Abstract
Background:
Asthma and allergy prevalence is increasing in United States children. In utero exposure to chemicals used in personal care products and plastics may contribute to increase in these diseases.
Methods:
We quantified urinary concentrations of eight phthalate metabolites and bisphenol A in mothers twice during pregnancy in 1999–2000 in Salinas, California. We assessed probable asthma, aeroallergies, eczema, and spirometry in their age 7 children, and measured T helper 1 and T helper 2 cells in blood at ages 2, 5, and 7 (N=392). We employed Bayesian Model Averaging to select confounders from additional biomarkers measured in this population and controlled for them in logistic and linear regressions.
Results:
Monocarboxyisooctyl phthalate was associated with increased odds for probable asthma (odds ratio: 1.54, 95% CI: 1.12, 2.12), and with lower forced expiratory volume in one second (β: −0.09 liters, 95% CI: −0.15, −0.03) and forced expiratory flow from 25–75% of forced vital capacity (β: −7.06 liters/second, 95% CI: −11.04, −2.90). Several other associations were attenuated in final models that controlled for additional biomarkers.
Conclusion:
Monocarboxyisooctyl phthalate was associated with lower respiratory health after controlling for related chemical exposure, which suggests that confounding by multiple chemical exposures should be considered in future research.
Keywords: Asthma, bisphenol A, diethylhexyl phthalate, endocrine disruptor, environmental exposure, hypersensitivity, Th1-Th2 Balance
Introduction
Rates of childhood asthma, aeroallergy, and eczema have increased substantially since the 1960s and have plateaued since the 1990s1,2. Currently in the United States 8.3–15.9% children estimated to have asthma3,4, 24.6% estimated to have aeroallergies5, and 14.3% estimated to have eczema5. Respiratory infections, dietary deficiencies, damp or moldy housing, air conditioning, and increased hygiene have all been associated with increases in asthma or inhalant allergies in recent decades6,7. Environmental chemicals may also be causing increases in these diseases. Air pollution, environmental tobacco smoke, heavy metals, and pesticides have been hypothesized as affecting asthma or inhalant allergies6–11. Studies in animals and humans suggest that exposure to certain environmental chemicals found in and plastics, such as high molecular weight (HMW) phthalates and bisphenol A (BPA), may also be responsible for some of the increased incidence of disease12,13. Benzylbutyl phthalate (BBzP), di-isononyl phthalate (DiNP), di-isodecyl phthalate (DiDP), and di(2-ethylhexyl) phthalate (DEHP) are widely used as plasticizers in food packages, building materials, medical devices, vinyl flooring, and paints. BPA, used in polycarbonate plastics, can be found in certain food and beverage packaging, dental sealants, and receipts. BPA and most HMW phthalate metabolites were detected in ≥90% of Americans in 2013–201414.
Animal studies on these chemicals suggest associations with decreased lung function15, dermatitis16, and an increased T helper 2 cell (Th2) response17. Several longitudinal epidemiologic studies conducted in New York City, Ohio, Sweden, Spain, and Bulgaria reported associations between HMW phthalates and BPA and asthma or respiratory symptoms, aeroallergy, and eczema12, but results have conflicted on cytokines12, which may play a role in asthma and allergy development18,19. T helper 1 (Th1) cells release cytokines such as interferon gamma (IFN-y), interleukin-2 (IL-2), and IL-3 and act through cell-mediated immunity to attack intracellular viruses and bacteria. Th2 cells release cytokines such as IL-4, IL-5, and IL-6 and are involved in allergic and inflammatory immunity20. A higher ratio of Th2 cytokines to Th1 cytokines has been associated with increased risks of aeroallergy and asthma18,19. Further research on this topic would enhance the epidemiologic literature on plasticizing chemicals and atopy.
Plasticizing chemicals can cross the placental barrier21,22 and prenatal exposures may have greater impact on immune system functioning than later exposures23. We measured urinary concentrations of eight phthalate metabolites and BPA at two timepoints during pregnancy. Our primary objective was to analyze associations with asthma, aeroallergies, eczema, and lung function at age seven, and our secondary objective was to analyze associations with T helper 1 cell (Th1) and Th2 cell percentages in whole blood at ages 2, 5, and 7. This is the first study to examine associations between in utero concentrations of these biomarkers and cytokine levels in childhood, and one of the first studies to examine atopic illnesses and cytokine levels in the same study.
Methods
Study population.
The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) has followed children living in the Salinas Valley, California from birth to age 1624. Women attending first prenatal care visits at local clinics were screened for eligibility in 1999–2000. Women were invited to participate if they spoke Spanish or English, were ≤20 weeks’ pregnant, were ≥18 years old, qualified for MediCal (low income health insurance), and planned to deliver at the county hospital. Participant mothers were primarily Mexican-born, Spanish-speaking, and lived with or were farmworkers. Of the 601 women enrolled, 531 were followed through a live birth. Of these, 517 children had at least one prenatal high molecular weight phthalate or BPA measurement (101 children each were missing both prenatal samples for MBzP MCNP, MCOP, MCPP, and metabolites of DEHP, and 27 children were missing both prenatal samples for BPA). Of the 517 children with at least one prenatal biomarker measurement, 167 were missing data on probable asthma; 180 were missing data on aeroallergies; 181 were missing data on eczema; 219 did not have measurements for FEV1; 249 each did not have measurements for FVC, FEV1/FVC, or FEF25–75%; and 281, 288, and 265 did not have cytokines measurements at ages 2, 5, and 7 respectively. There were 392 children with data on at least one prenatal biomarker and one outcome. This study conforms to recognized ethical standards and was approved by the Office for the Protection of Human Subjects (OPHS) at UC Berkeley. The Centers for Disease Control and Prevention (CDC) deferred to the OPHS. Mothers gave written informed consent and children verbally assented at age 7.
Questionnaire data collection. Mothers completed interviewer-administered, structured questionnaires in English or Spanish twice during pregnancy, at delivery, and when children were 6 months, 1, 2, 3.5, 5, and 7 years old. During the first pregnancy interview, questionnaires asked about maternal age, education, country of birth, years lived in the U.S.A, parity, family income, and family history of asthma (mother, father, or siblings).
Exposure assessment.
Mothers provided spot urine samples at two interviews during pregnancy (first interview: mean 13 weeks gestation, range 5 to 27; second interview: mean 26 weeks gestation, range 21 to 40). Phthalate- and BPA-free polypropylene urine cups were used to collect urine, which was aliquoted into glass vials and stored at −80°C until shipment to the CDC.
We used solid phase extraction coupled with isotope dilution high performance liquid chromatography-tandem mass spectrometry to quantify BPA and eight phthalate metabolites: monobenzyl phthalate (MBzP) [a metabolite of BBzP]; four metabolites of DEHP [mono-2-ethylhexyl phthalate, mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, and mono-(2-ethyl-5-carboxypentyl) phthalate]; mono(carboxyoctyl) phthalate [MCOP; a metabolite of DiNP]; mono(carboxynonyl) phthalate (MCNP) [a metabolite of DiDP]; and mono(3-carboxypropyl) phthalate (MCPP) [a metabolite of several HMW phthalates and a minor metabolite of dibutyl phthalate]. Analytic methods have been published previously for phthalates25 and BPA26. Concentrations were reported in ng/mL of urine and limits of detection ranged from 0.2 ng/mL – 0.5 ng/mL. Concentrations below the limits of detection were assigned the instrument-reading values, if available, or an imputed value below the limits of detection selected randomly from the log-normal distribution using maximum likelihood estimation27. We combined mono-2-ethylhexyl phthalate, mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, and mono-(2-ethyl-5-carboxypentyl) phthalate to create a molar sum of DEHP metabolites (∑DEHP).
A hand-held refractometer (National Instrument Company Inc., Baltimore, Maryland) was used to measure urinary specific gravity. We imputed urinary specific gravity based on urinary creatinine for 81 samples missing specific gravity measurements by regressing specific gravity on creatinine and multiplying the coefficient by existing creatinine values to generate missing specific gravity values. We used the formula (analyte concentration*(1.024 – 1))/(sample specific gravity–1) to correct for urinary dilution28.
Outcome Assessment.
The primary outcomes for this study were probable asthma, aeroallergies, and eczema at age 7, and lung function at age 7. We defined “probable asthma” at age 7 as currently taking asthma medication or having two or more of the following criteria: any current respiratory symptom, doctor diagnosis of asthma at any age, or a positive bronchodilator test.A child was considered to have been diagnosed with asthma if the mother had reported a doctor’s diagnosis at any visit, and to have respiratory symptoms at age 7 if mothers reported any of the following based on the International Study of Asthma and Allergies in Childhood questionnaire29: tightness in the chest and difficulty breathing; wheezing or whistling in the chest; trouble sleeping, speaking, running, or playing because of wheezing, whistling, shortness of breath, or coughing without a cold; or an asthma attack. Mothers were also asked if their child was currently taking asthma medication.Spirometry was administered at age 7 using three identical EasyOne spirometers that were calibrated daily. Children performed up to eight expiratory maneuvers, lasting at least three seconds. Two physicians with experience in pediatric spirometry verified all maneuvers, and the best verified maneuver was used for analyses. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC, and forced expiratory flow from 25–75% of FVC (FEF25%−75%) were measured from each maneuver.
Children whose mothers reported they had respiratory symptoms at age 7 (n=54) were offered a bronchodilator test, for which they inhaled albuterol and repeated spirometry 20 minutes later. A positive test was defined as 12%+ improvement from their best FEV1 or FVC before the bronchodilator.
A child was considered to have eczema at age 7 if their mother reported a doctor’s diagnosis of “eczema or an allergic skin rash” in the last year, and a child was considered to have aeroallergies if their mother reported “runny or itchy eyes apart from colds,” “sneezing or a runny nose apart from colds,” or a doctor’s diagnosis of “hay fever or allergic rhinitis” in the last year.
Cytokine detection.
A secondary outcome of this study was the percentage of Th1 and Th2 cytokine producing cells, and their ratio. Flow cytometry was used to detect Th1 and Th2 cells in unfrozen pediatric whole blood collected at ages 2, 5, and 7. Methods have been previously described30. We divided the number of Th1 and Th2 cells by the total number of CD4+ cells to obtain the percentage of Th1 and Th2 cells (Th1% and Th2%), respectively. Th1:Th2 cell ratio was defined as Th1% divided by Th2%.
Statistical analysis. We averaged urinary biomarker concentrations across pregnancy and used specific gravity corrected values in all analyses. We analyzed the first and second biomarker measurements separately in sensitivity analyses. Biomarker concentrations were examined as continuous log2-transformed variables. In initial analyses, separate models were constructed for each biomarker; subsequent analyses included several biomarkers in the same model to explore confounding.
Probable asthma, aeroallergies, and eczema were analyzed as binary variables using logistic regression, and lung function and Th1 and Th2 levels were analyzed as continuous variables using linear regression or generalized estimating equations. FEV1 and FVC were not transformed; FEV1/FVC, FEF25–75%, Th1%, Th2%, and Th1:Th2 cell ratio were assessed as continuous log10-transformed variables. Thus, for each two-fold increase in biomarker concentration, regression coefficients represent mean or percent difference in outcomes. To determine the longitudinal association of cytokine variables at 2, 5, and 7 years of age, we used generalized estimating equations with Gaussian specification and an exchangeable correlation structure, including interaction terms for child age.
In demographically adjusted models, we included maternal age at birth, parity, household income as a proportion of poverty at baseline, and family history of asthma as confounders, as determined a priori via directed acyclic graphs. We then generated fully adjusted models controlling for additional chemical exposure. In addition to the HMW phthalates and BPA discussed in this paper, we aimed to control for metabolites of low molecular weight phthalates (monoethyl phthalate, mono-n-butyl phthalate, mono-isobutyl phthalate) and phenols (methyl paraben, propyl paraben, triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, benzophenone-3) that were measured in the same urine samples. To identify the most important variables to include, we used Bayesian Model Averaging (BMA)31, which estimates models for all possible combinations of the specified biomarkers with an outcome, and weights models by their posterior model probability while adjusting for demographic covariates to determine how influential given variables are on an outcome. BMA produces Posterior Inclusion Probabilities, which are measurements of each variable’s influence on the outcome relative to other variables in the BMA model. We conducted BMA and chose the three most influential variables (those with the highest Posterior Inclusion Probabilities) to include in fully adjusted regression models for each outcome.
We conducted Generalized Additive Models (GAMs) for all associations to test for linearity of relationships. We controlled for the same covariates as in fully adjusted models and allowed for three equivalent degrees of freedom. Relationships were considered linear if the P-value was above 0.05.
BMA analyses were conducted in R32 (Vienna, Austria) with the BMS package33. Descriptive statistics and regressions were conducted in Stata 14 (College Station, TX).
Results
Most mothers were aged 18–29 at enrollment (76%), lived below 100% of the federal poverty index (62%), and were multiparous (61%); most children had no family history of asthma (90%) (Table 1). All plasticizer biomarkers were detected in over 90% of urine samples (Table 2). Correlations ranged from 0.71 (MCNP and MCOP) to 0.05 (MCOP and ∑DEHP) (Figure 1).
Table 1.
Demographic Characteristics of the Study Population: CHAMACOS Children in Salinas, California (1999–2008)
| Characteristics at time of pregnancy | N | (%) |
|---|---|---|
| Maternal age | ||
| 18–24 | 174 | (44.7) |
| 25–29 | 123 | (31.6) |
| 30–34 | 62 | (15.9) |
| 35+ | 30 | (7.7) |
| Household income as a proportion of poverty | ||
| <100% | 240 | (61.2) |
| >100% | 152 | (38.8) |
| Maternal education | ||
| 6th grade or less | 174 | (44.7) |
| 7th-12th grade | 136 | (35.0) |
| High school graduate or greater | 79 | (20.3) |
| Maternal country of birth | ||
| United States | 48 | (12.3) |
| Mexico | 334 | (85.9) |
| Other | 7 | (1.8) |
| Years mother has lived in the United States | ||
| Five or fewer | 195 | (50.1) |
| Six to ten | 96 | (24.7) |
| Eleven or more | 101 | (26.0) |
| Parity | ||
| First child | 126 | (32.4) |
| Second child | 117 | (30.1) |
| Third child or greater | 146 | (37.5) |
| Child’s mother, father, or siblings have asthma history | ||
| No | 354 | (90.3) |
| Yes | 38 | (9.7) |
CHAMACOS: Center for the Health Assessment of Mothers and Children of Salinas
Table 2.
Distribution of Specific Gravity Corrected Phthalate Monoester Metabolites and BPA in CHAMACOS Maternal Prenatal Urine Collected at Two Time Points in Pregnancy in Salinas, California (1999–2000)
| % > LOD |
Average of two measurements |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | LOD | Early Pregnancy† |
Late Pregnancy‡ |
Geo. Mean |
10th% | 25th% | 50th% | 75th% | 90th% | 95th% |
| MBzP (ng/mL) | 0.3 | 98.3% | 98.9% | 8.9 | 2.5 | 4.9 | 9.2 | 17.7 | 28.3 | 40.1 |
| MCNP (ng/mL) | 0.2 | 97.5% | 97.4% | 2.3 | 1.0 | 1.5 | 2.3 | 3.4 | 5.2 | 7.6 |
| MCOP (ng/mL) | 0.2 | 97.0% | 96.8% | 3.8 | 1.5 | 2.4 | 3.9 | 5.5 | 8.7 | 11.6 |
| MCPP (ng/mL) | 0.2 | 90.3% | 95.1% | 2.2 | 0.8 | 1.4 | 2.4 | 3.8 | 5.1 | 7.1 |
| ∑DEHP (umol/mL) | 0.2–0.5 | 97.0%§ | 97.0%§ | 0.2 | 0.1 | 0.1 | 0.2 | 0.4 | 0.6 | 0.8 |
| BPA (ug/mL) | 0.4 | 97.4% | 97.8% | 1.5 | 0.6 | 0.9 | 1.3 | 2.2 | 3.7 | 5.8 |
BPA: Bisphenol A; CHAMACOS: Center for the Health Assessment of Mothers and Children of Salinas; DEHP: Di(2-ethylhexyl) phthalate; LOD: limit of detection; MBzP: Monobenzyl phthalate; MCNP: Mono(carboxynonyl) phthalate; MCOP: Monocarboxyisooctyl phthalate; MCPP: Mono(3-carboxypropyl) phthalate; MEHP: Mono(2-ethylhexyl) phthalate; OR: Odds ratio
Mean = 13 weeks gestation
Mean = 26 weeks gestation
MEHP used as proxy for ∑DEHP in % > LOD
LODs for metabolites of DEHP were 0.5 for MEHP and 0.2 for MEHHP, MEOHP, and MECCP
Figure 1.
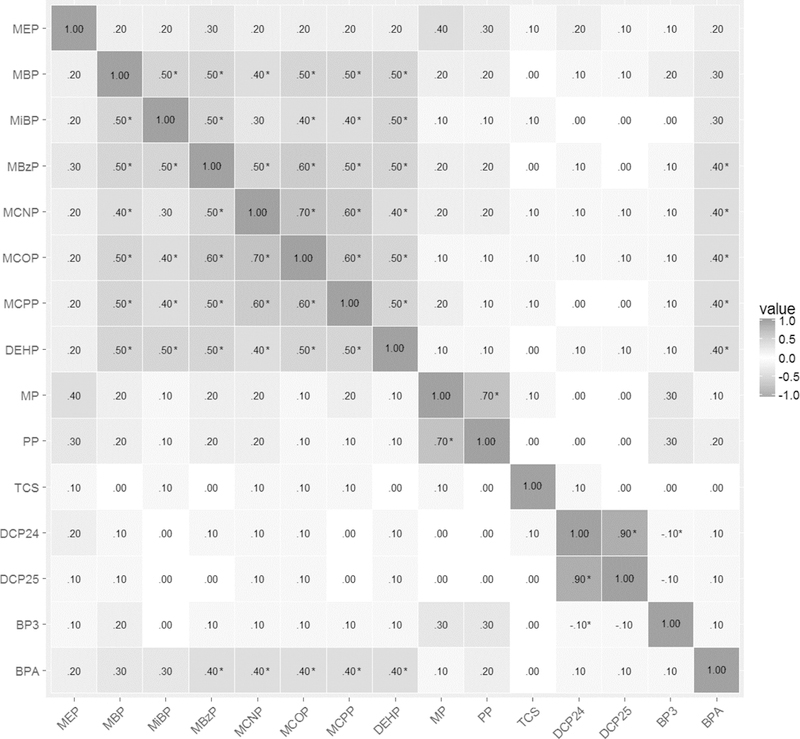
Correlations of log2 specific gravity corrected phthalate, paraben, and other phenol urinary concentrations of CHAMACOS mothers during pregnancy
*P<0.05
We categorized 37 children (11%) with probable asthma, 87 children (25%) with aeroallergies, and 23 children (7%) with eczema (Table 3). Th1:Th2 cell ratios and Th1% increased as children aged, while Th2% stayed stable with age (Table 3).
Table 3.
Respiratory Symptom, Spirometry, and Cytokine Data in CHAMACOS Participants in Salinas, California (2006–2008)
| Maternal report | N | (%) |
|---|---|---|
| Probable Asthma† | 37 | (11%) |
| Any current symptoms | 54 | (15%) |
| Any current asthma medication use | 22 | (6%) |
| Past diagnosis of asthma | 62 | (18%) |
| Positive bronchodilator test‡ | 10 | (3%) |
| Aeroallergy symptoms or diagnosis | 87 | (25%) |
| Eczema diagnosis | 23 | (7%) |
| Spirometry | N | Mean (Standard Deviation) |
| Best maneuver (all children) | ||
| FEV1 | 300 | 1.8 (0.5) liters |
| FVC | 270 | 2.1 (0.6) liters |
| FEV1/FVC | 270 | 0.9 (0.1) |
| FEF25–75 | 270 | 2.5 (0.9) liters/second |
| Cytokine variable | N | Mean (Standard Deviation) |
| Age 2 | ||
| Th1:Th2 cell ratio | 239 | 6.13 (5.58) |
| % CD4 cells | 239 | 32.9 (7.5) |
| % Th1 cells | 239 | 4.0 (3.0) |
| % Th2 cells | 239 | 0.9 (0.7) |
| Age 5 | ||
| Th1:Th2 cell ratio | 231 | 14.40 (44.53) |
| % CD4 cells | 231 | 29.9 (7.3) |
| % of Th1 cells | 231 | 6.8 (3.2) |
| % of Th2 cells | 231 | 1.1 (0.8) |
| Age 7 | ||
| Th1:Th2 cell ratio | 254 | 22.51 (36.29) |
| % CD4 cells | 254 | 33.8 (10.2) |
| % of Th1 cells | 254 | 10.0 (5.9) |
| % of Th2 cells | 254 | 0.9 (0.7) |
CHAMACOS: Center for the Health Assessment of Mothers and Children of Salinas; FEF25–75%: Forced expiratory flow from 25–75% of forced vital capacity; FEV1: Lower forced expiratory volume in one second; FVC: Forced vital capacity; SD: Standard Deviation
Defined as: currently taking asthma medications or any two of current respiratory symptoms, diagnosis of asthma, positive bronchodilator test
Completed only by children with respiratory symptoms (N=42)
The Posterior Inclusion Probabilities for each outcome are shown in Supplemental Table S1. The top three ranked biomarkers were MCOP, propyl paraben, and 2,4-dichlorophenol for probable asthma; MCPP, BPA, and MCOP for aeroallergies; MCPP, mono-isobutyl phthalate, and mono-n-butyl phthalate for eczema; MCOP, mono-n-butyl phthalate, and monoethyl phthalate for FEV1; MCOP, MCNP, and monoethyl phthalate for FVC; MBzP, MCOP, and BPA for FEV1/FVC; MCOP, monoethyl phthalate, and triclosan for FEF25–75%; mono-isobutyl phthalate, triclosan, and mono-n-butyl phthalate for Th1:Th2 ratio; methyl paraben, 2,4-dichlorophenol, and propyl paraben for Th1%; and mono-n-butyl phthalate, methyl paraben, and monoethyl phthalate for Th2%.
Table 4 shows prenatal maternal urinary MCOP concentrations were associated with increased odds for probable asthma in fully adjusted models (OR: 1.54, 95% CI: 1.12, 2.12) and increased odds for aeroallergies in demographically adjusted models (OR: 1.28, 95% CI: 1.01, 1.61). BPA (OR: 1.34, 95% CI: 1.04, 1.71) and MCPP concentrations (OR: 1.35, 95% CI: 1.06, 1.72) were associated with increased odds for aeroallergies in demographically adjusted models. MBzP also showed an association with aeroallergies in fully adjusted models and MCNP concentrations showed associations with increased odds for probable asthma and aeroallergies in demographically adjusted models. There were no associations with eczema.
Table 4.
Association of Log 2 Prenatal Urinary Biomarker Concentrations and Asthma Outcomes in CHAMACOS Children in Salinas, California (1999–2008)
| Probable asthma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Demographically adjusted† | Fully adjusted‡ | |||||||
| Biomarker | N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI |
| MBzP | 336 | 0.99 | 0.77, 1.29 | 333 | 1.05 | 0.79, 1.38 | 329 | 0.88 | 0.63, 1.24 |
| MCNP | 336 | 1.34 | 0.98, 1.84 | 333 | 1.38 | 0.97, 1.97 | 329 | 1.21 | 0.71, 2.06 |
| MCOP | 336 | 1.37 | 1.04, 1.80 | 333 | 1.42 | 1.03, 1.94 | 329 | 1.54 | 1.12, 2.12 |
| MCPP | 336 | 1.24 | 0.91, 1.70 | 333 | 1.30 | 0.92, 1.85 | 329 | 1.15 | 0.75, 1.74 |
| ∑DEHP | 336 | 0.99 | 0.72, 1.36 | 333 | 1.02 | 0.73, 1.43 | 329 | 0.81 | 0.53, 1.23 |
| BPA | 346 | 1.04 | 0.75, 1.45 | 343 | 1.19 | 0.83, 1.71 | 329 | 1.03 | 0.68, 1.55 |
| Aeroallergies | |||||||||
| Crude | Demographically adjusted† | Fully adjusted§ | |||||||
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| MBzP | 327 | 1.01 | 0.84, 1.21 | 324 | 1.03 | 0.85, 1.25 | 321 | 0.81 | 0.63, 1.04 |
| MCNP | 327 | 1.24 | 0.98, 1.56 | 324 | 1.25 | 0.98, 1.61 | 321 | 1.01 | 0.70, 1.44 |
| MCOP | 327 | 1.22 | 0.99, 1.52 | 324 | 1.28 | 1.01, 1.61 | 321 | 1.09 | 0.82, 1.45 |
| MCPP | 327 | 1.30 | 1.04, 1.64 | 324 | 1.35 | 1.06, 1.72 | 321 | 1.20 | 0.90, 1.61 |
| ∑DEHP | 327 | 1.07 | 0.85, 1.34 | 324 | 1.12 | 0.89, 1.42 | 321 | 0.91 | 0.68, 1.21 |
| BPA | 334 | 1.24 | 0.99, 1.56 | 331 | 1.34 | 1.04, 1.71 | 321 | 1.19 | 0.91, 1.57 |
| Eczema | |||||||||
| Crude | Demographically adjusted† | Fully adjusted¶ | |||||||
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| MBzP | 326 | 0.98 | 0.71, 1.36 | 323 | 1.00 | 0.72, 1.40 | 323 | 1.34 | 0.86, 2.09 |
| MCNP | 326 | 0.91 | 0.60, 1.39 | 323 | 0.94 | 0.61, 1.45 | 323 | 1.17 | 0.70, 1.96 |
| MCOP | 326 | 0.99 | 0.68, 1.46 | 323 | 1.03 | 0.69, 1.55 | 323 | 1.39 | 0.87, 2.20 |
| MCPP | 326 | 0.79 | 0.58, 1.07 | 323 | 0.77 | 0.56, 1.07 | 323 | 0.83 | 0.57, 1.21 |
| ∑DEHP | 326 | 0.96 | 0.64, 1.44 | 323 | 0.96 | 0.64, 1.45 | 323 | 1.25 | 0.79, 1.98 |
| BPA | 333 | 0.86 | 0.56, 1.31 | 330 | 0.89 | 0.57, 1.39 | 320 | 1.00 | 0.61, 1.63 |
BPA: Bisphenol A; CHAMACOS: Center for the Health Assessment of Mothers and Children of Salinas; CI: Confidence Interval; DEHP: Di(2-ethylhexyl) phthalate; MBzP: Monobenzyl phthalate; MCNP: Mono(carboxynonyl) phthalate; MCOP: Monocarboxyisooctyl phthalate; MCPP: Mono(3-carboxypropyl) phthalate; OR: Odds ratio Separate models created for each chemical
Models control for maternal age, parity, household income as a proportion of poverty at baseline, child’s family history of asthma, and maternal education
Models control for maternal age, parity, household income as a proportion of poverty at baseline, child’s family history of asthma, maternal education, monocarboxyisooctyl phthalate, propyl paraben, 2,4-dichlorophenol
Models control for maternal age, parity, household income as a proportion of poverty at baseline, child’s family history of asthma, maternal education, mono(3-carboxypropyl) phthalate, bisphenol A, monocarboxyisooctyl phthalate
Models control for maternal age, parity, household income as a proportion of poverty at baseline, child’s family history of asthma, maternal education, mono(3-carboxypropyl) phthalate, mono-isobutyl phthalate, mono-n-butyl phthalate
Table 5 shows associations between urinary biomarker concentrations and spirometry measures. For each two-fold increase in biomarker concentration, regression coefficients represent mean (FEV1 and FVC) or percent difference (FEV1/FVC and FEF25–75%) in outcomes. MCOP concentrations were associated with lower FEV1 (β: −0.09 liters, 95% CI: −0.15, −0.03) and FEF25–75% (Percent difference: −7.06 liters/second, 95% CI: −11.04, −2.90) in fully adjusted models, as well as lower FVC (β: −0.07 liters, 95% CI: −0.13, 0.00) in demographically adjusted models. MCNP concentrations also showed associations with lower FEV1 (β: −0.05 liters, 95% CI: −0.11, 0.01) and lower FEF25–75% (Percent difference: −5.29 liters/second, 95% CI: −9.74, −0.61) in demographically adjusted models. MBzP concentrations also showed associations with lower FEV1 (β: −0.04 liters, 95% CI: −0.09, 0.00) and lower FEF25–75% (Percent difference: −4.23 liters/second, 95% CI: −7.72, −0.60) in demographically adjusted models.
Supplemental Table S2 shows no associations in generalized estimating equation models of cytokines across ages 2, 5, and 7, with P-values for interaction by age. For each two-fold increase in biomarker concentration, regression coefficients in this table represent percent difference in outcomes.
Supplemental Table S3 shows the results of GAM analyses testing for linearity. All associations demonstrate linear relationships.
Sensitivity analyses examining the first and second biomarker measurements separately yielded results similar to the main models with averaged values (data not shown). Correlations ranged from 0.29 for MBzP to 0.16 for MCOP.
Discussion
We found that prenatal urinary concentrations of MCOP were associated with increased odds for probable asthma and with lower lung function at age 7, after controlling for demographic factors and related chemical exposures, and were associated with increased odds for aeroallergies in demographically adjusted models. MCNP concentrations were associated with increased odds for probable asthma and aeroallergies and with lower lung function in demographically adjusted models. MBzP concentrations were associated with lower lung function, and MCPP and BPA concentrations were associated with increased odds for aeroallergies in demographically adjusted models.
Previous studies of chemical exposures and respiratory and allergic outcomes have examined one biomarker at a time, failing to account for multiple exposures. We used BMA variable selection to identify potentially confounding biomarkers to control for, and results from these fully adjusted models showed markedly fewer associations than models adjusted for demographic confounders only. When estimates were higher in fully adjusted models compared to demographically adjusted models, such as with MCOP and probable asthma, we believe this indicates reduced confounding by the presence of other, related chemical biomarkers. Results from demographically adjusted models resemble those from other studies to a greater extent than do our fully adjusted models, suggesting that other studies’ findings may be partially due to confounding by co-exposure to other chemicals.
Our strongest findings were with MCOP, a phthalate metabolite on which there have been few studies. Our results agree with the Dampness in Buildings and Health study that observed an association between living with polyvinyl chloride flooring, which may be a source of exposure to the parent compounds of MCOP and MCPP, at ages 1–3 and increased risk for asthma and aeroallergies, but not eczema, at ages 6–834. Our results also agree with the cross-sectional associations found between MCOP, MCNP, and MCPP and asthma in participants aged 18 and over in the 2005–2006 NHANES study35. However, a French birth cohort study found no association between prenatal urinary concentrations of these chemicals and asthma or FEV1 at age 536.
Our study found many null associations, which could indicate a true lack of association or low power to detect associations due to low sample size. Specifically, unlike several other studies12, we did not find associations of in utero exposures to DEHP, Benzylbutyl phthalate, or BPA and probable asthma or allergy risk. One of these studies reported associations with early childhood but not in utero BPA37 which suggests that early childhood exposure to BPA may be more impactful on childhood lung health and may explain our null results.
Our study found little evidence of associations of prenatal maternal urinary concentrations of metabolites of high molecular weight phthalates or BPA with Th1 and Th2 profiles in childhood. No other studies have examined the effect of these plasticizing chemicals on Th1 and Th2 function in childhood, and only a small number have examined prenatal exposure and cytokine function in cord blood. One study found no association between prenatal maternal urinary concentrations of high molecular weight phthalates or BPA and cord blood Th2 cytokine concentrations in infants64, while another found an association between prenatal maternal blood concentrations of BPA and higher Th2 cytokines in cord blood71. However, T helper cell-related immunity changes drastically from birth to age two87, and these previous studies may not be appropriate comparisons.
One limitation of our study is that although the focus of this paper was atopic illnesses, some of our probable asthma cases may be non-atopic and our inability to differentiate atopic versus non-atopic cases obscures any differentiation by etiology. Our population is from a largely low-income Mexican-American farm working community, and may not be comparable to other study populations. Our sample size was also relatively small, which may have resulted in lower power to detect differences. Additionally, as in all studies, Type I and Type II errors may have occurred. Our employment of BMA as a variable selection tool is a strength, though it should be noted that BMA does not adequately account for collinearity among variables. Although few of our included biomarkers were highly correlated, many are used in similar products and this is not corrected for in BMA. Intraclass correlation coefficients (ICCs) for these chemicals are generally low in our population (high molecular phthalates range from 0.14 to 0.37 and the ICC for BPA is 0.16), indicating high variability between samples. However, unlike many previous studies, our exposure estimates are based on two measures of urinary chemical concentrations during pregnancy rather than one. Our urinary biomarker measurements at two prenatal timepoints are also a strength because gestational exposure to environmental chemicals may influence lung development and function more substantially than later exposures39. Our comprehensive evaluation of atopic diseases in childhood is also a strength. We measured cytokine levels at several ages, spanning important periods in juvenile immune system development, and we measured lung function. We used an integrated classification of probable asthma based on a number of asthma-related variables, including bronchodilator responsiveness. However, while our probable asthma and aeroallergy case definitions were based on both symptoms and medical management, we were unable to apply the same rigor to our eczema cases, which were based only on maternal report of a doctor diagnosis of eczema in their children.
We observed that prenatal maternal urinary concentrations of biomarkers of exposure to HMW phthalates, particularly MCOP, a metabolite of DiNP, are associated with increased odds of probable asthma and aeroallergies at age 7, and with lower lung function at age 7. The potential clinical relevance of the slightly lower lung function of the exposed children is difficult to assess at one time point, but reduced lung function in childhood has been associated with lower lung function and increased risk of respiratory illness in adulthood. We found limited evidence that prenatal urinary concentrations of BPA are associated with increased odds of aeroallergies at age 7, and did not confirm findings of other studies that showed associations between BPA and respiratory symptoms. This is the first study to examine the relationship between prenatal maternal urinary concentrations of any high molecular weight phthalate or BPA and cytokine measurements in childhood. It is also one of the first studies to evaluate associations between in utero exposures to MCOP, MCNP, or MCPP and childhood asthma, aeroallergies, or eczema, and one of the first studies to analyze atopic illnesses and cytokines concurrently. Future research should measure IgE in children along with asthma status in order to distinguish atopic versus non-atopic asthma and any associations with prenatal chemical exposure that are differential by this distinction. In addition, other classes of CD4+ cells such as Th17 cells and regulatory T cells should be evaluated for their associations with prenatal exposure to the chemicals in this study to further characterize the behavior of the childhood immune system in conjunction with these chemicals.
Supplementary Material
Acknowledgements:
We would like to acknowledge Xiaoyun Ye, Manori Silva, and Tao Jia for their work on biomarker measurement, and Eric Coker for his counsel on Bayesian Model Averaging methods.
This work was supported by the Environmental Protection Agency (grants R82670901, RD83171001); and the National Institute for Environmental Health Sciences (grants P01 ES009605, 1RC2 ES018792, R01 ES021369, R21 ES024909, R24 ES028529).
Footnotes
The authors declare no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
Material in the electronic repository: Supplemental Table S1. Posterior Inclusion Probabilities for All Phthalate Metabolites, Parabens, and Other Phenols Included in Bayesian Model Averaging in CHAMACOS Children in Salinas, California (1999–2008)
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Supplement 3):S131–S145. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KD, Howie LD, Akinbami OJ. Trends in allergic conditions among children: United States, 1997–2011. 2013. [PubMed] [Google Scholar]
- 3.CDC. National Health and Nutrition Examination Survey Data. US Department of Health and Human Services; 2013–2014. [Google Scholar]
- 4.CDC. National Current Asthma Prevalence (2016). Most Recent Asthma Data. 2018. [Google Scholar]
- 5.CDC. National Health and Nutrition Examination Survey Data. US Department of Health and Human Services; 2005–2006. [Google Scholar]
- 6.Dick S, Friend A, Dynes K, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ open. 2014;4(11):e006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebert CS, Pillsbury HC. Epidemiology of allergy. Otolaryngologic clinics of North America. 2011;44(3):537–548. [DOI] [PubMed] [Google Scholar]
- 8.Huang S-K, Zhang Q, Qiu Z, Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. Journal of thoracic disease. 2015;7(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patelarou E, Tzanakis N, Kelly FJ. Exposure to indoor pollutants and wheeze and asthma development during early childhood. International journal of environmental research and public health. 2015;12(4):3993–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S-N, Hsieh C-C, Kuo H-F, et al. The effects of environmental toxins on allergic inflammation. Allergy, asthma & immunology research. 2014;6(6):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo Y, Perzanowski MS. Allergic sensitization and the environment: latest update. Current allergy and asthma reports. 2014;14(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Current environmental health reports. 2015;2(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130(2):453–460 e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (March, 2018). Atlanta, GA, USA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 15.Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environmental Health. 2012;11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen S, Li J, You H, et al. Oral exposure to diisodecyl phthalate aggravates allergic dermatitis by oxidative stress and enhancement of thymic stromal lymphopoietin. Food and Chemical Toxicology. 2017;99:60–69. [DOI] [PubMed] [Google Scholar]
- 17.Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A Prenatally or in Adulthood Promotes T^ sub H^ 2 Cytokine Production Associated with Reduction of CD4^ sup+^ CD25^ sup+^ Regulatory T Cells. Environmental health perspectives. 2008;116(4):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes PJ. Th2 cytokines and asthma: an introduction. Respiratory research. 2001;2(2):64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India: Official Organ of Indian Chest Society. 2010;27(2):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volcheck GW. Overview of the Human Immune Response In: Clinical Allergy. Springer; 2008:1–39. [Google Scholar]
- 21.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. American Journal of Obstetrics & Gynecology. 2010;202(4):393 e391–393. e397. [DOI] [PubMed] [Google Scholar]
- 22.Mose T, Mortensen GK, Hedegaard M, Knudsen LE. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reproductive Toxicology. 2007;23(1):83–91. [DOI] [PubMed] [Google Scholar]
- 23.Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environmental health perspectives. 2000;108(Suppl 3):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland NT. CHAMACOS, A Longitudinal Birth Cohort Study: Lessons from the Fields. Journal of Children’s Health. 2003;1(1):3–27. [Google Scholar]
- 25.Silva MJSE, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography. 2007(860):106–112. [DOI] [PubMed] [Google Scholar]
- 26.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL. Normalization of urinary drug concentrations with specific gravity and creatinine. Journal of Analytical Toxicology. 2009;33(1):1–7. [DOI] [PubMed] [Google Scholar]
- 29.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 30.Duramad P, McMahon CW, Hubbard A, Eskenazi B, Holland NT. Flow cytometric detection of intracellular TH1/TH2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1452–1458. [PubMed] [Google Scholar]
- 31.Madigan D, Raftery AE, Volinsky C, Hoeting J. Bayesian model averaging. Paper presented at: Proceedings of the AAAI Workshop on Integrating Multiple Learned Models, Portland, OR1996. [Google Scholar]
- 32.Team RC. R: A language and environment for statistical computing. 2013. [Google Scholar]
- 33.Zeugner S Bayesian model averaging with BMS. Tutorial to the R-package BMS 1e30. 2011. [Google Scholar]
- 34.Larsson M, Hägerhed‐Engman L, Kolarik B, et al. PVC–as flooring material–and its association with incident asthma in a Swedish child cohort study. Indoor air. 2010;20(6):494–501. [DOI] [PubMed] [Google Scholar]
- 35.Strassle PD, Smit LA, Hoppin JA. Endotoxin enhances respiratory effects of phthalates in adults: Results from NHANES 2005–6. Environmental research. 2018;162:280–286. [DOI] [PubMed] [Google Scholar]
- 36.Vernet C, Pin I, Giorgis-Allemand L, et al. In Utero Exposure to Select Phenols and Phthalates and Respiratory Health in Five-Year-Old Boys: A Prospective Study. Environmental Health Perspectives. 2017;125(9):097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donohue KM, Miller RL, Perzanowski MS, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. Journal of Allergy and Clinical Immunology. 2013;131(3):736–742. e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burchard EG, Silverman EK, Rosenwasser LJ, et al. Association between a sequence variant in the IL-4 gene promoter and FEV1 in asthma. American journal of respiratory and critical care medicine. 1999;160(3):919–922. [DOI] [PubMed] [Google Scholar]
- 39.Fetal Duijts L. and infant origins of asthma. European journal of epidemiology. 2012;27(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


