Abstract
We asked whether coordinated voluntary movement of the lower limbs could be regained in an individual having been completely paralyzed (>4 yr) and completely absent of vision (>15 yr) using a novel strategy – transcutaneous spinal cord stimulation at selected sites over the spinal vertebrae with just one week of training. We also asked whether this stimulation strategy could facilitate stepping assisted by an exoskeleton (EKSO, EKSO Bionics) that is designed so that the subject can voluntarily complement the work being performed by the exoskeleton. We found that spinal cord stimulation enhanced the level of effort that the subject could generate while stepping in the exoskeleton. In addition, stimulation improved the coordination patterns of the lower limb muscles resulting in a more continuous, smooth stepping motion in the exoskeleton. These stepping sessions in the presence of stimulation were accompanied by greater cardiac responses and sweating than could be attained without the stimulation. Based on the data from this case study it appears that there is considerable potential for positive synergistic effects after complete paralysis by combining the overground stepping in an exoskeleton, a novel transcutaneous spinal cord stimulation paradigm, and daily training.
I. Introduction
Results in mice [1, 2], rats [3, 4], cats [5, 6], and humans [7, 8] with motor complete paralysis have shown that the lumbosacral spinal circuitry can be neuromodulated using a combination of electrical epidural stimulation [9, 10], pharmacological interventions [11], and locomotor training [9, 12] to enable weight-bearing standing [8, 13], stepping [14, 15], voluntary movements [7, 8] and bladder control [16]. We recently demonstrated the ability to noninvasively neuromodulate the lumbosacral neural circuitry to induce locomotor-like movements in healthy subjects using electromagnetic stimulation [17]. Similar responses were observed using transcutaneous electrical spinal cord stimulation having a unique waveform that minimizes pain and discomfort in both healthy [18] and paralyzed (under review) subjects.
Over the past several years, robotic therapy has been tested as a method to improve locomotion in paralyzed subjects with varying results. An assist-as-needed (AAN) paradigm was tested in mice using a specially designed robotic treadmill with arms to move the legs in a trajectory with an allowable error window [2]. EKSO Bionics is a battery powered wearable bionic suit that enables individuals with lower extremity weakness to stand and step overground with partial weight bearing and reciprocal gait. The EKSO GT robotic exoskeleton used in this study is a class I medical device (United States FDA) that has the potential to facilitate functional rehabilitation. The variable assist mode offered by the EKSO allows active involvement of subjects having minimal voluntary ability and minimal supraspinal control [7, 8], even in patients with motor complete paralysis. Based on the effort applied by the subject, the onboard computer will provide the necessary assistance to complete the step cycle. The EKSO has several tilt and position sensors that maintain the position of various joints within a fized trajectory. We hypothesized that tonic transcutaneous electrical spinal cord stimulation can be used to re-engage the spinal circuitry to facilitate stepping in the EKSO and to progressively recover voluntary control of specific joint movements after complete paralysis.
II. Methods
A. Clinical assessment and patient information
The UCLA Review Board approved all procedures. The subject was a 38-year-old man at the time of the experiment. He lost his eyesight at the age of 22, and 4 years prior to the experiment fell from a second floor onto a concrete floor damaging his spinal cord at the T9 and L1 vertebral levels. He was assessed clinically as motor and sensory complete (AIS A). The subject had owned the EKSO bionics suit for ~2 years and had completed ~180,000 steps prior to the experiment. He signed an informed consent form. All experimental procedures were approved by the Institutional Review Board of the University of California, Los Angeles.
B. Training and testing procedures
The subject stepped in the EKSO for 1 hr/day in the active (with voluntary effort) mode with a 5 min warm-up in the passive (without voluntary effort) mode. The 1 hr session was divided into three 20-min laps (40 m in length). During the first lap, the stimulation was delivered at the T11 vertebral segment (30 Hz), the second involved stimulation at the Co1 vertebral level (5 Hz), and the third lap consisted of simultaneous stimulation at T11 and Co1. Blood pressure and heart rate were recorded at the end of each lap. At the end of 5 days of training, stepping ability was assessed while the subject walked in the EKSO with and without stimulation at T11 and/or Co1 in both a passive and an active mode. The following day, voluntary ability to perform movements in specific joints was assessed with and without stimulation at T11 and/or Co1 with the subject being in a supine position.
C. EMG and kinematic recordings
During every session, bipolar EMG surface electrodes were used to record bilaterally on the soleus, medial gastrocnemius (MG), tibialis anterior (TA), rectus femoris (RF) and vastus lateralis (VL) [18]. EMG signals were amplified differentially (bandwidth of 10 Hz to 5 KHz) and acquired at 10 KHz using a 16 channel hard-wired A/D board and a customized LabVIEW software (National Instruments, TX) acquisition program.
III. RESULTS
Based on the subject’s feedback, stimulation at T11 resulted in a feeling of ‘tension’ in all proximal lower limb muscles. The ‘tension’ was felt during sitting and increased when stepping (passive mode) in the EKSO. The tension greatly increased when the subject started stepping in the active mode. Tension was not felt with stimulation at Co1 when sitting and was minimal during passive stepping. During active stepping, however, the subject reported high levels of a tingling sensation in his entire leg, especially in the distal muscles, ankle joint and sole of the foot. During stimulation at T11 + Co1, the subject reported tension and tingling in the entire leg. At the end of each 1-hr training session, perspiration was observed in different parts of the upper and lower back, the gluteus muscles, and the calf muscles. This was the first time the subject reported perspiration below the level of lesion since his spinal injury. During the 1-hr training session, the average heart rate increased from 75 to 110 beats/sec and the systolic and diastolic pressure changed from 95 to 72 and 140 to 89 mm of Hg, respectively.
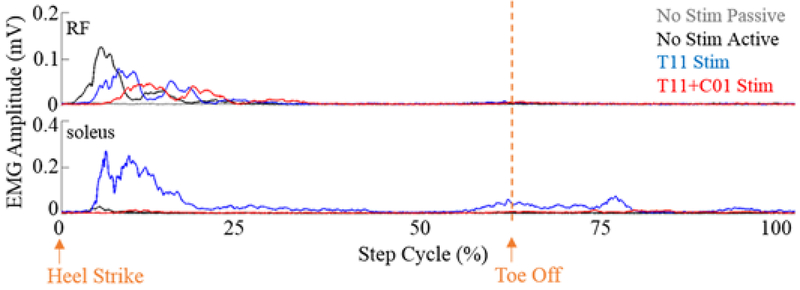
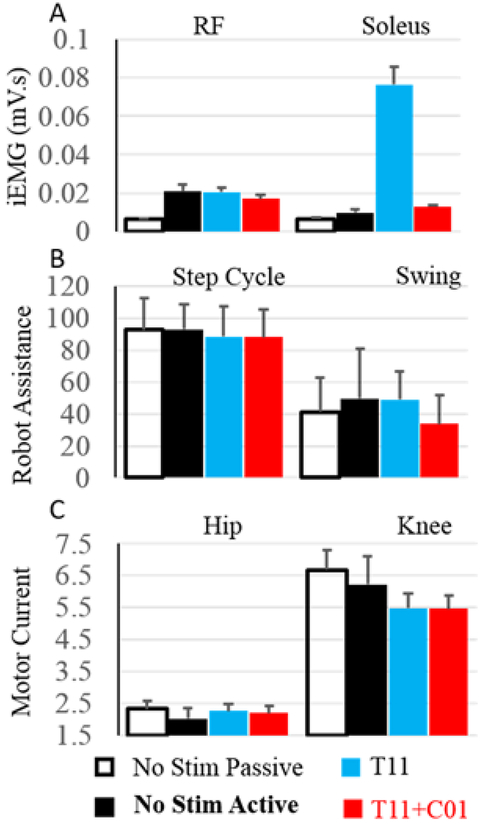
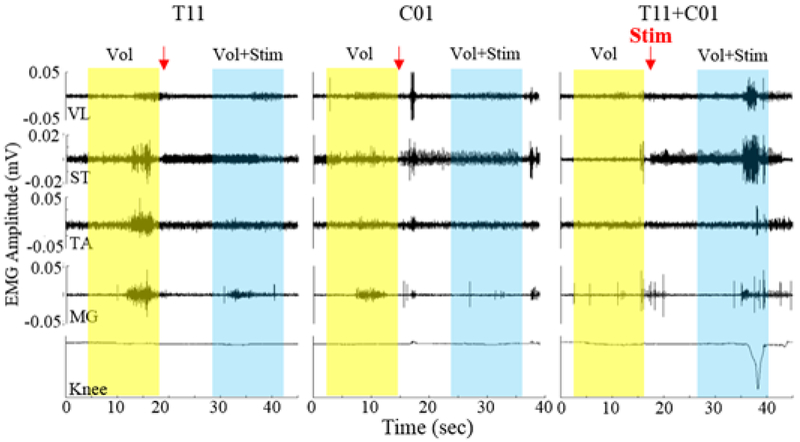
The EMG activity was higher during active compared to passive stepping without stimulation, especially in the RF (Fig. 1). With T11 stimulation the EMG activity was increased in the soleus muscles compared to No Stim with the activation being delayed in RF and lasting longer (Fig. 1). The assistance provided by the robot to maintain the path during the swing phase was greater during active compared to passive stepping without stimulation (Fig. 2B) even though the current drawn by the knee and hip motors were reduced, probably due to reduced movement precision without stimulation. The robot assistance and motor current were lower during stimulation at T11 and T11+Co1 compared to no stimulation. These lower values with stimulation could be attributed to the increased sense of feeling in the legs during active stepping. The EMG activation pattern in the RF during T11+Co1 was further delayed compared to T11 burst lasting longer. To further examine changes in the neural networks, we tested the subject’s ability to voluntarily move specific joints with and with stimulation at T11 and/or Co1 (Fig. 3). When the subject attempted to flex his knee with no stimulation (Fig. 3 yellow highlight), there was co-activation of the MG and soleus with the TA muscle. Little activation was observed in the proximal muscles. The EMG activity in all muscles was lower with stimulation at T11 or Co1 when the subject attempted to flex (Fig. 3, blue highlight). Although EMG activity was observed in all muscles, no change in knee angle was observed either with or without stimulation at T11 or Co1. During stimulation at T11+Co1, however, the subject was successful in flexing his knee completely and there were higher levels of EMG activity in all proximal muscles compared to stimulation at either site alone.
Figure 1:
Mean EMG activity (30 consecutive steps) from the RF and soleus muscles during a normalized step cycle with and without stimulation at T11 and T11+Co1 during active (with voluntary effort) and a passive (without voluntary effort) mode.
Figure 2:
A) mean±SD(30 steps) iEMG from the RF and soleus muscles while stepping in the EKSO. B) mean±SD assistance provided by the EKSO during stepping under different conditions. C) mean±SD current drawn by the knee motor while stepping in the EKSO under different conditions.
Figure 3:
Bandpass filtered EMG with the subject in supine position while attempting to flex the left knee with and without stimulation at T11 and/or Co1.
IV. DISCUSSION
A. Noninvasive activation of the spinal cord
We have developed a novel method for noninvasively neuromodulating the spinal cord using painless transcutaneous stimulation using a special form of electrical pulses at a high frequency [17]. This method enabled the activation of all leg muscles in a coordinated manner to aid in the performance of stepping in the EKSO as well as when performing voluntary tasks. This method has been shown to be effective in inducing locomotor-like activity in non-injured [18] and SCI (under review) subjects when their legs were placed in a gravity-neutral position. As the subject is in a vertical position, combination partial weight bearing and activation of spinal neural networks via stimulation greatly enhances locomotor as well as autonomic functions.
B. Incongruity of clinical and physiological assessments of completeness of paralysis
We have reported changes in the physiological state of the spinal cord in 4 out of 4 clinically motor complete subjects implanted with a 16-electrode epidural array over the L1-S1 spinal levels within weeks of implantation [7, 8]. The results show recovery and progressive improvement in volitional motor control as a result of daily motor training, but only in the presence of epidural stimulation. This increase in excitability was sufficiently close to the motor threshold so that the newly evolved supraspinal descending input to the lumbosacral spinal cord was sufficient to reach motor threshold. Kakulas [19] reported a remarkable finding in the study of 564 human cadavers with SCI. He studied variables such as axonal lesions, traumatic demyelination-remyelination, and quantification of white matter tracts. Surprisingly, many of the cadavers had a proportion of their spinal cord white matter remaining across the level of lesion even though they were completely paralyzed as assessed clinically. The changes in both locomotor and autonomic systems reported by the subject under the influence of stimulation and descending cortical control suggests that the spinal cord of a paralyzed subject clinically diagnosed at AIS A can be physiologically neuromodulated to a functional state
V. Conclusion
We have shown we can successfully neuromodulate the spinal cord neural circuitry controlling overground stepping in the EKSO via transcutaneous spinal cord stimulation and minimal descending control after complete paralysis. This change in excitability also enables voluntary control of legs, cardiovascular function, and thermoregulation.
Acknowledgments
* This research was funded by NIH U01EB15521, R01EB007615 the Christopher & Dana Reeve Foundation and the F. M. Kirby Foundation. The research was conducted in the UCLA Clinical and Translational Research Center (CTRC) supported by National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124. This was also supported by RFBR grant No 13-04-12030 ofi-m as well as the Russian Scientific Fund project No 14-45-00024.
Footnotes
Appendix
VRE, along with (DCL, RRR and YG) hold shareholder interest in NeuroRecovery Technologies, the company providing the electric stimulator for this study. VRE, DCL, RRR and YG hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and it’s subsidiaries.
References
- [1].Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, and Edgerton VR, "Plasticity of functional connectivity in the adult spinal cord," Philos Trans R Soc Lond B Biol Sci, vol. 361, pp. 1635–46, September 29 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, et al. , "Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training," J Neurosci, vol. 25, pp. 11738–47, December 14 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, et al. , "Transformation of nonfunctional spinal circuits into functional states after the loss of brain input," Nat Neurosci, vol. 12, pp. 1333–42, October 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, et al. , "Restoring voluntary control of locomotion after paralyzing spinal cord injury," Science, vol. 336, pp. 1182–5, June 1 2012. [DOI] [PubMed] [Google Scholar]
- [5].Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, et al. , "Somatosensory control of balance during locomotion in decerebrated cat," J Neurophysiol, vol. 107, pp. 2072–82, April 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Musienko PE, Zelenin PV, Lyalka VF, Gerasimenko YP, Orlovsky GN, and Deliagina TG, "Spinal and supraspinal control of the direction of stepping during locomotion," J Neurosci, vol. 32, pp. 17442–53, November 28 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Angeli CA, Edgerton VR, Gerasimenko YP, and Harkema SJ, "Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans," Brain, vol. 137, pp. 1394–409, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. , "Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study," Lancet, vol. 377, pp. 1938–47, June 4 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, et al. , "Step training reinforces specific spinal locomotor circuitry in adult spinal rats," J Neurosci, vol. 28, pp. 7370–5, July 16 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, et al. , "Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input," J Neurosci, vol. 28, pp. 7774–80, July 30 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, et al. , "Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries," J Neurosci, vol. 31, pp. 9264–78, June 22 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barbeau H and Rossignol S, "Enhancement of locomotor recovery following spinal cord injury," Curr Opin Neurol, vol. 7, pp. 517–24, December 1994. [DOI] [PubMed] [Google Scholar]
- [13].Gad P, Choe J, Nandra MS, Zhong H, Roy RR, Tai YC, et al. , "Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats," J Neuroeng Rehabil, vol. 10, p. 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rossignol S, Dubuc R, and Gossard JP, "Dynamic sensorimotor interactions in locomotion," Physiol Rev, vol. 86, pp. 89–154, January 2006. [DOI] [PubMed] [Google Scholar]
- [15].Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, et al. , "Retraining the injured spinal cord," J Physiol, vol. 533, pp. 15–22, May 15 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gad PN, Roy RR, Zhong H, Lu DC, Gerasimenko YP, and Edgerton VR, "Initiation of bladder voiding with epidural stimulation in paralyzed, step trained rats," PLoS One, vol. 9, p. el08184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerasimenko Y, Gorodnichev R, Machueva E, Pivovarova E, Semyenov D, Savochin A, et al. , "Novel and direct access to the human locomotor spinal circuitry," J Neurosci, vol. 30, pp. 3700–8, March 10 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gorodnichev RM, Pivovarova EA, Pukhov A, Moiseev SA, Savokhin AA, Moshonkina TR, et al. , "[Transcutaneous electrical stimulation of the spinal cord: non-invasive tool for activation of locomotor circuitry in human]," Fiziol Cheloveka, vol. 38, pp. 46–56, Mar-Apr 2012. [PubMed] [Google Scholar]
- [19].Kakulas BA, "A review of the neuropathology of human spinal cord injury with emphasis on special features," J Spinal Cord Med, vol. 22, pp. 119–24, Summer 1999. [DOI] [PubMed] [Google Scholar]