Summary
Alzheimer's disease (AD) is characterized by extensive neurodegeneration and inflammation in selective brain areas, linked to severely disabling cognitive deficits. Before full manifestation, different stages appear with progressively increased brain pathology and cognitive impairment. This significantly extends the time lag between initial molecular triggers and appearance of detectable symptoms. Notably, a number of studies in the last decade have revealed that in the early stage of mild cognitive impairment, events that appear in contrast with neuronal distress may occur. These have been reproduced in vitro and in animal models and include increase in synaptic elements, increase in synaptic and metabolic activity, enhancement of neurotrophic milieu and changes in glial cell reactivity and inflammation. They have been interpreted as compensatory responses that could either delay disease progression or, in the long run, result detrimental. For this reason, these mechanisms define a new and previously undervalued window of opportunity for intervention. Their importance resides especially in their early appearance. Directing efforts to better characterize this stage, in order to identify new pharmacological targets, is an exciting new avenue to future advances in AD research.
Keywords: beta‐amyloid, cognitive impairment, compensation, glial reactivity, neurodegeneration
1. INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative condition characterized by extensive neuronal damage and death, with consequent progressive decline in cognition, up to the total loss of self‐sufficiency in all basic skills. AD includes rare familial forms with an early onset and a more common sporadic form that primarily affects the elderly. The increased average age of the world's population, makes it a major emergency for modern society. From a molecular point of view, two main factors play a major role in the pathophysiology of AD, beta amyloid peptide (Aβ) accumulation and hyper‐phosphorylation of tau protein. Although the latter is receiving increasing attention, Aβ has been assigned a leading role in triggering the sequence of events that result in the serial stages of AD development.1, 2, 3 Aβ is prone to aggregation into species of progressively higher molecular weight, ranging from oligomeric (oAβ) to protofibrillar and fibrillar, with different relevance as pathological effectors. To date, oAβ is acknowledged as the most synaptotoxic aggregate.4, 5 oAβ specifically interacts with a variety of receptors on the surface of both neuronal and glial cells, evoking signaling cascades that in turn modify the cellular profile of gene transcription/protein expression.6, 7, 8, 9 In general, research on Aβ toxicity has focused mainly on its detrimental effects, culminating with significant declines in synaptic activity, neuronal metabolism, neurotrophic factors, as well as increased glial activation/inflammation. However, a more attentive look at the literature of the last two decades, points out the existence of a biphasic trend where all these reductions are preceded by transient paradoxical rises.10, 11 These were described in AD patients and replicated in animal and in vitro models and were interpreted as compensatory responses to initial damage. Compensation likely contributes to central nervous system (CNS) resilience, conferring the ability to “tolerate” greater amounts of Aβ and delay appearance of symptoms. This would be in agreement with the lack of a constant correlation between levels of Aβ pathology and actual degree of cognitive impairment.12
Thus, since the strategies that focused on late interventions on AD were all unsuccessful, focusing on the early compensatory mechanisms represent an alternative therapeutic strategy that deserves more attention for future research in the field. We will here present an up‐to‐date summary on paradoxical compensatory responses in AD, individually reviewing the main biological processes involved.
2. SYNAPTIC ELEMENTS
In a modern perspective, with increased knowledge of underlying molecular mechanisms, AD can be described as a “synaptic failure”.13, 14 Synapses are in fact key elements in AD, and there is a clear correlation between their dysfunction or loss and onset of dementia.14, 15, 16, 17 Focusing specifically on changes in synaptic structure and function during disease progression is thus of paramount importance for therapeutic developments. The first observations of a biphasic trend of expression for synaptic components, with an early but transient increase, followed by reduction as neurodegeneration progresses, date a couple of decades back. A number of studies, carried out on human subjects and transgenic animal models, started to report what was then described as an “unexpected” or “paradoxical” increase in glutamatergic, cholinergic, and GABAergic presynaptic bouton density.18, 19, 20, 21, 22 This always appeared upstream of extensive neurodegeneration and before appearance of important cognitive deficits. Furthermore, a compensatory upregulation of hippocampal 5‐HT1A receptor density was shown in the early stage of MCI, whereas a dramatic decline was found at later stages of AD.23 Such compensatory increase in receptor density, evaluated by positron emission tomography (PET), was observed in the hippocampus (HC) of an experimental rat model, as a consequence of induced reduction in serotonin levels.24
The same biphasic pattern of expression was later shown for a number of synaptic components, for which reduction is a late‐stage event in different regions of postmortem AD or demented elderly brains. Some examples are presynaptic proteins synaptophysin (SYP), synaptopbrevin, SNAP‐25, rab 3A, and postsynaptic proteins such as syntaxin, drebrin, and PSD‐95.25, 26, 27, 28, 29 This observation was reproduced in AD animal models30, 31, 32 and in vitro models.33, 34, 35 In early stages, again coincident with mild cognitive symptoms, many of these were found to be transiently increased.26, 29, 36 Among others, synaptic vesicle component SYP has been a distinctive marker for evaluating synaptic conditions. In general, SYP increase is associated with improved synaptic function. Higher levels of SYP correlated with yet normal cognition in older people displaying extensive A brain pathology, compared to cognitively impaired AD patients.29 In addition, in 3xTg‐AD animal brains, SYP region‐ and time‐selective upregulation appeared even after an initial loss of synaptic components.37 This compensatory rise was related to partial recovery of cognitive functions, followed by SYP loss as symptoms worsened. In our own recent studies, in a model of slow‐developing neuronal damage, obtained by exposing rat organotypic hippocampal slices to sub‐lethal concentrations of oAβ42, we showed an initial compensatory increase in SYP levels and vesicle recycling.35 A similar trend was evident for postsynaptic density protein PSD‐95.
Altogether, these results confirm the existence of a combined attempt to compensate for reduction in synaptic connections with an overexpression of the main players involved, as here discussed.
3. NEURAL ACTIVATION
The compensatory structural changes induced by Aβ toxicity in synapses, find a correlate in intensification of neural activity, as seen by functional magnetic resonance imaging (fMRI) studies.38 In particular, hyperactivation appeared in selective brain areas and exclusively where Aβ accumulation was detected, as shown by fMRI studies comparing MCI patients and normal subjects with or without Aβ deposits.39, 40, 41, 42, 43 In the presence of neuronal hyperactivity, cognition was transiently ameliorated, although this effect was only evident below a threshold degree of neuronal damage. In agreement, in MCI patients, hyperactivation shifted among different brain areas as disease progressed, coming into play to selectively compensate specific compromised functions depending on disease severity.44 The compensatory and beneficial nature of neural hyperactivation finds support in the observation that nondemented older individuals, carrying the ApoE 4 allele, achieved memory, and learning performances comparable to their matched 3 counterparts by activating compensatory mechanisms.45
Also in animal models of AD, neuronal hyperactivity occurs early during disease development, even before plaque appearance.46, 47 Based on animal and in vitro studies using sub‐lethal concentrations of Aβ for slow development of neuronal damage, early improvements in synaptic plasticity and memory were proposed to be mediated by an increased rate of neurotransmitter release. With time, however, this would be responsible for excitotoxic damage and decline of these functions.48
Unexpectedly, several molecules not exclusively linked to neuronal activation, also show a biphasic pattern of expression during disease progression. For example, in presymptomatic AD mouse models, a compensatory mechanism acts through upregulation of nitric oxide (NO) synthase and recruitment of NO. In turn, NO signaling would increase calcium responses and rescue synaptic plasticity. This phenomenon once again appears transient and counteracts maladaptive synaptic depression that takes places later on as AD progresses.49 Similarly, Chol‐1α gangliosides, selectively expressed in cholinergic neurons, are increased in the frontal lobes of AD patients as a compensatory event aimed at preserving cholinergic transmission.50
The real significance of compensatory mechanisms involving neural activation as a protective strategy in AD is still a rather controversial issue. While on the one hand improved neural activity is indicative of a rescued function, hyperactivation can in the long run lead to excitotoxicity, potentially exacerbating neuronal damage.
4. BRAIN METABOLIC ACTIVITY
According to recent research, transient compensatory responses in early AD include increased brain glucose metabolism, as determined by [18F]‐fluoro‐deoxyglucose PET (FDG‐PET) scans.51, 52, 53, 54 However, discrepancies emerged regarding the real correlation between rate of glucose consumption and the degree of Aβ deposition. FDG‐PET analysis in a group of MCI subjects with different levels of Aβ pathology, revealed that increased metabolic rate in selective cortical areas was limited to subjects with lower Aβ levels. Therefore compensation is an early event followed by a decline in brain metabolism as Aβ load worsens.51 In a different study, brain metabolism was correlated directly to Aβ load in MCI, but inversely in AD.54 Despite apparently discordant results on the correlation with levels of brain pathology, both studies showed that metabolic compensation was protective and delayed MCI to AD conversion. A biphasic pattern of glucose metabolism was also reported in Down syndrome patients, wherein a stage preceding dementia was characterized by hypermetabolism that decreased progressively as disease developed.55 In contrast, such positive correlation was not confirmed in a recent study enrolling a heterogeneous population of cognitively impaired patients. In this case, hippocampal hypermetabolism was interpreted as a maladaptive, detrimental event, rather than a beneficial compensatory response.56 Results obtained in animal models appear in line with clinical data, as a peak in brain metabolic rate was described in Tg2576 AD mice at 7 months of age, progressively decreasing to reach wild type levels by 19 months of age.57 Curiously, authors negatively interpreted these data as a limitation to translatability of the animal model, although actually in line with the compensatory response observed in patients.
Hypermetabolism in MCI was also associated with insulin resistance (IR), an established risk factor for AD,58 in a study relating IR with FDG metabolism in AD‐vulnerable brain areas.59 IR was associated, in a region‐specific fashion, with hypermetabolism in MCI‐progressors to AD as opposed to MCI‐stable subjects, and with hypometabolism in AD. Although the role of IR is currently unclear, such biphasic trend observed selectively in MCI progressors is suggestive of a transient compensatory effect induced by IR during AD pathogenesis.
Quite interestingly, glucose hypermetabolism was also shown to underlie the impact of education on the degree of tolerance to Aβ before appearance of cognitive symptoms.52 In fact, intellectual enrichment delayed the rate of cognitive decline in AD patients, compared to lower degree of education.60 As the level of education is not always taken into account, this finding could be relevant to explain discrepancies among studies.
5. NEUROTROPHIC MILIEU
Neurotrophins are a family of small peptides comprising brain‐derived neurotrophic factor (BDNF), nerve growth factor (NGF) and neurotrophins‐(NT) 3 and 4/5, each acting through selective receptors. Neurotrophins impact CNS development and homeostasis, as well as synaptic modeling and function,61 so that any imbalance in the neurotrophic environment will inevitably take a toll on neuronal function and survival. A plethora of studies have explored BDNF expression in brain and plasma from patients at different disease stages, from MCI to early and advanced AD, as well as in animal models and in vitro studies, yielding extremely variable results (extensively reviewed in 62, 63). As mentioned before, variability likely derives from the stages of progression of disease, which may be difficult to compare considered the now well‐acknowledged lack of correspondence between degree of pathology and degree of cognitive symptoms in AD. In particular, the transient nature of compensatory responses makes them potentially difficult to identify. Nevertheless, it is again possible to discern a trend of biphasic expression of BDNF, where a compensatory increase appears early and is followed by a drop in advanced AD.62 Increased serum levels of BDNF correlated with slower worsening of cognitive symptoms in a study comparing slow vs fast declining AD patients.64 Intriguingly, early administration of the orally bioavailable neurotrophic compound P021, able to induce BDNF expression, rescued cognitive impairment in different animal models of aging and AD.65 This is a promising result that identifies the support of the neurotrophic milieu during compensatory phases as a potential new strategy.
Increased expression was described for NGF and its pro‐form. However, this was a late event in advanced AD, when BDNF levels were already declined.66, 67, 68, 69, 70 Thus, it implicates mechanisms other than a compensatory survival attempt.
6. OXIDATIVE STRESS RESPONSES
Oxidative stress, deriving from imbalance between production and removal of reactive oxygen species (ROS), is an early event in AD.71, 72 Due to its high oxygen consumption, the brain is exposed to particularly high ROS concentrations. With ageing, alterations in the ability to counteract oxidative stress make the brain even more prone to accumulate ROS, which promote neuronal damage and death.73 In agreement, levels of expression/activity of antioxidant enzymes, such as Cu/Zn‐ and Mn‐superoxide dismutase (SOD), were reported to be decreased in AD brains compared to control subjects.74, 75, 76 Interestingly, a compensatory increase in the expression of Mn‐SOD and glutathione reductase (GSSG‐R) proteins was described in MCI.77 Even in AD patients, protein and mRNA levels of glutathione peroxidase, GSSG‐R and catalase were increased, compared to control subjects, in selective areas also characterized by increased lipid peroxidation.78, 79, 80 Altogether these results are suggestive of a compensatory local rise in response to increased ROS levels, which may even persist through AD and precede loss of antioxidant function. Accordingly, expression of the anti‐oxidant heme oxygenase‐1 was increased in temporal cortex and HC in MCI as well as AD and this negatively correlated with cognitive performances.81 Once again, this implies attempts to realize compensatory mechanisms against oxidative stress. Evidence that a generalized compensatory response is able to actually contrast oxidative stress comes from studies on AD patients. Evaluation at different stages of pathology showed that oxidative damage was highest at early stages but decreased with progressive A deposition and concurrent disease progression. Of note, this correlation appeared more significant in ApoE 4 carriers.82
An additional source of oxidative damage in AD is the presence and redox state of copper and iron.83 Accordingly, the copper‐binding protein ceruloplasmin, responsible for copper entry in the brain and for the redox state of iron, was increased in selective AD‐vulnerable brain areas, suggesting a compensatory response to locally increased oxidative stress.84
Finally, lysosomal activation and autophagy emerged as antioxidant protective mechanisms that also undergo a compensatory induction early in AD.85, 86 However, later in disease progression as the load exceeds lysosomal clearance capability, they become unsuccessful.87
7. NEUROGENESIS
Adult neurogenesis continues throughout adult life in the dentate gyrus (DG) of the HC and in the subventricular zone (SVZ) of the mammalian brain. Here, newly generated neurons are integrated into local circuitries, where they play a role in plasticity of the HC and olfactory system.88, 89 Ageing and disease modify the neurogenic potential of the brain, with a general trend toward its decline.90 Impairment in neurogenesis is counteracted by a compensatory increase as observed in patients and in AD animal models. Likewise, in the ageing brain, declining neuronal functions are compensated in individuals with preserved neurogenesis.91
Postmortem brain studies of AD patients showed increased hippocampal neurogenesis in DG and CA1.92 A more detailed study further showed that alterations in neurogenesis vary differently during specific phases of the neurogenic process and in selective neurogenic niches, depending also on the stage of progression of AD. In particular, progenitor stem cells in the DG decreased in early AD stages, an effect contrasted by a compensatory increase of transit‐amplifying cells.93 Accordingly, increased neurogenesis appeared consistently during AD progression in animal models although at variable stages of disease. Enhanced proliferation and increased expression of immature neuronal markers were shown in both the DG and SVZ of APPswe/PS1dE9 mice at 3 months of age. These changes preceded amyloid deposition and neuronal loss, indicative of early compensatory responses likely triggered by early neuronal dysfunction.94 More studies on different genetic mice models of AD confirmed a compensatory increase of neurogenesis in younger animals, followed by decreased hippocampal adult neurogenesis at older ages.95, 96, 97 In contrast, a similar increase was also reported in older animals, already affected by memory impairment and Aβ deposits.98 It is interesting to note that increased proliferation of progenitors was not always followed by an actual development of new cells into mature and functional network‐integrated neurons.93, 99 On the whole, these data show that despite the lack of a uniform time/regional pattern of activation of compensatory neurogenesis, its promotion may represent an important defense mechanism against disease progression. Accordingly, drugs and hormones that are known to upregulate neurogenesis, such as the acethylcholinesterase inhibitor galantamine, the NMDA antagonist memantine, estrogen and allopregnenolone or the dopamine D2/3 receptor agonist pramipexole, have a great anti‐neurodegenerative potential as from in vitro and in vivo studies.100, 101, 102, 103, 104
Of note, as evidenced for other responses, the contribution of environmental enrichment in potentiating compensation was demonstrated also for neurogenesis, where it rescued reduced survival and maturation of newly generated neurons.105
8. GLIAL CELLS
It has long been established that glial cells not only provide structural support to neurons, but also hold an active role in neuronal development and function. At the synaptic level, neuronal and glial cells closely interact, with glia directly modulating synaptic transmission.106, 107
Microglia show two phases of activation that occur early and late in disease progression. Longitudinal PET studies on MCI vs AD patients over a 14‐month follow‐up, showed in fact in MCI the appearance of an early peak of an anti‐inflammatory, protective microglial phenotype. However, as amyloid load increases and probably exceeds its clearance capacity, a secondary activation begins that switches microglia to a pro‐inflammatory phenotype.108 It is known that overexpression of the transmembrane triggering receptor expressed on myeloid cells 2 (TREM2) favors an anti‐inflammatory phenotype in primary microglia exposed to increasing amyloid concentrations. Accordingly, a compensatory upregulation of TREM2 occurs during disease progression in a transgenic AD mouse, and this correlates with improved pathology and cognition.109 Of note, TREM‐2 overexpression, at further disease stages, failed to provide neuroprotection and did not correlate with improvement of neuropathology or cognitive impairment,110 suggesting that TREM‐2‐mediated protection yields significance only at a particular disease phase, likely depending on microglial functional condition.
What happens in microglia is sometimes recapitulated in astrocytes and both cell types ameliorate neurotrophic environment in models of AD. For instance, astrocytic and microglial production of BDNF is significantly increased around plaques in transgenic mice111 and selectively in rat primary microglial cells exposed to sub‐lethal concentrations of oligomeric Aβ42.112
With regard to astrocytes, several controversies still exist on the real function of their reactivity in AD pathogenesis.10 The appearance of a reactive astrocytic phenotype is a relatively early event occurring before the appearance of Aβ deposits both in AD mouse models113 and in AD patients.114 Such astrogliosis represents a compensatory event and in fact dampening of this activation increased the number of dystrophic neurites115 and accelerated plaque formation in APP/PS1 mice.116
In contrast, others described the appearance of hypertrophic astrocytes with increased GFAP levels only as a consequence of Aβ deposition and only surrounding plaques at late disease stages in transgenic mice.117, 118 Interestingly, these divergent responses appeared to be region specific. The same group reported in fact the coexistence of hyper/hypotrophic astrocytes in the HC, but not in the entorhinal cortex (EC) where only the hypotrophic phenotype was detected at all disease stages.117, 119, 120 According to the Authors, the hypotrophy observed in EC may contribute to the higher vulnerability of this area to AD pathology,120 confirming that the lack of reactive gliosis correlates with a negative outcome. Another study analyzed astroglial reactivity in the post mortem EC region of subjects with AD pathology associated (AD‐D) or not (AD‐N) with dementia.121 Both were characterized by an increased number of GFAP+astrocytes in layer I/II compared to normal brain. However, in AD‐N subjects, these displayed thicker and longer processes, indicative of glial reactivity, together with enhanced glutamate transporter‐1 (GLT‐1) expression. As these astrocytes play an important role in preservation of synaptic transmission to CA1, their selective reactivity in the AD‐N group was suggested as a compensatory mechanism in response to synaptic damage.121
Of note, astrocytic compensation in old 3xTg‐AD mice and AD patients also involves membrane Kir6.2 channels, whose function results in enhanced glutamate uptake.122
Finally, in line with the hypothesis of astrogliosis coming into play after Aβ deposition/later into disease development, exposure to Aβ in vitro, or the presence of Aβ deposits in TgCNRD8 AD animals, modified astrocytic bioenergetics through upregulation of the glycolytic enzyme 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase (PFKFB3). This increases neuronal supply of lactate as an alternative energy source, under conditions of impaired oxidative phosphorylation processes. Notably, inhibition of PFKFB3 made astrocytes vulnerable to Aβ toxicity.118
9. CONCLUSIONS
Compensatory mechanisms in AD have been well documented over the years. They appear early, transiently, and selectively in brain areas affected by the disease, comprising a wide spectrum of biological processes involved in brain function and homeostasis. These are all somehow linked to one another and give life to a concerted series of actions: strengthening of synaptic structure is linked to upregulation of synaptic activity, also contributed to by neurogenesis, with delivery of new neurons into the disrupted network. Concurrently, brain metabolism increases and protective antioxidant activity is potentiated. All the while, glial cells are activated to contribute putting into place all these responses. Although most likely originating as an attempt to contrast conditions harmful to neurons, it is not yet clear whether the final outcome of compensation can indeed be a positive one. On the one hand, data seem to suggest that compensatory responses succeed in promoting resilience to pathology progression. On the other hand, paradoxical increases in processes like neural activity or autophagy may also lead to exacerbation of neuronal damage. The existence of different thresholds of neuronal resistance to insults has been described as cognitive reserve. 123 This has a primary role in determining an individual's ability to activate compensation against incipient brain decline in AD (Figure 1). So, in our hands, it seems logical to infer that enhancement of compensatory events will most likely be an effective strategy, making it mandatory to shift attention to the cellular mechanisms at the heart of the axis cognitive reserve‐compensation‐resilience. In perspective, exploiting the beneficial fallouts of compensation, while containing the negative, will offer new grounds for disease modifying or even preventive approaches in AD.
Figure 1.
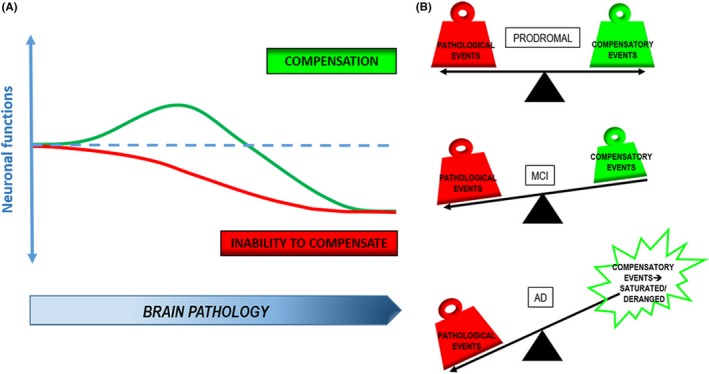
Compensation as a neuroprotective strategy in AD. A, As Aβ/tau brain pathology increases, compensatory events come into play to counteract the decline in neuronal functions and confer increased tolerance to brain pathology. Inability to adopt compensatory responses results in an earlier decline. B, Compensatory events evenly balance pathological ones in early prodromal stages of disease, but they begin to fail due to overload of pathology in MCI until they are no longer able to efficiently contrast pathology, or even become detrimental, in severe AD
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by institutional funding to MAS (55090193).
Merlo S, Spampinato SF, Sortino MA. Early compensatory responses against neuronal injury: A new therapeutic window of opportunity for Alzheimer's Disease? CNS Neurosci Ther. 2019;25:5–13. 10.1111/cns.13050
REFERENCES
- 1. Cline EN, Bicca MA, Viola KL, et al. The amyloid‐beta oligomer hypothesis: beginning of the third decade. J Alzheimers Dis. 2018;64(s1):S567‐S610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8(6):595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chong FP, Ng KY, Koh RY, et al. Tau proteins and tauopathies in alzheimer's disease. Cell Mol Neurobiol. 2018;38(5):965‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh DM, Selkoe DJ. A beta oligomers ‐ a decade of discovery. J Neurochem. 2007;101(5):1172‐1184. [DOI] [PubMed] [Google Scholar]
- 5. Park J, Jang M, Chang S. Deleterious effects of soluble amyloid‐beta oligomers on multiple steps of synaptic vesicle trafficking. Neurobiol Dis. 2013;55:129‐139. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Sun Z, Cao Q, et al. Role of amyloid beta protein receptors in mediating synaptic plasticity. Biomed Rep. 2017;6(4):379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith LM, Strittmatter SM. Binding sites for amyloid‐beta oligomers and synaptic toxicity. Cold Spring Harb Perspect Med. 2017;7(5):pii: a024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salazar SV, Strittmatter SM. Cellular prion protein as a receptor for amyloid‐beta oligomers in Alzheimer's disease. Biochem Biophys Res Commun. 2017;483(4):1143‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdier Y, Zarandi M, Penke B. Amyloid beta‐peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer's disease. J Pept Sci. 2004;10(5):229‐248. [DOI] [PubMed] [Google Scholar]
- 10. De Strooper B, Karran E. The cellular phase of Alzheimer's disease. Cell. 2016;164(4):603‐615. [DOI] [PubMed] [Google Scholar]
- 11. Bobkova N, Vorobyov V. The brain compensatory mechanisms and Alzheimer's disease progression: a new protective strategy. Neural Regen Res. 2015;10(5):696‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arenaza‐Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90(15):695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nimmrich V, Ebert U. Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta‐amyloid. Rev Neurosci. 2009;20(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 14. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789‐791. [DOI] [PubMed] [Google Scholar]
- 15. Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572‐580. [DOI] [PubMed] [Google Scholar]
- 16. Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56(1):127‐129. [DOI] [PubMed] [Google Scholar]
- 17. Koffie RM, Hyman BT, Spires‐Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27(40):10810‐10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossor MN, Garrett NJ, Johnson AL, et al. A post‐mortem study of the cholinergic and GABA systems in senile dementia. Brain. 1982;105(Pt 2):313‐330. [DOI] [PubMed] [Google Scholar]
- 20. Hu L, Wong TP, Cote SL, et al. The impact of Abeta‐plaques on cortical cholinergic and non‐cholinergic presynaptic boutons in Alzheimer's disease‐like transgenic mice. Neuroscience. 2003;121(2):421‐432. [DOI] [PubMed] [Google Scholar]
- 21. Bell KF, de Kort GJ, Steggerda S, et al. Structural involvement of the glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology. Neurosci Lett. 2003;353(2):143‐147. [DOI] [PubMed] [Google Scholar]
- 22. DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51(2):145‐155. [DOI] [PubMed] [Google Scholar]
- 23. Truchot L, Costes SN, Zimmer L, et al. Up‐regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69(10):1012‐1017. [DOI] [PubMed] [Google Scholar]
- 24. Zimmer L, Rbah L, Giacomelli F, et al. A reduced extracellular serotonin level increases the 5‐HT1A PET ligand 18F‐MPPF binding in the rat hippocampus. J Nucl Med. 2003;44(9):1495‐1501. [PubMed] [Google Scholar]
- 25. Sze CI, Troncoso JC, Kawas C, et al. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(8):933‐944. [DOI] [PubMed] [Google Scholar]
- 26. Counts SE, Nadeem M, Lad SP, et al. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65(6):592‐601. [DOI] [PubMed] [Google Scholar]
- 27. Ishibashi K, Tomiyama T, Nishitsuji K, et al. Absence of synaptophysin near cortical neurons containing oligomer Abeta in Alzheimer's disease brain. J Neurosci Res. 2006;84(3):632‐636. [DOI] [PubMed] [Google Scholar]
- 28. Reddy PH, Mani G, Park BS, et al. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7(2):103–117; discussion 173‐80. [DOI] [PubMed] [Google Scholar]
- 29. Head E, Corrada MM, Kahle‐Wrobleski K, et al. Synaptic proteins, neuropathology and cognitive status in the oldest‐old. Neurobiol Aging. 2009;30(7):1125‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oakley H, Cole SL, Logan S, et al. Intraneuronal beta‐amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26(40):10129‐10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutten BP, Van der Kolk NM, Schafer S, et al. Age‐related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am J Pathol. 2005;167(1):161‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30(14):4845‐4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dominguez‐Prieto M, Velasco A, Vega L, et al. Aberrant co‐localization of synaptic proteins promoted by Alzheimer's disease amyloid‐beta peptides: protective effect of human serum albumin. J Alzheimers Dis. 2017;55(1):171‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau CF, Ho YS, Hung CH, et al. Protective effects of testosterone on presynaptic terminals against oligomeric beta‐amyloid peptide in primary culture of hippocampal neurons. Biomed Res Int. 2014;2014:103906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merlo S, Spampinato SF, Capani F, et al. Early beta‐amyloid‐induced synaptic dysfunction is counteracted by estrogen in organotypic hippocampal cultures. Curr Alzheimer Res. 2016;13(6):631‐640. [DOI] [PubMed] [Google Scholar]
- 36. Mukaetova‐Ladinska EB, Garcia‐Siera F, Hurt J, et al. Staging of cytoskeletal and beta‐amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer's disease. Am J Pathol. 2000;157(2):623‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baazaoui N, Flory M, Iqbal K. Synaptic compensation as a probable cause of prolonged mild cognitive impairment in Alzheimer's disease: implications from a transgenic mouse model of the disease. J Alzheimers Dis. 2017;56(4):1385‐1401. [DOI] [PubMed] [Google Scholar]
- 38. Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickerson BC, Sperling RA. Large‐scale functional brain network abnormalities in Alzheimer's disease: insights from functional neuroimaging. Behav Neurol. 2009;21(1):63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elman JA, Oh H, Madison CM, et al. Neural compensation in older people with brain amyloid‐beta deposition. Nat Neurosci. 2014;17(10):1316‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Putcha D, Brickhouse M, O'Keefe K, et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non‐demented elderly adults. J Neurosci. 2011;31(48):17680‐17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yap KH, Ung WC, Ebenezer E, et al. Visualizing hyperactivation in neurodegeneration based on prefrontal oxygenation: a comparative study of mild alzheimer's disease, mild cognitive impairment, and healthy controls. Front Aging Neurosci. 2017;9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamalainen A, Pihlajamaki M, Tanila H, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28(12):1889‐1903. [DOI] [PubMed] [Google Scholar]
- 44. Clement F, Belleville S. Effect of disease severity on neural compensation of item and associative recognition in mild cognitive impairment. J Alzheimers Dis. 2012;29(1):109‐123. [DOI] [PubMed] [Google Scholar]
- 45. Bondi MW, Houston WS, Eyler LT, et al. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stargardt A, Swaab DF, Bossers K. The storm before the quiet: neuronal hyperactivity and Abeta in the presymptomatic stages of Alzheimer's disease. Neurobiol Aging. 2015;36(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 47. Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid‐beta for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2012;109(22):8740‐8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koppensteiner P, Trinchese F, Fa M, et al. Time‐dependent reversal of synaptic plasticity induced by physiological concentrations of oligomeric Abeta42: an early index of Alzheimer's disease. Sci Rep. 2016;6:32553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chakroborty S, Kim J, Schneider C, et al. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer's disease mice. J Neurosci. 2015;35(17):6893‐6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fukami Y, Ariga T, Yamada M, et al. Brain gangliosides in Alzheimer's disease: increased expression of cholinergic neuron‐specific gangliosides. Curr Alzheimer Res. 2017;14(6):586‐591. [DOI] [PubMed] [Google Scholar]
- 51. Ashraf A, Fan Z, Brooks DJ, et al. Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging. 2015;42(3):447‐458. [DOI] [PubMed] [Google Scholar]
- 52. Arenaza‐Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017;59:72‐79. [DOI] [PubMed] [Google Scholar]
- 53. Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332‐1339. [DOI] [PubMed] [Google Scholar]
- 54. Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29(47):14770‐14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haier RJ, Alkire MT, White NS, et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology. 2003;61(12):1673‐1679. [DOI] [PubMed] [Google Scholar]
- 56. Apostolova I, Lange C, Maurer A, et al. Hypermetabolism in the hippocampal formation of cognitively impaired patients indicates detrimental maladaptation. Neurobiol Aging. 2018;65:41‐50. [DOI] [PubMed] [Google Scholar]
- 57. Luo F, Rustay NR, Ebert U, et al. Characterization of 7‐ and 19‐month‐old Tg2576 mice using multimodal in vivo imaging: limitations as a translatable model of Alzheimer's disease. Neurobiol Aging. 2012;33(5):933‐944. [DOI] [PubMed] [Google Scholar]
- 58. Merlo S, Spampinato S, Canonico PL, et al. Alzheimer's disease: brain expression of a metabolic disorder? Trends Endocrinol Metab. 2010;21(9):537‐544. [DOI] [PubMed] [Google Scholar]
- 59. Willette AA, Modanlo N, Kapogiannis D, et al. Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64(6):1933‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amieva H, Mokri H, Le Goff M, et al. Compensatory mechanisms in higher‐educated subjects with Alzheimer's disease: a study of 20 years of cognitive decline. Brain. 2014;137(Pt 4):1167‐1175. [DOI] [PubMed] [Google Scholar]
- 61. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Song JH, Yu JT, Tan L. Brain‐derived neurotrophic factor in Alzheimer's disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52(3):1477‐1493. [DOI] [PubMed] [Google Scholar]
- 63. Tanila H. The role of BDNF in Alzheimer's disease. Neurobiol Dis. 2017;97(Pt B):114‐118. [DOI] [PubMed] [Google Scholar]
- 64. Laske C, Stellos K, Hoffmann N, et al. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 2011;14(3):399‐404. [DOI] [PubMed] [Google Scholar]
- 65. Baazaoui N, Iqbal K. A novel therapeutic approach to treat Alzheimer's disease by neurotrophic support during the period of synaptic compensation. J Alzheimers Dis. 2018;62(3):1211‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hellweg R, Gericke CA, Jendroska K, et al. NGF content in the cerebral cortex of non‐demented patients with amyloid‐plaques and in symptomatic Alzheimer's disease. Int J Dev Neurosci. 1998;16(7–8):787‐794. [DOI] [PubMed] [Google Scholar]
- 67. Narisawa‐Saito M, Wakabayashi K, Tsuji S, et al. Regional specificity of alterations in NGF, BDNF and NT‐3 levels in Alzheimer's disease. Neuroreport. 1996;7(18):2925‐2928. [DOI] [PubMed] [Google Scholar]
- 68. Hock C, Heese K, Hulette C, et al. Region‐specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain‐derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57(6):846‐851. [DOI] [PubMed] [Google Scholar]
- 69. Crutcher KA, Scott SA, Liang S, et al. Detection of NGF‐like activity in human brain tissue: increased levels in Alzheimer's disease. J Neurosci. 1993;13(6):2540‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peng S, Wuu J, Mufson EJ, et al. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63(6):641‐649. [DOI] [PubMed] [Google Scholar]
- 71. Su B, Wang X, Nunomura A, et al. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5(6):525‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith MA, Zhu X, Tabaton M, et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19(1):363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol. 1999;9(1):133‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marcus DL, Thomas C, Rodriguez C, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998;150(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 75. Richardson JS. Free radicals in the genesis of Alzheimer's disease. Ann N Y Acad Sci. 1993;695:73‐76. [DOI] [PubMed] [Google Scholar]
- 76. Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69(2):155‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sultana R, Piroddi M, Galli F, et al. Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem Res. 2008;33(12):2540‐2546. [DOI] [PubMed] [Google Scholar]
- 78. Lovell MA, Ehmann WD, Butler SM, et al. Elevated thiobarbituric acid‐reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology. 1995;45(8):1594‐1601. [DOI] [PubMed] [Google Scholar]
- 79. Aksenov MY, Tucker HM, Nair P, et al. The expression of key oxidative stress‐handling genes in different brain regions in Alzheimer's disease. J Mol Neurosci. 1998;11(2):151‐164. [DOI] [PubMed] [Google Scholar]
- 80. Kato K, Kurobe N, Suzuki F, et al. Concentrations of several proteins characteristic of nervous tissue in cerebral cortex of patients with Alzheimer's disease. J Mol Neurosci. 1991;3(2):95‐99. [DOI] [PubMed] [Google Scholar]
- 81. Schipper HM, Bennett DA, Liberman A, et al. Glial heme oxygenase‐1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27(2):252‐261. [DOI] [PubMed] [Google Scholar]
- 82. Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8):759‐767. [DOI] [PubMed] [Google Scholar]
- 83. Castellani RJ, Honda K, Zhu X, et al. Contribution of redox‐active iron and copper to oxidative damage in Alzheimer disease. Ageing Res Rev. 2004;3(3):319‐326. [DOI] [PubMed] [Google Scholar]
- 84. Loeffler DA, LeWitt PA, Juneau PL, et al. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res. 1996;738(2):265‐274. [DOI] [PubMed] [Google Scholar]
- 85. Butler D, Nixon RA, Bahr BA. Potential compensatory responses through autophagic/lysosomal pathways in neurodegenerative diseases. Autophagy. 2006;2(3):234‐237. [DOI] [PubMed] [Google Scholar]
- 86. Bendiske J, Bahr BA. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis–an approach for slowing Alzheimer disease? J Neuropathol Exp Neurol. 2003;62(5):451‐463. [DOI] [PubMed] [Google Scholar]
- 87. Bordi M, Berg MJ, Mohan PS, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy. 2016;12(12):2467‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Toda T, Gage FH. Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2017. 10.1007/s00441-017-2735-4 [DOI] [PubMed] [Google Scholar]
- 89. Apple DM, Solano‐Fonseca R, Kokovay E. Neurogenesis in the aging brain. Biochem Pharmacol. 2017;141:77‐85. [DOI] [PubMed] [Google Scholar]
- 90. Toda T, Parylak SL, Linker SB, et al. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2018. 10.1038/s41380-018-0036-2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007;257(5):271‐280. [DOI] [PubMed] [Google Scholar]
- 92. Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101(1):343‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perry EK, Johnson M, Ekonomou A, et al. Neurogenic abnormalities in Alzheimer's disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol Dis. 2012;47(2):155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jin K, Galvan V, Xie L, et al. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF‐APPSw, Ind) mice. Proc Natl Acad Sci USA. 2004;101(36):13363‐13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lopez‐Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J Alzheimers Dis. 2007;12(3):229‐240. [DOI] [PubMed] [Google Scholar]
- 96. Unger MS, Marschallinger J, Kaindl J, et al. Early changes in hippocampal neurogenesis in transgenic mouse models for Alzheimer's disease. Mol Neurobiol. 2016;53(8):5796‐5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gan L, Qiao S, Lan X, et al. Neurogenic responses to amyloid‐beta plaques in the brain of Alzheimer's disease‐like transgenic (pPDGF‐APPSw, Ind) mice. Neurobiol Dis. 2008;29(1):71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu Y, He J, Zhang Y, et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer's disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009;19(12):1247‐1253. [DOI] [PubMed] [Google Scholar]
- 99. Krezymon A, Richetin K, Halley H, et al. Modifications of hippocampal circuits and early disruption of adult neurogenesis in the tg2576 mouse model of Alzheimer's disease. PLoS One. 2013;8(9):e76497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Merlo S, Calafiore M, Vancheri C, et al. Astrocyte‐like cells as a main target for estrogen action during neuronal differentiation. Mol Cell Neurosci. 2007;34(4):562‐570. [DOI] [PubMed] [Google Scholar]
- 101. Merlo S, Canonico PL, Sortino MA. Distinct effects of pramipexole on the proliferation of adult mouse sub‐ventricular zone‐derived cells and the appearance of a neuronal phenotype. Neuropharmacology. 2011;60(6):892‐900. [DOI] [PubMed] [Google Scholar]
- 102. Jin K, Xie L, Mao XO, et al. Alzheimer's disease drugs promote neurogenesis. Brain Res. 2006;1085(1):183‐188. [DOI] [PubMed] [Google Scholar]
- 103. Wang JM, Singh C, Liu L, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2010;107(14):6498‐6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185‐190. [DOI] [PubMed] [Google Scholar]
- 105. Pfeffer A, Munder T, Schreyer S, et al. Behavioral and psychological symptoms of dementia (BPSD) and impaired cognition reflect unsuccessful neuronal compensation in the pre‐plaque stage and serve as early markers for Alzheimer's disease in the APP23 mouse model. Behav Brain Res. 2018;347:300‐313. [DOI] [PubMed] [Google Scholar]
- 106. Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421‐431. [DOI] [PubMed] [Google Scholar]
- 107. Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural Plast. 2013;2013:627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fan Z, Brooks DJ, Okello A, et al. An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain. 2017;140(3):792‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jiang T, Tan L, Zhu XC, et al. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer's disease. Neuropsychopharmacology. 2014;39(13):2949‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jiang T, Wan Y, Zhang YD, et al. TREM2 overexpression has No improvement on neuropathology and cognitive impairment in aging APPswe/PS1dE9 mice. Mol Neurobiol. 2017;54(2):855‐865. [DOI] [PubMed] [Google Scholar]
- 111. Burbach GJ, Hellweg R, Haas CA, et al. Induction of brain‐derived neurotrophic factor in plaque‐associated glial cells of aged APP23 transgenic mice. J Neurosci. 2004;24(10):2421‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Merlo S, Spampinato SF, Beneventano M, et al. The contribution of microglia to early synaptic compensatory responses that precede beta‐amyloid‐induced neuronal death. Sci Rep. 2018;8(1):7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Heneka MT, Sastre M, Dumitrescu‐Ozimek L, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Carter SF, Scholl M, Almkvist O, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C‐deuterium‐L‐deprenyl: a multitracer PET paradigm combining 11C‐Pittsburgh compound B and 18F‐FDG. J Nucl Med. 2012;53(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 115. Kamphuis W, Kooijman L, Orre M, et al. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild‐type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer's disease. Glia. 2015;63(6):1036‐1056. [DOI] [PubMed] [Google Scholar]
- 116. Kraft AW, Hu X, Yoon H, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27(1):187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Olabarria M, Noristani HN, Verkhratsky A, et al. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia. 2010;58(7):831‐838. [DOI] [PubMed] [Google Scholar]
- 118. Fu W, Shi D, Westaway D, et al. Bioenergetic mechanisms in astrocytes may contribute to amyloid plaque deposition and toxicity. J Biol Chem. 2015;290(20):12504‐12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kulijewicz‐Nawrot M, Verkhratsky A, Chvatal A, et al. Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer's disease. J Anat. 2012;221(3):252‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yeh CY, Vadhwana B, Verkhratsky A, et al. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer's disease. ASN Neuro. 2011;3(5):271‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kobayashi E, Nakano M, Kubota K, et al. Activated forms of astrocytes with higher GLT‐1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep. 2018;8(1):1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Griffith CM, Xie MX, Qiu WY, et al. Aberrant expression of the pore‐forming KATP channel subunit Kir6.2 in hippocampal reactive astrocytes in the 3xTg‐AD mouse model and human Alzheimer's disease. Neuroscience. 2016;336:81‐101. [DOI] [PubMed] [Google Scholar]
- 123. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]


