Abstract
Background
Despite advances in therapy, heart failure (HF) patients have significant symptom burden and poor quality of life. However, data on palliative care (PC) utilization in this population are scarce. We sought to assess national trends in PC utilization in patients admitted with acute HF.
Methods
Adults hospitalized with HF without acute coronary syndrome were identified in the National inpatient sample. PC was identified using ICD‐9‐CM‐Code V66.7. Trends in PC utilization, its predictors and its association with length‐of‐stay and cost were assessed.
Results
A total of 939 680 HF patients were hospitalized with HF between 2003 and 2014. Of those,1.2% received PC during the hospitalization, with an upward trend in the use of PC over time (0.12% in 2003 to 3.6% in 2014, P < 0.001). Compared with patients who did not receive PC, those who had PC were older (79 ± 12 vs 69 ± 16 years), and had higher prevalence of Caucasian race (73.4% vs 51.8%), coronary disease (45.6% vs 39.3%), chronic renal disease (79.3% vs 42.8%), and pulmonary hypertension (28.3% vs 15.1%) (P < 0.001). In‐hospital mortality (35.2% vs 2.2%), length‐of‐stay (9 ± 13 days vs 6 ± 6, P < 0.001), cost ($19 984 ± 42 922 vs $11 921 ± 18 175), and non‐home discharges (46% vs 19.2%) (P < 0.001) were higher in the PC group. In‐hospital mortality in PC group trended downward over time (69% in 2003 vs 29% in 2014, P < 0.001).
Conclusion
PC is being utilized in an increasing but overall small number of patients hospitalized with HF. Further research is needed to identify the optimal role and timing of PC in HF patients.
Keywords: heart failure, palliative care
Heart failure (HF) is a major and growing public health burden, which affects approximately 6.5 million adults in the United States.1 It is projected that >8 million Americans older than 18 years of age will have HF by 2030, a substantial increase of 46% from 2012.1 Data also suggest an increase in the incidence and prevalence of HF because of the continuous aging of the population and the improved survival of HF patients.1 Despite advances in medical therapy, HF carries significant physical and psychological symptom burden on both patients and their families.2 Palliative care (PC) has a positive impact on quality of life for patient with advanced HF by improving the burden of symptoms, reducing rehospitalization, and decreasing anxiety and depression.3, 4, 5 Current guidelines from the American College of Cardiology/American Heart Association recommend strong consideration of PC as an integral part of multidisciplinary CHF management, and hospitalizations for HF represent an opportunity to involve PC team.6 We sought to assess the contemporary trends and utilization of PC in patients hospitalized with HF in the United States using a national representative database.
1. METHODS
The national inpatient sample (NIS) was used to derive patient relevant information between January first 2003 and December 31st 2014. The NIS is the largest publicly available all‐payer administrative claims‐based database and contains information about patient discharges from approximately 1000 non‐federal hospitals in 45 states. It contains clinical and resource utilization information on 5 to 8 million discharges annually, with safeguards to protect the privacy of individual patients, physicians, and hospitals. These data are stratified to represent approximately 20% of US inpatient hospitalizations across different hospital and geographic regions (random sample). National estimates of the entire US hospitalized population were calculated using the Agency for Healthcare Research and Quality sampling and weighting method.
1.1. Study's population
Patients >18‐year‐old with a principle admission diagnosis of acute HF (International Classification of Diseases‐Ninth Revision‐Clinical Modification [ICD‐9‐CM] codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.13, 40 491, 404.93, 428) were identified in the NIS. PC was identified using the ICD‐9‐CM procedure, code (V66.7 [PC encounter]). This code has been shown to have high specificity and positive predictive value for identifying PC in large administrative databases.7, 8, 9 Patients with discharge diagnosis of acute coronary syndrome or acute myocardial infarction were excluded to eliminate a potentially confounding group of patients. Advanced HF was defined as major or extreme (APR‐DRG) severity score. Patients were then divided into two groups: HF with PC and HF without PC. Baseline patient characteristics of both groups are described. The trends of PC in patients with HF during the 12‐year study period were assessed. In addition, in‐hospital morbidity, mortality, length of stay, and resource utilization were compared between the two groups. We also investigated PC predictors using univariate and multivariate logistic regression models. The following variables were included in the logistic regression model (age, sex, race, hypertension, diabetes, coronary artery disease, cardiogenic shock, peripheral vascular disease, dyslipidemia, chronic renal failure, hypornatremia, chronic pulmonary disease, liver disease, depression, pulmonary hypertension, anemia, prior sternotomy, atrial fibrillation, conduction disorder, smoking, prior pacer/defibrillator, prior stroke, mechanical ventilation, teaching hospital status, rural location, hospital size, median household income, primary payer, and geographic location).
1.2. Statistical analysis
Descriptive statistics presented as frequencies with percentages for categorical variables and as means with SDs for continuous variables. Baseline characteristics were compared using a Pearson χ 2 test for categorical variables and an independent‐samples t test for continuous variables. Univariate and multivariate logistic regression was performed to estimate odds ratios (OR) with 95% confidence intervals (CI) to determine predictors of PC referral following admission with HF. Trends over years were assessed using Cochran‐Armitage test. A type I error rate of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 24 (IBM Corporation, Armonk, New York).
2. RESULTS
A total of 939 680 patients admitted with acute HF were included in this analysis. Of those, 1.2% received PC during the hospitalization (Figure S1, Supporting Information). The rate of PC increased during the study period, with a clear trend toward its utilization in patients with lower in‐hospital mortality (Figure 1). Patients who received PC were older (79 ± 12 vs 69 ± 16 years, P < 0.001), Caucasians (73.4% vs 51.8%, P < 0.001), and had a higher prevalence of coronary artery disease (45.6% vs 39.3%, P < 0.001), chronic renal disease (79.3% vs 42.8%, P < 0.001), pulmonary hypertension (28.3% vs 15.1%, P < 0.001), and other key comorbidities compared with those who did not receive PC (Table 1).
Figure 1.
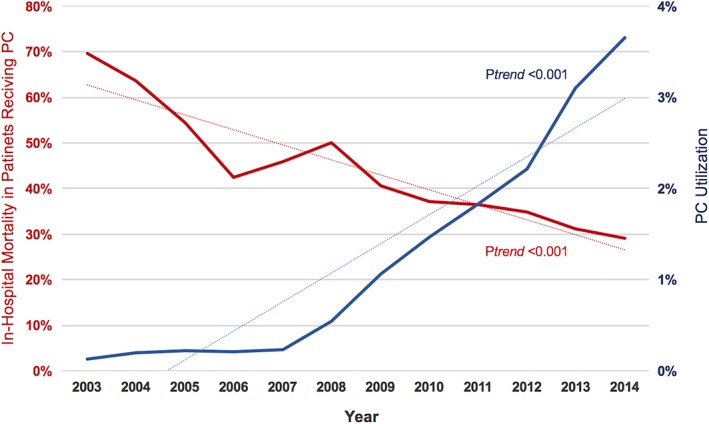
Temporal trends in palliative care utilization and in‐hospital mortality in patients admitted with acute heart failure between 2003 and 2014. PC, palliative care
Table 1.
Baseline characteristics, and in‐hospital outcomes of patients who are admitted with heart failure stratified by the occurrence of palliative care encounter
| Baseline characteristics | Non‐PC (n = 188991) (NE = 928093) | PC (n = 2338) NE = 11587) | P‐value | ||
|---|---|---|---|---|---|
| Age (mean, SD) | 69 ± 16 | 79 ± 12 | <0.001 | ||
| Age | <0.001 | ||||
| 18‐40 (n, %) | 15 589 | 8.2% | 35 | 1.5% | |
| 41‐65 | 55 264 | 29.2% | 270 | 11.5% | |
| 65‐85 | 88 296 | 46.7% | 1180 | 50.5% | |
| >85 | 29 842 | 15.8% | 853 | 36.5% | |
| Female | 100 269 | 53.1% | 1172 | 50.1% | 0.005 |
| Race | <0.001 | ||||
| White | 82 720 | 51.8% | 1593 | 73.4% | |
| Black | 54 772 | 34.3% | 347 | 16.0% | |
| Hispanic | 14 229 | 8.9% | 121 | 5.6% | |
| Diabetes mellitus | 84 686 | 44.8% | 910 | 38.9% | <0.001 |
| Hypertension | 3879 | 2.1% | 47 | 2.0% | 0.857 |
| Coronary artery disease | 74 062 | 39.2% | 1065 | 45.6% | <0.001 |
| Peripheral vascular disease | 22 157 | 11.7% | 348 | 14.9% | <0.001 |
| Dyslipidemia | 65 865 | 34.9% | 888 | 38.0% | <0.001 |
| Chronic renal failure | 80 077 | 42.4% | 1854 | 79.3% | <0.001 |
| Hyponatremia | 13 294 | 7.0% | 480 | 20.5% | <0.001 |
| Chronic pulmonary disease | 61 694 | 32.6% | 767 | 32.8% | 0.868 |
| Metastatic cancer | 1048 | 0.6% | 51 | 2.2% | <0.001 |
| Liver disease | 4499 | 2.4% | 131 | 5.6% | <0.001 |
| Depression | 13 499 | 7.1% | 204 | 8.7% | 0.003 |
| Pulmonary hypertension | 28 136 | 14.9% | 661 | 28.3% | <0.001 |
| Anemia | 61 190 | 32.4% | 940 | 40.2% | <0.001 |
| Prior sternotomy | 20 872 | 11.0% | 399 | 17.1% | <0.001 |
| Atrial fibrillation/flutter | 55 655 | 29.4% | 1191 | 50.9% | <0.001 |
| Conduction disorders | 9220 | 4.9% | 133 | 5.7% | 0.071 |
| Smoking | 38 707 | 20.5% | 429 | 18.3% | <0.001 |
| Prior defibrillator | 9142 | 4.8% | 299 | 12.8% | <0.001 |
| Prior pacemaker | 13 490 | 7.1% | 232 | 9.9% | <0.001 |
| Prior stroke | 8597 | 4.5% | 173 | 7.4% | <0.001 |
| Do‐Not‐Resuscitate orders | 3461 | 1.8% | 898 | 38.4% | <0.001 |
| Severity Subclasses | <0.001 | ||||
| Minor | 15 153 | 8.1% | 31 | 1.3% | |
| Moderate | 76 112 | 40.5% | 300 | 12.8% | |
| Major | 81 396 | 43.3% | 1222 | 52.3% | |
| Extreme | 15 298 | 8.1% | 785 | 33.6% | |
| Cardiogenic Shock | 1371 | 0.7% | 207 | 8.9% | <0.001 |
| Teaching | 79 653 | 42.3% | 1377 | 59.0% | <0.001 |
| Rural | 20 647 | 11.0% | 173 | 7.4% | <0.001 |
| Median household income no (%) | <0.001 | ||||
| 1. 0‐25th percentile | 68 198 | 36.9% | 564 | 24.4% | |
| 2. 26‐50th percentile | 47 278 | 25.6% | 575 | 24.9% | |
| 3. 51‐75th percentile | 39 345 | 21.3% | 611 | 26.5% | |
| 4. 76‐100th percentile | 30 159 | 16.3% | 557 | 24.1% | |
| Bed size | 0.043 | ||||
| Small | 24 135 | 12.8% | 263 | 11.3% | |
| Medium | 50 275 | 26.7% | 611 | 26.2% | |
| Large | 113 862 | 60.5% | 1461 | 62.6% | |
| Insurance status | <0.001 | ||||
| Medicare/medicaid | 148 808 | 78.7% | 1982 | 84.8% | |
| Private insurance | 25 664 | 13.6% | 219 | 9.4% | |
| Self‐pay/no charge | 9863 | 5.2% | 32 | 1.4% | |
| Hospital region | <0.001 | ||||
| Northeast | 32 289 | 17.1% | 441 | 18.9% | |
| Midwest | 42 064 | 22.3% | 547 | 23.4% | |
| South | 85 305 | 45.1% | 849 | 36.3% | |
| West | 29 333 | 15.5% | 501 | 21.4% | |
| In‐hospital mortality | 4086 | 2.2% | 823 | 35.2% | <0.001 |
| Mechanical ventilation | 1387 | 0.7% | 66 | 2.8% | <0.001 |
| Non‐home discharges | 35 419 | 19.2% | 697 | 46.0% | <0.001 |
| Length of stay (mean, SD) | 6 ± 6 | 9 ± 13 | <0.001 | ||
| Hospitalization cost ($) | 11 921 ± 18 175 | 19 984 ± 42 922 | <0.001 | ||
Abbreviations: N, number; PC, palliative care; $; dollar.
In a multivariate logistical regression analysis, the strongest predictors of referring to PC were: older age (OR 14.17, 95% CI 9.53‐21.09 for age > 85, and OR 6.18, 95% CI 4.18‐9.15 for age 65‐85 [reference age 18‐40]), cardiogenic shock (OR 6.17, 95% CI 5.15‐7.40), chronic renal failure (OR 4.19, 95% CI 3.75‐4.68), and mechanical ventilation (OR 2.49, 95% CI 1.85‐3.35). Other independent predictors of PC utilization included hyponatremia, liver disease, prior defibrillator implantation, pulmonary hypertension, atrial fibrillation, teaching hospital status, medium or large hospital size, private payer, and higher median household income (Table S1, Supporting Information). Racial minorities were less likely to receive PC than Caucasian patients, respectively: (OR 0.56, 95% CI 0.49‐0.64) for African‐American vs Caucasian, and (OR 0.53, 95% CI 0.43‐0.65) for Hispanic vs Caucasian. Geographic differences in PC utilization were also observed with higher utilization in hospitals located in the West (OR 1.59, 95% CI 1.38‐1.83).
In‐hospital mortality was higher in patients who received PC vs those who did not (35.2% vs 2.2%, P < 0.001), but this disparity decreased significantly over time (Figure 2). Patients who received PC had longer hospitalizations (9 ± 13 vs 6 ± 6, P < 0.001), higher rates of non‐home discharges (46% vs 19.2%, P < 0.001) and accrued higher total hospital cost ($19 984 ± 42 922 vs $11 921 ± 18 175, P < 0.001) (Table 1, Figures 3 and 4).
Figure 2.
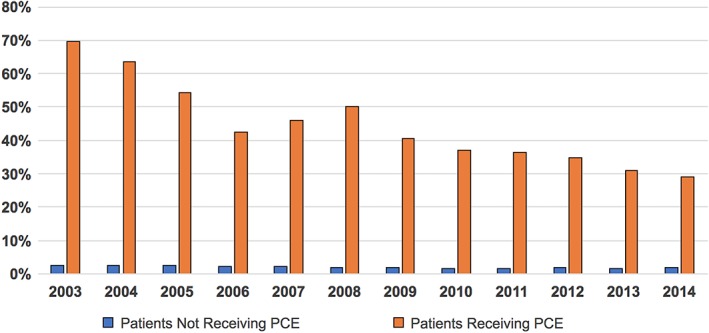
In‐hospital mortality among heart failure patients stratified by the utilization of palliative care. PCE, palliative care encounter
Figure 3.
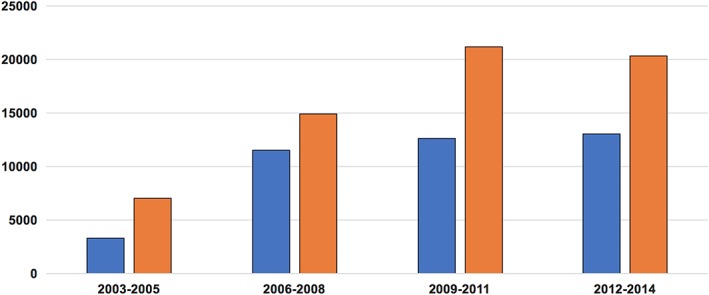
Trends in hospital cost among patients among patients who received palliative care compared with those who did not. HF, heart failure
Figure 4.
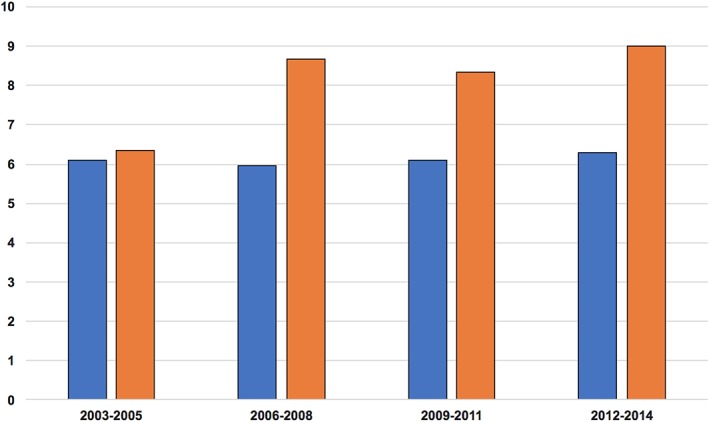
Trends in hospital length of stay among heart failure patients who received palliative care and those who did not
3. DISUSSION
The main findings of the present investigation are: (a) in contemporary US practice, PC is utilized in a small but increasing number of patients hospitalized with HF. (b) Certain patient and hospital specific factors were predictive of utilization of PC in HF patients. (c) Although patients receiving PC have high in‐hospital mortality, this might be a result of a referral bias; perhaps more decompensated patients were referred to PC while those with milder disease were not because they were inherently expected to have lower mortality. The mortality rates among PC patients, however, decreased over time suggesting a temporal trend that a wider range of disease severity and lower risk population received PC in the latter years.
PC services have the potential to positively impact the health and quality of life of patients with HF and should be integrated as an ongoing key component of their care.4, 10 Hospitalizations for HF serve as an opportunity to assess, introduce, and provide PC alongside optimal medical management in a multi‐disciplinary comprehensive model of care.11 Indeed, an inpatient PC model for patients with acute HF has been associated with short‐term improvement in symptom burden, quality of life, and depressive symptoms.12 However, data on PC utilization in HF patients are limited. In a study of 4474 veteran patients admitted with HF, 338 (7.6%) received PC during the hospitalization with doubling of the utilization rates between 2007 and 2013.13 Similarly, our study showed a significant upward trend in PC utilization among patient hospitalized with HF in the United States between 2003 and 2014. Despite this trend, only a minority of patients (3.6% in 2014) received in‐hospital PC. This is in comparison with 6.2% of patients admitted with stroke, 11.9% of patients admitted with cancer, 13.1% of hospitalized patients receiving prolonged ventilation, and 16.7% of patients who suffered an out‐of‐hospital cardiac arrest.7, 8, 14, 15 Hence, examination of factors associated with PC in the HF population is warranted.
Our study documents several patient‐specific clinical predictors of PC utilization including older age, cardiogenic shock, chronic renal failure, and mechanical ventilation. Those predictors are intuitive and are in‐line with what has been observed in other studies on PC utilization in patients admitted with other advanced illnesses.16, 17 However, several demographic elements and wealth indicators were also independently associated with more or less PC utilization in our study suggesting that PC might also be subject to racial and social disparities. This was most evident in the lower rates of PC utilization among racial minorities (37% and 42% less PC utilization among African‐American and Hispanic patients compared to Caucasian patients, respectively). Although cultural differences might play a role, further research is needed to assess potential underlying causes in this racial disparity. There were also substantial variations in the use of PC across hospitals. Large and teaching hospitals were more likely to offer PC to hospitalized patients. This may be partially because of the limited access to PC at small and non‐teaching hospitals. Nonetheless, variations also existed when hospitals were stratified based on their geographic location. Patients admitted with HF to hospitals in the West were 39% more likely to utilize PC services compared with those admitted to hospitals in the Northeast. This is similar to the findings of Singh et al, who found that hospitals in the West are 50% more likely to offer PC services to patients admitted with acute stroke.7 This suggests a multifactorial interplay in the in‐between‐hospitals variations in PC utilization, which might include cultural differences in different parts of the United States.
Other important factors that may play a role in PC utilization among HF patients are: knowledge as to when to initiate PC interventions, uncertainty regarding patient goals and prognosis, and the shortage of specialist PC providers.18, 19, 20 In a national survey on physician attitudes toward end‐stage HF, only 16% of the physician respondents were confident in predicting 6‐month mortality, and inpatient volume was a predictor of increased confidence.20 Whether using risk models to predict in‐hospital mortality at the time of hospitalization will increase PC utilization remains to be studied.4
The substantial in‐hospital mortality (35%) among PC recipients as compared to overall cohort points to the fact that PC is mostly utilized in patients with advanced and late/decompensated stages of HF. Nonetheless, in‐hospital mortality decreased over time among these patients suggesting a trend to extend PC services to lower risk patients with higher chances of surviving the hospitalization. Patients who received PC had longer hospitalizations and higher cost of care and these differences persisted over time. This is in contrary to a similar analysis in patients admitted with stroke, in whom patients who received PC had shorter hospitalizations and lower cost compared with those who did not receive PC.7 We speculate that this difference can be explained by the earlier seeking of PC services among patients admitted with stroke because of the recognizable morbidity and mortality of stroke admissions.21 Nonetheless, 1‐year mortality rates among patients admitted with HF are also substantial (25%‐50%)13, 22, 23 suggesting that HF hospitalizations do represent an excellent opportunity to offer PC for these patients. Indeed, in one study, 80% of the patients with HF were hospitalized in the last 6 months of their lives, and those months were associated with significant resource utilization and cost.24 PC discussion with HF patients, especially those admitted with advanced stages, may aid shared decision making with patients regarding their prognosis and goals of care which could lead to reductions in unplanned hospitalizations and health care costs.25, 26, 27, 28
Our study has a number of limitations: (a) The NIS is an administrative database that gathers data for billing purposes and can be limited by erroneous coding. Because the code used for PC (V66.7) is not linked to reimbursement, its documentation may be less reliable. It is possible that our observations indicate an increase in the accurate coding of PC rather than a true increase in the use of PC over time. However, the temporal increase in PC observed in concordant with other studies, suggesting a proportionate use of the PC code.7, 29 (b) The documentation of PC does not include specifics of the timing or extent of PC services received. Nonetheless, our study mainly aims to understand the general trends and predictors of PC utilization among the growing HF population, which can be delineated in the current study despite those limitations. Further studies are needed to assess the role of PC in HF patients, and to tackle the barriers in its use. (c) The NIS provides comprehensive assessment of events and procedure that occur during the hospitalization. It does not capture PC that may have occurred in the outpatient setting. Hence, it does not allow differentiation between patients who have had PC prior to the admission and those who received PC as new patients. (d) The PC utilization code is usually used only for dedicated PC teams and is not coded for PC encounter provided by the primary teams or cardiologists which is also one of the reasons why it is under coded.
In conclusion, PC is being utilized in an increasing but overall small number of patients hospitalized because of HF. Those patients have distinctive clinical profile, and higher in‐hospital morbidity, mortality, and cost. Evidence of sex and race‐related disparity in PC utilization in HF patients arose in this study. Further research is needed to assess the impact of PC on short‐ and long‐term outcomes in HF patients and the barriers in utilization.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
FIGURE S1 Study flow chart
TABLE S1 Univariate and multivariate logistic regression analysis for predictors of receiving a palliative care encounter in patients admitted with acute heart failure between 2003 and 2014
Alqahtani F, Balla S, Almustafa A, Sokos G, Alkhouli M. Utilization of palliative care in patients hospitalized with heart failure: A contemporary national perspective. Clin Cardiol. 2019;42:136–142. 10.1002/clc.23119
Sudarshan Balla and Mohamad Alqahtani contributed equally to this manuscript.
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke Statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345(20):1435‐1443. [DOI] [PubMed] [Google Scholar]
- 3. Zhou K, Mao Y. Palliative care in heart failure : a meta‐analysis of randomized controlled trials. Herz. 2018. 10.1007/s00059-017-4677-8. [DOI] [PubMed] [Google Scholar]
- 4. Rogers JG, Patel CB, Mentz RJ, et al. Palliative Care in Heart Failure: the PAL‐HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017;70(3):331‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu MLR, De Venecia TA, Goyal A, et al. Psychiatric conditions as predictors of rehospitalization among African American patients hospitalized with heart failure. Clin Cardiol. 2017;40(11):1020‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147‐e239. [DOI] [PubMed] [Google Scholar]
- 7. Singh T, Peters SR, Tirschwell DL, Creutzfeldt CJ. Palliative Care for Hospitalized Patients with Stroke: Results from the 2010 to 2012 National Inpatient Sample. Stroke. 2017;48(9):2534‐2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albaeni A, Chandra‐Strobos N, Eid SM. Palliative care utilization following out‐of‐hospital cardiac arrest in the United States. Resuscitation. 2018;124:112‐117. [DOI] [PubMed] [Google Scholar]
- 9. Feder SL, Redeker NS, Jeon S, et al. Validation of the ICD‐9 diagnostic code for palliative care in patients hospitalized with heart failure within the veterans health administration. Am J Hosp Palliat Care. 2018;35(7):959‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Widera E, Pantilat SZ. Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3(4):247‐251. [DOI] [PubMed] [Google Scholar]
- 11. Warraich HJ, Xu H, DeVore AD, et al. Trends in hospice discharge and relative outcomes among Medicare patients in the get with the guidelines‐heart failure registry. JAMA Cardiol. 2018;3(10):917‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134‐142. [DOI] [PubMed] [Google Scholar]
- 13. Mandawat A, Heidenreich PA, Mandawat A, Bhatt DL. Trends in palliative care use in veterans with severe heart failure using a large National Cohort. JAMA Cardiol. 2016;1(5):617‐619. [DOI] [PubMed] [Google Scholar]
- 14. Chatterjee K, Goyal A, Kakkera K, Harrington S, Corwin HL. National Trends (2009‐2013) for palliative care utilization for patients receiving prolonged mechanical ventilation. Crit Care Med. 2018;46:1230‐1237. [DOI] [PubMed] [Google Scholar]
- 15. Wiskar KJ, Celi LA, McDermid RC, et al. Patterns of palliative care referral in patients admitted with heart failure requiring mechanical ventilation. Am J Hosp Palliat Care. 2018;35(4):620‐626. [DOI] [PubMed] [Google Scholar]
- 16. Rush B, Hertz P, Bond A, McDermid RC, Celi LA. Use of palliative care in patients with end‐stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151(1):41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rush B, Walley KR, Celi LA, Rajoriya N, Brahmania M. Palliative care access for hospitalized patients with end‐stage liver disease across the United States. Hepatology. 2017;66(5):1585‐1591. [DOI] [PubMed] [Google Scholar]
- 18. Psotka MA, McKee KY, Liu AY, Elia G, de Marco T. Palliative care in heart failure: what triggers specialist consultation? Prog Cardiovasc Dis. 2017;60(2):215‐225. [DOI] [PubMed] [Google Scholar]
- 19. Ziehm J, Farin E, Schafer J, Woitha K, Becker G, Koberich S. Palliative care for patients with heart failure: facilitators and barriers ‐ a cross sectional survey of German health care professionals. BMC Health Serv Res. 2016;16(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hauptman PJ, Swindle J, Hussain Z, Biener L, Burroughs TE. Physician attitudes toward end‐stage heart failure: a national survey. Am J Med. 2008;121(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 21. Fitzpatrick J, Mavissakalian M, Luciani T, Xu Y, Mazurek A. Economic impact of early inpatient palliative care intervention in a community hospital setting. J Palliat Med. 2018;21:933‐939. [DOI] [PubMed] [Google Scholar]
- 22. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355(3):260‐269. [DOI] [PubMed] [Google Scholar]
- 23. Tsuchihashi‐Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese cardiac registry of heart failure in cardiology (JCARE‐CARD). Circ J. 2009;73(10):1893‐1900. [DOI] [PubMed] [Google Scholar]
- 24. Unroe KT, Greiner MA, Hernandez AF, et al. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000‐2007. Arch Intern Med. 2011;171(3):196‐203. [DOI] [PubMed] [Google Scholar]
- 25. Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative Care in Heart Failure: rationale, evidence, and future priorities. J Am Coll Cardiol. 2017;70(15):1919‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyers DE, Goodlin SJ. End‐of‐life decisions and palliative Care in Advanced Heart Failure. Can J Cardiol. 2016;32(9):1148‐1156. [DOI] [PubMed] [Google Scholar]
- 27. Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative care interventions for patients with heart failure: a systematic review and meta‐analysis. J Palliat Med. 2017;20(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative Care for Patients with heart failure. Int Heart J. 2018;59:503‐509. [DOI] [PubMed] [Google Scholar]
- 29. Gentsch C, Lichteiner M, Feer H. Behavioral effects of yohimbine and chlordiazepoxide: dependence on the rat's previous familiarization with the test conditions. Neuropsychobiology. 1989;22(2):101‐107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Study flow chart
TABLE S1 Univariate and multivariate logistic regression analysis for predictors of receiving a palliative care encounter in patients admitted with acute heart failure between 2003 and 2014


