Abstract
The existence of a tetrafascicular intraventricular conduction system remains debatable. A consensus statement ended up with some discrepancies and, despite agreeing on the possible existence of an anatomical left septal fascicle, the electrocardiographic and vectorcardiographic characteristics of left septal fascicular block (LSFB) were not universally accepted. The most important criteria requested to confirm the existence of LSFB is its intermittent nature. So far, our group has published cases of transient ischemia‐induced LSFB and phase 4 or bradycardia‐dependent LSFB. Finally, anatomical, anatomopathological, histological, histopathological, electrocardiographic, vectorcardiographic, body surface potential mapping, and electrophysiology studies support the fact that the left bundle branch divides into three fascicles or a “fan‐like interconnected network.”
Keywords: intraventricular conduction system, left septal fascicle, left septal fascicular block
1. INTRODUCTION
The left bundle branch (LBB) has a central role in normal cardiac function. According to prevailing literature, including textbooks, the LBB is composed of an anterior and a posterior fascicle. The existence of the septal fascicle has been debated. Growing scientific evidence has emerged during the last years supporting the concept of a trifascicular structure, not as an exception, but as the rule.
We published the first case in the literature of left septal fascicular block (LSFB) of the LBB caused by percutaneous implantation of a self‐expanding aortic valve prosthesis. The fact that the actual ECG phenomenon LSFB was transient is crucial for the understanding of the nature of the conduction disorder. In addition, we want to emphasize the fact that LSFB was associated with left anterior fascicular block (LAFB). Hence, the patient had a left bifascicular block consisting of LAFB + LSFB.1, 2, 3, 4, 5 We have presented several cases indicating that LSFB is not necessarily a rare ECG finding. Although well known from textbooks and other sources, isolated left posterior fascicular block (LPFB) without right bundle branch block (RBBB) is a very rare finding.
LSFB has been described in the following scenarios1, 2, 3, 4, 5: proximal obstruction of the left anterior descending (LAD) coronary artery with or without acute coronary syndrome and with the Wellens syndrome6; exercise‐induced during the treadmill test,7 chronic Chagasic myocarditis8; Kearns‐Sayre syndrome9; self‐expandable percutaneous transcatheter aortic valve implantation1; diabetes10; and aberrant conduction in healthy individuals.11
As data on LSFB is missing, so far there is no epidemiological data to establish the exact prevalence of all four main blocks of the left His system. However, our observations indicate that LSFB is much more frequent than isolated LPFB. During 10 years of studying these phenomena, we identified 18 cases of LSFB and only two cases of isolated LPFB,12 It is extremely rare to see isolated LPFB,13 which is much more frequent when associated with RBBB.14
LAFB is by far the most frequent left fascicular block. In 2254 patients with chronic heart failure, LAFB was found in 154 patients, while only 14 had LPFB.15 Left bundle branch block (LBBB) was the most frequent intraventricular conduction delay (n = 532), while 134 patients had RBBB, 87 had combined BBB, and 131 nonspecific intraventricular conduction delay.
In patients referred for stress echocardiogram, complete LBBB was found in 0.8% (1% in the normal population).16
In a study dealing with electrocardiography/vector cardiogram characteristics of LPFB, Lopes et al17 reported a prevalence of this conduction delay of 7.43 per 1000 (of 7000 consecutive cases studied).
1.1. Anatomical aspects
In 1906, Dr. Tawara18 showed that the trunk of the LBB splits into three fascicles. In Tawara's original monograph describing the LBB, septal fibers interposing between left anterior fascicle (LAF) and left posterior fascicle (LPF) may be seen. Tawara's pioneering work on the conduction system: “The Conduction System of the Mammalian Heart” still serves as an invaluable reference.
That the LBB divides into three fascicles or “fan‐like interconnected network” has been shown in anatomical, anatomopathological,19 histological, histopathological,19 electrocardiographic,1, 2, 3, 4, 5 vectorcardiographic,3 exercise testing, and epicardial activation studies on experimentally induced subdivision block of the LBB, electrical endocardial mapping, electrophysiology studies,20 in vitro, and experimental studies. The LBB originates at the crest of the muscular interventricular septum (IVS), just distal to the membranous septum. It arises in a fanlike fashion that descends inferiorly along the left ventricular (LV) septal surface beneath the noncoronary cusp of the aortic valve. The LBB branches into three fascicles: (a) The LAF is directed to the anterolateral papillary muscle (ALPM); (b) The LPF is directed to the posteromedial papillary muscle; (c) The left septal fascicle (LSF) is a central fascicle extending to the midseptal region.
1.2. Electrophysiological observations
Durrer et al21 demonstrated that the following three endocardial areas are synchronously excited from 0 to 5 ms after the LV activity potential: (a) high on the anterior paraseptal wall just below the attachment of the ALPM where the LAF ends; (b) central on the left surface of the IVS where the LSF ends; and (c) in the left inferior two‐thirds of the IVS. The experiments showed that the initial ventricular activation takes place in the three points corresponding to the site where the three left fascicles end. As the vectors resulting from the activation of the regions that depend on the LAF (the anterior paraseptal wall of the LV) and the LPF (posterior paraseptal wall of the LV) have opposite directions, they cancel each other. Thus, the only vector that manifests is the LSF.
Numerous terms have been used when referring to the LSF: left septal, third, left‐middle fibers, middle septal fiber, centroseptal fascicle, septal, medial division, left anterior‐medial division, anterior‐medial ramulus, anterior median branch of the LBB of His, and others. Demoulin and Kulbertus's pathological studies reinforced this finding.
The Demoulin and Kulbertus diagrammatic sketches of the left‐sided conduction system (observed in 20 normal human hearts) clearly show a predominant feature of three fascicles within the LBB.22
1.3. Historical aspects
The terminology of hemiblocks was criticized for the first time in 1973 by Hecht et al. These authors coined the terms divisional/fascicular blocks as being more appropriate, since it was clear that the LBB splits into three and not into two branches. Yet, as the authors stated, Rosenbaum's model of trifascicular ventricular conduction, consisting of the right bundle branch (RBB), the LAF and LPF, prevails.
Perrin et al,20 when discussing LSFB, wrote paraphrasing Einstein: “Everything should be made as simple as possible, but no simpler.” These authors presented a case, where they found that not all patterns of ventricular conduction are captured by Rosenbaum's conception. A man of 43 years underwent an electrophysiological study for premature ventricular complexes associated with LV dysfunction. The baseline ECG showed intermittent LAFB and absent septal Q waves. With the catheter nestled in the right aortic sinus facing the left/right commissure, a fascicular signal was recorded, presumed LBB/LAF‐onset 28‐ms pre‐His and 35‐ms pre‐QRS. Pacing captured the fascicle without local myocardial capture. The resultant QRS was narrow (98 ms) with a normal frontal axis but prominent anterior QRS forces (Figure 1). The authors reasoned that activation in the fascicle traveled both anterograde to the Purkinje network subtended by the LAF and retrograde to the bifurcation of RBB and LBB and thence (anterograde) to the LPF and RBB. This hypothesis explains the narrow QRS (activation of the RBB) and normal frontal axis (activation of LAF and LPF), but not the appearance of prominent anterior QRS forces. The authors suspected that the patient, in addition, had a delay or block in his LSF accompanying delay in his LAF at baseline. Pacing the LAF proximate to its termination “compensated” for its slow conduction, but the LSF could only be activated by the signal passing retrograde in the LAF and then anterograde along the length of the LSF (where it was blocked or met significant delay). Prominent anterior QRS forces may have many causes including LSFB. The case was presented in relation to the authors' comments: “We share the authors' desire that a tetrafascicular conception of intraventricular conduction should ultimately prevail. The trifascicular Rosenbaum's model is simple, but simpler than true.” (Figure 2). Dr. de Pádua expressed this very succinctly: “If hemiblocks do exist, they are only two—if a third one is postulated, hemiblocks do not exist!”.
Figure 1.
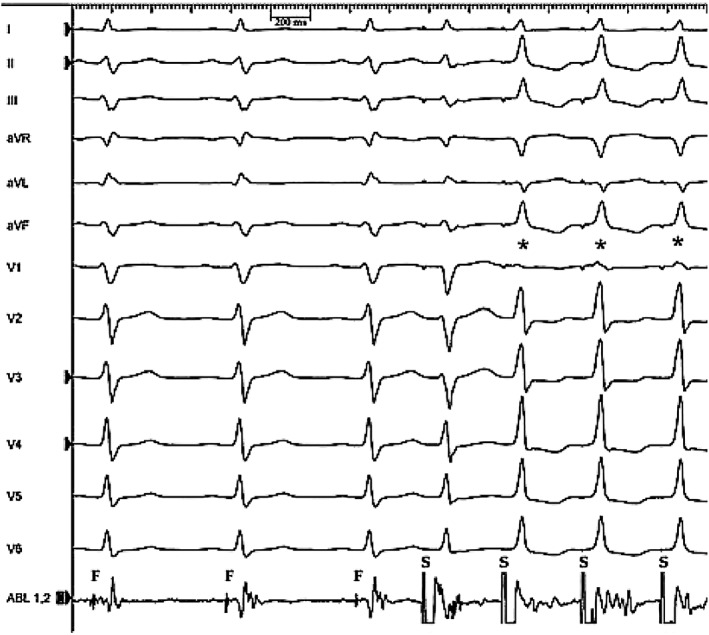
Electrophysiological study of a 43‐year old man. ECG recorded while pacing (S) from the ablation catheter resting in the right coronary sinus. The first three sinus beats show a typical pattern of LAFB with a QRS duration of 110 ms; each QRS is preceded by a fascicular signal (F). The final three beats (*) result from capture of the left septal fascicle—note prominent anterior QRS forces (reproduced with permission). ECG, electrocardiography; LAFB, left anterior fascicular block
Figure 2.
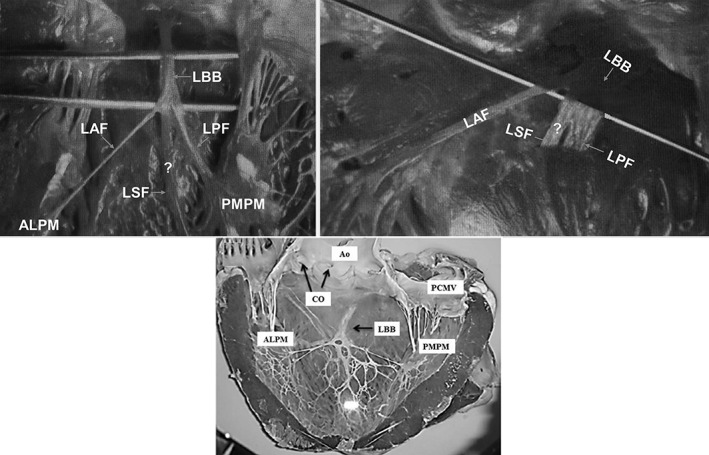
A, Figure from Rosenbaum's book figure 23, page 72. B, Figure from Rosenbaum's book page 77. C, Visualization of the endocardial surface of ungulates showing LBB and its fascicles (reproduced with permission of anatomical science international). ALPM, anterolateral papillary muscle; Ao, Aorta; CO, coronary ostium; LAF, left anterior fascicle; LBB, left bundle branch; LPF, left posterior fascicle; LSF, left septal fascicle; PCMV, posterior cuspid of mitral valve; PMPM, posteromedial papillary muscle
1.4. Prominent anterior forces
The ECG criteria for LSFB have been previously published and are not presented in detail in this paper. The critical point is a high R wave (>15 mm) in lead V2, which should raise the suspicion of LSFB, especially when the ECG phenomenon is transient. However, other causes of prominent anterior QRS forces, such as right ventricular hypertrophy, septal hypertrophy, or lateral wall myocardial infarction have to be excluded. Also lead switch has to be considered in the ECG diagnosis.
Figure 3 illustrates the main ECG features and Table 1 summarizes the ECG criteria of LBBB, LSFB, LAFB, and LPFB.
Figure 3.
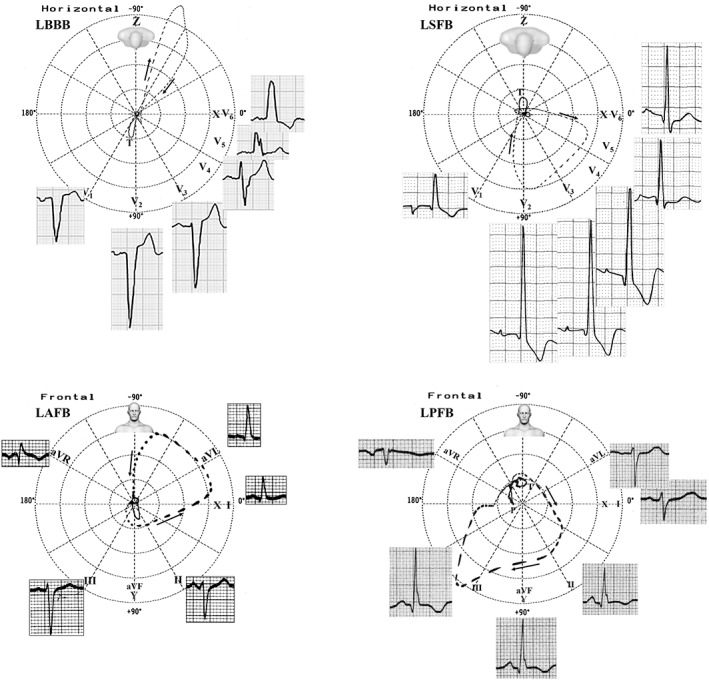
LBBB and LSFB have their main ECG/VCG features in the horizontal plane, on the other hand, LAFB and LPFB in the frontal plane. ECG, electrocardiography; LAFB, left anterior fascicular block; LBBB, left bundle branch block; LPFB, left posterior fascicular block; LSFB, left septal fascicular block; VCG, vector cardiogram
Table 1.
Main ECG criteria of LBB, LSFB, LAFB and LPFB
| ECG criteria | |
|---|---|
| LBBB | Supraventricular command (if the rhythm is sinus, the PR interval is ≥120 ms); QRS duration ≥120 ms in adults, ≥100 ms age 4 to 16 years, and ≥ 90 ms in children <4 years of age; QRS complexes in right precordial leads (V1 and V2) total or predominantly negative: rS, QS, or qrS; monophasic, broad notched, or slurred R wave, recorded slowly in the left leads: I, aVL, V5 and V6; prolonged ventricular activation time in left leads (≥50 ms); ST‐segment and T‐wave vectors are directed opposite to the mean QRS vector with QRS/ST‐T angle near 180°. |
| LSFB | Only in the precordial leads: Normal QRS duration or with a minor increase (up to 110 ms); increased ventricular activation time in V1/V2 ≥ 35 ms; R wave voltage of V1 ≥ 5 mm; R/S ratio in V1 and V2 > 2; S wave depth in V1 < 5 mm; possible small (embryonic) q wave in V2 and V3 or V1 and V2; R wave of V2 > 15 mm; R wave “in crescendo” from V1 to V3 and decreasing from V5 to V6; the absence of q wave in left precordial leads V5, V6 and in lead I; intermittent PAF during a hyperacute phase of myocardial infarction, or during an exercise stress test in patients with severe myocardial ischemia (Uchida 2006), and during early atrial extrastimuli with some degree of ventricular aberration (Hoffman 1976); appearance of intermittent, rate‐dependent q wave in V1 and V2. |
| LAFB | Extreme shift of SÂQRS in the left superior quadrant (beyond 30° up to −90°); QRS duration <120 ms; rS in II, III and aVF; SIII > SII; qR pattern in I and aVL; prolonged R‐peak time in aVL (≥45 ms). |
| LPFB | QRS axis between +80° and +140° in adults; rS pattern in leads I and aVL; qR pattern in III, aVF and II; RIII > RII; prolonged ventricular activation time in aVF (≥35 ms). |
Abbreviations: ECG, electrocardiography; LAFB, left anterior fascicular block; LBB, left bundle branch; LBBB, left bundle branch block; LPFB, left posterior fascicular block; LSFB, left septal fascicular block; PAF, prominent anterior forces.
Note: The diagnosis is always clinical‐electrocardiographic, because it is necessary to rule out right ventricular hypertrophy, a vertical heart in slender subjects and a large lateral infarction, QRS duration ≤110 ms, and broad QRS loop with clockwise rotation and maximal vector near +110° (+80° to +140°).
1.4.1. Differential diagnosis of LSFB with other causes of prominent anterior QRS forces
PAF in the ECG occurs when the voltage of the R wave in any precordial lead of the anterior or anteroseptal wall from V1 (+115°) to V4 (+47°) is greater than the normal maximal limit for gender and age. In the presence of PAF in the anterior wall (tall R waves) in the right and/or middle precordial leads V1 through V3 or V4, certain entities need to be considered in the differential diagnosis.23 PAF is observed in only 1% of normal subjects.24 The two most frequent differential diagnoses are normal variant with marked counterclockwise rotation of the heart around the longitudinal axis25 and athlete's heart. Other background factors are26: misplaced precordial leads24, 27; lateral myocardial infarction (previously known as strictly posterior)28; vectorcardiographic right ventricular hypertrophy; diastolic LV hypertrophy29, 30; RBBB31, 32; ventricular pre‐excitation with accessory pathways in a posterior location33; hypertrophic cardiomyopathy34; cardiomyopathy associated with Duchenne muscular dystrophy35, 36; endomyocardial fibrosis37; dextroposition of the heart8; LSFB; and a combination of the above.
Figure 4 shows the main anatomical variants of LSF.
Figure 4.
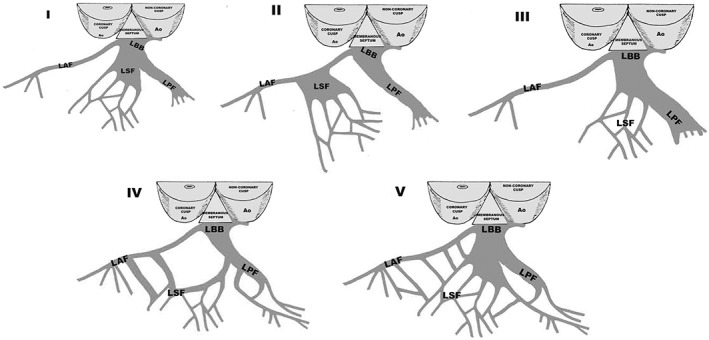
Variation in LBB anatomy. I: LSF originates from the main LBB; II: LSF originates from the LAF; III: LSF originates from the LPF; IV: LSF originates concomitantly from the LAF and LPF; V: LSF is a “fan‐like interconnecting network.” LAF, left anterior fascicle; LBB, left bundle branch; LPF, left posterior fascicle; LSF, left septal fascicle
1.5. Clinical implications
The main point with this paper is to put forward the need for a change of concepts. The clinical importance of the ECG finding needs to be better evaluated in the future. We already know that in acute coronary syndrome, a culprit lesion in the proximal LAD should be suspected when the ECG findings are compatible with LSFB. When the concept of a trifascicular LBB will be generally accepted, new important clinical information will emerge.
2. CONCLUSION
Concerning the pathogenesis of the so‐called hemiblocks, the LBB is generally considered as an anatomically bifascicular system. However, growing evidence points to the fact that this concept may be erroneous. The data of anatomical, anatomopathological, histological, histopathological, electrocardiographic, vectorcardiographic, exercise testing, endocardial mapping, electrophysiology and in vitro studies, and experimental studies indicate that this description is oversimplified. Indeed, in nearly all presented anatomic‐histopathological cases, a central radiation or, at least, or “fan‐like interconnected network” over the midseptal area exists. The LV Purkinje system, therefore, in most cases, appears to be constituted of three main peripheral networks. Consequently, the structure and function of the left intraventricular conduction system should be reappraised. Due to the heavy evidence accumulated by us and other teams, we believe that it is time to change the nomenclature.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Pérez‐Riera AR, Barbosa‐Barros R, Daminello‐Raimundo R, de Abreu LC, Nikus K. The tetrafascicular nature of the intraventricular conduction system. Clin Cardiol. 2019;42:169–174. 10.1002/clc.23093
REFERENCES
- 1. Perez‐Riera AR, Barbosa‐Barros R, Cabral de Oliveira MF, et al. Transient left anterior and septal fascicular blocks after self‐expandable percutaneous transcatheter aortic valve implantation. Ann Noninvasive Electrocardiol. 2018;e12553 10.1111/anec.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez‐Riera AR, Barbosa‐Barros R, Daminello‐Raimundo R, et al. Transient left septal fascicular block and left anterior fascicular block as a consequence of proximal subocclusion of the left anterior descending coronary artery. Ann Noninvasive Electrocardiol. 2018;e12546 10.1111/anec.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez‐Riera AR, Barbosa‐Barros R, Daminello‐Raimundo R, et al. Electro‐vectorcardiographic demonstration of bifascicular block associated with ventricular preexcitation. Ann Noninvasive Electrocardiol. 2018;e12550 10.1111/anec.12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perez‐Riera AR, Barbosa‐Barros R, Lima Aragao W, et al. Transient left septal fascicular block in the setting of acute coronary syndrome associated with giant slurring variant J‐wave. Ann Noninvasive Electrocardiol. 2018;23:e12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez‐Riera AR, Barbosa‐Barros R, Penachini da Costa de Rezende Barbosa M, et al. Transient left septal and anterior fascicular block associated with type 1 electrocardiographic Brugada pattern. J Electrocardiol. 2018;51:145‐149. [DOI] [PubMed] [Google Scholar]
- 6. Riera AR, Ferreira C, Ferreira Filho C, et al. Wellens syndrome associated with prominent anterior QRS forces: an expression of left septal fascicular block? J Electrocardiol. 2008;41:671‐674. [DOI] [PubMed] [Google Scholar]
- 7. Uchida AH, Moffa PJ, Riera AR, Ferreira BM. Exercise‐induced left septal fascicular block: an expression of severe myocardial ischemia. Indian Pacing Electrophysiol J. 2006;6:135‐138. [PMC free article] [PubMed] [Google Scholar]
- 8. Perez Riera AR, Ferreira C, Ferreira Filho C, et al. Electrovectorcardiographic diagnosis of left septal fascicular block: anatomic and clinical considerations. Ann Noninvasive Electrocardiol. 2011;16:196‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riera AR, Kaiser E, Levine P, et al. Kearns‐Sayre syndrome: electro‐vectorcardiographic evolution for left septal fascicular block of the his bundle. J Electrocardiol. 2008;41:675‐678. [DOI] [PubMed] [Google Scholar]
- 10. Magnacca M, Valesano G, Rizzo G, Trotti F, Pagetto A, Boverio R. Diagnostic value of electrocardiogram in septal fascicular conduction disorders of the left branch in diabetics. Minerva Cardioangiol. 1988;36:361‐363. [PubMed] [Google Scholar]
- 11. Acunzo RS, Konopka IV, Sanchez RA, et al. Right bundle branch block and middle septal fiber block with or without left anterior fascicular block manifested as aberrant conduction in apparent healthy individuals: electro‐vectorcardiographic characterization. J Electrocardiol. 2013;46:167‐172. [DOI] [PubMed] [Google Scholar]
- 12. Pérez‐Riera AR, Barbosa‐Barros R, Baranchuk A. Left Septal Fascicular Block: Characterization, Differential Diagnosis and Clinical Significance. London, UK: Springer; 2016. [Google Scholar]
- 13. Medrano GA, Brenes CP, De Micheli A, et al. Block of the posterior subdivision of the left bundle branch of his. J Electrocardiol. 1970;3:309‐315. [DOI] [PubMed] [Google Scholar]
- 14. Rosenbaum MB, Elizari MV, Lazzari JO. Los Hemibloqueos. Buenos Aires, Argentina: Editora Paidos; 1967. [Google Scholar]
- 15. Cinca J, Mendez A, Puig T, et al. on behalf of the investigators of the Spanish Heart Failure Network (REDINSCOR). Differential clinical characteristics and prognosis of intraventricular conduction defects in patients with chronic heart failure. Eur J Heart Fail. 2013;15:877‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Supariwala AA, Po JR, Mohareb S, et al. Prevalence and long‐term prognosis of patients with complete bundle branch block (right or left bundle branch) with normal left ventricular ejection fraction referred for stress echocardiography. Echocardiography. 2015;32:483‐489. [DOI] [PubMed] [Google Scholar]
- 17. Lopes VM, Miguel JM, dos Reis DD, et al. Left‐posterior hemiblock. Clinical and vectorcardiographic study of twenty cases. J Electrocardiol. 1974;7:197‐214. [DOI] [PubMed] [Google Scholar]
- 18. Tawara S. Das Reizleitungssystsem des Saeugetierherzens: eine anatomhistologische Studie ueber die Atrioventriculaer Buendel und die Purkinjeschen Faden. Jena, Germany: Gustav Fischer; 1906. [Google Scholar]
- 19. Demoulin JC, Kulbertus HE. Left hemiblocks revisited from the histopathological viewpoint. Am Heart J. 1973;86:712‐713. [DOI] [PubMed] [Google Scholar]
- 20. Perrin MJ, Keren A, Green MS. Electrovectorcardiographic diagnosis of left septal fascicular block. Ann Noninvasive Electrocardiol. 2012;17:157‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durrer D, van Dam RT, Freud GE, et al. Total excitation of the isolated human heart. Circulation. 1970;41:899‐912. [DOI] [PubMed] [Google Scholar]
- 22. Kulbertus H. Significance of segmental blocks of the left branch of the bundle of His. Bull Acad R Med Belg. 1973;128:481‐493. [PubMed] [Google Scholar]
- 23. Zema MJ. Electrocardiographic tall R waves in the right precordial leads. Comparison of recently proposed ECG and VCG criteria for distinguishing posterolateral myocardial infarction from prominent anterior forces in normal subjects. J Electrocardiol. 1990;23:147‐156. [DOI] [PubMed] [Google Scholar]
- 24. Mattu A, Brady WJ, Perron AD, Robinson DA. Prominent R wave in lead V1: electrocardiographic differential diagnosis. Am J Emerg Med. 2001;19:504‐513. [DOI] [PubMed] [Google Scholar]
- 25. Mori H, Kobayashi S, Mohri S. Electrocardiographic criteria for the diagnosis of the left septal fascicular block and its frequency among primarily elderly hospitalized patients. Nihon Ronen Igakkai Zasshi. 1992;29:293‐297. [DOI] [PubMed] [Google Scholar]
- 26. Ferst JA, Chaitman BR. The electrocardiogram and the athlete. Sports Med. 1984;1:390‐403. [DOI] [PubMed] [Google Scholar]
- 27. MacKenzie R. Tall R wave in lead V1. J Insur Med. 2004;36:255‐259. [PubMed] [Google Scholar]
- 28. McManus K, Condos G, Lin A. Chest pain in a patient with a tall R wave in V1. BMJ Case Rep. 2014;2014. pii: bcr2014205923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabrera E, Gaxiola A. Diagnostic contribution of the vectorcardiogram in hemodynamic overloading of the heart. Am Heart J. 1960;60:296‐317. [DOI] [PubMed] [Google Scholar]
- 30. Donoso E, Sapin SO, Braunwald E, Grishman A. A study of the electrocardiogram and vectorcardiogram in congenital heart disease. II. Vectorcardiographic criteria for ventricular hypertrophy. Am Heart J. 1955;50:674‐693. [DOI] [PubMed] [Google Scholar]
- 31. Baydar ID, Walsh TJ, Massie E. A vectorcardiographic study of right bundle branch block with the frank lead system. clinical correlation in ventricular hypertrophy and chronic pulmonary disease. Am J Cardiol. 1965;15:185‐194. [DOI] [PubMed] [Google Scholar]
- 32. Chen CH, Kawai C, Sakurai T, et al. The RSR' pattern in right chest leads in hypertrophic cardiomyopathy: vectorcardiographic analysis. Jpn Circ J. 1980;44:734‐739. [DOI] [PubMed] [Google Scholar]
- 33. Chung KY, Walsh TJ, Massie E. Wolff‐Parkinson‐White syndrome. Am Heart J. 1965;69:116‐133. [DOI] [PubMed] [Google Scholar]
- 34. Perez‐Riera AR, de Lucca AA, Barbosa‐Barros R, et al. Value of electro‐vectorcardiogram in hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2013;18:311‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Secchi MB, Wu SC, Obbiassi M, Oltrona L, Folli G. Electro‐vectorcardiographic study in Duchenne de Boulogne progressive muscular dystrophy. Arch Mal Coeur Vaiss. 1982;75:1297‐1309. [PubMed] [Google Scholar]
- 36. Yotsukura M, Yamamoto A, Kajiwara T, et al. QT dispersion in patients with Duchenne‐type progressive muscular dystrophy. Am Heart J. 1999;137:672‐677. [DOI] [PubMed] [Google Scholar]
- 37. Tobias NM, Moffa PJ, Pastore CA, et al. The electrocardiogram in endomyocardial fibrosis. Arq Bras Cardiol. 1992;59:249‐253. [PubMed] [Google Scholar]


