Dear Editor,
Anti‐N‐methyl‐d‐aspartate receptor (anti‐NMDAR) encephalitis, which is the most common type of autoimmune encephalitis, usually manifests as a clinical spectrum including psychiatric symptoms, cognitive disorders, epilepsy, and decreased levels of consciousness, autonomic dysfunction with or without teratoma. The confirmed major mechanism is dysfunction of NMDARs due to specific antibody‐mediated internalization.1 Antibodies as a kind of IgG are secreted by plasma cells which are differentiated from B cells as effector cells of the humoral immune system. The management is focused on immunotherapy and removal of tumor. Approximately 47% of patients, especially those without tumors, received no clinical improvement after first‐line treatments including prednisone, intravenous immunoglobulins (IVIg), and plasmapheresis.2
As a target drug against CD20+ B cell, rituximab has been shown the therapeutic effect on anti‐NMDAR encephalitis.3 The presumed mechanism is to delete the plasma cells indirectly by depletion of the pre‐B cells and mature B cells to reduce antibody production. However, the long‐lived plasma cells continue to produce antibodies,4 which may be the reason why some patients still do not improve after receiving rituximab treatment. Based on the above, we reasonably propose that the combined therapy targeting B cells and plasma cells may be the choice of treating severe and refractory anti‐NMDAR encephalitis. In this article, we evaluated the short‐term efficacy of this combined therapy and monitored the percentage of B cells and plasma cells. This is the first report using it in China.
1. CASE 1
A 23‐year‐old woman presented with seizure, sleep disorder, and pre‐infection a month before was diagnosed as anti‐NMDAR encephalitis for NMDAR antibodies 1:10 in cerebrospinal fluid (CSF). The results of lumbar puncture and cranial MRI were normal. She removed the right ovary 9 years ago because of right ovarian pedicle twist and did not find teratoma and other tumors through imaging. After treatments with high dose methylprednisolone (1000 mg × 3 d and half reduction) and IVIg (0.4 g/kg/d × 5 d) for 2 cycles, she was healed and continued to take oral prednisone (60 mg daily and gradually reduced) after discharge. When prednisone was reduced to 10 mg, she relapsed with a rapidly progressive personality disorder, emotional lability, hallucinations, irritability, seizure, and cognitive impairment and visited our hospital. At admission, she was conscious but mentally abnormal, and not cooperative in the physical and cognitive examination. The modified Rankin scale (mRS) was 5. Antibody titers rose to 1:32 in CSF and serum. Electroencephalogram (EEG) showed widely slow wave with partial fast wave rhythm, sharp wave or sharp slow wave released. She began to be treated with IVIg and methylprednisolone again, but the effect was not obvious. After full informed consent, she received 100 mg rituximab weekly for 4 times3 with CD19 B lymphocytes% declined from 13.2% to 0. There was a little improvements in seizure but still with paroxysmal muscle tension increased, involuntary movements, and hallucinations. The mRS was at 4. Due to lack of clinical improvement and mRS score remained at 4 for one month after rituximab, a more aggressive treatment regime was instigated. She was treated with bortezomib for 1.3 mg/m2 subcutaneous injection at days 1, 4, 8, and 11 and followed by a 10‐day rest as one cycle. Her mental state was rapidly improved when finished 2 cycles of bortezomib and had significant improvement in cognitive function (MMSE:25/30 and Moca 21/30) with mRS score at 2. Antibody titers declined to 1:10 in CSF and serum. After 6 months of follow‐up, the symptoms including seizures, hallucinations involuntary movement basically disappeared. The cognitive scores (MMSE:29/30, Moca27/30) and EEG were normal. She has returned to the campus with only very small dose of prednisone and mycophenolate mofetil to reduce the risk of relapse.
2. CASE 2
A 54‐year‐old woman presented to our hospital with acute onset fever, mental disorder, seizure, paroxysmal disturbance of consciousness, and involuntary facial movement for 10 days. Infective encephalitis was ruled out, and NMDAR antibody was detected in both the CSF and the serum with titers of 1:32. Then, she received first‐line treatment including methylprednisolone and IVIg for 2 cycles. No neoplasm was found. During this period, she was transferred to the ICU for central hypoventilation, status epilepticus, severe pulmonary infection, and bilateral pneumothorax. After actively supporting treatment, she left the ventilator but unconscious all day and irritated especially at night. Then, she received rituximab the same dosage as above and CD19 B lymphocytes% declined to 0. But she was in stupor basically with mRS score at 5. About one month later, she received bortezomib for 1 cycle with the same dosage of patient 1. She gradually recovered her consciousness and could walk with the help of others. After 6 months of follow‐up, she can communicate with families, complete simple daily activities such as eating by herself, and cooperate with cognitive examinations although still with serious cognitive impairment (MMSE:10). The mRS score was 3. Regrettably, they refused to review the antibody titers of serum and CSF.
3. DISCUSSION
We described two cases of severe anti‐NMDAR encephalitis which had no immediate response to first‐line immunotherapy and the remission did occur after the combined treatments of rituximab and bortezomib. The percentage of CD19+ B lymphocytes and CD138+ plasma cells decreased to almost 0 in both two patients (Figure 1 and 2) without significant rebound during the six months of follow‐up. The mRS scores of both patients have also improved (Figure 2). To the best of our knowledge, this is the first report on simultaneous monitoring of B cells and plasma cells. No obvious side effects have been found during the follow‐up.
Figure 1.
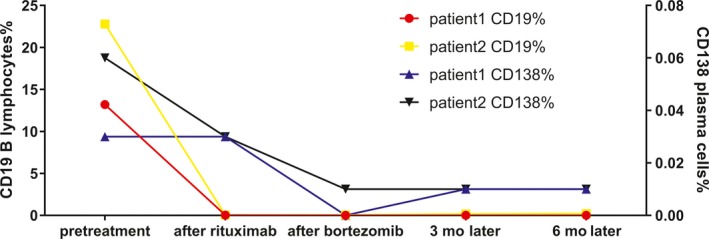
The changes in percentage of CD 19 + B lymphocytes and CD 138 + plasma cells in two patients
Figure 2.
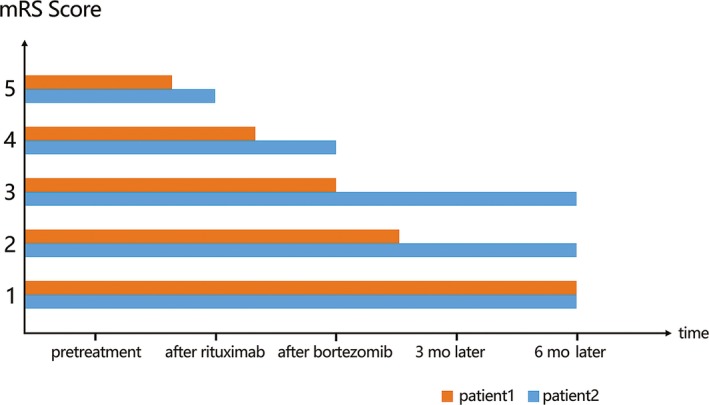
The changes in mRS scores in two patients
Since anti‐NMDAR encephalitis is mainly mediated by humoral immunity, treatments targeting B cells and plasma cells are therapeutic.2 Rituximab is a monoclonal antibody directed against the CD20 surface antigen expressed on pre‐B cells, mature B cells, and memory B cells, but not plasma cells.5 Preliminary data6 suggested that the protracted clinical course of anti‐NMDAR encephalitis is due to antibody production by long‐lived plasma cells. However, long‐lived plasma cells are not affected by conventional immunosuppressive drugs such as steroids or cyclophosphamide, or by B‐cell depletion.7 Bortezomib, as a proteasome inhibitor, it blocks the activation of antiapoptotic nuclear factor kappa B (NF‐κB) and activates the terminal unfolded protein response leading to apoptosis. It was associated with a significant depletion of short‐ and long‐lived plasma cells in peripheral blood and bone marrow.8 In past two years, there are a few reports of bortezomib for the treatment of severe anti‐NMDAR encephalitis9 , 10 and they confirmed that it could reduce antibody titers. Monitoring of CD138+ plasma cells in peripheral blood in our study confirmed its targeted therapeutic effect on plasma cells. Based on the above, we can speculate that the combination of rituximab and bortezomib can reduce the production of pathogenic antibodies by reducing the proliferation and differentiation of B cells and plasma cells. But both two drugs have limited penetration of blood‐brain barrier especially the bortezomib, so it is still unknown whether they can affect the synthesis of intrathecal antibodies.
Researches have shown that for antibody‐related autoimmune diseases, the combined treatment strategy targeting B cells and plasma cells is superior to B‐cell depletion therapy alone.5, 8 Based on our clinical observations, we propose that it also can improve the prognosis of severe and refractory anti‐NMDAR encephalitis. More large researches are needed to confirm the efficacy and safety.
DISCLOSURE
The authors report no conflict of interests in this work.
AUTHOR CONTRIBUTIONS
All authors contributed toward medical record, drafting and revising the article, and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
It was supported by the Natural Science Foundation of China (Grant No NSFC81671183).
REFERENCES
- 1. Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice‐Gordon RJ. Acute mechanisms underlying antibody effects in anti‐N‐methyl‐D‐aspartate receptor encephalitis. Ann Neurol. 2014;76:108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang BJ, Wang CJ, Zeng ZL, Yang Y, Guo SG. Lower dosages of rituximab used successfully in the treatment of anti‐NMDA receptor encephalitis without tumour. J Neurol Sci. 2017;377:127‐132. [DOI] [PubMed] [Google Scholar]
- 4. Nauen DW. Extra‐central nervous system target for assessment and treatment in refractory anti‐N‐methyl‐d‐aspartate receptor encephalitis. J Crit Care. 2017;37:234‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofmann K, Clauder AK, Manz RA. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front Immunol. 2018;9:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malviya M, Barman S, Golombeck KS, et al. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody. Ann Clin Translat Neurol. 2017;4:768‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long‐lived autoreactive plasma cells drive persistent autoimmune inflammation. Nature Rev Rheumatol. 2011;7:170‐178. [DOI] [PubMed] [Google Scholar]
- 8. Alexander T, Cheng Q, Klotsche J, et al. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in SLE but does not target their precursors. Eur J Immunol. 2018;48(9):1573‐1579. [DOI] [PubMed] [Google Scholar]
- 9. Scheibe F, Pruss H, Mengel AM, et al. Bortezomib for treatment of therapy‐refractory anti‐NMDA receptor encephalitis. Neurology. 2017;88:366‐370. [DOI] [PubMed] [Google Scholar]
- 10. Behrendt V, Krogias C, Reinacher‐Schick A, Gold R, Kleiter I. Bortezomib Treatment for patients with anti‐N‐methyl‐d‐aspartate receptor encephalitis. JAMA Neurol. 2016;73:1251‐1253. [DOI] [PubMed] [Google Scholar]


