Abstract
Background
Takotsubo syndrome (TTS) is characterized by acute, transient systolic dysfunction of the left ventricle not attributed to coronary artery disease (CAD).
Hypothesis
There are differences in hospital outcomes in patients admitted with TTS based on their gender.
Methods
The National Inpatient Sample database was searched for patients admitted with a principal diagnosis of TTS from 2006 to 2014 using the ICD9‐CM code 429.83. Using Pearson's χ 2 and Student's t test analyses, the P‐value was calculated for differences among baseline characteristics of patients. Multivariate regression models were then created to adjust for potential confounders.
Results
A total of 39 662 admissions with TTS were identified, 91.7% female and 8.3% male with mean age of 66.5 and 61.6 years, respectively. The incidence of TTS increased progressively from 2006 to 2014. Female patients were more likely to have hypertension, hypothyroidism, or depression. Males were more likely to use tobacco, or have known CAD. Males had almost 4‐fold higher probability of in‐hospital mortality compared to females (3.7% vs 1.1%; P<0.001). Certain complications including cardiogenic shock, ventricular fibrillation/tachycardia, and acute kidney injury were more common in males.
Conclusions
There are distinct gender differences in clinical characteristics of patients admitted with TTS. Although TTS is more common in females, it is associated with higher morbidity and mortality in males.
Keywords: gender, stress‐induced cardiomyopathy, Takotsubo
1. BACKGROUND
Takotsubo syndrome (TTS) is characterized by acute, transient systolic dysfunction of the left ventricle not attributed to coronary artery disease (CAD). Since its initial report in 1991, TTS has gained widespread recognition as an important cause of acute heart failure.1 The criteria for diagnosing TTS include electrocardiographic changes and regional wall motion abnormalities on cardiac imaging, typically extending beyond single coronary artery distribution. Absence of other conditions such as obstructive CAD, pheochromocytoma, and myocarditis are also required for diagnosis.2, 3 TTS is thought to be precipitated by excess sympathetic activity, often secondary to a stressful event. Coronary vasospasm, microcirculatory disorders, and decreased levels of estrogen have also been proposed as potential causes.4, 5
Epidemiological studies have found that 2% of all patients presenting to the hospital with suspected acute coronary syndrome (ACS) are diagnosed with TTS. Outcome studies of patients presenting with ACS, who underwent percutaneous coronary intervention (PCI), have demonstrated a greater incidence of in‐hospital mortality and complications in women, despite adjusting for possible confounders, including age and comorbidities.6, 7 This gender difference in ACS outcome following PCI has been further observed in younger, premenopausal women.8 While multiple studies have examined the impact of gender in ACS outcome, few exist for TTS. This condition is more common in older postmenopausal women and historically was initially described in Asian populations.9 Nevertheless, because of increased awareness and availability of diagnostic tools, the number of cases in other populations has expanded greatly.10 Given its increasing incidence, more population studies are needed to better understand the impact of gender differences in patients presenting with this serious cardiovascular condition. In the current study, we used the National Inpatient Sample (NIS) from 2006 to 2014 to determine the impact of gender on in‐hospital outcomes.
2. METHODS
Institutional Review Board (IRB) review and approval was not required as the NIS is a publically available database that contains de‐identified patient information. The NIS contains all‐payer data on hospital inpatient stays from States participating in the Healthcare Cost and Utilization Project. The NIS database is a sample of discharges from the United States and contains data about 7 to 8 million discharges per year. The NIS data is originated from billing data submitted by hospitals to statewide data organizations across the United States and contains discharge‐level weights to calculate national estimates for discharges. Before 2012, a 20% probability sample of all hospitals within each stratum is collected, all discharges from these hospitals are recorded and then weighted to ensure that they are nationally representative. Starting 2012, the NIS sample design was changed, and now the design includes a 20% of discharges among all the hospitals in the NIS universe. As many as 30 discharge diagnoses and 15 procedures are recorded for each patient by using the International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD‐9‐CM).
We included admissions with a principal diagnosis of Stress‐induced cardiomyopathy (ICD‐9‐CM: 429.83) from 2006 to 2014 across the United States, which has been used in prior studies to identify this syndrome.11, 12 Patients were excluded if they were younger than 18 years of age and if data relating to mortality, age or gender was missing or omitted (Supplemental Figure 2). Study variables such as mortality, length of hospital stay, and total hospitalization costs were provided within the NIS for each patient hospitalization. Intensive care unit (ICU) admission is not reported in the NIS database and a surrogate variable (eg, intubation) was created based on procedures that require an ICU setting to identify patients that were admitted to the ICU during that hospitalization.
Comorbidities were also provided by the NIS database using the Agency for Healthcare Research and Quality (AHRQ) comorbidity software. The AHRQ comorbidity software identifies coexisting medical conditions that are not directly related to the principal diagnosis, and are likely to have originated prior to the hospital stay. Comorbidities were recognized by their corresponding ICD‐9‐CM diagnosis code and the diagnosis related group in effect on the date of discharge (Supplemental Table 1).
The statistical analyses were performed using a general‐purpose statistical software package called STATA 13.0. NIS is based on a complex sampling design that includes stratification, clustering and weighting. We used a survey analysis that accounted for stratification and clustering, as well as the weights for each hospital discharge. Pearson's χ 2 and Student's t test analyses were used to calculate the P‐value for the differences among the baseline characteristics of patients. Multivariate regression models were created to adjust for potential confounders using backward elimination and variables with a P < 0.2 were included in the model. Potential confounders obtained from the NIS data included age, race/ethnicity, patient's comorbidities (using the Charlson Comorbidity Index [CCI] for administrative data), coronary catheterization, known CAD, hospital location (rural or urban), geographic region (Northeast, Midwest, West, or South), length of stay, hospital teaching status, and hospital bed size (ie, small, medium, and large). We performed a sensitivity analysis to evaluate differences in our outcomes before and after 2012 to assess if the NIS sampling design had an impact in our study population; we found no statistical significance for in‐hospital mortality, ICU admission, ventricular fibrillation or tachycardia, and major bleeding.
3. RESULTS
We identified 39 662 admissions with stress‐induced cardiomyopathy from 2006 to 2014:36 354 (91.7%) were female and 3304 (8.3%) were male. The incidence of stress‐induced cardiomyopathy increased progressively in both genders from 2006 to 2014. In the female subgroup, there were 306 encounters for TTS in 2006 compared to 6750 cases in 2014. In the male subgroup, there were only nine cases of TTS in 2006 compared to 625 cases in 2014 (Figure 1). Throughout these same years, the overall incidence of TTS was highest in the month of June for males (10.6%) and September for females (9.9%). The lowest overall incidence was recorded in February for both genders (6.4% and 7.0% for males and females, respectively) (Supplemental Figure 1).
Figure 1.
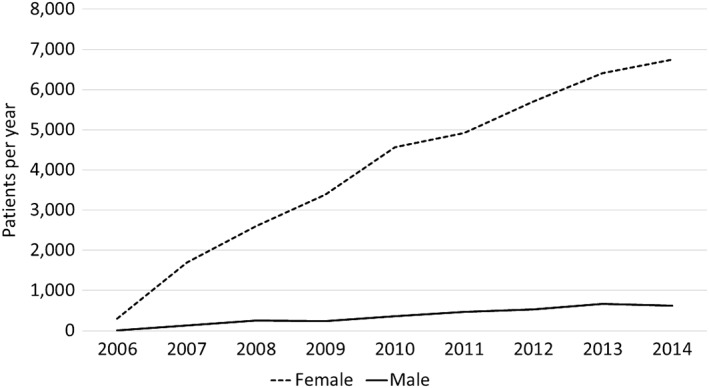
Reported cases of Takotsubo syndrome by year
3.1. Baseline characteristics of patient with stress‐induced cardiomyopathy by gender
The mean age for females was significantly higher than that of males (66.5 vs 61.6 years; P < 0.001), and more females were over the age of 75 (14.7% vs 11.7%; P < 0.001). In the male subgroup, 21.2% of patients were younger than 35 years compared to 9.5% in the female subgroup. For both genders, Caucasian was the predominant race; interestingly, in the male subgroup African‐American was the second most common race, while in females it was Hispanic. Female patients had a significantly higher prevalence of hypertension (63.9% vs 58.4%; P = 0.004), hypothyroidism (18.6% vs 6.5%; P < 0.001), and depression (16.5% vs 9.7%; P < 0.001). On the other hand, males had a higher prevalence of tobacco use (40.1% vs 31.4%; P < 0.001), known CAD (43.7% vs 36.3%; P < 0.001), prior PCI (6.0% vs 4.0%; P = 0.009), prior coronary artery bypass grafting (CABG) surgery (2.7% vs 1.1%; P < 0.001), and liver disease (3.2% vs 1.5%; P < 0.001). There was no significant gender difference in the prevalence of diabetes mellitus, end stage renal disease, anemia, fluid and electrolyte disorders, prior myocardial infarction, congestive heart failure, obesity (body mass index [BMI] ≥ 30 kg/m2), chronic pulmonary disease, peripheral vascular disease, atrial fibrillation, and prior stroke or transient ischemic attack (TIA) (Table 1). Also, no significant differences were observed between males and females in median household income, elective admissions, and hospital teaching status. More males were discharged to nursing home facilities (9.6% vs 8.1%; P = 0.013) or transferred to another hospital (2.5% vs 1.3%; P = 0.013), while more females had Medicaid or Medicare as their primary insurance (63.2% vs 58.1%; P = 0.003).
Table 1.
Baseline characteristics of patient with stress‐induced cardiomyopathy by gender
| Male (%) | Female (%) | P value | |
|---|---|---|---|
| Number of patients | 8.3 (3366) | 91.7 (37 097) | |
| Age (mean ± SD) | 61.6 ± 16.2 | 66.5 ± 12.5 | <0.001 |
| 18‐34 years | 21.2 | 9.5 | |
| 35‐54 years | 33.2 | 36.1 | |
| 55‐74 years | 33.9 | 39.6 | |
| >75 years | 11.7 | 14.7 | |
| Race | <0.001 | ||
| Caucasian | 78.9 | 83.4 | |
| African‐American | 9.5 | 5.6 | |
| Hispanic | 4.4 | 6.0 | |
| Asian | 2.3 | 1.5 | |
| Other | 4.9 | 3.5 | |
| Comorbidities | |||
| Hypertension | 58.4 | 63.9 | 0.004 |
| Diabetes mellitus | 16.8 | 18.9 | 0.178 |
| End stage renal disease | 1.2 | 0.7 | 0.171 |
| Congestive heart failure | 23.7 | 26.3 | 0.144 |
| Obesity (BMI ≥30 kg/m2) | 6.8 | 9.3 | 0.031 |
| Chronic pulmonary disease | 23.0 | 25.6 | 0.146 |
| Peripheral vascular disease | 7.4 | 6.4 | 0.311 |
| Hypothyroidism | 6.5 | 18.6 | <0.001 |
| Depression | 9.7 | 16.5 | <0.001 |
| Smoking | 40.1 | 31.4 | <0.001 |
| Prior MI | 6.0 | 6.2 | 0.889 |
| Prior PCI | 6.0 | 4.0 | 0.009 |
| Prior CABG | 2.7 | 1.1 | <0.001 |
| Prior stroke/TIA | 5.8 | 5.7 | 0.957 |
| Known CAD | 43.7 | 36.3 | <0.001 |
| Carotid artery disease | 1.0 | 1.0 | 0.982 |
| Atrial fibrillation | 12.8 | 12.2 | 0.619 |
| Anemia | 10.0 | 11.5 | 0.260 |
| Cancer | 5.0 | 2.7 | 0.001 |
| Liver disease | 3.2 | 1.5 | <0.001 |
| Fluid and electrolyte disorders | 22.3 | 22.1 | 0.911 |
| Other neurological disorders | 8.6 | 7.2 | 0.194 |
| CCI | |||
| 0 | 48.7 | 44.1 | 0.013 |
| 1 | 29.9 | 31.3 | |
| 2 | 13.9 | 18.1 | |
| ≥3 | 7.5 | 6.4 | |
| Other characteristics | |||
| Teaching hospital | 57.8 | 56.6 | 0.528 |
| Median household income | 0.596 | ||
| US$ 1‐39 999 | 24.3 | 22.6 | |
| US$ 40 000‐50 999 | 25.1 | 25.5 | |
| US$ 51 000‐65 999 | 26.7 | 26.1 | |
| US$ more than 66, 000 | 23.9 | 25.8 | |
| Elective admission | 6.4 | 5.8 | 0.540 |
| Primary payer | 0.003 | ||
| Medicare/medicaid | 58.1 | 63.2 | |
| Private insurance | 32.3 | 30.3 | |
| Self‐pay/other | 9.6 | 6.6 | |
| Discharge disposition | 0.013 | ||
| Home | 79.5 | 83.9 | |
| Nursing home/facility | 9.6 | 8.1 | |
| Against medical advice | 0.4 | 0.3 | |
| Transfer to another hospital | 2.5 | 1.3 | |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCI, Charlson Comorbidity Index; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
3.2. In‐hospital outcomes of patients with stress‐induced cardiomyopathy by gender
As shown in Table 2, the in‐patient mortality was significantly higher among males admitted with TTS (3.7% vs 1.1%; P < 0.001). Male patients had almost four times higher in‐hospital mortality compared to female patients (Adjusted Odds Ratio [OR]: 3.89, 95% confidence interval [CI] 2.41‐6.24, P < 0.001).
Table 2.
In‐hospital outcomes of patients with stress‐induced cardiomyopathy by gender
| Outcome | Male (%) | Female (%) | Adjusted odds ratio* | 95% CI | P value |
|---|---|---|---|---|---|
| Mortality | 3.7 | 1.1 | 3.89 | 2.41‐6.24 | <0.001 |
| ICU admission | 10.9 | 5.0 | 2.12 | 1.57‐2.86 | <0.001 |
| Cardiogenic shock | 6.9 | 4.0 | 1.51 | 1.03‐2.06 | 0.032 |
| Acute respiratory failure | 9.6 | 6.5 | 1.31 | 0.97‐1.78 | 0.083 |
| Ventricular fibrillation or tachycardia | 6.2 | 3.5 | 1.52 | 1.07‐2.16 | 0.02 |
| AKI | 9.9 | 5.9 | 1.93 | 1.44‐2.59 | <0.001 |
| Ischemic stroke/TIA | 1.0 | 1.1 | 0.92 | 0.41‐2.03 | 0.832 |
| Major bleeding | 2.8 | 1.7 | 1.59 | 0.96‐2.62 | 0.072 |
| Coronary catheterization | 86.9 | 88.5 | 0.88 | 0.60‐1.23 | 0.51 |
| Mean hospital costs (US$) | $14 474 | $12 160 | — | — | 0.019 |
| LOS ≥5 days | 26.0 | 23.2 | 1.34 | 1.11‐1.62 | 0.002 |
| Median length of stay (days [IQR]) | 3 (2‐5) | 3 (2‐4) | — | — | <0.001 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; LOS, lenght of stay; TIA, transient ischemic attack.
OR adjusted for age, race/ethnicity, patient's comorbidities (using the Charlson Comorbidity Index [CCI] for administrative data), coronary catheterization, coronary artery disease, teaching hospital, elective admission, hospital location (rural or urban), geographic region (Northeast, Midwest, West, or South), length of stay, hospital teaching status, and hospital bed size.
In terms of in‐hospital complications, male patients with TTS had a significantly higher probability of cardiogenic shock (6.9% vs 4.0%; OR: 1.51, 95% CI 1.03‐2.06, P = 0.03), ventricular fibrillation or tachycardia (6.2% vs 3.5%; OR: 1.52, 95% CI 1.07‐2.16, P = 0.02), acute kidney injury (9.9% vs 5.9%; OR: 1.93, 95% CI 1.44‐2.59, P < 0.001), intensive care unit admission (10.9% vs 5.0%; OR: 2.12, 95% CI 1.57‐2.86, P < 0.001), and length of stay exceeding 5 days (26.0% vs 23.2%; OR: 1.34, 95% CI 1.11‐1.62, P = 0.002) compared to female patients. No significant difference was observed in the incidence of in‐hospital acute respiratory failure, stroke, coronary angioplasty, or major bleeding (Table 2). The mean hospital cost was higher in males (14 474 vs 12 160; P = 0.014).
4. DISCUSSION
Since its initial description in Japan in 1990, TTS has gained worldwide recognition and resultantly a distinct and sharp increase in the reported incidence was seen over the recent years. Although female predominance of TTS has been confirmed in multiple studies, gender differences in clinical characteristics have not been adequately studied. The current analysis revealed important gender‐specific associations among patients admitted with TTS and demonstrated markedly higher rates of in‐hospital complications and mortality in males compared to females.
Prior epidemiological and clinical studies changed our understanding of the effects of age, gender, and race on the incidence, morbidities and mortality of patients with TTS. Single center studies and studies performed in smaller samples showed effects of gender on mortality in patients with TTS.10, 13, 14, 15, 16 A large sample size and the continuous yearly sampling of cases are the important features of the current study compared to older studies typically reporting a limited number of cases. By pooling the samples from 2006 to 2014, we aimed to create a statistically powerful and representative sample of admissions with TTS and re‐evaluate the effects of gender on the mortality of patient with TTS. Our results show that the reported incidence of TTS in the United States increased 14‐fold from 2006 to 2014 (Figure 1). This finding could be attributed to increasing knowledge and understanding of TTS as well as widespread availability of imaging modalities leading to increased recognition of this clinical entity.
Similar to prior studies, we found that over 90% of TTS cases occurred in women and female patients on average were older than male patients. A significant number of male patients were younger than 35 years of age at the time of diagnosis (21.2% of males vs 9.5% of females). The pathogenesis of this condition is poorly understood, but current models favor a hypersympathetic response to stress with excess of catecholamines as the primary driving factor. It is believed that this catecholamine overload results in cardiotoxicity and microvascular dysfunction which leads to myocardial stunning.17, 18, 19 Others have suggested that estrogen may have a cardioprotective role in stress‐induced cardiomyopathy during reproductive age,20, 21 leading to a higher incidence of TTS in older postmenopausal women as estrogen levels continue to decline. Some authors have postulated that estrogen may enhance transcription of cardioprotective factors, which protect against the toxic effects of catecholamines and resulting oxidative stress.22
In addition to being older, the female subgroup had significantly higher incidence of hypertension, depression, and hypothyroidism. A noteworthy 18.6% of all females with TTS had hypothyroidism (vs 6.5% of males), which is an important association considering the reported prevalence of hypothyroidism in the United States of 4.6%.23 In a previous study, Mahajan et al24 found both subclinical hypothyroid and hypothyroid patients to have autonomic dysfunction with the sympathetic reactivity being affected in most cases. This suggests that hormonal imbalances, other than estrogen, may also play an important role in precipitating stress‐induced cardiomyopathy. In contrast, males exhibited a higher prevalence of tobacco use, liver disease, known CAD, prior PCI, and CABG. Another noteworthy finding is the high prevalence of underlying CAD in our TTS patient population (43.7% in males vs 36.3% in females). The prevalence of CAD in patients with TTS has been reported to range from 10% to 61% in prior studies.25, 26 These findings have been challenging the notion that CAD necessarily excludes the diagnosis of TTS and it is now increasingly accepted that the two conditions are not mutually exclusive, but can exist coincidently in many cases.27
The current study demonstrates higher in‐hospital mortality in males, despite the younger average age. The in‐hospital mortality rates seen in this study are consistent with previous studies, which demonstrate mortality rates ranging from 0% to 8%, similar to that found in acute myocardial infarction.9, 10, 14, 15, 20, 21, 23 Males also had higher probability of acute complications such as ICU admissions, cardiogenic shock, ventricular fibrillation or tachycardia, and acute kidney injury compared to females. Length of hospital stay of more than 5 days and hospital costs were also higher in men. Prior studies have shown that the majority of adverse events in patients with TTS happen in those with underlying comorbid conditions.28, 29 Higher mortality rates in younger patients with physical stressors has been previously reported in the InterTAK Registry study by Templin et al25 as well and might partially explain the higher rates of mortality in the generally younger male patients in our study. In addition, the observed differences in baseline comorbidities between genders could also represent a driving factor for the higher in‐hospital morbidity and mortality and higher hospital costs in male patients presenting with TTS. Younger male patients have been reported to suffer from TTS mostly in the setting of physical as opposed to emotional stressors and TTS in many of these cases has been associated with stimulant drug use.30, 31 Multicenter, prospective studies focusing on risk factors and predictors of morbidity and mortality in TTS patients are required in order to fully explore the factors responsible for higher mortality in younger patients and in male gender.
5. STUDY LIMITATIONS
There are several limitations to the current study due to the nature and well‐described short‐comings of NIS database.16 The mortality data does not distinguish the cardiac and noncardiac causes of death. In addition, the study is limited to in‐hospital outcomes only and the outcome data are not available for patients after hospital discharge. Although TTS has been traditionally described as an acute myocardial injury long‐term derangements in myocardial function have also been demonstrated.8 TTS was identified by an ICD‐9 code as a principal diagnosis; other studies have used a similar approach but no validation specific to TTS has been performed. In addition, it is not possible to reliably distinguish in‐hospital complications from comorbidities with this administrative database. The lack of information about laboratory results, medications, and diagnostic test results such as echocardiographic imaging is also a limitation, as we are not able to identify TTS patterns and because certain imaging characteristics correlate with adverse outcomes in TTS.32 The study reports hospital encounters, not individual patients; recurrent TTS has been reported but this is unlikely to skew the results of the current study. Finally, the change in the sampling design may significantly affect the study results but the findings of sensitivity analysis suggest robustness of the study conclusions.
6. CONCLUSION
TTS is an acute cardiovascular condition characterized by transient regional systolic left ventricular dysfunction. The current study described important gender differences in clinical characteristics of patients with TTS. Although TTS is less common in men it is associated with a marked increase in in‐hospital morbidity and mortality.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
DISCLOSURE OF INTERESTS
Authors have nothing to disclose.
Supporting information
Table S1 Variables by ICD 9 code.
Figure S1 Reported monthly incidence of Takotsubo syndrome.
Figure S2 Flowchart of study population.
Lemor A, Ramos‐Rodriguez AJ, De La Villa R, et al. Impact of gender on in‐hospital outcomes in patients with Takotsubo syndrome: A nationwide analysis from 2006 to 2014. Clin Cardiol. 2019;42:13–18. 10.1002/clc.23109
REFERENCES
- 1. Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754‐2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408‐417. [DOI] [PubMed] [Google Scholar]
- 3. Kawai S, Kitabatake A, Tomoike H, Takotsubo Cardiomyopathy G. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71(6):990‐992. [DOI] [PubMed] [Google Scholar]
- 4. Yoshikawa T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol. 2015;182:297‐303. [DOI] [PubMed] [Google Scholar]
- 5. Ndrepepa G, Kufner S, Mayer K, et al. Sex differences in the outcome after percutaneous coronary intervention – a propensity matching analysis. Cardiovasc Revasc Med. 2018. S1553‐8389(18)30212‐4. 10.1016/j.carrev.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 6. Potts J, Sirker A, Martinez SC, et al. Persistent sex disparities in clinical outcomes with percutaneous coronary intervention: insights from 6.6 million PCI procedures in the United States. PLoS One. 2018;13(9):e0203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahil K, Dhaval K, Tanush G, et al. Temporal trends and sex differences in revascularization and outcomes of ST‐segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol. 2015;66(18):1973‐1975. [DOI] [PubMed] [Google Scholar]
- 8. Sharkey SW, Maron BJ. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ J. 2014;78(9):2119‐2128. [DOI] [PubMed] [Google Scholar]
- 9. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472‐479. [DOI] [PubMed] [Google Scholar]
- 10. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;10(4):311‐316. [DOI] [PubMed] [Google Scholar]
- 11. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21(2):203‐214. [PubMed] [Google Scholar]
- 12. Stollberger C, Finsterer J. Why does takotsubo ("broken heart syndrome") affect more females than males? Int J Cardiol. 2011;147(1):175‐176. [DOI] [PubMed] [Google Scholar]
- 13. Ueyama T, Ishikura F, Matsuda A, et al. Chronic estrogen supplementation following ovariectomy improves the emotional stress‐induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J. 2007;71(4):565‐573. [DOI] [PubMed] [Google Scholar]
- 14. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489‐499. [DOI] [PubMed] [Google Scholar]
- 15. Mahajan AS, Lal R, Dhanwal DK, Jain AK, Chowdhury V. Evaluation of autonomic functions in subclinical hypothyroid and hypothyroid patients. Indian J Endocrinol Metab. 2013;17(3):460‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee PH, Song JK, Sun BJ, et al. Outcomes of patients with stress‐induced cardiomyopathy diagnosed by echocardiography in a tertiary referral hospital. J Am Soc Echocardiogr. 2010;23(7):766‐771. [DOI] [PubMed] [Google Scholar]
- 17. Hurst RT, Prasad A, Askew JW III, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3(6):641‐649. [DOI] [PubMed] [Google Scholar]
- 18. Nef HM, Moellmann H, Akashi YJ, Hamm CW. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol. 2010;7(4):187‐193. [DOI] [PubMed] [Google Scholar]
- 19. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 26 July 2014. 6(7): 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress‐induced cardiac responses in the animal model of Tako‐tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S117‐S119. [DOI] [PubMed] [Google Scholar]
- 21. Brinjikji W, El‐sayed AM, Salka S. In‐hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 22. Sestini S, Pestelli F, Leoncini M, et al. The natural history of takotsubo syndrome: a two‐year follow‐up study with myocardial sympathetic and perfusion G‐SPECT imaging. Eur J Nucl Med Mol Imaging. 2017;44(2):267‐283. [DOI] [PubMed] [Google Scholar]
- 23. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807‐1816. [DOI] [PubMed] [Google Scholar]
- 24. Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 25. Khera R, Krumholz HM. With great power comes great responsibility: big data research from the National inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929‐938. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed S, Ungprasert P, Ratanapo S, Hussain T, Riesenfeld EP. Clinical characteristics of takotsubo cardiomyopathy in North America. N Am J Med Sci. 2013;5(2):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Medina de Chazal H, Giuseppe Del Buono M, Keyser‐Marcus L, et al. Cardiomyopathy diagnosis and treatment. J Am Coll Cardiol. 2018;72(16):1955‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khera R, Light‐mcgroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164(1):66‐71. e61. [DOI] [PubMed] [Google Scholar]
- 31. Winchester DE, Ragosta M, Taylor AM. Concurrence of angiographic coronary artery disease in patients with apical ballooning syndrome (tako‐tsubo cardiomyopathy). Catheter Cardiovasc Interv. 2008;72:612‐616. [DOI] [PubMed] [Google Scholar]
- 32. Alfonso CE. Takotsubo cardiomyopathy and coronary artery disease: a meaningful coincidence? J Am Heart Assoc. 2016;5(12):e005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Variables by ICD 9 code.
Figure S1 Reported monthly incidence of Takotsubo syndrome.
Figure S2 Flowchart of study population.


