BMI <20 kg/m2 | 1.71 | 1.02‐2.85 | 0.04 | 1.62 | 0.75‐3.49 | 0.22 |
| No coronary revascularization or thrombolysis | 1.59 | 1.31‐1.93 | <0.001 | 1.30 | 1.01‐1.68 | 0.04 |
| Creatinine (per log unit if ≥1.2 mg/dL)a , b | 2.14 | 1.61‐2.85 | <0.001 | 1.64 | 1.05‐2.56 | 0.03 |
| EQ‐5D: 0 | 1 | — | — | 1 | — | — |
| 1 | 1.11 | 0.86‐1.43 | 0.43 | 0.86 | 0.62‐1.20 | 0.37 |
| ≥2 | 1.41 | 1.14‐1.75 | 0.002 | 1.22 | 0.93‐1.60 | 0.14 |
| Hemoglobina < 11 g/dL | 1.37 | 0.98‐1.92 | 0.06 | 1.55 | 1.00‐2.41 | 0.05 |
| <13 g/dL | 1.26 | 1.01‐1.57 | 0.04 | 1.33 | 0.99‐1.78 | 0.06 |
| ≥13 g/dL | 1 | — | — | 1 | — | — |
| Prior cardiac disease | 1.44 | 1.18‐1.76 | <0.001 | 2.01 | 1.56‐2.59 | <0.001 |
| Previous COPD | 1.54 | 1.18‐2.01 | 0.002 | 1.92 | 1.37‐2.68 | <0.001 |
| Glucose (per 100 mg/dL if ≥140 mg/dL)a , c | 1.20 | 1.06‐1.36 | 0.004 | 1.29 | 1.09‐1.53 | 0.003 |
| On diuretics at discharge | 1.22 | 0.98‐1.51 | 0.07 | 1.39 | 1.05‐1.84 | 0.02 |
| On aldosterone inhibitor at discharge | 1.39 | 1.09‐1.78 | 0.008 | 1.30 | 0.91‐1.88 | 0.15 |
| In‐hospital cardiac complications | 1.28 | 1.05‐1.57 | 0.02 | 1.26 | 0.96‐1.65 | 0.10 |
| Diagnosis of STEMI | 1.02 | 0.83‐1.25 | 0.86 | 0.91 | 0.70‐1.19 | 0.50 |
| Killip class: I | 1 | — | — | 1 | — | — |
| II | 1.32 | 1.02‐1.70 | 0.03 | 0.76 | 0.50‐1.15 | 0.19 |
| III–IV | 1.30 | 0.93‐1.82 | 0.13 | 1.09 | 0.68‐1.74 | 0.72 |
MACCE, defined as death, non‐fatal MI and non‐fatal stroke.
At admission.
Example, creatinine 2.4 mg/dL, HR is 2.14 compared with creatinine ≤1.2 mg/dL during year 1 follow‐up.
Example, glucose 240 mg/dL, HR is 1.2 compared with glucose ≤140 mg/dL during year 1 follow‐up.
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; EQ‐5D, EuroQol five‐dimension questionnaire; HR, hazard ratio; MI, myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Cumulative probability of MACCE stratified by six risk groups (based on the 2‐year mortality EPICOR risk score) was estimated for each follow‐up time period, as illustrated in Figure S1. Table S3 shows the occurrence of MACCE in the different risk groups based on the EPICOR 2‐year mortality risk score by type of ACS (STEMI vs NSTE‐ACS) for each time period.
4. DISCUSSION
Using a prospective, international, real‐world cohort study of consecutive patients hospitalized for an ACS within 24 hours of symptom onset who survived to discharge (EPICOR registry), we observed a continuous incidence of MACCE during the 2‐year follow‐up, although there was a higher mortality during the first year compared than the second year of follow‐up, an observation that is consistent with other cohort studies.3 By contrast, the rate of non‐fatal MI and non‐fatal stroke did not substantially differ between the first and second year after ACS. Remarkably, we found that absence of revascularization (PCI, coronary artery bypass graft or thrombolysis) is associated with a higher incidence of MACCE not only during year 1 but also in year 2. Using the 18 predictors of the EPICOR risk score model, we failed to identify specific risk factors associated with poor prognosis beyond 1 year post‐ACS. There was a tendency for the use of diuretics at discharge to be associated with MACCE during year 2, but not year 1 (P = 0.02 and 0.07, respectively), but no strong conclusions can be drawn from this observation. Some differences were found across factors between year 1 and 2: half of them were significantly associated to both time periods, while among the remaining, most of them were only associated to outcomes in year 1.
Data from a large Swedish registry including 108 315 post‐MI patients with long‐term follow‐up revealed a cumulative rate of a cardiovascular composite endpoint (cardiovascular death, recurrent MI, and stroke) of 18.3% in the first year after MI, 9.0% in the subsequent year and 20.0% in the following 3 years.3 Similarly, the APOLLO study,18 which recruited 1‐year post‐MI patients aged ≥65, showed an adjusted risk mortality over the subsequent 3 years ranging from 12.8% to 19.5% across four different countries. Data from randomized clinical trials show substantially lower mortality rates in the first year after the index MI.19, 20 For example, in the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54 (PEGASUS‐TIMI 54) study, the 3‐year mortality risk was 5.2% in the aspirin group.6 By contrast, the rates of non‐fatal MI and non‐fatal stroke did not substantially differ between the first and second year after ACS. These findings are also in line with observations from previous reports of long‐term follow‐up after ACS,3 and might be explained because high‐risk patients often experience events or die during the first year of MI and are therefore no longer at risk for non‐fatal events. Our data indicate that despite current treatments, there is a residual risk in a large number of patients after ACS, even after an uneventful first year after discharge. The question about the risk of ischemic events beyond a post‐ACS period of stabilization might be answered by an ongoing trial: long‐term risk, clinical management, and healthcare resource utilization of stable coronary artery disease in post‐MI patients (TIGRIS, NCT01866904). This large trial is a multicenter, observational, prospective, longitudinal study enrolling patients with a history of MI 1 to 3 years before study entry and who are at high risk of developing atherothrombotic events.21
In our study, the type of ACS (STEMI vs NSTE‐ACS) was not associated with a higher risk of events at either year 1 or 2. However, lack of revascularization, a recognized independent risk factor for MACCE,14 was a major risk factor for future events regardless of type of ACS. This might be related both to the coronary anatomy and patient‐related risk factors but underscores the importance of revascularization for long‐term ischemic risk. Our findings agree with previous studies, which also showed that patients who did not undergo revascularization have an increased risk of subsequent cardiovascular events,22 either in year 1 or year 2.3 Although the benefit of reperfusion has been well described in patients with STEMI, the long‐term impact of revascularization in NSTE‐ACS patients is less established.23 Our observations point to a continuous lower risk for MACCE in revascularized in patients with NSTE‐ACS.
There is a need to identify risk factors for long‐term events to inform decisions on the duration and intensity of secondary prevention measures.24 By using valid clinical prediction models, clinicians can accurately advise patients about their prognosis and how this translates into treatment decisions. We used the EPICOR 2‐year mortality risk score model and applied it for the identification of specific risk factors for MACCE during first and second year post‐ACS. We were unable to identify a relevant subset of risk factors for year 2 events. This information is relevant in the light of studies showing that prolonging dual antiplatelet therapy (DAPT) for >12 months can improve outcomes,6, 25 and that analysis of prolonged DAPT use in EPICOR is ongoing. Although it is sensible that some risk factors under investigation were associated with outcomes beyond year 1 because of their “acute” effect (ie, Killip class, in‐hospital cardiac complications or ejection fraction), we cannot rule out a lack of power for detecting an effect in year 2, in which there were fewer outcomes. Previous attempts to identify specific risk factors for “stable” post‐ACS have also failed — the Swedish registry reported the same risk factors (older age, diabetes, lack of revascularization for the index MI, and a prior history of MI, stroke, heart failure or UA) for ischemic events in the 2 follow‐up periods.3
4.1. Strengths and limitations
There are several methodological limitations that should be mentioned. In patients with more than one event during follow‐up, only the first event was considered. Most patients were censored during the last follow‐up interview (24 months ±2 weeks). We did not evaluate the influence of discharge medication and length of secondary prevention therapies on outcomes. The selection of site investigators was not random and central adjudication of outcomes was not used in the EPICOR study. Some comparisons in risk factors between year 1 and 2 might be underpowered and hence some true associations may have been undetected. The EPICOR 2‐year risk score predicts mortality but not non‐fatal outcomes. A major strength of our study is that we assessed a comprehensive set of patients and practice patterns, as this analysis derives from a large international database that includes subjects from different health systems treated in different hospital settings. We also assessed various risk factors which were previously described to predict 2‐year mortality in the same population.
5. CONCLUSIONS
In post‐ACS, there is a continuous risk for MACCE during the 2‐year follow‐up. Event rate for MACCE was higher in year 1 than in year 2, primarily driven by a higher mortality rate in year 1. Rates of non‐fatal MI and stroke did not change between years 1 and 2. Several easily measured patient characteristics were predictive for ACS‐related MACCE during both years 1 and 2, although some lost their predictive value over time. As we were unable to identify specific risk factors for events occurring after an uneventful first year, secondary prevention measures should therefore be continued especially in higher‐risk patients who can be identified by the EPICOR 2‐year mortality risk score.
CONFLICTS OF INTEREST
Xavier Rossello has nothing to disclose. Stuart J. Pocock has received research and statistical consulting honoraria from AstraZeneca. Héctor Bueno has received advisory/consulting fees from Abbott, AstraZeneca, Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Eli Lilly, Novartis, Pfizer, Sanofi, and Servier, and grants from AstraZeneca. Frans van de Werf has received consulting fees and research grants from Boehringer Ingelheim and Merck, and consulting fees from Roche, Sanofi‐Aventis, AstraZeneca, and The Medicines Company. Nicolas Danchin has received fees for lectures or consulting from Amgen, AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi‐Sankyo, Eli‐Lilly, MSD, Novo‐Nordisk, Pfizer, Sanofi, and Servier, and research grants from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, Eli‐Lilly, Merck, Pfizer, and Sanofi. Lieven Annemans received honoraria from AstraZeneca, Bayer, and BMS. Jesús Medina is an employee of AstraZeneca. Uwe Zeymer has received honoraria from AstraZeneca, Bayer, BMS, Daiichi Sankyo, Eli Lilly, Pfizer, Novartis, Medicines Company, Sanofi, and Amgen.
Supporting information
Table S1. Incidence of major adverse cardiac and cerebrovascular events (MACCE) and of mortality; event rates per 100 person‐years at risk by intervals of time
Table S2. Univariable analysis of incidence rate of MACCE by risk factor subgroup
Table S3. Occurrence of MACCE in the different risk groups according to EPICOR (long‐tErm follow uP of antithrombotic management patterns In acute CORonary syndrome) 2‐year mortality risk, by type of index acute coronary syndrome
Figure S1. Cumulative probability of major adverse cardiac and cerebrovascular events (MACCE) over time stratified by risk group at (A) year 1 of follow‐up after discharge, and (B) year 2 of follow‐up (MACCE‐free in year 1). The same risk categories are displayed in both plots. Risk classification was estimated for each patient using the EPICOR 2‐year mortality risk score.14 Abbreviation: EPICOR, long‐term follow‐up of antithrombotic management patterns In acute CORonary syndrome
ACKNOWLEDGEMENTS
Medical writing support was provided by Carl V Felton PhD, Paragon, Knutsford, Cheshire, UK, and funded by AstraZeneca. Xavier Rossello has received support from the SEC‐CNIC CARDIOJOVEN fellowship program. EPICOR was funded by AstraZeneca. Being a non‐interventional study, no drugs were supplied or funded.
Rossello X, Bueno H, Pocock SJ, et al. Predictors of all‐cause mortality and ischemic events within and beyond 1 year after an acute coronary syndrome: Results from the EPICOR registry. Clin Cardiol. 2019;42:111–119. 10.1002/clc.23116
Funding information EPICOR was funded by AstraZeneca.
REFERENCES
- 1. Rossello X, Pocock SJ, Julian DG. Long‐term use of cardiovascular drugs: challenges for research and for patient care. J Am Coll Cardiol. 2015;66:1273‐1285. [DOI] [PubMed] [Google Scholar]
- 2. Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in long‐term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10‐year trend analysis. J Am Coll Cardiol. 2008;51:1247‐1254. [DOI] [PubMed] [Google Scholar]
- 3. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36:1163‐1170. [DOI] [PubMed] [Google Scholar]
- 4. Fox KAA, Carruthers KF, Dunbar DR, et al. Underestimated and under‐recognized: the late consequences of acute coronary syndrome (GRACE UK‐Belgian study). Eur Heart J. 2010;31:2755‐2764. [DOI] [PubMed] [Google Scholar]
- 5. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA. 2004;291:2727‐2733. [DOI] [PubMed] [Google Scholar]
- 6. Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791‐1800. [DOI] [PubMed] [Google Scholar]
- 7. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706‐1717. [DOI] [PubMed] [Google Scholar]
- 8. Menzin J, Wygant G, Hauch O, Jackel J, Friedman M. One‐year costs of ischemic heart disease among patients with acute coronary syndromes: findings from a multi‐employer claims database. Curr Med Res Opin. 2008;24:461‐468. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen F, Butrymovich V, Kelbaek H, et al. Short‐ and long‐term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64:2101‐2108. [DOI] [PubMed] [Google Scholar]
- 10. Pocock S, Bueno H, Licour M, et al. Predictors of one‐year mortality at hospital discharge after acute coronary syndromes: a new risk score from the EPICOR (long‐tErm follow uP of antithrombotic management patterns in acute CORonary syndrome patients) study. Eur Heart J Acute Cardiovasc Care. 2015;4:509‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835‐842. [DOI] [PubMed] [Google Scholar]
- 12. Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST‐segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557‐2567. [DOI] [PubMed] [Google Scholar]
- 13. Bueno H, Danchin N, Tafalla M, Bernaud C, Annemans L, van de Werf F. EPICOR (long‐tErm follow‐up of antithrombotic management patterns in acute CORonary syndrome patients) study: rationale, design, and baseline characteristics. Am Heart J. 2013;165:8‐14. [DOI] [PubMed] [Google Scholar]
- 14. Pocock SJ, Huo Y, Van de Werf F, et al. Predicting two‐year mortality from discharge after acute coronary syndrome: an internationally‐based risk score. Eur Heart J Acute Cardiovasc Care. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53‐72. [DOI] [PubMed] [Google Scholar]
- 16. Rossello X, Huo Y, Pocock S, et al. Global geographical variations in ST‐segment elevation myocardial infarction management and post‐discharge mortality. Int J Cardiol. 2017;245:27‐34. [DOI] [PubMed] [Google Scholar]
- 17. Bueno H, Rossello X, Pocock S, et al. Regional variations in hospital management and post‐discharge mortality in patients with non‐ST‐segment elevation acute coronary syndrome. Clin Res Cardiol. 2018;107:836‐844. [DOI] [PubMed] [Google Scholar]
- 18. Rapsomaniki E, Thuresson M, Yang E, et al. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors of myocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2016;2:172‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045‐1057. [DOI] [PubMed] [Google Scholar]
- 20. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001‐2015. [DOI] [PubMed] [Google Scholar]
- 21. Westermann D, Goodman SG, Nicolau JC, et al. Rationale and design of the long‐term rIsk, clinical manaGement, and healthcare resource utilization of stable coronary artery dISease in post‐myocardial infarction patients (TIGRIS) study. Clin Cardiol. 2017;40:1197‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hvelplund A, Galatius S, Madsen M, et al. Significance of the invasive strategy after acute myocardial infarction on prognosis and secondary preventive medication: a nationwide study of 6364 women and 11,915 men. J Invasive Cardiol. 2012;24:19‐24. [PubMed] [Google Scholar]
- 23. Schiele F, Gale CP, Bonnefoy E, et al. Quality indicators for acute myocardial infarction: a position paper of the acute cardiovascular care association. Eur Heart J Acute Cardiovasc Care. 2017;6:34‐59. [DOI] [PubMed] [Google Scholar]
- 24. Goodman SG, Nicolau JC, Requena G, et al. Longer‐term oral antiplatelet use in stable post‐myocardial infarction patients: insights from the long term rIsk, clinical manaGement and healthcare resource utilization of stable coronary artery dISease (TIGRIS) observational study. Int J Cardiol. 2017;236:54‐60. [DOI] [PubMed] [Google Scholar]
- 25. Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Incidence of major adverse cardiac and cerebrovascular events (MACCE) and of mortality; event rates per 100 person‐years at risk by intervals of time
Table S2. Univariable analysis of incidence rate of MACCE by risk factor subgroup
Table S3. Occurrence of MACCE in the different risk groups according to EPICOR (long‐tErm follow uP of antithrombotic management patterns In acute CORonary syndrome) 2‐year mortality risk, by type of index acute coronary syndrome
Figure S1. Cumulative probability of major adverse cardiac and cerebrovascular events (MACCE) over time stratified by risk group at (A) year 1 of follow‐up after discharge, and (B) year 2 of follow‐up (MACCE‐free in year 1). The same risk categories are displayed in both plots. Risk classification was estimated for each patient using the EPICOR 2‐year mortality risk score.14 Abbreviation: EPICOR, long‐term follow‐up of antithrombotic management patterns In acute CORonary syndrome


