Abstract
Background
Surgical myectomy (SM) and Alcohol septal ablation (ASA) are effective therapies for patients with hypertrophic cardiomyopathy who remain symptomatic despite medical therapy. A plethora of data has recently emerged on the long‐term outcomes of these procedures. We hence sought to perform an updated meta‐analysis comparing both procedures.
Methods
Studies reporting long‐term (>3‐years) outcomes of SM and/or ASA were included. The primary endpoint was all‐cause mortality. Secondary endpoints included cardiovascular mortality, sudden cardiac death (SCD), reintervention, and complications including death, pacemaker implantation, and stroke.
Results
Twenty‐two ASA cohorts (n = 4213; follow‐up = 6.6‐years) and 23 SM cohorts (n = 4240; follow‐up = 6.8‐years) were included. Septal myectomy was associated with higher periprocedural mortality and stroke (2% vs 1.2%, P = 0.009 and 1.5% vs 0.8% P = 0.013, respectively), but ASA was associated with more need of pacemaker (10% vs 5%, P < 0.001). During long‐term follow‐up, all‐cause mortality, cardiovascular mortality, and sudden cardiac death rates were 1.5%, 0.4%, and 0.3% per person‐year in the ASA group and 1.1%, 0.5%, and 0.3% per person‐year in the SM group (P = 0.21, P = 0.53, P = 0.43), respectively. Repeat septal reduction intervention(s) were more common after ASA (11% vs 1.5%, P < 0.001).
Conclusion
Compared with SM, ASA is associated with lower periprocedural mortality and stroke but higher rates of pacemaker implantations and reintervention. However, there was no difference between ASA and SM with regards to long‐term all‐cause mortality, cardiovascular mortality, or SCD.
Keywords: alcohol septal ablation, hypertrophic obstructive cardiomyopathy, sudden cardiac death, surgical myectomy
1. INTRODUCTION
Septal reduction therapies have revolutionized the management of hypertrophic obstructive cardiomyopathy (HOCM) in patients who remain symptomatic despite optimal medical therapy.1, 2 Septal myectomy (SM) is still considered as the first line septal reduction method in patients who are deemed appropriate surgical candidates and when surgical expertise is available.3 However, alcohol septal ablation, first introduced by Sigwart et al. in 1995, has emerged as a feasible alternative to SM with safety and efficacy demonstrated in multiple studies.1, 4 Nonetheless, concerns arose about the long‐term outcomes of ASA due to the potential of increased propensity to life‐threatening arrhythmia induced by the ablation scar.5, 6 Although early data with ASA were reassuring, debates on the optimal choice of septal reduction methods continues, especially in light of the lack of randomized trials providing head‐to‐head comparison between ASA and SM.7 A previous meta‐analysis by Liebregts et al. including 24 observational studies demonstrated similar long‐term mortality rates following SM vs ASA.8 However, a large number of studies reporting long‐term outcomes of SM and ASA have since been published. We sought to perform a comprehensive systematic review and an updated meta‐analysis comparing transcatheter and surgical septal reduction techniques in patients with HOCM.
2. METHODS
We conducted a literature search of PubMed, Embase, and Cochrane library from inception through May 5, 2018. Our search strategy is presented in Table S1, Supporting Information. We utilized the “related articles” function in PubMed to find relevant articles, which were missed by the initial search. In addition, reference lists of included studies were hand searched to further locate relevant articles that were missed by keyword searches and the “related articles” function. Our search and meta‐analysis was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Protocols (PRISMA‐P) Statement 2015.9
Titles and abstracts of studies retrieved by the initial search were screened by two authors (MO and BK). Consequently, the full texts of the potentially relevant articles were reviewed to determine if the study fulfill the inclusions criteria. The initial database search retrieved 3203 articles. After excluding duplicates, 2550 articles were screened for eligibility by reading the title and abstract of the study. A total of 174 studies were screened using the predetermined inclusions criteria to assess eligibility. We only included studies that reported ≥3 years of follow‐up for the primary endpoint. In case of multiple publications from the same cohort, we only included the most recently published studies. In the study of Collis et al. only surgical myectomy group was included as the other groups had different surgical interventions (Mitral valve replacement and repair).10 In the study by Lai et al. we only included the modified Morrow group as the other group reported outcomes for less than 3 years duration.11 In the study of Sorajja et al. we only included the ASA cohort as the SM cohort was part of the study by Ommen et al. during the same period.29, 42 Quality of the included studies was assessed by two different authors (KO and HA) using the Modified Newcastle‐Ottawa Scale (MNOS) Table S1. Details of the study selection process are reported following the PRISMA guidelines in Figure 1.
Figure 1.
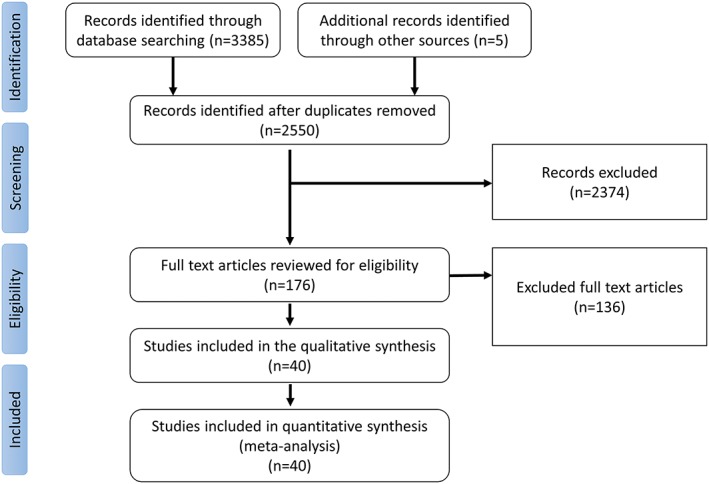
The preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow diagram
The primary outcome of interest was long‐term all‐cause mortality, secondary outcomes included long‐term sudden cardiac death (SCD) (defined as mortality which was classified as SCD by the included studies and excluding resuscitated SCD and defibrillator shocks), long‐term cardiovascular death (death due to heart failure), reintervention (need for repeat SM or ASA), periprocedural mortality (defined as mortality within the first 30 days of the procedure), complete heart block requiring pacemaker implantation and periprocedural complications (stroke, cardiac tamponade, ventricular septal defects, and coronary artery dissections) .
Effect estimates were extracted from each study in the form of events in dichotomous data and mean or medians for continuous data. These were directly extracted from the article or calculated indirectly based on the available data presented in the text of the article. The effect measures were pooled together using the random effect model. Heterogeneity between studies was explored by Cochran Q statistic (P < 0.05) and I‐squared (I 2) statistic. All statistical tests were two‐sided and P values <0.05 were considered statistically significant. Funnel plots were examined for publication bias (Figure S1) and potential sources of heterogeneity were investigated using meta‐regression. We also conducted subgroup analysis in which we only included comparative studies (studies which reported data for alcohol septal ablation and surgical myectomy) and excluded single‐arm studies. All statistical analyses were conducted with Comprehensive Meta‐analysis software professional version 3.3.070.
3. RESULTS
A total of 40 studies including 22 ASA studies4, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 and 23 SM cohorts10, 11, 18, 25, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 were included. The ASA cohort contained 4213 patients with a mean follow‐up of 6.6 ± 2.8 years. The myectomy cohort included 4240 patients with a mean follow‐up of 6.8 ± 2.6 years. Patients in the ASA group were older than SM group with a mean age of 54 ± 6 years in the ASA group compared to 45 ± 9 years in the SM group (P < 0.001). Detailed study‐level characteristics are shown in Table S1.
Periprocedural death occurred in 44 patients (1.2%, 95%CI 0.9%‐1.6%) in the ASA group and 76 patients (2%, 95%CI 1.4%‐3.1%) in the SM group (P = 0.009). Periprocedural rates of stroke and cardiac tamponade were higher after SM than after ASA; (1.5%, 95%CI 1%‐2.3% vs 0.8%, 95%CI 0.5%‐1.3%, P = 0.013), and (1.8%, 95%CI 0.8%‐3.6% vs 1.3%, 95%CI 0.9%‐1.7%, P = 0.14), respectively. Permanent pacemaker implantation secondary to complete heart block was required in 409 (10%) patients in the ASA group compared to 180 (5%) patients in the SM group P < 0.001. There were a few reported events of ventricular septal defects and coronary arteries dissection, hence no comparative analysis of these events was performed.
Long‐term all‐cause mortality was reported by all the included studies. There was a total of 450 deaths in the ASA group compared to 350 deaths in the myectomy group during the follow‐up period. Pooled all‐cause mortality rate was 1.5% per person‐year in the ASA group and 1.1% per person‐year in the SM group (P = 0.10). (Figure 2 ). Severe heterogeneity was present for all‐cause mortality in the ASA group (I 2 = 76%; P < 0.001) and SM group (I 2 = 86%; P < 0.001). Meta‐regression revealed that age, duration of follow‐up and difference in study quality played an important role in the heterogeneity. In the ASA group studies with older cohort, shorter duration of follow‐up and higher quality as reported by higher MNOS score reported higher rate of all‐cause mortality (R 2 = 0.63, I 2‐residual = 50%, P for heterogeneity = 0.07). (Figures S2, S3, and S4 ). In the SM group, higher all‐cause mortality rates were reported by earlier publications. (R 2 = 0.16, I 2‐residual = 83%, P for heterogeneity <0.001). (Figure S5).
Figure 2.
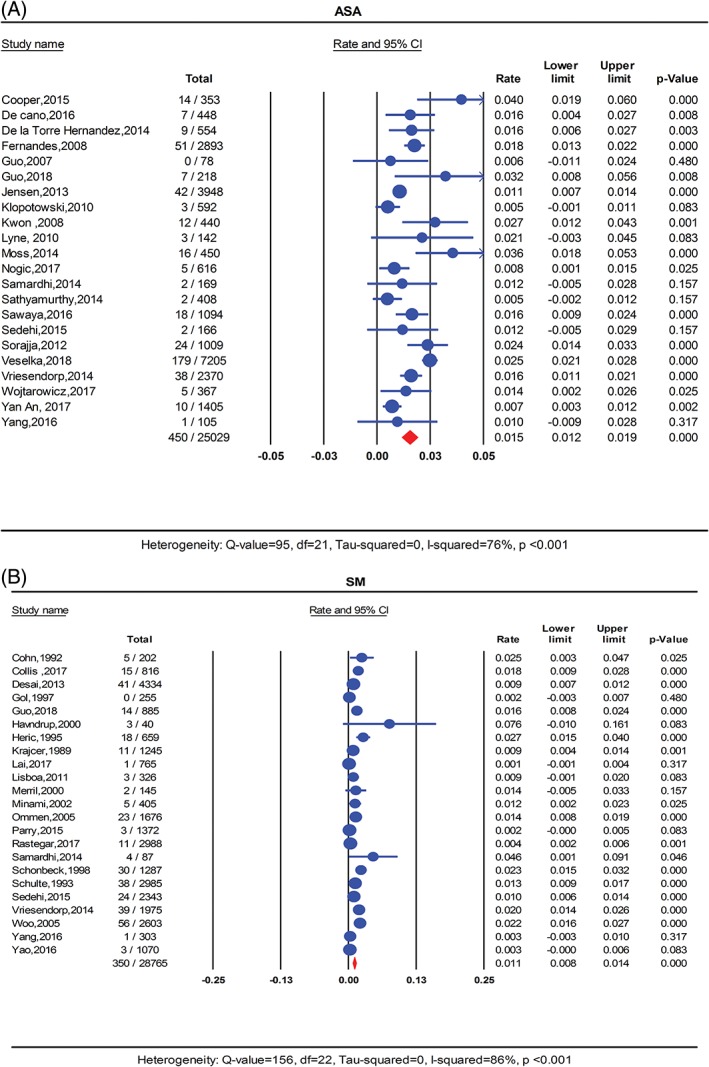
Forest plot for pooled all‐cause mortality rate per person‐year in A, ASA and B, SM. ASA, alcohol septal ablation; SM, surgical myectomy
Cardiovascular mortality data were available from 21 studies in the ASA and 21 studies in the SM group. There was a total of 88 cardiovascular deaths in the ASA group compared to 179 cardiovascular deaths in the SM group. Pooled cardiovascular mortality rate was 0.4% per person‐year in the ASA cohort and 0.5% per person‐year in the SM group (P = 0.53). (Figure 3 ). Mild heterogeneity was detected in the ASA cohort (I 2 = 32%, P = 0.08) but severe heterogeneity was observed in the SM group (I 2 = 85%, P < 0.001). In a meta‐regression analysis, there was no interaction between any of the study covariates and cardiovascular mortality heterogeneity in the ASA group. In the SM cohort, studies published earlier reported higher rate of mortality than recent studies (R 2 = 0.33, I 2‐residual = 78%, P for heterogeneity<0.001). (Figure S6).
Figure 3.
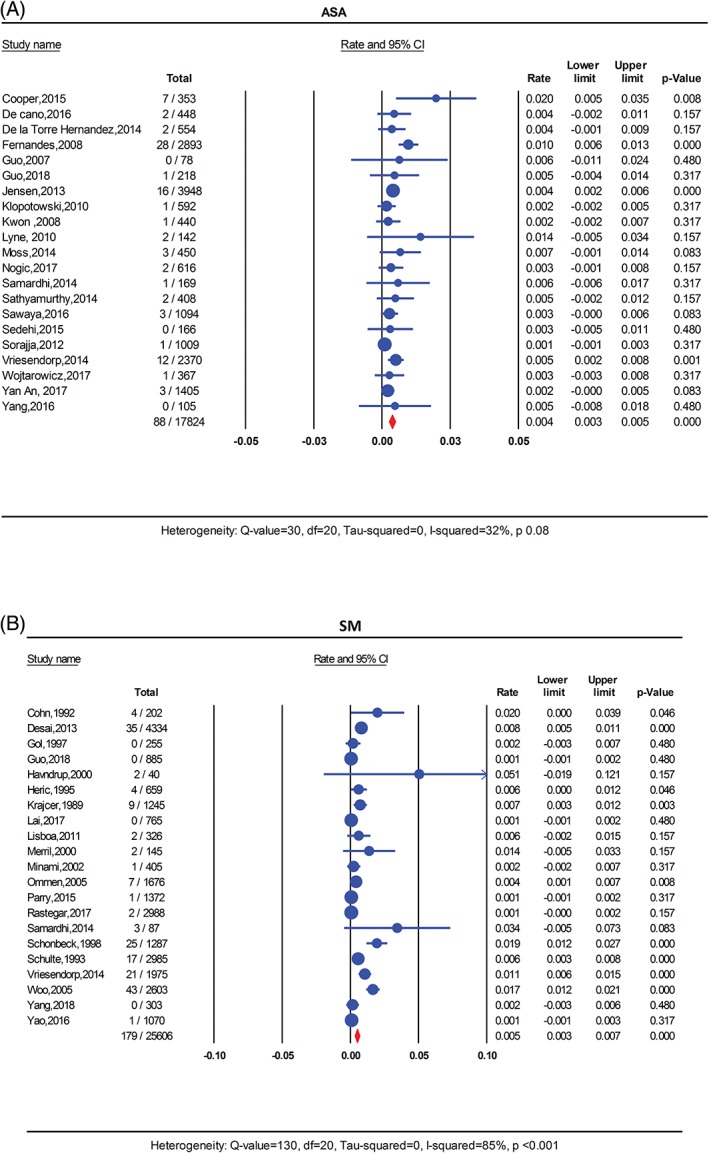
Forest plot for pooled cardiovascular mortality rate per person‐year in A, ASA and B, SM. ASA, alcohol septal ablation; SM, surgical myectomy
Sudden cardiac death data was available in 17 studies in the ASA cohort and 19 studies in the SM cohort. There was a total of 48 events in the ASA compared to 84 events in the SM group. The pooled SCD rate was similar at 0.3% for the ASA group and the SM group. (Figure 4) Mild heterogeneity was detected in the ASA cohort (I 2 = 26%, P = 0.13) and severe heterogeneity in the SM group (I 2 = 65%, P < 0.001). In meta‐regression analysis for the ASA cohort, there was a tendency for higher SCD rate in studies with shorter duration of follow‐up and higher alcohol volume injected during ablation (R 2 = 0.82, I 2‐residual = 7%, P for heterogeneity = 0.3). (Figure S7 and S8 ). In the SM cohort, studies published earlier reported higher rate of SCD than recent studies (R 2 = 0.39, I 2‐residual = 47%, P for heterogeneity = 0.01). (Figure S9 ).
Figure 4.
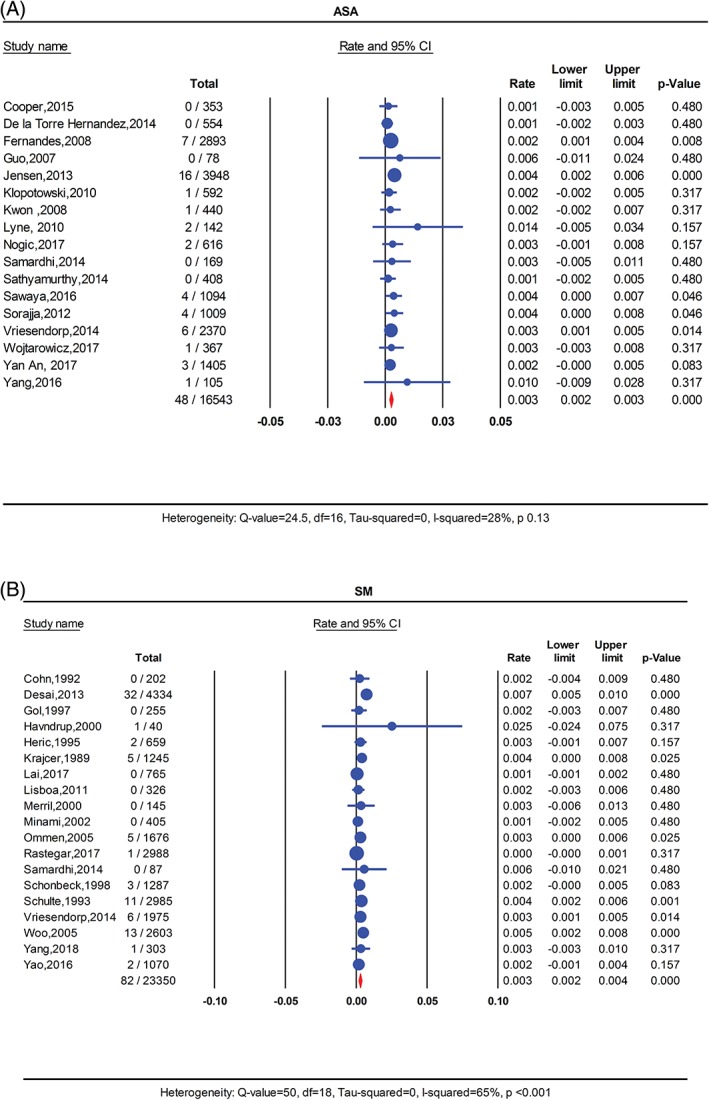
FIGURE 4 Forest plot for pooled sudden cardiac death rate per person‐year in A, ASA and B, SM. ASA, alcohol septal ablation; SM, surgical myectomy
The need for repeat septal reduction interventions (ASA or SM) was higher in the ASA group with 398 patients requiring reinterventions (11.6%) compared to only 51 patients in the SM group (1.5%) (P < 0.001). (Figure S10).
In the subgroup analysis involving only comparative studies, there was no significant difference between the two groups in term of all‐cause mortality, cardiovascular mortality or sudden cardiac death rates (risk difference = 0.0%, 95%, CI = −0.8‐0.8, I 2 = 17%, P = 0.3) (risk difference = −0.2%, 95% CI = −1‐0.7, I 2 = 41%, P = 0.17), (risk difference = 0.1%, 95% CI = −0.2‐0.3, I 2 = 0%, P = 0.7), respectively. (Figure S11).
4. DISCUSSION
The management of HOCM patients who remain symptomatic despite medical treatments continues to be challenging for providers. Surgical myectomy has been the gold standard septal reduction therapy because of its safety and effectiveness in patients who are deemed suitable for surgery.3 However, the excellent data of SM come from center of excellence with experienced surgeons and high surgical volume limiting their generalizability.21, 49, 50 In a nationwide survey of SM and ASA, a clear relationship was observed between annual SM volume and outcomes with patients in the lowest tertile (SM volume) hospitals experiencing significantly higher mortality after SM (adjusted odds ratio, 3.11; 95% CI, 1.98‐4.89).51 Alcohol septal ablation has faced heightened scrutiny despite the promising early reports on its mid‐ and long‐term outcomes4, 52 because of the feared effects of the septal scar in young patients and the higher likelihood of needing a permanent pacemaker. Nonetheless, there is now growing long‐term evidence of the safety and efficacy of ASA even in younger patients53 This is coupled with an increasing volume of ASA procedures performed worldwide. Indeed, it is estimated that the number of ASA procedures performed in the last 10 years outnumbered the sum of all SM procedures done in the last 50 years.3 In addition, there are now emerging data on the optimal ASA techniques and their associated learning curve confirming the increasing interest in this procedure.4, 54, 55, 56
One meta‐analysis comparing long‐term outcomes of SM and ASA (24 studies including 4804 patients) has previously been published.8 In this analysis, Liebregts et al. found similar long‐term rates of mortality and SCD in patients undergoing SM or ASA. However, a large number of studies have been reported since, and hence our updated meta‐analysis aims to assess the long‐term safety and efficacy of ASA in light of these emerging data. Our meta‐analysis included 40 observational studies that reported long‐term outcomes of 8453 patients undergoing SM or ASA. The findings of our analysis confirm the long‐term safety of ASA as an alternative to SM. Although SM resulted in more durable results evident by the lower need for reintervention, it was associated with significantly higher periprocedural mortality, stroke and cardiac tamponade compared with ASA. Nonetheless, long‐term outcomes (all‐cause mortality, cardiovascular mortality, and SCD) were comparable between the two groups suggesting that ASA remains a viable option for symptomatic HOCM patients.
4.1. Limitations
This analysis has number of limitations that needs to be mentioned. First, there is no randomized clinical trial comparing ASA vs SM. Hence, all studies included in our meta‐analysis are observational and are therefore subject to the known limitations of observational studies. Second, there was significant heterogeneity between the included studies, likely because of the differences in patient's risk profile and treatment preferences. Patients in the ASA were older and had higher prevalence of morbid conditions. Nonetheless, our findings of lower perioperative morbidity and mortality after ASA, and comparable long‐term outcomes after ASA or SM despite the higher risk profile of patients in the ASA arm supports our main findings that ASA has an important role in the treatment of symptomatic HOCM patients. Third, there was a paucity of studies reporting the status of patient's symptoms and functional status. Hence, we used the rate of reintervention as a surrogate for clinical efficacy. However, the decision to reintervene might be subject to other factors that are not captured by this analysis. Fourth, reporting bias is an important limitation of this analysis, as it is likely that the current literature emerges from centers performing a large number of either ASA or SM or those with positive outcomes. Moreover, techniques of ASA and SM vary significantly among centers and may have undergone refinements overtime. This, along with the increasing experience might explain our finding of improved surgical outcomes overtime. Fifth, most of the SM studies were performed earlier than the ASA ones which may explain the higher perioperative mortality compared to the ASA group.
In conclusion, this updated meta‐analysis including >9000 patients undergoing ASA or SM, ASA was associated with lower rates of periprocedural mortality, stroke and cardiac tamponade but higher rates of permanent pacemaker implantations and reintervention. However, there was no difference between ASA and SM with regards to long‐term all‐cause mortality, cardiovascular mortality or SCD. These data are supportive of ASA as feasible alternative to SM in selected patients.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Table S1: Search Strategy
Figure S1: Funnel Plot for (A) ASA all‐cause mortality (B) SM all‐cause mortality
Table S2: Baseline characteristics of the studies included in the meta‐analysis
Figure S2: Meta‐regression for interaction of Age with all‐Cause mortality in the ASA group
Figure S3: Meta‐regression for interaction of duration of follow‐up with all‐Cause mortality in the ASA group
Figure S4: Meta‐regression for interaction of Modified Newcastle‐Ottawa Scale (MNOS) with all‐Cause mortality in the ASA group
Figure S5: Meta‐regression for interaction of year of publication with all‐cause mortality in the SM group.
Figure S6: Meta‐regression for interaction of year of publication with cardiovascular mortality in the SM group
Figure S7: Meta‐regression for interaction of duration of follow‐up with sudden cardiac death in the ASA group
Figure S8: Meta‐regression for interaction of Injected Alcohol Volume with sudden cardiac death in the ASA group
Figure S9: Meta‐regression for interaction of year of publication with sudden cardiac death in the SM group
Figure S10: Forest Plot for pooled reintervention rate (A) ASA (B)SM
Figure S11: Forest plot comparing ASA to SM (A) All‐cause mortality, (B) Cardiovascular mortality, (C) Sudden cardiac death
Osman M, Kheiri B, Osman K, et al. Alcohol septal ablation vs myectomy for symptomatic hypertrophic obstructive cardiomyopathy: Systematic review and meta‐analysis. Clin Cardiol. 2019;42:190–197. 10.1002/clc.23113
REFERENCES
- 1. Sigwart U. Non‐surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346:211‐214. [DOI] [PubMed] [Google Scholar]
- 2. Wigle ED, Chrysohou A, Bigelow WG. Results of ventriculomyotomy in muscular subaortic stenosis. Am J Cardiol. 1963;11:572‐586. [DOI] [PubMed] [Google Scholar]
- 3. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212‐e260. [DOI] [PubMed] [Google Scholar]
- 4. Veselka J, Faber L, Jensen MK, et al. Effect of institutional experience on outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Can J Cardiol. 2018;34:16‐22. [DOI] [PubMed] [Google Scholar]
- 5. Hess OM, Sigwart U. New treatment strategies for hypertrophic obstructive cardiomyopathy: alcohol ablation of the septum: the new gold standard? J Am Coll Cardiol. 2004;44:2054‐2055. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Nishimura RA. Surgical septal myectomy versus alcohol septal ablation. Circulation. 2014;130:1617‐1624. [DOI] [PubMed] [Google Scholar]
- 7. Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy Will there ever be a randomized trial? J Am Coll Cardiol. 2007;50:831‐834. [DOI] [PubMed] [Google Scholar]
- 8. Liebregts M, Vriesendorp PA, Mahmoodi BK, Schinkel AFL, Michels M, ten Berg JM. A systematic review and meta‐analysis of long‐term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Failure. 2015;3:896‐905. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1). 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai Y, Guo H, Li J, Dai J, Ren C, Wang Y. Comparison of surgical results in patients with hypertrophic obstructive cardiomyopathy after classic or modified morrow septal myectomy. Medicine (Baltimore). 2017;96:e9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorajja P, Ommen SR, Holmes DR Jr, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012;126:2374‐2380. [DOI] [PubMed] [Google Scholar]
- 12. Ommen SR, Maron BJ, Olivotto I, et al. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470‐476. [DOI] [PubMed] [Google Scholar]
- 13. An SY, Yang YJ, Hang F, Wang ZM, Fan CM. Procedural complication and long term outcomes after alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: data from China. Sci Rep. 2017;7:9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper RM, Shahzad A, McShane J, Stables RH. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: safe and apparently efficacious but does reporting of aggregate outcomes Hide less‐favorable results, experienced by a substantial proportion of patients? J Invasive Cardiol. 2015;27:301‐308. [PubMed] [Google Scholar]
- 15. de l THJM, Masotti Centol M, Lerena Saenz P, et al. Effectiveness and safety beyond 10 years of percutaneous transluminal septal ablation in hypertrophic obstructive cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2014;67:353‐358. [DOI] [PubMed] [Google Scholar]
- 16. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow‐up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008;1:561‐570. [DOI] [PubMed] [Google Scholar]
- 17. Fortunato de Cano S, Nicolas Cano M, Costa R, et al. Long‐term clinical follow‐up of patients undergoing percutaneous alcohol septal reduction for symptomatic obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2016;88:953‐960. [DOI] [PubMed] [Google Scholar]
- 18. Guo H, Wang P, Xing Y, et al. Delayed electrocardiographic changes after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy. J Electrocardiol. 2007;40:356.e1‐356.e6. [DOI] [PubMed] [Google Scholar]
- 19. Guo HC, Li JH, Jiang TY, et al. Comparison of clinical effects between percutaneous transluminal septal myocardial ablation and modified morrow septal Myectomy on patients with hypertrophic cardiomyopathy. Chin Med J (Engl). 2018;131:527‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013;99:1012‐1017. [DOI] [PubMed] [Google Scholar]
- 21. Klopotowski M, Chojnowska L, Malek LA, et al. The risk of non‐sustained ventricular tachycardia after percutaneous alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2010;99:285‐292. [DOI] [PubMed] [Google Scholar]
- 22. Kwon DH, Kapadia SR, Tuzcu EM, et al. Long‐term outcomes in high‐risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. JACC Cardiovasc Interv. 2008;1:432‐438. [DOI] [PubMed] [Google Scholar]
- 23. Lyne JC, Kilpatrick T, Duncan A, Knight CJ, Sigwart U, Fox KM. Long‐term follow‐up of the first patients to undergo transcatheter alcohol septal ablation. Cardiology. 2010;116:168‐173. [DOI] [PubMed] [Google Scholar]
- 24. Moss TJ, Krantz MJ, Zipse MM, et al. Left ventricular systolic function following alcohol septal ablation for symptomatic hypertrophic cardiomyopathy. Am J Cardiol. 2014;113:1401‐1404. [DOI] [PubMed] [Google Scholar]
- 25. Nogic J, Koh Y, Bak M, Gooley RP, Meredith IT, McCormick LM. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a 16‐year Australian single Centre experience. Hear Lung Circ. 2018;27(12):1446‐1453. [DOI] [PubMed] [Google Scholar]
- 26. Samardhi H, Walters DL, Raffel C, et al. The long‐term outcomes of transcoronary ablation of septal hypertrophy compared to surgical myectomy in patients with symptomatic hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Interv. 2014;83:270‐277. [DOI] [PubMed] [Google Scholar]
- 27. Sathyamurthy I, Nayak R, Oomman A, et al. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy—8 years follow up. Indian Heart J. 2014;66:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sawaya FJ, Louvard Y, Spaziano M, et al. Short and long‐term outcomes of alcohol septal ablation with the trans‐radial versus the trans‐femoral approach. A single center‐experience. Int J Cardiol. 2016;220:7‐13. [DOI] [PubMed] [Google Scholar]
- 29. Sedehi D, Finocchiaro G, Tibayan Y, et al. Long‐term outcomes of septal reduction for obstructive hypertrophic cardiomyopathy. J Cardiol. 2015;66:57‐62. [DOI] [PubMed] [Google Scholar]
- 30. Vriesendorp PA, Liebregts M, Steggerda RC, et al. Long‐term outcomes after medical and invasive treatment in patients with hypertrophic cardiomyopathy. JACC Heart Failure. 2014;2:630‐636. [DOI] [PubMed] [Google Scholar]
- 31. Wojtarowicz A, Kornacewicz‐Jach Z. Alcohol septal ablation in hypertrophic cardiomyopathy utilizing a longitudinal 17‐year study (mean 10.8). Observation follow‐ups taken at a single medical Centre. Cardiol J. 2017;24:125‐130. [DOI] [PubMed] [Google Scholar]
- 32. Yang YJ, Fan CM, Yuan JQ, et al. Effectiveness of alcohol septal ablation versus transaortic extended myectomy in hypertrophic cardiomyopathy with midventricular obstruction. J Interv Cardiol. 2016;29:619‐627. [DOI] [PubMed] [Google Scholar]
- 33. Collis R, Watkinson O, O'Mahony C, et al. Long‐term outcomes for different surgical strategies to treat left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Eur J Heart Fail. 2018;20:398‐405. [DOI] [PubMed] [Google Scholar]
- 34. Cohn LH, Trehan H, Collins JJ Jr. Long‐term follow‐up of patients undergoing myotomy/myectomy for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 1992;70:657‐660. [DOI] [PubMed] [Google Scholar]
- 35. Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209‐216. [DOI] [PubMed] [Google Scholar]
- 36. Gol MK, Emir M, Keles T, et al. Septal myectomy in hypertrophic obstructive cardiomyopathy: late results with stress echocardiography. Ann Thorac Surg. 1997;64:739‐745. [DOI] [PubMed] [Google Scholar]
- 37. Havndrup O, Pettersson G, Kjeldsen K, Bundgaard H. Outcome of septal myectomy in patients with hypertrophic obstructive cardiomyopathy. Scand Cardiovasc J. 2000;34:564‐569. [DOI] [PubMed] [Google Scholar]
- 38. Heric B, Lytle BW, Miller DP, Rosenkranz ER, Level HM, Cosgrove DM. Surgical management of hypertrophic obstructive cardiomyopathy. J Thorac Cardiovasc Surg. 1995;110:195‐208. [DOI] [PubMed] [Google Scholar]
- 39. Krajcer Z, Leachman RD, Cooley DA, Coronado R. Septal myotomy‐myomectomy versus mitral valve replacement in hypertrophic cardiomyopathy. Ten‐year follow‐up in 185 patients. Circulation. 1989;80:I57‐I64. [PubMed] [Google Scholar]
- 40. Lisboa LAF, Dallan LAO, Pomerantzeff PMA, Oliveiras SA, Jatene FB, Stolf NAG. Long‐term results of septal myectomy in the treatment of obstructive hypertrophic cardiomyopathy. Brazilian J Cardiovasc Surg. 2011;26:86‐92. [DOI] [PubMed] [Google Scholar]
- 41. Merrill WH, Friesinger GC, Graham TP Jr, et al. Long‐lasting improvement after septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2000;69:1732‐1735. [DOI] [PubMed] [Google Scholar]
- 42. Minami K, Woltersdorf H, Kleikamp G, Böthig D, Koertke H, Koerfer R. Long‐term results after myectomy in 64 patients with hypertrophic obstructive cardiomyopathy (HOCM). Morphological and hemodynamic aspects. J Cardiovasc Surg (Torino). 2000;41:801‐806. [PubMed] [Google Scholar]
- 43. Parry DJ, Raskin RE, Poynter JA, et al. Short and medium term outcomes of surgery for patients with hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2015;99:1213‐1219. [DOI] [PubMed] [Google Scholar]
- 44. Rastegar H, Boll G, Rowin EJ, et al. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the tufts experience. Ann Cardiothorac Surg. 2017;6:353‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schönbeck MH, Brunner‐La Rocca HP, Vogt PR, et al. Long‐term follow‐up in hypertrophic obstructive cardiomyopathy after septal myectomy. Ann Thorac Surg. 1998;65:1207‐1214. [DOI] [PubMed] [Google Scholar]
- 46. Schulte HD, Borisov K, Gams E, Gramsch‐Zabel H, Lösse B, Schwartzkopff B. Management of symptomatic hypertrophic obstructive cardiomyopathy—long‐term results after surgical therapy. Thorac Cardiovasc Surg. 1999;47:213‐218. [DOI] [PubMed] [Google Scholar]
- 47. Steggerda RC, Damman K, Balt JC, Liebregts M, ten Berg JM, van den Berg MP. Periprocedural complications and long‐term outcome after alcohol septal ablation versus surgical myectomy in hypertrophic obstructive cardiomyopathy. JACC Cardiovasc Interv. 2014;7:1227‐1234. [DOI] [PubMed] [Google Scholar]
- 48. Woo A, Williams WG, Choi R, et al. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033‐2041. [DOI] [PubMed] [Google Scholar]
- 49. Yao L, Li L, Lu XJ, Miao YL, et al. Long‐term clinical and echocardiographic outcomes of extensive septal myectomy for hypertrophic obstructive cardiomyopathy in Chinese patients. Cardiovasc Ultrasound. 2016;14(18). 10.1186/s12947-016-0060-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kotkar KD, Said SM, Dearani JA, Schaff HV. Hypertrophic obstructive cardiomyopathy: the Mayo Clinic experience. Ann Cardiothorac Surg. 2017;6:329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siregar S, de Heer F, Groenwold RHH, et al. Trends and outcomes of valve surgery: 16‐year results of Netherlands cardiac surgery National Database. Eur J Cardiothorac Surg. 2014;46:386‐397. discussion 397. [DOI] [PubMed] [Google Scholar]
- 52. Veselka J, Jensen MK, Liebregts M, et al. Long‐term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the euro‐ASA registry. Eur Heart J. 2016;37:1517‐1523. [DOI] [PubMed] [Google Scholar]
- 53. Liebregts M, Faber L, Jensen MK, et al. Outcomes of alcohol septal ablation in younger patients with obstructive hypertrophic cardiomyopathy. JACC Cardiovasc Interv. 2017;10:1134‐1143. [DOI] [PubMed] [Google Scholar]
- 54. Alkhouli M, Sajjad W, Lee J, et al. Prevalence of non‐left anterior descending septal perforator culprit in patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. Am J Cardiol. 2016;117:1655‐1660. [DOI] [PubMed] [Google Scholar]
- 55. Kim LK, Swaminathan RV, Looser P, et al. Hospital volume outcomes after septal Myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US Nationwide inpatient database, 2003‐2011. JAMA Cardiol. 2016;1:324‐332. [DOI] [PubMed] [Google Scholar]
- 56. Sorajja P, Binder J, Nishimura RA, et al. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2013;81:E58‐E67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Search Strategy
Figure S1: Funnel Plot for (A) ASA all‐cause mortality (B) SM all‐cause mortality
Table S2: Baseline characteristics of the studies included in the meta‐analysis
Figure S2: Meta‐regression for interaction of Age with all‐Cause mortality in the ASA group
Figure S3: Meta‐regression for interaction of duration of follow‐up with all‐Cause mortality in the ASA group
Figure S4: Meta‐regression for interaction of Modified Newcastle‐Ottawa Scale (MNOS) with all‐Cause mortality in the ASA group
Figure S5: Meta‐regression for interaction of year of publication with all‐cause mortality in the SM group.
Figure S6: Meta‐regression for interaction of year of publication with cardiovascular mortality in the SM group
Figure S7: Meta‐regression for interaction of duration of follow‐up with sudden cardiac death in the ASA group
Figure S8: Meta‐regression for interaction of Injected Alcohol Volume with sudden cardiac death in the ASA group
Figure S9: Meta‐regression for interaction of year of publication with sudden cardiac death in the SM group
Figure S10: Forest Plot for pooled reintervention rate (A) ASA (B)SM
Figure S11: Forest plot comparing ASA to SM (A) All‐cause mortality, (B) Cardiovascular mortality, (C) Sudden cardiac death


