Abstract
This systematic review and meta‐analysis sought to summarize the available evidence on the use of transcatheter aortic valve replacement (TAVR) in patients with Native Aortic Valve Regurgitation (NAVR) and compare outcomes between first and second generation valves. Owing to the improvements in transcatheter heart valve design and procedural success, TAVR has become increasingly performed in broader aortic valve pathologies. We searched Medline, Embase, Cochrane, and Scopus databases from 2007 to 2018 and performed a systematic review on reports with at least 10 patients with aortic valve regurgitation undergoing TAVR procedure. The main outcome of interest was all‐cause mortality at 30 days. A total of 638 patients across 12 studies were included. Mean age ranged from 68 to 84. Society of Thoracic Surgeons score ranged from 5.4% to 13.1% and Logistic EuroSCORE ranged from 18.2% to 33%. The incidence rate of all‐cause mortality at 30 days was found to be 11% (95% CI 7%‐16%; I 2 = 20.86%). All‐cause mortality at 30 days for first generation valves had an incidence rate of 15% (95% CI 10%‐20%; I 2 = 10%) compared to 7% (95% CI 3%‐13%; I 2 = 37%) in second generation valves with subgroup interaction analysis P = 0.059. Device success incidence rate in second generation valves was 92% (95% CI 83%‐99%; I 2 = 67%) vs 68% (95% CI 59%‐77%; I 2 = 53%) in first generation valves with P = 0.001. TAVR appears to be a feasible treatment choice for NAVR patients at high risk for surgical valve replacement. Second generation valves show promising results in terms of short‐term outcomes.
Keywords: aortic regurgitation, meta‐analysis, systematic review, transcatheter aortic valve replacement
1. INTRODUCTION
According to the Euro Heart Survey, approximately 13% of patients with native valve disease suffer from isolated aortic regurgitation (AR).1 Surgical aortic valve replacement remains the standard of care in symptomatic patients, patients with left ventricular dilatation or decreased left ventricular function2 and pharmacologic therapy is limited to symptomatic patients who are not candidates for surgery or to treat patients with severe AR in preparation for surgery.
Transcatheter aortic valve replacement (TAVR) has become the standard of care for the management of high‐risk patients with aortic stenosis (AS). Since the first in human aortic valve replacement in 20023 and owing to the improvements in device design and procedural success, TAVR has become increasingly performed in broader aortic valve pathologies, other than aortic stenosis.4, 5, 6 Limited data is available regarding the performance of TAVR in patients with native aortic valve regurgitation (NAVR).
Initial experiences with TAVR for AR patients using first generation valves, majority of which used CoreValve system, showed high risk for valve dislocation, residual AR and need for second valve implantation. Using second generation valves, such as Jenavalve, was found to overcome some of the technical challenges with early generation valves.
A systematic review and meta‐analysis published in 2016 by Franzone et al has summarized the available evidence up to 15 February 2016.7 There have been multiple studies published since then. Thus, we conducted this systematic review and meta‐analysis as an update to the previous work and to compare the outcomes between first vs second generation valves.
2. METHOD
This is systematic review and meta‐analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.8 A protocol was developed a priori by the authors and is available in Supporting information Appendix S1.
2.1. Search strategy
A comprehensive search of several databases from 2007 to January 22nd 2018, English language was conducted. The databases included Ovid MEDLINE Epub Ahead of Print, Ovid Medline In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study's principle investigator. Controlled vocabulary supplemented with keywords was used to search for studies of TAVR for aortic valve regurgitation. The actual strategy is available in Appendix S2. Previous systematic reviews were also reviewed for cross‐referencing.
2.2. Study selection
Two reviewers screened the titles and abstracts of the identified references in an independent and blinded manner. The full‐texts were then retrieved and evaluated for inclusion by the same independent blinded reviewers and establishing consensus solved disagreements. Studies were eligible if they met the following criteria: (a) Patients with pure native aortic regurgitation (AR); (b) studies including patients with pure degenerative AR within a larger cohort of patients undergoing TAVR because of severe aortic stenosis; (c) reports of a minimum of 10 patients; (d) abstracts or conference presentations reporting procedural characteristics, and clinical outcomes of interest; and (e) reports written in English language. We excluded case reports as well as reports with aortic valve replacement due to regurgitation in failed aortic prosthesis, endocarditis, aortic dissection, or in patients with left ventricular assist device (LVAD). When multiple publications reported results from one center, we identified the most comprehensive reports and used them to avoid inflation of our results.
2.3. Data extraction
When reported, the following data were sought from included studies: patients' characteristics including number of patients included in the report, age, gender, New York Heart Association (NYHA) class, aortic regurgitation grade (moderate or severe), concomitant moderate or severe mitral stenosis, aortic annulus diameter and ascending aortic diameter, left ventricular ejection fraction, Society of Thoracic Surgeon and Logistic EuroSCORE, and comorbidities. Procedure characteristics including device type, size and access site were also extracted when available. Follow‐up duration was also extracted when reported. Two reviewers extracted the data and disagreements were solved with establishing consensus.
2.4. Outcomes of interest
Out main outcome of interest was all‐cause mortality at 30 days. Other outcomes were extracted, when available, all‐cause mortality at the end of follow‐up period for each study, cardiovascular mortality at 30 days and end of follow up, cerebrovascular accidents, myocardial infarction, acute kidney injury, major bleeding, major vascular complication, pacemaker implantation, moderate or severe AR and paravalvular leak, second valve required, conversion to SAVR and devices success.9
2.5. Risk of bias assessment
We planned to assess the risk of bias in randomized controlled trials and non‐randomized comparative studies using the Cochrane Risk of Bias Tool10 and the Newcastle Ottawa Scale,11 respectively. For non‐comparative studies and case series, we used the tool suggested by Murad et al.12
2.6. Data synthesis
We extracted the number of events and total for each study arm to generate an incidence rate (event rate) and 95% confidence interval [CI] using the Freeman Turkey double arcsine method.13 Incidence rates were pooled using a random effects model.14 Heterogeneity between studies was assessed using the I 2 statistic.15 All analyses were performed using STATA.16
3. RESULTS
Our search protocol identified 2608 references. After screening titles and abstracts, 39 reports were selected for full‐text evaluation. We were able to identify three reports that met our inclusion criteria but had to be excluded from the analysis due to study centers overlap with other included studies and the risk of data inflation.17, 18, 19 Finally, 12 studies were identified as eligible and included in our systematic review20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 (Figure 1). In total, 638 patients with native pure aortic regurgitation identified across the 12 studies. Patients and procedural characteristics in addition to outcomes are summarized in Table 1. For more comprehensive summary of the included studies, with the excluded studies because of centers overlap, refer to the Table S1.
Figure 1.
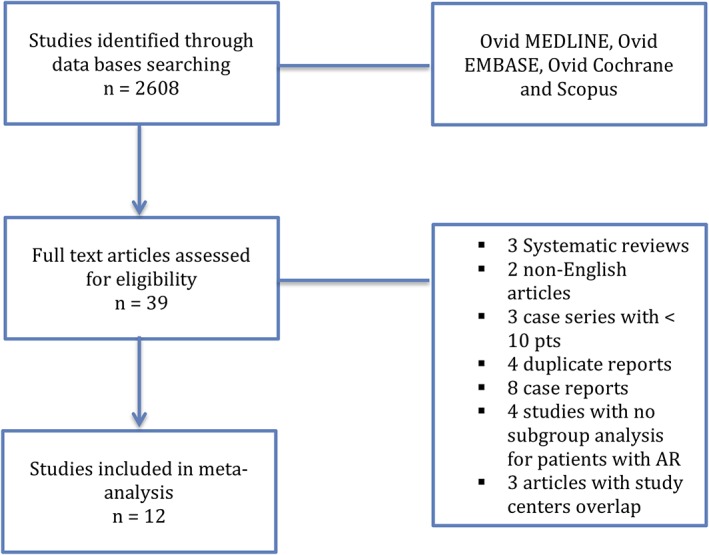
Flow chart of manuscript selection
Table 1.
Main clinical, procedural and outcomes of included studies
| Author, year | Patient (n) | Age (y) | Male (n) | Logistic euro‐score (% ± SD) | Logistic euro‐score II (% ± SD) | STS score (% ± SD) | Device | All cause mortality (30 d) | Device success | Pacemaker rate | Follow‐up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roy et al21 | 43 | 75.3 ± 8.8 | 20 | 26.9 ± 17.9 | — | 10.2 ± 5.3 | Corevalve (43) | 4 | 32 | 7 | 12 |
| Koschyk et al18 | 10 | 68 | 7 | 26.1 | — | — | JenaValve (10) | — | — | — | 3‐7 |
| Rossi et al20 | 16 | 84 ± 2.6 | 9 | 33 | — | — | CoreValve (16) | — | — | 7 | 36 |
| Schlingloff et al23 | 10 | 79 ± 9 | 6 | 28.3 ± 17.1 | — | 6.74 ± 11.1 | JenaValve (10) | 3 | 10 | 2 | 12 |
| Seiffert et al25 | 31 | 73.8 ± 9.1 | 20 | 23.6 ± 14.5 | 9.3 ± 6.4 | 5.4 ± 3.6 | JenaValve (31) | 4 | 30 | 2 | 6 |
| Testa et al27 | 26 | 73.0 ± 10.0 | 16 | 24.0 ± 8.0 | — | 13.1 ± 2.0 | CoreValve (26) | 6 | 20 | 3 | 12 |
| Freker et al17 | 22 | 80.0 ± 5.6 | 12 | 25 ± 18.0 | — | — | CoreValve (21) SapienXT (1) |
5 | 18 | 6 | 12 |
| Schofer et al24 | 11 | 74.7 ± 12.9 | 4 | 19.9 ± 7.1 | — | 8.84 ± 8.9 | Direct flow (11) | 1 | 11 | 1 | 1 |
| Munoz‐Garcia et al19 | 16 | 73.1 ± 17 | — | 18.2 ± 8.9 | — | — | CoreValve (16) | 1 | — | — | 36.5 |
| Sawaya et al22 | 78 | 74 ± 10 | 46 | 20.4 ± 11.8 | — | 6.7 ± 4.8 | CoreValve (33) Evolut R (5) JenaValve (23) direct flow (6) Lotus (6) SAPIEN XT (4) SAPIEN 3 (1) |
11 | 55 | 12 | 1 |
| Yoon et al28 | 331 | 74.4 ± 12.2 | 172 | — | 9.8 ± 10.7 | 6.7 ± 6.7 | Sapien XT (9) Sapien 3 (41) CoreValve (110) Evolut R (50) JenaValve (64) direct flow (35) J‐valve (1) engager (7) portico (3) Acurate (5) Lotus (6) |
36 | 246 | 51 | 12 |
| Zhu et al29 | 44 | 73.8 ± 5.6 | 31 | 25.4 ± 5.3 | — | 9.1 ± 3.6 | J‐valve (44) | 1 | — | 2 | 6 |
Abbreviations: d, day; n, number; SD, standard deviation.
3.1. Baseline characteristics
The mean age ranged from 68 to 84 ± 2.6. Society of Thoracic surgeons score was reported in eight studies with mean ranged from 5.4 ± 3.6 to 13.1 ± 2.0. Logistic EuroSCORE was reported in 11 of the included studies and it ranged from 18.2 ± 8.9 to 33 while Logistic EuroSCORE II was only reported in two studies with mean ranging from 9.3 ± 6.4 to 9.8 ± 10.7. Access site was reported in 540/638 patients, 9/12 studies, and consisted of 351 femoral, 154 apical, 20 subclavian, 12 aortic, and 3 carotid.
3.2. Risk of bias assessment
The risk of selection and reporting biases were unclear in more than half of the included studies. The method of selecting patient patients for TAVR or the specific criteria they met to be eligible for this intervention was not reported in some of the included studies. All studies have reported at least outcomes at the end of the follow up period post valve replacement. Some studies failed to report outcomes according to Valve Academic Research Consortium‐2 (VARC‐2) criteria. Figure S1 summarizes the risk of bias for each individual study using the tool suggested by Murad et al.12
3.3. Outcomes
Our main outcome of interest was all‐cause mortality at 30 days. The incidence rate of patients who did not survive until the 30 days benchmark was 11% (95% confident interval (CI) from 7% to 16%; I 2 = 35%) (Figure 2). All‐cause mortality at the end of the follow up period for all studies had incidence rate of 17% (95% CI from 11% to 24%; I 2 = 59%). Cardiovascular mortality at 30 days and at the end of follow‐up period had incidence rates of 7% (95% CI, from 4% to 10%; I 2 = 35%) and 9% (95% CI, from 4% to 15%; I 2 = 67%), respectively. Incidence rate of cerebrovascular accidents, reported in nine studies, was 2% (95% CI, from 1% to 3%; I 2 = 0%). Device success was reported in eight studies with incidence rate of 84% (95% CI, from 75% to 91%; I 2 = 72%) and conversion to surgical aortic valve replacement had incidence rate of 2% (95% CI, from 0% to 3%; I 2 = 0%).
Figure 2.
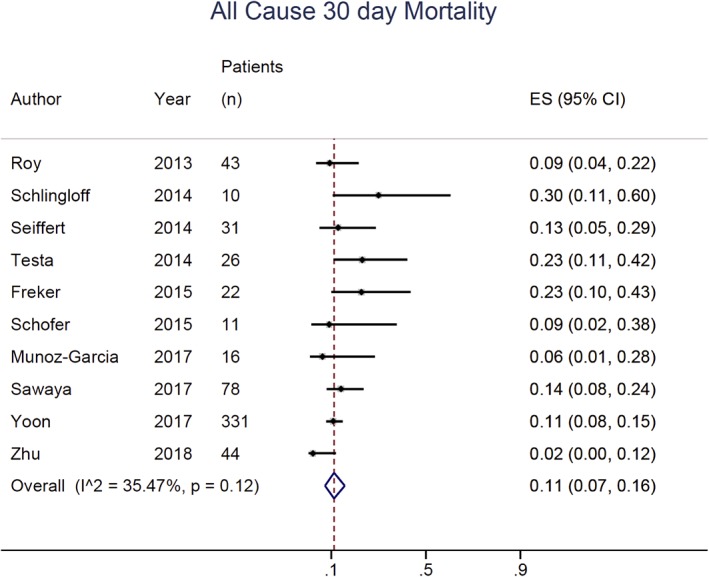
Forest plot with individual and pooled event rate for the all‐cause mortality at 30 day
Other outcomes were extracted from the included studies, when reported, and are listed in Table 2 .
Table 2.
Mata‐analysis of TAVR in AR endpoints
| Endpoints | No. of studies | No. of events | Incidence rate (95% CI) | I 2, % |
|---|---|---|---|---|
| All‐cause mortality, 30 days | 10 | 72/612 | 11% (7%‐16%) | 35.47 |
| All cause mortality | 12 | 130/638 | 17% (11%‐24%) | 59.17 |
| Cardiovascular mortality, 30 days | 6 | 45/553 | 7% (4%‐10%) | 34.61 |
| Cardiovascular mortality | 6 | 68/553 | 9%(4%‐15%) | 67.11 |
| Cerebrovascular accidents | 9 | 19/602 | 2% (1%‐3%) | 0 |
| Acute kidney injury | 6 | 49/549 | 9% (5%‐14%) | 55.01 |
| Major bleeding | 6 | 56/553 | 8% (3%‐15%) | 77.90 |
| Major vascular complication | 4 | 22/447 | 4% (2%‐7%) | 11.63 |
| Myocardial infarction | 5 | 0/160 | 0% (0%‐1%) | 0 |
| Pacemaker implantation | 10 | 93/612 | 14% (9%‐20%) | 45.70 |
| Moderate or severe AR | 5 | 54/484 | 14% (7%‐21%) | 61.06 |
| > mild paravalvular leak | 4 | 8/117 | 5% (0%‐17%) | 74.07 |
| Second valve required | 9 | 74/556 | 9% (4%‐15%) | 64.72 |
| Conversion to SAVR | 9 | 17/552 | 2% (0%‐3%) | 0 |
| Device success | 8 | 422/552 | 84% (75%‐91%) | 71.62 |
Abbreviation: CI, confidence interval.
3.4. Baseline characteristics of patients that received first vs second device generations
We performed a subgroup analysis to compare outcomes between first vs second generation valves (Table 3) The mean age ranged from 73 ± 10 to 84 ± 2.6 in patients who had first generation valves implanted vs 68 to 79 ± 9 in patients with second generation valves implanted. The mean Logistic EuroScore ranged from 18.2 ± 8.9 to 33 in first generation compared to 18.5 ± 13.2 to 28.3 ± 17.1 in second generation valves. Logistic EuroScore II mean was 11.7 ± 12.9 in first generation valves compared to 8.9 ± 9.4 to 9.3 ± 6.4 in second generation valves. The mean STS score in second generation valves ranged from 5.4 ± 3.6 to 9.1 ± 3.6 compared to 7.6 ± 6.7 to 13.1 ± 2.0 in first generation valves.
Table 3.
Subgroups pooled proportion with interaction analysis
| Endpoints | Early generation | New generation | Subgroup interaction analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of events | Incidence rate (95% CI) | I 2, % | No. of studies | No. of events | Incidence rate (95% CI) | I 2, % | ||
| P value | |||||||||
| All cause mortality, 30 days | 6 | 40/263 | 15% (10%‐20%) | 9.95% | 6 | 31/349 | 7% (3%‐13%) | 37.03% | 0.059 |
| All cause mortality | 7 | 68/279 | 24% (19%‐29%) | 0.71% | 7 | 60/359 | 13% (5%‐22%) | 68.60% | 0.052 |
| Cardiovascular mortality, 30 days | 4 | 23/225 | 9% (4%‐15%) | 39.71% | 4 | 20/328 | 6% (3%‐9%) | 0% | 0.209 |
| Cardiovascular mortality | 4 | 40/225 | 15% (8%‐24%) | 61.04% | 4 | 24/328 | 6% (3%‐10%) | 15.20% | 0.035 |
| Cerebrovascular accidents | 6 | 6/263 | 1% (0%‐4%) | 0% | 5 | 13/339 | 2% (0%‐5%) | 35.4% | 0.921 |
| Acute kidney injury | 4 | 22/221 | 9% (5%‐15%) | 24.09% | 4 | 27/328 | 9% (3%‐18%) | 73.14% | 0.988 |
| Major bleeding | 4 | 31/225 | 12% (6%‐20%) | 53.27% | 4 | 25/328 | 5% (0%‐12%) | 72.37% | 0.095 |
| Major vascular complication | 4 | 12/194 | 5% (2%‐9%) | 0% | 2 | 10/256 | 4% (1%‐6%) | — | 0.239 |
| Myocardial infarction | 3 | 0/85 | 0% (0%‐2%) | — | 2 | 0/75 | 0% (0%‐3%) | — | 0.894 |
| Pacemaker implantation | 5 | 40/226 | 19% (11%‐29%) | 52.4% | 5 | 41/308 | 10% (5%‐17%) | 37.66% | 0.109 |
| Moderate or severe AR | 5 | 45/231 | 19% (14%‐24%) | 0% | 2 | 9/253 | 3% (1%‐6%) | — | <0.001 |
| > mild paravalvular leak | 2 | 7/42 | 16% (6%‐29%) | — | 2 | 1/75 | 1% (0%‐5%) | — | 0.003 |
| Second valve required | 5 | 52/241 | 21% (16%‐27%) | 0% | 6 | 34/315 | 9% (6%‐13%) | 0% | 0.001 |
| Conversion to SAVR | 4 | 7/204 | 2% (0%‐6%) | 16.74% | 6 | 10/318 | 1% (0%‐4%) | 0% | 0.889 |
| Device success | 5 | 163/247 | 68% (59%‐77%) | 52.74% | 5 | 258/305 | 92% (83%‐99%) | 66.81% | 0.001 |
Abbreviation: CI, confidence interval.
The valve type was reported in all 638 patients underwent TAVR for NAVR. Out of these patients 44% received a first generation valve, CoreValve (265) and Sapien XT (14), and 56% had a second generation valve implanted, Acurate (5), Direct flow (52), Engager (7), Evolut R (55), JenaValve (138), J‐Valve (45), lotus (12), Portico (3), and Sapien 3 (42).
3.5. Outcomes of first vs second generation valves
Upon comparing all‐cause mortality at 30 days between the two groups, the incidence rate was 15% (95% CI from 10% to 20%; I 2 = 10%) in first generation valves compared to 7% (95% CI from 3% to 13%; I 2 = 37%) in the second generation valves with subgroup interaction test P = 0.059.32 The incidence rate of all‐cause mortality at the end of the follow‐up was found to be higher in first generation compared to second generation valves, 24% (95% CI from 19% to 29%; I 2 = 0.7%) vs 13% (95% CI from 5% to 22%; I 2 = 69%) with subgroup interaction analysis P = 0.052. While the difference between the incidence rates in the two groups was not statistically significant (P = 0.209) for cardiovascular mortality at 30 days, it was found to be significant at the end of follow‐up period (P = 0.035) with incidence rates of 15% (95% CI from 8% to 24%; I 2 = 61%) vs 6% (95% CI from 3% to 10%; I 2 = 15%).
Conversion to SAVR was lower in second generation valves, 1% (95% CI from 0% to 4%; I 2 = 0%), compared to first generation valves, 2% (95% CI from 0% to 6%; I 2 = 17%), with interaction analysis P = 0.889. When comparing device success between first and second generation valves, we found that the incidence rate of success was higher in second generation, 92% (95% CI from 83% to 99%; I 2 = 67%) compared to first generation valves, 68% (95% CI from 59% to 77%; I 2 = 53%), with P value of 0.001. Comparisons of other outcomes of interest are listed in Table 3 .
4. DISCUSSION
In the Euro Heart Survey on Valvular Heart Disease, AR comprises 13.3% of patients with single left‐sided valve heart disease. The main three etiologies for aortic regurgitation were degenerative (50.3%), congenital (15.2%) and rheumatic (15.2%).1 Despite that surgical aortic valve replacement remains the treatment of choice in patients with AR,33, 34, 35 only 32% of patients with aortic regurgitation, as a single valve disease, underwent surgical replacement.1 Comorbidities and advance age were the most frequent inhibitive factors from surgical intervention resulting in an annual mortality rate of 10% to 20%.1, 7
Patients with predominant AR are not considered to be candidates for TAVR according to current guidelines.33 However, advancement in valve technology and accumulated experiences lead to the experiment in the off‐label use of TAVR to treat patients with valvular diseases other than severe aortic stenosis.36
Operative mortality in AR patients undergoing surgical valve replacement was reported at 3.4%.1 Aortic stenosis patients included in PARTNER trial had all‐cause mortality at 30 days of 3.4% with mean STS risk score reported at 11.4.37 Our meta‐analysis has shown that all‐cause mortality at 30 days has an incidence rate of 11% with STS score ranging from 5.4 to 13.1%. The incidence rate of all‐cause mortality at 30‐days drops to 7% when using a second generation valve. The higher mortality in NAVR patients undergoing TAVR is speculated to be due to most patients included in this meta‐analysis were deemed high surgical risk; had depressed LVEF, concomitant moderate or severe mitral regurgitation and dilated aortic diameter in AR patients.
The incidence rate of cerebrovascular accidents in our analysis was 2% with no significant difference between first and second generation valves, compared to 5% at 30 days and 7.8% at 1 year in PARTNER trial.37 The rate of embolism, including transient ischemic attacks, post‐surgical replacement in the Euro Heart Survey on Valvular Heart Disease was reported at 2.5%. The absence of aortic valve calcium and the rare need for valvuloplasty have potentially allowed for lower risk for thromboembolic events in AR patients undergoing TAVR.
Owing to its larger annular size, CoreValve was predominately used in first generation valves as most patients with pure AR have large annulus size. Second generation valves have offered features like self‐positioning, repositionability, and fixation mechanism to improve anchoring, as seen in JenaValve and J‐Valve clipping onto the native leaflets. These features have helped lowering the rate of second valve implantation, post implantation moderate or severe AR and paravalvular leak compared to what were observed when using a first generation valve.18, 24, 30 Additionally, these features could explain the higher device success rate in second generation valves when compared to first generation, 92% vs 68%, respectively.
Finally, a systematic review and meta‐analysis published in 2016 by Franzone et al7 has summarized the available evidence up to 15 February 2016. There have been multiple studies published since then and by including them in our analysis we were able to include 638 patients, compared to 237 in the previous meta‐analysis. We were able to perform a subgroup analysis to compare the outcomes between first and second generation valves and assess the quality of the evidence in this meta‐analysis.
4.1. Study limitations
Our systematic review and meta‐analysis has multiple limitations. First, all the studies were observational single‐arm studies without direct comparison to an alternative with an inherently high risk of bias. Second, the type of valves used varied in generation, design, features and implantation techniques, which could have led to heterogeneity among included studies. Third, the subgroup analysis results are based on indirect comparisons rather than direct head‐to‐head comparative studies and they should be interpreted with caution. Fourth, there was inconsistent reporting of baseline characteristics and outcomes between the studies. Fifth, longer follow up is needed to determine the durability of implanted valves and the rate of progression of trivial and mild post‐implantation paravalvular leak or aortic regurgitation and their effect on the device outcomes. Sixth, our analysis has included different access sites without assessing outcomes per site. This was due to the included studies not reporting outcomes per access site and hence we were not able to perform a sensitivity analysis to test if this would affect the results, especially procedure related mortality rate. Seventh, up to our knowledge, the first published case report for TAVR in AR was in 201038 and there has been rapid evolution in the field over the past few years and growing operators experience which also might explain the improvement in outcomes with the second valve generation.39 Finally, publication bias might have affected our report of published data as reports of negative outcomes or from small centers have higher likelihood of not being able to publish their results.40
5. CONCLUSION
The present systematic review and meta‐analysis is a comprehensive review of the literature with the largest number of patients with native aortic valve regurgitation undergoing TAVR. TAVR appears to be a viable option for high surgical risk or inoperable patients with NAVR.
CONFLICTS OF INTEREST
Dr. De Marchena is a local PI in Medtronic CoreValve trials. The other authors have no conflict of interest to declare.
Supporting information
Appendix S1. TAVR in AR: A systematic review and meta‐analysis
Appendix S2. Ovid.
TABLE S1. Summary of included studies.
FIGURE S1. Supporting figure.
Haddad A, Arwani R, Altayar O, Sawas T, Murad MH, de Marchena E. Transcatheter aortic valve replacement in patients with pure native aortic valve regurgitation: A systematic review and meta‐analysis. Clin Cardiol. 2019;42:159–166. 10.1002/clc.23103
REFERENCES
- 1. Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on Valvular heart disease. Eur Heart J. 2003;24(13):1231‐1243. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of Valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42(4):S1‐S44. [DOI] [PubMed] [Google Scholar]
- 3. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006‐3008. [DOI] [PubMed] [Google Scholar]
- 4. Yousef A, Froeschl M, Hibbert B, Burwash IG, Labinaz M. Transcatheter aortic valve implantation: current and evolving indications. Can J Cardiol. 2016;32(2):266‐269. [DOI] [PubMed] [Google Scholar]
- 5. Praz F, Windecker S, Huber C, Carrel T, Wenaweser P. Expanding indications of transcatheter heart valve interventions. JACC Cardiovasc Interv. 2015;8(14):1777‐1796. [DOI] [PubMed] [Google Scholar]
- 6. Kanjanahattakij N, Horn B, Vutthikraivit W, et al. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: a systematic review and meta‐analysis. Clin Cardiol. 2018;41(7):896‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franzone A, Piccolo R, Siontis GCM, et al. Transcatheter aortic valve replacement for the treatment of pure native aortic valve regurgitation: a systematic review. JACC Cardiovasc Interv. 2016;9(22):2308‐2317. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research Consortium‐2 consensus document (VARC‐2). Eur J Cardiothorac Surg. 2012;42(5):S45‐S60. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses
- 12. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23(2):60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974‐978. [DOI] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2017 StataCorp . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 17. Anwaruddin S, Desai N, Reardon MJ, et al. Self‐expanding TAVR for treatment of aortic insufficiency: an analysis of the STS/ACC TVT registry. J Am Coll Cardiol. 2017;70(18 Supplement 1):B16‐B17. [Google Scholar]
- 18. De Backer O, Pilgrim T, Sondergaard L, et al. Transcatheter aortic valve replacement for isolated severe native aortic valve regurgitation‐results from the TAVR‐NAVR registry. J Am Coll Cardiol. 2017;70(18 Supplement 1):B184. [Google Scholar]
- 19. Silaschi M, Conradi L, Wendler O, et al. The JUPITER registry: one‐year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. 2017;24:24. [DOI] [PubMed] [Google Scholar]
- 20. Frerker C, Schewel J, Schewel D, et al. Expansion of the indication of transcatheter aortic valve implantation‐‐feasibility and outcome in "off‐label" patients compared with "on‐label" patients. J Invasive Cardiol. 2015;27(5):229‐236. [PubMed] [Google Scholar]
- 21. Koschyk D, Seiffert M, Conradi L, et al. Transcatheter aortic valve implantation for non‐calcified pure aortic insufficiency ‐ initial results and follow‐up. Catheter Cardiovasc Interv. 2014;83(Supplement 1):S211‐S212. [Google Scholar]
- 22. Munoz‐Garcia E, Munoz‐Garcia M, AJ AJMG, et al. Clinical impact of patients with pure native aortic valve regurgitation undergoing transcatheter aortic valve replacement. Eur J Heart Failure. 2017;19(Supplement 1):453. [Google Scholar]
- 23. Rossi ML, Barbaro C, Pagnotta P, Lucarelli C, Gasparini G, Presbitero P. TCT‐755 trancatheter aortic valve implantation in patients with pure severe native aortic regurgitation: results after 3 year of follow‐up. J Am Coll Cardiol. 2014;64(11 Supplement):B220‐B221. [Google Scholar]
- 24. Roy DA, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol. 2013;61(15):1577‐1584. [DOI] [PubMed] [Google Scholar]
- 25. Sawaya FJ, Deutsch M‐A, Seiffert M, et al. Safety and efficacy of Transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: results from an international registry study. JACC Cardiovasc Interv. 2017;10(10):1048‐1056. [DOI] [PubMed] [Google Scholar]
- 26. Schlingloff F, Schafer U, Frerker C, Schmoeckel M, Bader R. Transcatheter aortic valve implantation of a second‐generation valve for pure aortic regurgitation: procedural outcome, haemodynamic data and follow‐up. Interact Cardiovasc Thorac Surg. 2014;19(3):388‐393. [DOI] [PubMed] [Google Scholar]
- 27. Schofer J, Nietlispach F, Bijuklic K, et al. Transfemoral implantation of a fully repositionable and retrievable transcatheter valve for noncalcified pure aortic regurgitation. JACC Cardiovasc Interv. 2015;8(14):1842‐1849. [DOI] [PubMed] [Google Scholar]
- 28. Seiffert M, Bader R, Kappert U, et al. Initial German experience with Transapical implantation of a second‐generation Transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv. 2014;7(10):1168‐1174. [DOI] [PubMed] [Google Scholar]
- 29. Testa L, Latib A, Rossi M, De Marco F, De Carlo M, Fiorina C, et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry 2014. 739–45. [DOI] [PubMed]
- 30. Yoon S‐H, Schmidt T, Bleiziffer S, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol. 2017;70(22):2752‐2763. [DOI] [PubMed] [Google Scholar]
- 31. Zhu L, Guo Y, Wang W, et al. Transapical transcatheter aortic valve replacement with a novel transcatheter aortic valve replacement system in high‐risk patients with severe aortic valve diseases. J Thorac Cardiovasc Surg. 2017;15:15. [DOI] [PubMed] [Google Scholar]
- 32. Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis In: Egger M, Smith GD, Altman DG, eds. Systematic Reviews in Health Care. 2008. 10.1002/9780470693926.ch15. [DOI] [Google Scholar]
- 33. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252‐289. [DOI] [PubMed] [Google Scholar]
- 34. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451‐2496. [DOI] [PubMed] [Google Scholar]
- 35. Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007;28(2):230‐268. [DOI] [PubMed] [Google Scholar]
- 36. Hira RS, Vemulapalli S, Li Z, et al. Trends and outcomes of off‐label use of transcatheter aortic valve replacement: insights from the NCDR STS/ACC TVT registry. JAMA Cardiol. 2017;2(8):846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364(23):2187‐2198. [DOI] [PubMed] [Google Scholar]
- 38. Ducrocq G, Himbert D, Hvass U, Vahanian A. Compassionate aortic valve implantation for severe aortic regurgitation. J Thorac Cardiovasc Surg. 2010;140(4):930‐932. [DOI] [PubMed] [Google Scholar]
- 39. Barbanti M, Buccheri S, Rodes‐Cabau J, et al. Transcatheter aortic valve replacement with new‐generation devices: a systematic review and meta‐analysis. Int J Cardiol. 2017;245:83‐89. [DOI] [PubMed] [Google Scholar]
- 40. Fanelli D. Do pressures to publish increase scientists' bias? An empirical support from US states data. PLoS One. 2010;5(4):e10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. TAVR in AR: A systematic review and meta‐analysis
Appendix S2. Ovid.
TABLE S1. Summary of included studies.
FIGURE S1. Supporting figure.


