Abstract
Tumor cells interact with each other, and their surroundings, using a variety of mechanisms to promote virtually all aspects of cancer progression. One such form of intercellular communication that has been attracting considerable attention from the cancer community and the pharmaceutical industry in recent years involves the ability of cancer cells to generate multiple distinct types of non-classical secretory vesicles, generally referred to as extracellular vesicles (EVs). Microvesicles (MVs) represent one of the major classes of EVs and are formed as a result of the outward budding and fission of the plasma membrane. The other main class of EVs is exosomes, which are generated when multivesicular bodies (MVBs) fuse with the cell surface and release their contents into the extracellular space. Both MVs and exosomes have been shown to contain bio-active cargo, including proteins, metabolites, RNA transcripts, micro-RNAs, and DNA that can be transferred to other cancer cells and stimulate their growth, survival, and migration. However, cancer cell-derived EVs also play important roles in helping re-shape the tumor microenvironment to support tumor expansion and invasive activity, dampen immune responses, as well as enter the circulation to help promote metastatic spread. Here, we provide an overview of what is currently known regarding how the different classes of EVs are generated and contribute to various cancer cell phenotypes. Moreover, we highlight how some of the unique properties of EVs are being used for the development of novel diagnostic and clinical applications.
Keywords: Extracellular vesicles, microvesicles, shedding vesicles, exosomes, microparticles, cancer, stroma, invasion, metastasis, intercellular communication, tumor microenvironment, multi-vesicular body
General EV Characteristics
Malignant transformation involves the accumulation of genetic and epigenetic alterations that manifest themselves by hijacking and de-regulating the cellular machinery that controls normal cell growth, survival, attachment, and migration. Another cellular process that is frequently de-regulated in cancer is cell-to-cell communication, with the growth factor-growth factor receptor paracrine signaling paradigm serving as a prototypical example [1]. However, more recently a unique form of intercellular communication has been identified that appears to be as important as secreted growth factors in mediating disease progression. It involves the ability of cancer cells to generate and release multiple distinct types of non-classical secretory vesicles, collectively referred to as extracellular vesicles (EVs) [2–4]. These vesicles can function in an autocrine, paracrine, and even endocrine fashion, and have been shown to impact several different cancer cell phenotypes, ranging from increasing cell growth [5] to promoting metastasis [6,7]. What has made the study of EVs so intriguing is how they mediate their effects. EVs contain a wide-range of proteins and nucleic acids from their parental (donor) cells [2–4]. The protein cargo includes extracellular matrix proteins, cell adhesion proteins, cell-surface receptor tyrosine kinases, chaperones, cytosolic and nuclear signaling proteins, as well as DNA and RNA binding proteins. The types of nucleic acids that have been identified in EVs are similarly diverse and include RNA transcripts, microRNAs, long non-coding RNAs (lncRNAs), and even DNA. The EVs released by donor cancer cells then dock on to the surfaces of neighboring cells within the tumor micro-environment, or at distant sites, via direct EV-plasma membrane surface interactions or through ligand-receptor-mediated interactions [3]. These docking events have been shown to be sufficient to initiate signaling events in cells [8]. However, EVs are also ultimately taken-up by recipient cells by endocytosis, pinocytosis, and/or direct membrane fusion [3] and their bioactive contents released, further altering cellular processes and intracellular signaling activities [8]. In both cases, EVs derived from cancer cells cause recipient cells to undergo phenotypic changes that support tumor expansion and spread [2,4,9].
There are two broad classes of EVs that are generated by cells, and they can be distinguished from one another based on size and the mechanism through which they are formed. The first class of EVs is most often referred to as microvesicles (MVs), but they are also sometimes called oncosomes (when produced specifically by cancer or transformed cells), shedding vesicles, or ectosomes [2–4]. MV formation is initiated by the outward budding of the cell surface at lipid-raft-like domains [10], which are distinct regions along the plasma membrane characterized by high concentrations of certain lipids, i.e. cholesterol and glycosphingolipids, and proteins such as Flotillin-1 and 2, and several different glycosylphosphatidylinositol (GPI)-anchored proteins [11] (Figure 1A). The budding vesicles are then loaded with cargo and eventually pinched-off at the neck, releasing lipid-bilayer enclosed packages into the extracellular space that range in size from ~100–1000 nm [2–4]. Consistent with the idea that MVs originate from lipid rafts, protein markers of this structure (i.e. Flotillin-2) can be routinely detected in MVs derived from various types of cells [12–14]. Some of the other commonly used MV markers are shown in Figure 2.
Figure 1.
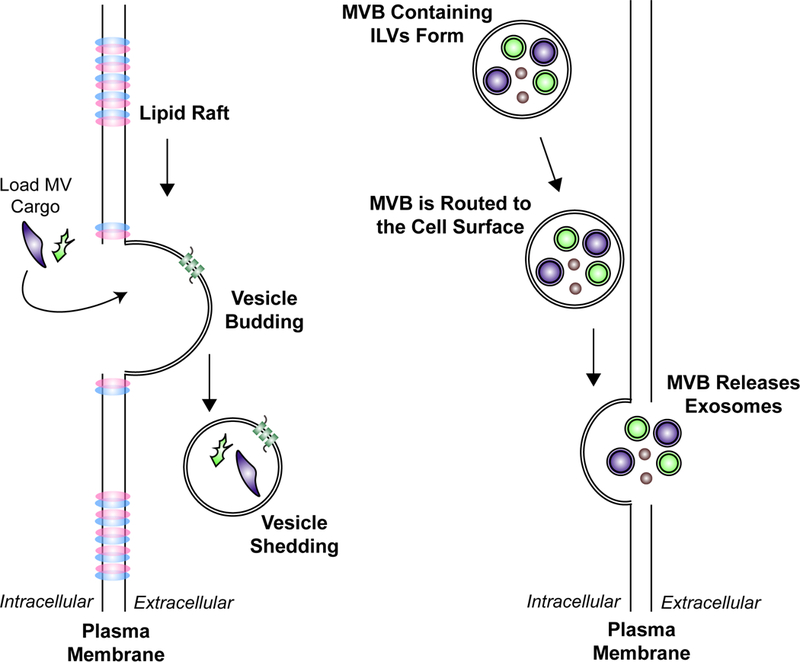
The mechanisms that give rise to the two major classes of EVs, MVs and exosomes. A) MV formation is initiated by the outward budding of the plasma membrane at lipid-raft-like domains. The MV is then loaded with various cargo, before it is shed into the extracellular space. The size of MVs range from 200–2000 nm. B) Exosomes represent the second major class of EVs. They are derived as MVBs containing ILVs are routed to the cell surface. The MVB then fuses with the plasma membrane and releases its contents. The ILVs that are released are referred to as exosomes. This class of EVs is typically 50–120 nm in diameter.
Figure 2.
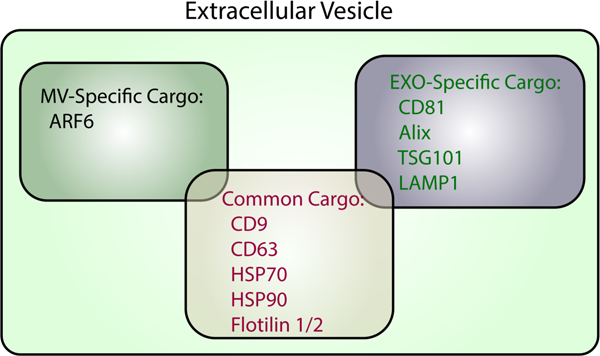
Proteins commonly found in MVs and exosomes. Proteins expressed exclusively in MVs (black), exosomes (green), or are common to both types of EVs (red) are listed.
Exosomes, represent the second major class of EVs [2–4]. Their formation is initiated when multivesicular bodies (MVBs) in the endo-lysosomal pathway accumulate intraluminal vesicles (ILVs) comprised of proteins and nucleic acids. However, instead of being trafficked to the lysosome for degradation, as typically occurs, some of the MVBs are routed to the cell surface [15,16]. At this point, the MVBs fuse with the plasma membrane and release their contents into the extracellular space [15–17]. The secreted ILVs, which typically range in size from ~50–120 nm, are termed exosomes (Figure 1B). Because exosomes are derived from MVBs, they contain proteins known to be involved in MVB function/trafficking including Alix and TSG101, as well as several proteins that play roles in membrane budding, i.e. CD9, CD63, and CD81 [2–4,18]. Many of these same proteins are considered classical exosome markers (Figure 2). However, there continues to be a good deal of debate in the field regarding which protein markers are the best to use to distinguish MVs from exosomes. This is primarily because various studies have shown that, depending on the approach used to isolate EVs, several of these proteins are present in both the MV and exosome fractions (Figure 2). As EV isolation approaches improve, and eventually become standardized, MV- and exosome-specific markers are expected to be identified.
It is worth noting that there are likely to be additional types of EVs, and sub-classes of MVs and exosomes. For example, a recent study showed, using asymmetric flow field-flow fractionation to precisely separate vesicles of different sizes, that at least some forms of cancer cells appear to generate large quantities of EVs, termed exomers, that are distinct from either MVs or exosomes [19]. Exomeres are even smaller than exosomes (20–50 nm) and do not express many of the traditional exosome and MV markers. However, whether this form of EV is common, and how exomeres are generated and the biological roles that they play, are currently unknown.
Mechanisms Regulating EV biogenesis
One area of emphasis in the study of EVs has been to understand how the biogenesis of MVs and exosomes is regulated. Due to the efforts of several research groups, there is now a basic understanding of how these two major classes of EVs are formed and released by cancer cells.
Regulation of MV Biogenesis
The ability of cells to generate MVs was initially observed in highly aggressive cancer cells. For example, the triple-negative MDAMB231 breast cancer cell line and the U87 glioblastoma cell line, were both shown to constitutively generate large amounts of MVs [14,20]. Due to their size, the budding of MVs from the surfaces of these cancer cells could be visualized by immunofluorescence microscopy, upon staining the cells for known MV-associated cargo proteins (Figure 2). The amounts and sizes of MVs released by MDAMB231 and U87 cells could also be determined by subjecting their conditioned medium to nanoparticle tracking analysis (NTA) [14,20]. In a pair of important studies carried-out by the Rak and Breakefield laboratories, it was shown that glioblastoma cells ectopically expressing EGFRvIII, an oncogenic form of the EGF receptor, generated many more MVs, compared to control cells [21,22]. It was subsequently shown that EGF-stimulation of HeLa cervical carcinoma cells, or DU145 prostate cancer cells, caused a dramatic increase in MV formation and release [14,23]. Interestingly, non-transformed cells and low grade cancer cells were found to generate far fewer MVs than their highly aggressive cancer cell counterparts [13,14,21,22]. These findings provided some of the earliest suggestions that malignant forms of cancer are particularly good at generating MVs, and their formation is under the control of at least some proteins known to cause cancer, such as the EGF receptor. They also raise some intriguing questions for further study, such as does the rate at which cancer cells grow correlate with MV production, and would treating cancer cells with therapies that inhibit cell cycle progression also block MV biogenesis?
The signaling pathway that mediates EGF receptor-stimulated MV biogenesis has been delineated [20]. This pathway starts with the EGF receptor stimulating the activation of the small GTPase RhoA. Ectopic expression of an activated form of RhoA in HeLa cervical carcinoma cancer cells markedly increased MV production, while depleting HeLa cells, as well as several other cancer cell lines, of RhoA using siRNAs inhibited MV formation. The signaling partners of RhoA that are essential for MV biogenesis include the Rho-associated, coiled-coil containing protein kinase (ROCK) and LIM kinase (LIMK). The actin de-polymerizing enzyme cofilin becomes inactivated when it is phosphorylated by LIMK, resulting in the accumulation of actin filaments and the necessary re-arrangements to the actin-cytoskeleton that promote MV budding. Consistent with these findings, one of the distinguishing features of MVs protruding from the surfaces of highly aggressive cancer cells is the presence of filamentous actin within the vesicles (14,20).
Another signaling pathway that promotes MV biogenesis requires the activation of the small GTPase, ADP-ribosylation factor 6 (Arf6). Ectopic expression of a dominant-negative form of Arf6 in melanoma cells was shown to lead to a build-up of MVs along their surfaces, suggesting Arf6 triggers a signaling pathway that is important for MV shedding [24]. It was then shown that ARF6 mediates these effects by the sequential activation of phospholipase D (PLD), the extracellular signal-regulated kinase (ERK), and the myosin light chain kinase (MLCK). At the necks of MVs, MLCK phosphorylates a key residue in the regulatory light chain of myosin (MLC) and stimulates the ability of myosin to associate with actin filaments. This, along with the recruitment of additional proteins, such as arrestin domain-containing protein-1 (ARRDC1), and the endosomal protein TSG101, to the necks of MVs is likely to contribute to the contraction, or “pinching off”, process that underlies MV fission and release [25].
Regulation of Exosome Biogenesis
Because exosomes are derived from the ILVs in MVBs, their biogenesis is heavily dependent on the mechanisms that regulate MVB maturation and trafficking [2,26]. The well-established endosomal sorting complexes required for transport (ESCRT) machinery play a crucial role in sorting membrane-associated proteins to ILVs as they are formed in MVBs [27]. Depleting the expression of many components of the multi-subunit ESCRT complex (i.e. TSG101) using shRNAs, effectively blocks ILV formation and correspondingly reduces the amount of exosomes released by a cell [28]. Moreover, many membrane-associated proteins that are sorted to ILVs in an ESCRT-dependent manner have also been identified as exosomal cargo [12,18], re-affirming that MVBs are a primary source of exosomes. However, the presence of large amounts of exosomal cargo that are distinct from the typical cargo found in classical ILVs, such as cytosolic/nuclear proteins, various RNA transcripts, as well as DNA, suggests there are likely additional mechanisms capable of sorting/trafficking these distinct cargo to MVBs that are destined to give rise to exosomes [2–5]. For example, one potentially interesting mechanism for delivering cytosolic proteins into exosomes involves endosomal microautophagy. This self-degradative process uses heat shock protein 70 (Hsc70) to deliver cytosolic proteins to MVBs [29]. Although these MVBs are typically thought to be trafficked to the lysosome and degraded, it is possible that some of them are used to generate exosomes containing cytosolic proteins. Consistent with this idea, Hsc70 is a major protein cargo in exosomes [30,31].
The trafficking of mature MVBs to the cell surface, and their fusion with the plasma membrane, are additional important processes involved in the biogenesis of exosomes [2,3]. Isoforms of the Ras-related protein, Rab27, were among the first proteins identified, using a short hairpin RNA (shRNA)-based screen, that helps mediate these effects [32]. Specifically, Rab27b knockdown was shown to result in the perinuclear accumulation of MVBs, suggesting that Rab27b was responsible for trafficking MVBs to the cell surface. On the other hand, depleting cells of Rab27a, or several of its effectors, prevented the ability of MVBs to efficiently fuse with the plasma membrane. Similarly, several components of the calcium-mediated exocytosis pathway, including synaptotagmin-7 and transient receptor potential channel 3 (TRPML3), have also been shown to promote the fusion of late MVBs and endo/lysosomal compartments with the cell membrane [33–35].
Compared to the well-established mechanisms that underlie MVB-plasma membrane fusion, much less is known regarding the identities of the proteins that determine which MVBs are directed to the cell surface, versus lysosomes. However, there is a growing appreciation that an important functional connection exists between lysosomal activity and exosome biogenesis. For example, in a study by Villarroya-Beltri et al., it was shown that conjugation of interferon-stimulated gene 15 (termed ISGylation) to TSG101, a major component of the ESCRT complex, negatively impacts exosome secretion by increasing the extent of lysosomal-mediated MVB degradation [36]. In another study, knocking down the expression of autophagy related gene 5 (aTg5) from cancer cells using shRNA was shown to inhibit the acidification and degradative activity of lysosomes, resulting in the increased production of exosomes that promoted metastasis [37]. In line with these findings, inhibiting lysosomal activity by treating cells with Chloroquine or Bafilomycin has been consistently shown to enhance exosome release [34,38,39]. Thus, there appears to be a finely tuned mechanism that couples lysosomal function to exosome biogenesis, such that under conditions where lysosomal activity is reduced, the MVBs that would normally be targeted for degradation in the lysosome are instead routed to the cell surface and their contents (the ILVs) released as exosomes.
Analysis of the cargo within the exosomes has revealed that alterations in the fate of MVBs are often accompanied by distinct changes in the exosomal content [34,38,39]. This has raised the possibility that the stage at which MVBs are transported to the plasma membrane can greatly impact the content of the exosomes (Figure 3).
Figure 3.
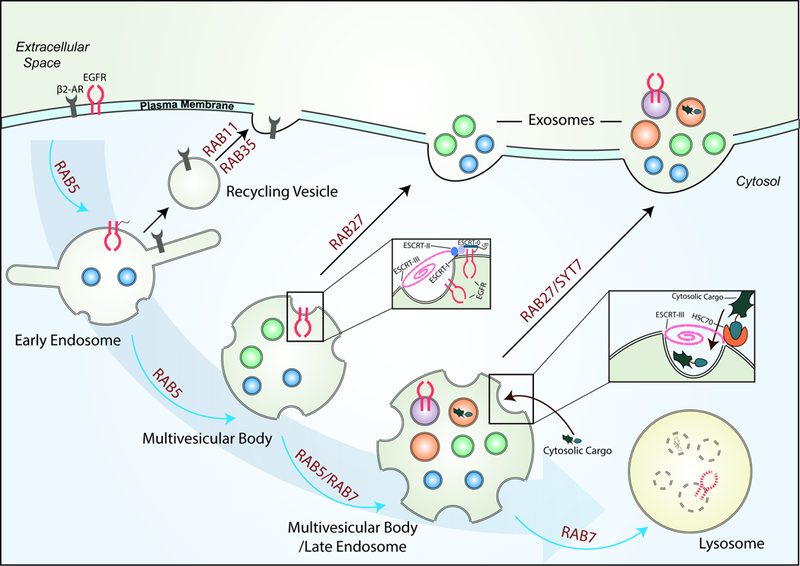
A model showing how the cargo in exosomes can potentially change. MVBs are part of the endocytic pathway, and they undergo a maturation process that includes the sorting of membrane and cytosolic proteins by the ESCRT complex and HSC70 on the limiting membrane of MVBs. This results in the incorporation of cargo proteins into the lumen of nascent ILVs. Many of the MVBs are then trafficked in Rab5- and Rab7-dependent mechanisms to lysosomes, where they are degraded. However, some MVBs can also be routed to the plasma membrane by Rab27 and SYT7, and their contents (i.e. ILVs) released as exosomes. The content of exosomes generated by a cell can potentially change depending on which point during their maturation process the MVBs are trafficked to the plasma membrane, as depicted.
EV Functions in Cancer
The release of MVs and exosomes by cancer cells has been implicated in nearly every aspect of cancer progression. For example, there are numerous studies showing that EVs generated by a cancer cell can enhance the growth, invasive activity, and therapy-resistance of another cancer cell [2–5]. Thus, it is easy to appreciate how the exchange of EVs between two cancer cells can directly impact tumor expansion. However, there is also growing evidence suggesting that EVs from aggressive cancer cells can help shape the tumor microenvironment by influencing the function of non-cancerous cell types. A classic example involves the ability of MVs derived from MDAMB231 breast cancer cells to cause fibroblasts and mammary epithelial cells, two major cell types found adjacent to breast tumor tissue, to acquire the characteristics of a transformed cell, including the ability to grow in low serum conditions and form colonies in soft agar [14]. Fibroblasts treated with exosomes and MVs from cancer cells have also been shown to become re-programed to secrete growth factors, cytokines, and extracellular matrix proteins that are then used by the primary tumor to further promote its growth and survival [40–42]. The effects of EVs on both recipient cancer cells and fibroblasts have been attributed to the transfer of specific EV cargo, including various oncogenes, extracellular matrix proteins, RNA transcripts, and microRNAs. The findings from many of these studies have been extensively covered in previous reviews [2–5], and so they will not be discussed further here.
Instead, we will focus on a few of the more recently described, and less extensively studied, roles for EVs in promoting oncogenic progression, beginning with EV-mediated tumor angiogenesis. The formation of new blood vessels at the site of a tumor is critical for supplying cancer cells with oxygen and the nutrients needed to sustain their growth, as well as for promoting metastatic spread [43]. There are now several lines of evidence suggesting that various proteins and microRNAs contained within, or along the surface of, MVs and exosomes are important contributors to tumor angiogenesis. One of the earliest studies highlighting such a role for EVs demonstrated that treatment of endothelial cells with MVs derived from glioblastoma cells stimulated their growth, migration, and ability to undergo tubulation, an in vitro readout of angiogenesis [44]. The MV cargo that mediated these effects was shown to be an activated form of the EGF receptor, with its transfer to endothelial cells resulting in the stimulation of intracellular signaling events that lead to the production of vascular endothelial growth factor (VEGF), a major player in blood vessel formation. The VEGF secreted by the endothelial cells then bound to VEGF receptors within the same cells and promoted angiogenesis. More recently, our group showed that MVs from breast cancer cell lines, as well as from cell cultures derived from patient-derived xenographs, generated MVs that contained a uniquely crosslinked form of VEGF [45]. The VEGF associated with these large vesicles was capable of inducing a strong and sustained activation of the VEGF receptor in endothelial cells and stimulated tumor angiogenesis. Moreover, this effect was found to be insensitive to the inhibitory actions of the monoclonal VEGF antibody Bevacizumab, providing a plausible explanation as to why some cancer patients treated with Bevacizumab to block tumor angiogenesis fail to respond to the therapy [46].
Immune cells represent another type of cell whose function has been recently shown to be significantly altered by cancer cell EVs. It has long been appreciated that the regions surrounding solid tumors are often immunosuppressed, which is believed to be critical for cancer cells to evade immune surveillance and continue growing [47]. However, how this effect is mediated is still not well understood. A recent study has revealed that EVs, particularly exosomes, may be at the root of this phenomenon [48]. Specifically, glioblastoma cells were shown to release exosomes that contained programmed death ligand-1 (PD-L1). This ligand can then bind to its corresponding receptor, programmed cell death protein-1 (PD1), expressed in T-cells and inactivate them, creating a tumor microenvironment that is depleted of a major functional immune cell type. Another recent study has now demonstrated that exosomes shed by aggressive melanoma cells may have a major role in mediating the immune suppression characteristic of this cancer [49]. It has also been shown that exosomes isolated from breast cancer patients express significantly higher levels of CD47, compared to exosomes from healthy individuals. What makes this finding intriguing is that CD47 serves as a “don’t eat me” signal by binding to signal-regulatory protein α (SIRPα), an inhibitory cell surface receptor expressed in various types of immune cells, including macrophages, monocytes, and T cells [50]. Thus, the net result of tumor cells producing exosomes that contain CD47 could be to dampen the immune response.
In addition to the various important roles played by EVs at the primary tumor site, exosomes from cancer cells are also released into the blood stream where they are believed to facilitate metastatic spread [6,7,51,52]. The specific step of this highly lethal process that exosomes are thought to regulate is the formation of the pre-metastatic niche, which involves preparing secondary sites for the arrival and propagation of circulating cancer cells [7,52]. It was shown that exosomes derived from melanoma cells contained elevated levels of the tyrosine kinase Met (c-Met). Some of these exosomes, when injected into mice, were taken-up by bone marrow progenitor cells, causing them to express c-Met and acquire a migratory phenotype. The “educated” bone marrow progenitors then traveled to the site of secondary colonization, in this case the lung, and promoted the generation of blood cell lineages, as well as increased blood vessel formation, making it more likely that melanoma cells would be able to colonize the lung [52].
Exosomes derived from different types of cancer cells that enter the blood stream of mice also tend to accumulate at future sites of metastasis [6,51]. This effect appears to be directed by the expression of specific combinations of integrins, a family of adhesion proteins, along the surfaces of exosomes. The exosomes decorated with integrins are capable of preferentially adhering to certain resident cells within a tissue by binding to the different extracellular matrix proteins they secrete. The exosomes are then taken-up by recipient cells and induce a number of changes at the secondary site that make it more receptive to a tumor cell, such as stimulating the production of growth factors and cytokines, promoting the recruitment of stromal cells, and generating a highly fibrotic environment. Although the findings linking exosomes to immune suppression and metastasis are provocative and thought provoking, it is still too early to definitively say that extracellular vesicles play a major role in mediating these aspects of cancer progression. As the function of exosomes are investigated further using additional animal models of immune suppression and metastatic spread, a clearer picture of how generally important EVs are in promoting the phenotypes of highly aggressive cancer cells will be generated.
Concluding Remarks
The field of EVs has entered an exciting time, with new discoveries and novel applications coming forth at a rapid pace. What were once intriguing speculations, namely the ability to use these vesicles as diagnostic markers and therapeutic vehicles, are now becoming realities. This has been particularly true for cancer, where investigators have been able to deliver shRNAs targeting oncogenic proteins like K-Ras to tumors [53] and to isolate EVs from the blood and tissue fluids of patients [2–5]. The latter efforts are now converging with the rapidly developing field of liquid biopsies for cancer diagnosis and hold great promise for facilitating the diagnosis of cancer and the ultra-sensitive assessment of the success of specific therapies. Given the continually expanding roles for EVs in a range of biological processes and pathological conditions aside from cancer, including developmental events, neuronal function, neurodegenerative disorders, the immune response, bacterial and viral infections, and aging, it seems that the ‘sky is the limit’ regarding the applications for these vesicles in diagnosis and treatment strategies.
Still, in order to take full advantage of these exciting possibilities, a number of important questions will need to be addressed. Perhaps first and foremost is the need to achieve a better biochemical understanding of the different classes of EVs that exist. Up until now, EVs have typically been divided into two sub-families, based on their sizes and the underlying mechanisms leading to their formation, namely exosomes which are generated through endosomal trafficking, and MVs which are produced and shed exclusively from the plasma membrane [2–5]. However, from the work of Thery and colleagues [12], it is now apparent that what previously were designated as exosomes and MVs cannot strictly be resolved based on vesicle size, as both of these types of EVs show some overlap in the 100–200 nm size range. Moreover, it is becoming clear that there are multiple classes of ‘small vesicles’, as well as a new type of biological nanoparticle identified by Lyden and colleagues and designated as exomeres [19]. Each of these different vesicle types appears to be enriched in specific protein and nucleic acid cargo, all of which has led to the suggestion that a more appropriate designation for these different classes of EVs would be to designate them as small and large vesicles. Certainly, a pressing question will be to see if there is uniformity between cell types in terms of the types of EVs that are designated, whether these vesicles can be defined biochemically by their specific types of protein and nucleic acid cargo, or, if the sizes and types of EVs generated will vary with cell type, such that the type of vesicle being studied will be very much cell context dependent.
Another question requiring serious attention concerns the nature of the mechanisms responsible for the loading of different EVs with protein and nucleic acid cargo. It is interesting that certain cargo (e.g. heat shock proteins) appear to be present in more than one type of EVs, whereas in many other cases, there appears to be a high degree of specificity regarding the proteins present in the different classes of vesicles [12,18]. What is even more puzzling are the findings that what appear to be identical types of MVs shed by different cancer cells can show an extraordinary degree of specificity regarding their protein cargo. Thus, vesicles generated and shed from the cell surface of one type of cancer cell may contain proteins that are lacking in what would appear to be the same class of vesicles shed by another type of cancer cell. How this protein specificity is achieved is still not understood. The same types of specificity appears to be true for the RNA and DNA content of EVs. While we are starting to at least gain some clues regarding RNA-binding proteins that might contribute to the loading of RNA into EVs [54,55], virtually nothing is known regarding how the DNA content is loaded [56].
Finally, a critically important area of future study will be the development of strategies to manipulate the formation of EVs, so as to block their actions in differ disease states. For example, the generation of different classes of EVs in cancer cells appears to be under the control of the cellular metabolic programs [20,57]. Therefore, the development of metabolic inhibitors that are specific for cancer cell enzymatic activities could provide a strategy for blocking the biogenesis of EVs in these cells and thereby provide viable strategies for blocking their actions in metastatic spread. Similarly, other proteins that regulate the formation of the actin filament base responsible for generating the larger vesicles (MVs) may offer potential therapeutic targets, as well as those proteins implicated in the generation of small vesicles from the endosomal-lysosomal trafficking pathways (i.e. Rab GTPases, tetraspanins, and ESCRT proteins) [2–4]. The value of these strategies are likely to expand beyond cancer, as senescent cells have now been shown to shed extracellular vesicles with cargo that confer senescence upon the recipient cells that they engage. On the other hand, there may well be important value in generating strategies to increase the production of extracellular vesicles from stem cells, if as suggested by early studies, these vesicles are able to confer functions that would benefit regenerative medicine [58].
Thus, there seems to be little question that the field of EVs has taken hold, describing an important phenomenon in biology and disease. We have attempted to provide an overview of this rapidly developing field, and despite important progress being made, there is still a significant body of work that needs to be corroborated and reproduced by other groups. While there remains much to do, the future is bright and likely will provide fresh new insights into fundamental processes of relevance to the life sciences as well as offer new ideas for diagnosing and treating a variety of disorders.
References
- 1.Sever R and Brugge JS (2015) Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 5, a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desrochers LM, Antonyak MA and Cerione RA (2016) Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev. Cell 37, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Niel G, D’Angelo G and Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G and Stoorvogel W (2013) Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R (2016) The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M., et al. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H and Lyden D (2016) Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French KC, Antonyak MA and Cerione RA (2017) Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 67, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EL Andaloussi S, Mäger I, Breakefield XO and Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–57. [DOI] [PubMed] [Google Scholar]
- 10.Del Conde I, Shrimpton CN, Thiagarajan P and López JA (2005) Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604–1611. [DOI] [PubMed] [Google Scholar]
- 11.Sezgin E, Levental I, Mayor S and Eggeling C (2017) The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B et al. (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 113, E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreger BT, Dougherty AL, Greene KS, Cerione RA and Antonyak MA (2016) Microvesicle cargo and function changes upon induction of cellular transformation. J. Biol. Chem. 291, 19774–19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL et al. (2011) Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. U. S. A. 108, 4852–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan BT, Teng K, Wu C, Adam M and Johnstone RM (1985)Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101, 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding C, Heuser J and Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J et al. (2018) Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 217, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greening DW, Xu R, Gopal SK, Rai A and Simpson RJ (2017) Proteomic insights into extracellular vesicle biology–defining exosomes and shed microvesicles. Expert Rev. Proteomics 14, 69–95. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H et al. (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Antonyak MA, Zhang J and Cerione RA (2012) RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A et al. (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624. [DOI] [PubMed] [Google Scholar]
- 22.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ et al. (2009) Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G et al. (2009) ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabhan JF, Hu R, Oh RS, Cohen SN and Lu Q (2012) Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. U. S. A. 109, 4146–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo M, Raposo G and Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. [DOI] [PubMed] [Google Scholar]
- 27.Schuh AL and Audhya A (2014) The ESCRT machinery: From the plasma membrane to endosomes and back again. Crit. Rev. Biochem. Mol. Biol. 49, 242–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P et al. (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126, 5553–5565. [DOI] [PubMed] [Google Scholar]
- 29.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A et al. (2011) Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 20, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Géminard C, Nault F, Johnstone RM and Vidal M (2001) Characteristics of the interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J. Biol. Chem. 276, 9910–9916. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S et al. (2015) Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. U. S. A. 112, E2497–E2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30. [DOI] [PubMed] [Google Scholar]
- 33.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K and Andrews NW (2000) Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 148, 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao Y, Li G, Zhang X, Xu H and Abraham SN (2015) A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell 161, 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N et al. (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 5, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O et al. (2016) ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 7, 13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo H, Chitiprolu M, Roncevic L, Javalet C, Hemming FJ, Trung MT et al. (2017) Atg5 disassociates the V1V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev. Cell 43, 716–730.e7. [DOI] [PubMed] [Google Scholar]
- 38.Vingtdeux V, Hamdane M, Loyens A, Gelé P, Drobeck H, Bégard S et al. (2007) Alkalizing drugs induce accumulation of amyloid precursor protein byproducts in luminal vesicles of multivesicular bodies. J. Biol. Chem. 282, 18197–18205. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ et al. (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 42, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webber J, Steadman R, Mason MD, Tabi Z and Clayton A (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70, 9621–9630. [DOI] [PubMed] [Google Scholar]
- 41.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R et al. (2015) Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 34, 290–302. [DOI] [PubMed] [Google Scholar]
- 42.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC et al. (2017) Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 170, 352–366.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potente M, Gerhardt H and Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887. [DOI] [PubMed] [Google Scholar]
- 44.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC and Rak J (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. U. S. A. 106, 3794–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF et al. (2017) A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 8, 14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortimer J, Zonder H and Pal SK (2012) Lessons learned from Avastin. Cancer Control 19, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabinovich GA, Gabrilovich D and Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H et al. (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W et al. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang Y et al. (2017) Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 121, 121–129. [DOI] [PubMed] [Google Scholar]
- 51.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK et al. (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF Melo SA et al. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R et al. (2017) Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl. Acad. Sci. U. S. A. 114, E8987–E8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R et al. (2016) The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling MicroRNA sorting. Cell Rep. 17, 799–808. [DOI] [PubMed] [Google Scholar]
- 56.Kalluri R and LeBleu VS (2016) Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb. Symp. Quant. Biol. 81, 275–280. [DOI] [PubMed] [Google Scholar]
- 57.Wu B, Liu J, Zhao R, Li Y, Peer J, Braun AL et al. (2018) Glutaminase 1 regulates the release of extracellular vesicles during neuroinflammation through key metabolic intermediate alpha-ketoglutarate. J. Neuroinflammation 15, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA and Antonyak MA (2016) Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 7, 11958. [DOI] [PMC free article] [PubMed] [Google Scholar]


