Summary
Aims
To examine whether early rise of neutrophil‐to‐lymphocyte ratio (NLR) after patient hospitalization correlates with 30‐day mortality in patients with spontaneous intracerebral hemorrhage (ICH).
Methods
This retrospective study included all patients receiving treatment for spontaneous ICH between January 2015 and September 2016 at the Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences in Shanghai, China. NLR was determined at admission (T1), at 24‐48 hours (T2) and 5‐7 days (T3). NLR and clinicopathologic features were compared between those who survived for >30 days vs not. Multivariate regression was used to identify risk factors for 30‐day mortality.
Results
A total of 275 subjects were included in the analysis: 235 survived for at least 30 days; the remaining 40 subjects died within 30 days. The patients who died within 30 days had higher ICH score, larger ICH volume, and lower GCS score (all P < 0.05). In comparison with the baseline (NLRT 1), NLR at 24‐48 hours (NLRT 2) and 5‐7 days (NLRT 3) was significantly higher in patients who died within 30 days (P < 0.05), but not in patients surviving for >30 days. In the multivariate analysis, the 30‐day mortality was associated with both NLRT 2 (OR 1.112, 95%CI 1.032‐1.199, P = 0.006) and NLRT 3 (OR 1.163, 95%CI 1.067‐1.268, P = 0.001). Spearman correlation analysis showed that both NLRT 2 and NLRT 3 correlated inversely with GCS score and positively with ICH score and ICH volume at the baseline.
Conclusions
Early rise of NLR predicts 30‐day mortality in patients with spontaneous ICH.
Keywords: inflammation, mortality, neutrophil‐to‐lymphocyte ratio, spontaneous intracerebral hemorrhage
1. INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) refers to nontraumatic rupture of primary intracranial blood vessels, allowing blood to flow into the brain parenchyma or cerebral ventricles, resulting in the formation of hematoma that causes neuronal and glial injury and subsequent inflammatory response.1 ICH carries high risk of morbidity and mortality.2
Inflammatory response in ICH is intimately associated with hematoma expansion.3 Neutrophil‐to‐lymphocyte ratio (NLR) is an important determinant of inflammatory response4 and has been proposed as a prognostic indicator in many clinical contexts, including short‐term morbidity and mortality after acute ischemic stroke, ST segment‐elevated myocardial infarction, and acute cardiac insufficiency.5, 6, 7 NLR has also been used as a predictor of long‐term morbidity and mortality in malignancy, autoimmune diseases, and metabolic syndrome.8, 9, 10, 11 In patients with ICH, NLR has been closely associated with 30‐day mortality and 90‐day disability as well as risk of cerebral hematoma enlargement.3, 12, 13, 14
NLR varies with severity of bleeding during hemorrhagic transformation after cerebral thrombolysis15 and with the duration between ICH onset and blood sampling.12, 16, 17 The fact that NLR changes with ICH progression raises the question of under what conditions.13 NLR is a useful prognostic indicator in ICH. In this study, we examined the relationship between NLR at different time points and 30‐day mortality in patients with spontaneous ICH.
2. METHODS
2.1. Study subjects
The initial screen included 307 patients receiving treating for spontaneous ICH at the Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences between January 2015 and September 2016. For inclusion in data analysis, subjects must meet the following criteria: (i) first attack of spontaneous ICH, verified by CT scans; 2) age ≥18 years. Subjects were excluded from analysis if (i) hospital admission was >24 hours after disease onset; (ii) comorbidity with hematologic disorders or a history of malignancy; (iii) use of immunosuppressants of anticoagulants; (iv) a history of infection within the past 2 weeks; (v) stroke within the past 6 months. The final analysis included 275 patients (75.7% men; age range, 27‐94 years). The diagnosis was established by computed tomography (CT) and conforms to the criteria of the American Heart Association and American Stroke Association18 in all 275 cases.
2.2. Study methods
Demographic information, medical history (eg, hypertension and diabetes), Glasgow coma scale (GCS) score, and head CT scan results were retrieved from medical records. Hemorrhage location was recorded as supra‐ vs infratentorial based on head CT. Hematoma volume was calculated using the ABC/2 software19 and the formula a × b × c × 1/2, where a refers to hematoma thickness, and b and c refer to the largest vertical diameters of the high‐intensity region on CT scan. ICH score was calculated based on clinical data and CT results.20
Venous blood was obtained at admission (T1; n = 275), at 24‐48 hours after admission (T2; n = 268), and at 5‐7 days after admission (T3; n = 256). For comparison, study subjects were divided artificially into a subgroup surviving for > 30 days (n = 235) vs a subgroup who died within 30 days (n = 40).
2.3. Statistical methods
Data were analyzed using SPSS 19.0 (IBM, Chicago, IL, USA). All continuous variables are expressed as median (interquartile range, IQR) and were analyzed using the nonparametric Mann‐Whitney test. Categorical data are expressed as frequencies and were analyzed using the chi‐squared test. Changes of NLR over time were examined using repeated‐measurements test. Spearman correlation was used to analyze factors associated with NLR. Multivariate logistic regression was used to identify risk factors for 30‐day mortality. P < 0.05 was considered statistically significant.
2.4. Ethical approval
The study protocol was approved by the Ethics Review Board of Jiading District Central Hospital (2017‐ZD‐03). All subjects were de‐anonymized. Written informed consent was waived by the Ethics Review Board.
3. RESULTS
A total of 275 subjects were included in the analysis: 235 survived for at least 30 days; the remaining 40 subjects died within 30 days. In comparison with those who survived for at least 30 days, subjects who died within 30 days had significantly lower GCS score, higher score, and hemorrhage volume (P < 0.05 for all; Table 1).
Table 1.
Clinicopathologic features in patients who survived or died within 30 d
| Survived (n = 235) | Died (n = 40) | P | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 178 (75.7) | 29 (72.5) | 0.660 |
| Age in yr, median (P25,P75) | 69 (53, 79) | 71 (52, 82) | 0.889 |
| Age ≥80 yr, n (%) | 57 (24.3) | 12 (30.0) | 0.439 |
| Comorbidities, n (%) | |||
| Hypertension | 171 (72.8) | 27 (67.5) | 0.493 |
| Diabetes | 29 (12.3) | 5 (12.5) | 0.977 |
| Smoking | 126 (53.6) | 25 (62.5) | 0.297 |
| Alcohol drinking | 97 (41.3) | 14 (35.0) | 0.454 |
| Atrial fibrillation | 49 (20.9) | 13 (32.5) | 0.103 |
| Cerebrovascular disease family history | 1 (0.4) | 0 (0.0) | 1.000 |
| Dyslipidemia | 62 (26.4) | 13 (32.5) | 0.422 |
| Duration from onset to hospitalization, h | 5.0 (2.0, 15.1) | 6.3 (2.7, 11.6) | 0.936 |
| Site of bleeding, n (%) | |||
| Supratentorial | 207 (88.1) | 33 (82.5) | 0.327 |
| Infratentorial | 28 (11.9) | 7 (17.5) | |
| ICH volume in mL, median (P25,P75) | 8.9 (3.3, 22.4) | 45.6 (20.1, 80.0) | <0.001 |
| ICH volume ≥30 mL, n (%) | 42 (17.9) | 27 (67.5) | <0.001 |
| Concurrent ventricular hemorrhage, n (%) | 58 (24.7) | 13 (32.5) | 0.296 |
| GCS score, median (P25,P75) | 14.0 (12.0, 15.0) | 8.0 (5.0, 11.0) | <0.001 |
| ICH score, median (P25,P75) | 1.0 (0.0, 2.0) | 3.0 (2.0, 3.0) | <0.001 |
| Blood pressure in mm Hg, median (P25,P75) | |||
| Systolic at admission | 163 (144, 184) | 170 (141, 190) | 0.492 |
| Diastolic at admission | 90 (78, 103) | 88 (74, 102) | 0.633 |
| Systolic at 24 h after admission | 148 (132, 160) | 147 (131, 158) | 0.571 |
| Diastolic at 24 h after admission | 82 (75, 91) | 87 (73, 92) | 0.990 |
| Drug use within first week | |||
| Mannitol | 168 (71.5) | 33 (82.5) | 0.147 |
| Hyperosmolar saline | 17 (7.2) | 3 (7.5) | 0.952 |
| Corticosteroids | 22 (9.4) | 7 (17.5) | 0.121 |
| Anti‐epileptic drugs | 7 (3.0) | 3 (7.5) | 0.165 |
| Subject number at various times | |||
| T1 | 275 | 0 | |
| T2 | 268 | 7 | |
| T3 | 256 | 19 | |
GCS, Glasgow coma scale; ICH, intracerebral hemorrhage.
The bold values indicate P < 0.05.
The two groups had comparable NLR at admission (Figure 1). In subjects who died within 30 days, early increase in NLR was apparent: NLR was 2.4 (1.4‐6.9) upon admission (T1), 11.3 (8.0‐19.4) at 24‐48 hours (T2), and 12.9 (3.2‐20.1) at 5‐7 days (T3; P = 0.037). In the subjects who survived for at least 30 days, NLR remained relatively stable: 3.3 (1.9‐6.1) at T1, 4.9 (2.8‐7.9) at T2, and 4.2 (2.5‐6.3) at T3 (P = 0.122). At both T2 and T3, NLR was significantly higher in those who died within 30 days (P < 0.05).
Figure 1.
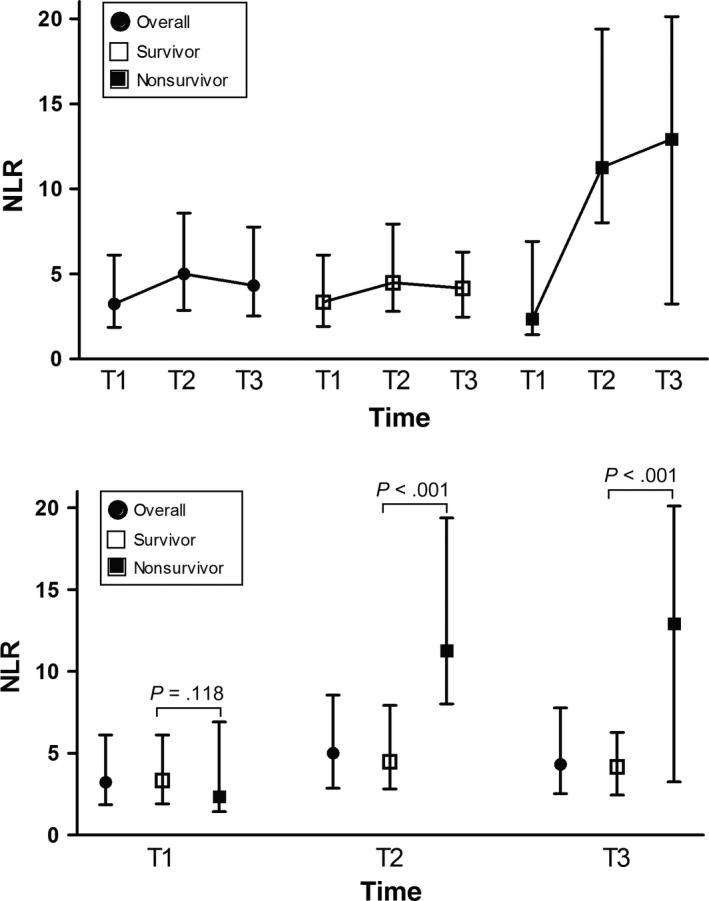
NLR over time in the subjects who died within 30 d versus in those surviving for >30 d. T1, at admission; T2, 24‐48 h after admission; T3, 5‐7 d after admission
In multivariate regression, ICH volume, NLRT2, and NLRT3 were independent risk factors for 30‐day mortality, even after adjustment for other variables (Table 2). NLRT1 was not associated with 30‐day mortality.
Table 2.
Potential factors associated with 30‐day mortality: results of multivariate regression
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Model 1 | ||||
| Male | 2.502 | 0.948 | 6.601 | 0.064 |
| Age | 1.005 | 0.978 | 1.033 | 0.718 |
| NLRT1 | 1.039 | 0.973 | 1.110 | 0.253 |
| ICH volume | 1.029 | 1.013 | 1.045 | <0.001 |
| GCS score | 0.838 | 0.740 | 0.948 | 0.005 |
| Infratentorial hemorrhage | 3.213 | 1.028 | 10.041 | 0.045 |
| Concurrent ventricular hemorrhage | 1.387 | 0.558 | 3.446 | 0.482 |
| Model 2 | ||||
| Male | 2.951 | 0.719 | 12.118 | 0.133 |
| Age | 1.013 | 0.970 | 1.058 | 0.546 |
| NLRT2 | 1.112 | 1.032 | 1.199 | 0.006 |
| ICH volume | 1.032 | 1.010 | 1.054 | 0.004 |
| GCS score | 0.892 | 0.747 | 1.066 | 0.209 |
| Infratentorial hemorrhage | 4.021 | 0.840 | 19.250 | 0.082 |
| Concurrent ventricular hemorrhage | 2.143 | 0.494 | 9.290 | 0.309 |
| Model 3 | ||||
| Male | 2.587 | 0.507 | 13.199 | 0.253 |
| Age | 1.008 | 0.958 | 1.060 | 0.771 |
| NLRT3 | 1.163 | 1.067 | 1.268 | 0.001 |
| ICH volume | 1.033 | 1.008 | 1.058 | 0.011 |
| GCS score | 0.926 | 0.752 | 1.141 | 0.471 |
| Infratentorial hemorrhage | 4.767 | 0.776 | 29.289 | 0.092 |
| Concurrent ventricular hemorrhage | 1.831 | 0.317 | 10.572 | 0.499 |
GCS, Glasgow coma scale; ICH, intracerebral hemorrhage; NLR, neutrophil‐to‐lymphocyte ratio.
The bold values indicate P < 0.05.
Spearman correlation analysis showed that NLRT2 and NLRT3 correlated positively with hemorrhage volume, ICH scores, and mortality, and negatively with GCS score (all P < 0.05; Table 3). NLRT1 did not correlate with any variables.
Table 3.
Correlation of NLR with clinical features at various time points
| NLRT1 | NLRT2 | NLRT3 | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Male | 0.112 | 0.064 | 0.086 | 0.205 | 0.070 | 0.377 |
| Age | 0.097 | 0.108 | 0.148 | 0.028 | 0.117 | 0.137 |
| ICH volume | 0.071 | 0.243 | 0.477 | <0.001 | 0.261 | 0.001 |
| Infratentorial hemorrhage | 0.017 | 0.778 | 0.120 | 0.075 | 0.065 | 0.413 |
| Concurrent ventricular hemorrhage | 0.103 | 0.089 | 0.110 | 0.101 | 0.033 | 0.679 |
| GCS score | −0.042 | 0.488 | −0.436 | <0.001 | −0.373 | <0.001 |
| ICH score | 0.032 | 0.593 | 0.339 | <0.001 | 0.271 | <0.001 |
| Death | 0.095 | 0.118 | 0.333 | <0.001 | 0.284 | <0.001 |
GCS, Glasgow coma scale; ICH, intracerebral hemorrhage; NLR, neutrophil‐to‐lymphocyte ratio; T1, at admission; T2, at 24‐48 h after admission; T3, at 5‐7 d after admission.
The bold values indicate P < 0.05.
4. DISCUSSION
The present study found early increase in NLR after admission in subjects who died within 30 days. NLR within the first week after admission (both 24‐48 hours and 5‐7 days), but not upon admission, closely correlated with 30‐day mortality.
Inflammatory response plays an important role in spontaneous ICH. Higher number of leukocytes, neutrophils, mononuclear cells, and other inflammatory cells correlates with poorer prognosis.21, 22, 23 As a surrogate marker for inflammation, NLR is not influenced by exercise and dehydration.24 In patients with spontaneous ICH, NLR is closely related to enlargement of hematoma17 and predicts 30‐day morbidity and mortality.13 Increased NLR is also related to increased 90‐day morbidity and mortality and decreased quality of life,14 and has been shown to be an independent risk for ICH development in diabetic patients.25 NLR increases with severity of hemorrhagic transformation and with time following cerebral thrombolysis.15 Given that fact that inflammation occurs through all stages in patients with spontaneous ICH,26 it is reasonable to speculate that NLR could reflect ICH progression. However, at what time point(s) NLR could serve as an ideal prognostic indicator remains unknown.
Consistent with those of previous reports,27, 28, 29 subjects who died within 30 days in our study had significantly higher ICH volume and ICH score and lower GCS score. Many factors may have contributed to the correlation between NLR and 30‐day mortality. A previous study showed that leukocytes start to accumulate around and infiltrate the hematoma as early as 5 hours after ICH.30 At 1‐3 days after ICH, macrophages start to infiltrate the hematoma, with a plateau at 7‐10 days after ICH.31 In the current study, both NLR and ICH volume increased at 24 hours after ICH; such changes may reflect infiltration of inflammatory cells around the hematoma. We also found that NLR positively correlated with ICH volume, which is an important determinant of ICH mortality.
In contrast to the early NLR increase in subjects who died within 30 days, patients who survived had relatively stable NLR level within the first week after hospitalization, emphasizing the need to monitor the dynamic changes in NLR within this period of time. Upon ICH, inflammatory cells that infiltrate the lesions and surrounding tissues activate glial cells, which in turn release pro‐inflammatory cytokines, thus forming a vicious cycle.32 Starting approximately 24 hours after ICH onset, the release of cytotoxic substances including hemoglobin, heme, and iron further aggravates the condition.33 With the progression of ICH, apoptosis of cells become apparent, thus worsening the inflammatory response.34 In the current study as well as an earlier report from this group of researchers, NLR at the time of admission (roughly 6 hours after disease onset) did not correlate with 30‐day mortality. Such findings possibly reflect delayed inflammatory response in elderly patients.35
The current study has several limitations. First, its retrospective design and relatively small sample prevent conclusions about the potentially causal relationships. Second, the study was conducted based on patients hospitalized in a secondary hospital away from major metropolitan centers. As a result, subjects in the current study had generally less severe conditions. Such a notion is supported by the considerably lower mortality at 14.5% vs 40%‐60% mortality in a previous report by Hemphill and colleagues.20 This discrepancy may limit the extrapolation of our findings to severe ICH patients. Lack of influence of patient age on mortality reflects, in our opinion, mostly due to the generally less ill patients in the current study. This speculation is supported by much lower mortality in our study vs that reported previously for ICH patients. For example, the mortality was 45% in a previous study by Hemphill and colleagues20 and only 14.5% in the current study despite of comparable age (66 ± 15 vs 66.4 ± 15.1 years of age; 21.7% vs 25.1% at >80 years). Intraventricular hemorrhage (IVH) is a proven risk factor of increased mortality and poor functional outcome.36 Lack of difference in the presence of IVH between the 2 groups could be due to the relatively low rate of IVH in the current study (25.8%) vs 55% in the Hemphill study,20 which in turn, could have attributed to the low mortality.
5. CONCLUSION
In summary, the current study indicated substantial rise in NLR within the first week after hospitalization, but only in those died within 30 days and not in those surviving beyond 30 days. Future prospective studies are needed to validate this finding. Also, the mechanisms underlying such an association need further investigation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The work was supported by New Key Subjects of Jiading District (2017‐ZD‐03), the Seed Fund (Natural Science Class) of Shanghai University of Medicine & Health Sciences (HMSF‐17‐21‐026), and Foundation of the Public Health Bureau of Jiading (2017‐KY‐09).
Wang F, Xu F, Quan Y, et al. Early increase of neutrophil‐to‐lymphocyte ratio predicts 30‐day mortality in patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. 2019;25:30–35. 10.1111/cns.12977
The first two authors contributed equally to this study.
Contributor Information
Shan‐You Hu, Email: hushanyou9@163.com.
Xiao Wu, Email: wx5187@163.com.
REFERENCES
- 1. Babu R, Bagley JH, Di C, Friedman AH, Adamson C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage‐induced secondary brain injury and as potential targets for intervention. Neurosurg Focus. 2012;32:E8. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8:355‐369. [DOI] [PubMed] [Google Scholar]
- 3. Gusdon AM, Gialdini G, Kone G, et al. Neutrophil‐Lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48:2589‐2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5‐14. [PubMed] [Google Scholar]
- 5. Xue J, Huang W, Chen X, et al. Neutrophil‐to‐lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:650‐657. [DOI] [PubMed] [Google Scholar]
- 6. Sawant AC, Adhikari P, Narra SR, Srivatsa SS, Mills PK, Srivatsa SS. Neutrophil to lymphocyte ratio predicts short‐ and long‐term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J. 2014;21:500‐508. [DOI] [PubMed] [Google Scholar]
- 7. Turfan M, Erdogan E, Tasal A, et al. Neutrophil‐to‐lymphocyte ratio and in‐hospital mortality in patients with acute heart failure. Clinics (Sao Paulo). 2014;69:190‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Kalhor N, Hu J, et al. Pretreatment neutrophil to lymphocyte ratio is associated with poor survival in patients with stage I‐III non‐small cell lung cancer. PLoS ONE. 2016;11:e0163397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uslu AU, Kucuk A, Sahin A, et al. Two new inflammatory markers associated with disease activity score‐28 in patients with rheumatoid arthritis: neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio. Int J Rheum Dis. 2015;18:731‐735. [DOI] [PubMed] [Google Scholar]
- 10. Kaya H, Ertas F, Islamoglu Y, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20:50‐54. [DOI] [PubMed] [Google Scholar]
- 11. Buyukkaya E, Karakas MF, Karakas E, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. 2014;20:159‐163. [DOI] [PubMed] [Google Scholar]
- 12. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47:1654‐1657. [DOI] [PubMed] [Google Scholar]
- 13. Wang F, Hu S, Ding Y, et al. Neutrophil‐to‐lymphocyte ratio and 30‐day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:182‐187. [DOI] [PubMed] [Google Scholar]
- 14. Tao C, Hu X, Wang J, Ma J, Li H, You C. Admission neutrophil count and neutrophil to lymphocyte ratio predict 90‐day outcome in intracerebral hemorrhage. Biomark Med. 2017;11:33‐42. [DOI] [PubMed] [Google Scholar]
- 15. Guo Z, Yu S, Xiao L, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflammation. 2016;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giede‐Jeppe A, Bobinger T, Gerner ST, et al. Neutrophil‐to‐lymphocyte ratio is an independent predictor for in‐hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis. 2017;44:26‐34. [DOI] [PubMed] [Google Scholar]
- 17. Sun Y, You S, Zhong C, et al. Neutrophil to lymphocyte ratio and the hematoma volume and stroke severity in acute intracerebral hemorrhage patients. Am J Emerg Med. 2017;35:429‐433. [DOI] [PubMed] [Google Scholar]
- 18. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032‐2060. [DOI] [PubMed] [Google Scholar]
- 19. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304‐1305. [DOI] [PubMed] [Google Scholar]
- 20. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891‐897. [DOI] [PubMed] [Google Scholar]
- 21. Sun W, Peacock A, Becker J, Phillips‐Bute B, Laskowitz DT, James ML. Correlation of leukocytosis with early neurological deterioration following supratentorial intracerebral hemorrhage. J Clin Neurosci. 2012;19:1096‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461‐467. [DOI] [PubMed] [Google Scholar]
- 23. Adeoye O, Walsh K, Woo JG, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:e107‐e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573‐577. [DOI] [PubMed] [Google Scholar]
- 25. Luo P, Li R, Yu S, et al. The relationship between neutrophil‐to‐lymphocyte ratio and intracerebral hemorrhage in type 2 diabetes mellitus. J Stroke Cerebrovasc Dis. 2017;26:930‐937. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25‐44. [DOI] [PubMed] [Google Scholar]
- 27. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy‐to‐use predictor of 30‐day mortality. Stroke. 1993;24:987‐993. [DOI] [PubMed] [Google Scholar]
- 28. Hu X, Zhang JH, Qin X. Risk factors of early death in patients with hypertensive intracerebral hemorrhage during hospitalization. Acta Neurochir Suppl. 2011;111:387‐391. [DOI] [PubMed] [Google Scholar]
- 29. Cho DY, Chen CC, Lee HC, Lee WY, Lin HL. Glasgow coma scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surg Neurol. 2008;70:628‐633. [DOI] [PubMed] [Google Scholar]
- 30. Mackenzie JM, Clayton JA. Early cellular events in the penumbra of human spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 1999;8:1‐8. [DOI] [PubMed] [Google Scholar]
- 31. Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871:57‐65. [DOI] [PubMed] [Google Scholar]
- 32. Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor‐kappa B signaling and cytokines. Stroke. 2007;38:753‐758. [DOI] [PubMed] [Google Scholar]
- 33. Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629‐652. [DOI] [PubMed] [Google Scholar]
- 34. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lively S, Schlichter LC. Age‐related comparisons of evolution of the inflammatory response after intracerebral hemorrhage in rats. Transl Stroke Res. 2012;3:132‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaberel T, Magheru C, Emery E. Management of non‐traumatic intraventricular hemorrhage. Neurosurg Rev. 2012;35:485‐495. [DOI] [PubMed] [Google Scholar]


