Summary
Aims
Usually, spinal cord injury (SCI) develops into a glial scar containing extracellular matrix molecules including chondroitin sulfate proteoglycans (CSPGs). Chondroitinase ABC (ChABC), from Proteus vulgaris degrading the glycosaminoglycan (GAG) side chains of CSPGs, offers the opportunity to improve the final outcome of SCI. However, ChABC usage is limited by its thermal instability, requiring protein structure modifications, consecutive injections at the lesion site, or implantation of infusion pumps.
Methods
Aiming at more feasible strategy to preserve ChABC catalytic activity, we assessed various stabilizing agents in different solutions and demonstrated, via a spectrophotometric protocol, that the 2.5 mol/L Sucrose solution best stabilized ChABC as far as 14 days in vitro.
Results
ChABC activity was improved in both stabilizing and diluted solutions at +37°C, that is, mimicking their usage in vivo. We also verified the safety of the proposed aqueous sucrose solution in terms of viability/cytotoxicity of mouse neural stem cells (NSCs) in both proliferating and differentiating conditions in vitro. Furthermore, we showed that a single intraspinal treatment with ChABC and sucrose reduced reactive gliosis at the injury site in chronic contusive SCI in rats and slightly enhanced their locomotor recovery.
Conclusion
Usage of aqueous sucrose solutions may be a feasible strategy, in combination with rehabilitation, to ameliorate ChABC‐based treatments to promote the regeneration of central nervous system injuries.
Keywords: axonal regeneration, chondroitinase ABC, chronic spinal cord injury, locomotor rehabilitation, thermal stabilization
1. INTRODUCTION
Spinal cord injury (SCI) is a complex and life‐disrupting event, resulting in a change that might be permanent. Unlike peripheral nervous system, axonal recovery in the spinal cord is thwarted by numerous barriers to successful axon regeneration, like the transitions of astrocytes into hypertrophic cells that produce biochemical signals inhibiting axonal recovery via upregulation of chondroitin sulfate proteoglycan (CSPGs).1 This reactive cell phenotype is an active player in the formation of the glial scar, a reactive cellular process whereby glial cells accumulate, surround, and seal in the site of injury.2, 3 After injury, CSPGs are rapidly upregulated, forming an inhibitory gradient that is highest at the center of the lesion and diminishes gradually into the penumbra.4 The members of the CSPGs family of molecules share 2 common structures: (i) one major core protein of the lectican family, which includes neural/glial antigen2 (NG2), and (ii) glycosylated chondroitin side chains (GAG), which differs from the core in size and complexity.
There is plenty of evidence that the inhibitory activity of CSPGs depends on the GAG components, that is why chondroitinase ABC (ChABC) administration, an enzyme attenuating this inhibition by digesting GAG chains of the protein core, has been proposed as a therapeutic approach.5, 6
The bacterial enzyme ChABC liberates GAGs from CSPG core proteins and was shown to promote a more permissive substrate for axonal outgrowth in vitro 7 and to enhance axonal regeneration in vivo.8, 9, 10, 11, 12 In the last decade, research made large use of ChABC in different models of SCI and in various combinatorial approaches to evaluate its impact in promoting motor functional repair and recovery.13, 14, 15, 16, 17, 18 Nevertheless, there are crucial limitations in the path to a clinical treatment; ChABC loses 50% of its enzymatic activity after 1 hour (h) of incubation at 37°C (by Morgan‐Elson reaction; Seikagaku, Japan) and as such its enzymatic activity is obliterated within 72 hours.19 Therefore, to bypass this hurdle, multiple injections of ChABC or infusion via mini‐pumps/catheters have been used to provide sustained local delivery of fresh ChABC in vivo. However, these infusion systems are invasive. Bellamkonda and his staff showed how to overcome the limited stability of ChABC. They reported a method to thermostabilize ChABC using the sugar trehalose: they determined the proper concentration of trehalose for stabilizing the enzymatic function of ChABC (2 U/0.5 mL; 250 ng) incubating for 4 weeks at 37°C; subsequently, they developed a system for delivery in vivo consisting of lipid hydrogel microtubes able to release thermostabilized ChABC for 6 weeks.1 In another work by M.M. Pakulska, the authors overcame the limited stability of ChABC by presenting a recombinant stabilized ChABC demonstrated to be active for at least 7 days.20 In contrast to mentioned articles, our work describes an alternative strategy to thermal stabilize the native ChABC both in vitro and in in vivo with no biomaterial implant involved. It is well known that carbohydrates stabilize the native state of proteins against chemical denaturants and temperature21; consequently, aqueous sugar solutions were used as stabilizers to retain the enzymatic functionality of ChABC. Standard stabilizing agents and different concentrations of sucrose were tested in vitro to stabilize ChABC at +37°C: 2.5 mol/L Sucrose solution best preserved the ChABC activity in vitro in both stabilizing and diluted solutions mimicking its usage in vivo.
However, treatment of SCI with only ChABC produced a modest regeneration and plasticity with low motor recovery reasoning that ChABC leads to random new connections. Instead, the formation of appropriate connections in the spinal cord may be need to be driven by functional rehabilitation.16
Thereby combining, in this study, sucrose‐stabilized ChABC injections and treadmill sessions, we demonstrated to enhance axonal regeneration 15 and hindlimb locomotor recovery, triggering an improved beneficial effect for SCI compared with the control group, thus showing the benefits of our moderately invasive combinatorial approach.
2. METHODS
2.1. Chemicals
Chondroitinase ABC (ChABC) from P. vulgaris, with specific activity of 50‐250 units/mg protein, and chondroitin‐4‐sulfate (C4S) were purchased from Sigma‐Aldrich (USA). Sucrose (Sucr) was purchased from Alfa Aesar (Germany). Saline (Sal) was purchased from Baxter (USA).
2.2. ChABC activity assay
The activity of ChABC was evaluated in vitro by determining its capacity to digest C4S to produce unsaturated disaccharide.22 Briefly, 50 μL of ChABC (0.3 U/mL) was placed into a Greiner 96‐well UV transparent plate (Greiner BioONe, Germany). 50 μL of 3 mg/mL C4S was then placed simultaneously into each well. The ChABC activity, relative amount of unsaturated disaccharide, was determined by ultraviolet (UV) absorbance at 232 nm using a TECAN Infinite M200 Pro spectrophotometer for 15 minutes at 37°C. The ChABC activity was calculated as a modified equation of Beer‐Lambert law:
where:
EmM, mini‐molar absorbance coefficient of unsaturated disaccharides (=5.1 for products from C4S);
ΔA232 nm/min test: optical density difference (measured at 232 nm) of unsaturated digested C4S at 15 minutes and at time zero; values are blanked with C4S substrate.
0.1: volume (in milliliter) of reaction mix used.
ChABC unit: quantity of enzyme that catalyzes the formation of 1 μmol of unsaturated disaccharides from C4S in 1 minute at 37°C and pH 8.0.
2.3. Stabilization and long‐term thermal stability assessments
All aqueous solutions used in this work were dissolved in saline. Briefly, the sucrose solutions were prepared and were mixed with ChABC (0.3 U/mL). Sucrose was tested at 2, 2.5, and 3 mol/L concentrations. To evaluate the thermal stability of ChABC in aqueous solutions, the enzymatic activity was determined after 1, 2, 3, 7, 10, and 14 days in an incubator at 37°C.
After incubation, for analysis of enzymatic activity against C4S, aliquots of stabilized ChABC (0.3 U/mL) were mixed with 50 μL of C4S (3 mg/mL), and enzymatic digestion was measured through a TECAN Infinite M200 Pro spectrophotometer for 15 minutes at 37°C (Figure 1A). Other potential ChABC stabilizers described in Figure S1 were tested in the same way.
Figure 1.
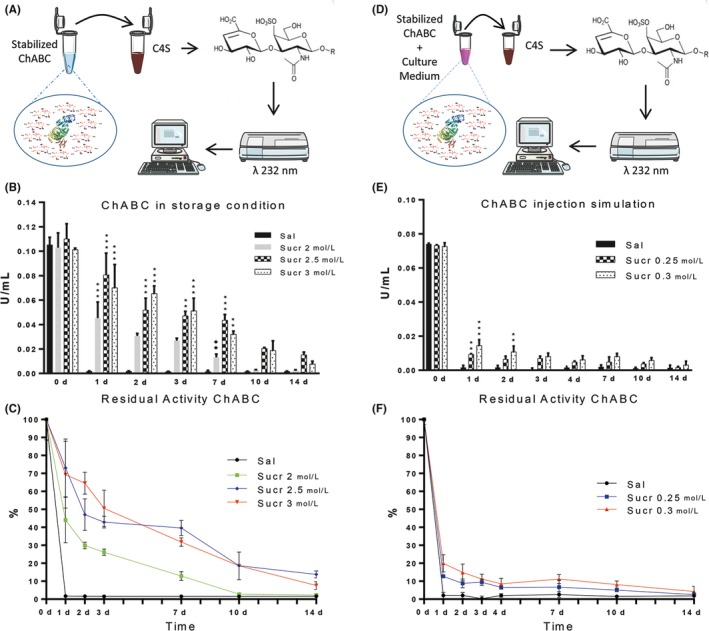
Enzymatic activity of ChABC in Sucr solutions in storage and in injection simulation conditions. A, Experimental protocol to assess ChABC activity through the detection of unsaturated disaccharide absorbance at 232 nm (see methods for details). B, Residual ChABC activity at 37°C in sucrose solutions (n = 5). At the beginning (day 0), results point out no significance difference between ChABC in Sal and Sucr 2 mol/L, Sucr 2.5 mol/L, Sucr 3 mol/L. After 1, 2, 3, and 7 d, the activity in Sucr 2.5 mol/L and Sucr 3 mol/L was significantly higher compared with Sal. C, ChABC activity in sucrose solutions expressed as percentage of the initial activity at day 0. D, ChABC in sucrose solutions were diluted in cell culture media (1‐10) to mimic in vivo dilution and interstitial fluid composition (n = 5). E, Results point out that after 1 and 2 d of incubation, ChABC activity in diluted sucrose solutions was importantly decreased but still significantly higher compared to Sal. F, Percentage of residual activity of ChABC in sucrose solutions. (P***<.001 and P** <.01 Sucrose solutions vs Saline solution; P♦♦<.01 Sucr 2.5 mol/L vs Sucr 2 mol/L; P••<.01 Sucr 0.3 mol/L 1 d vs 14 d)
To verify the effectiveness of the enzyme stability, it was performed an in vitro simulation to better mimic a scenario of injections in vivo, that is, where the injected solution is further diluted with body fluids containing inorganic salts, amino acids, vitamins, etc. 20 μL of ChABC (2 U/mL) in Sucr (at different concentrations) was added to 180 μL of well standardized DMEM/F12 cell culture medium, and incubated at 37°C for 1, 2, 3, 4, 7, 10, and 14 days. After incubation, 50 μL of mix of ChABC, Sucr, and culture medium was added to 50 μL of C4S (3 mg/mL), and enzymatic activity was measured as previously described (Figure 1D).
In all experiments, samples with no ChABC were used as blank and subtracted from the measured absorbance per each time point.
2.4. Sucrose solutions cytotoxicity
Neural precursors cultures are established and expanded as previously described.23, 24, 25 Briefly, murine neural precursors isolated from the subventricular zone (SVZ) of 8‐week‐old CD‐1 albino mice striata, at passage 10, were used. European Commission guidelines (EC Council 86/609, 1986) and Italian legislation (Decreto L.vo 116/92) for the care and use of laboratory animals have been observed.
NSC viability was quantified via Live/Dead kit (Molecular Probes). All cells used in this work were used 2 days after the last mechanical dissociation to obtain the maximum percentage of NSCs.
For NSC proliferation, cells were cultured in a medium containing fibroblast growth factor (ßFGF, 10 ng/mL) and epidermal growth factor (EGF, 20 ng/mL). Bulk cultures were generated by mechanically dissociating neurospheres and plating cells in a fresh medium containing 20 μL of Sucr 2.5 mol/L and 20 μL of Sucr 3 mol/L at the appropriate density (1 × 104 cells/cm2). After 24 hours, the cells were labeled with Live/Dead cell kit at 37°C for 1 hour and fluorescence measurements were obtained using a TECAN Infinite M200 Pro spectrophotometer (N = 3 independent experiments). The fluorescence emissions were acquired separately: Calcein at 530 ± 12.5 nm, and EtBr at 645 ± 20 nm.
For NSC differentiation, cells were cultured at the appropriate density (3 × 104 cells/cm2) in a medium in presence of FGF (10 ng/mL). After 3 days, the medium was shifted to a medium containing leukemia inhibitory factor (LIF, Chemicon) (20 ng/mL) and brain‐derived neurotrophic factor (BDNF, Peprotech) (20 ng/mL) to pursue the neuronal and glial population maturation in NSC progeny. After 7 and 14 days, the medium was shifted to a fresh medium containing 20 μL of Sucr 2.5 mol/L or 20 μL of Sucr 3 mol/L and cell viability was quantified at 1 and 4 days in vitro (DIV) using fluorescence microscope (N = 3 independent experiments). Live cells were stained with green Calcein‐AM, and dead cells were identified by nucleic acid red dye EtBr. Cell nuclei were stained with Hoechst 33342 (1:1000, Invitrogen).
2.5. Contusion SCI model
All procedures were carried out with protocols approved by Institutional Animal Care and Use Committee of the University of Milan‐Bicocca (IACUC 130/2014‐B) and were performed according to EC guidelines (86/609/EEC), to the Italian legislation on animal experimentation (Decreto L.vo 116/92). Rats were housed 2‐3 per cage, given free access to food and water, and kept on a 12/12 hours light/dark cycle.
Surgeries were performed under strict sterile conditions.
For lesion induction, 15 adult Sprague‐Dawley (SD) rats weighting 250‐275 g (Envigo Laboratories, Italy) were deeply anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). When unresponsive to toe pinch, the dorsal was shaved following incision of the dorsal skin, and a dorsal laminectomy was performed to expose the dura overlying the spinal cord at thoracic level T9‐T10. The vertebral column was stabilized by clamping the column at vertebra T8 and T11 and the lesion was inflicted by a 10 g rod dropped from 25 mm height (intermediate severity of injury) using a MASCIS Impactor device (WM Keck Centre for Collaborative Neuroscience, Rutgers University).
After contusion, the muscles were sutured and finally the skin was closed with wound clips. Rats were treated daily with analgesic (carprofen, 5 mg/kg) and antibiotic (enrofloxacin, 5 mg/kg). Animals were monitored for autophagia and their bladder was manually expressed until recovery of the voiding reflex.
2.6. Experimental groups and treatment
At 4 weeks after injury, chronically injured animals were randomly divided into 3 experimental groups as follows:
5 animals receiving injections of saline solution (control group);
5 animals receiving injections of ChABC (2 U/mL) in saline solution;
5 animals receiving injections of ChABC (2 U/mL) in SUCR 2.5 mol/L solution (treated group).
In more detail, all rats were anesthetized and the injured spinal cord was carefully re‐exposed. The dura mater located over the injury site was opened and 3 bilateral (6 in total) injections (3 μL per injection site at 1 μL/min) were performed around the lesion boundary. The gliotic scar was grossly distinguished from healthy spinal cord both in terms of color and brittleness. The injection sites were all restricted within the perimeter of the lesion, that is, 100 μm inward from the edge of the gliotic scar (2 rostral, 2 lateral, and 2 caudal) and at 100 μm in depth. Injections were performed with a Hamilton syringe (26‐gauge needle) secured to micromanipulator. After injections, the needle was kept in place for an additional minute to prevent reflux during needle exit.
2.7. Behavioral tests
Hindlimbs recovery was assessed using the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale.26 Briefly, this test involves placing the animal in an open field and evaluating the movement of both hindlimbs for individual joints movements, as well as paw posture, weight support, forelimb/hindlimb coordination, paw angle, and overall trunk stability. Scores were calculated according to the 0‐21 point BBB scale for each hindlimb and averaged to give an animal an overall score. Locomotor activity was evaluated on day 7 post‐injury and weekly, before and after treatment, until sacrifice. Each rat was observed and recorded with a digital video camera for 4 minutes.
2.8. Treadmill sessions
Inspired by previous publications,27 treadmill rehabilitation was initiated in all 3 groups from the first week after SCI to 2 weeks posttreatment and for a duration of 20 min/d for 5 days/week. In the first week post‐injury, the treadmill speed was set at 6 m/min. Along with BBB score improvements detected in all 3 groups, the treadmill speed for all animals was gradually increased to 7 m/min (second week), 8 m/min (third week) up to 9 m/min (from fourth to sixth weeks) throughout each training session until 2 weeks posttreatment.
2.9. Tissue processing, C4S digestion, and Neuroanatomical analysis
At the end of experiments, 6 weeks after injury, all rats were deeply anesthetized with an overdose of avertin (400 mg/kg). Animals were sacrificed by cardiac perfusion paraformaldehyde 4% (PFA) under terminal anesthesia. Once removed, The T8‐T12 spinal cord segments were explanted and were post‐fixed overnight in PFA 4% and tissue was cryopreserved in 30% sucrose. 16 μm‐thick longitudinal sections were cut serially via a cryostat, 3 per glass.
For C4S digestion, longitudinal sections (including the entire injury epicenter) were carefully taken from the glass slides and sonicated in PBS for 30 minutes. Spinal cord specimens were centrifuged at 956 g for 3 minutes. Similar to in vitro ChABC activity assay, samples were loaded on TECAN Infinite M200 Pro spectrophotometer for UV absorbance measurement (232 nm) of the digested C4S. Optical densities of readings were acquired and processed using GraphPad Prism 7 software.
For immunofluorescence staining, slices were washed with PBS, permeabilized with 0.1% Triton X‐100, and blocked with 10% normal goat serum. Afterward, slices were incubated overnight at 4°C with the following primary antibodies: anti‐βIII‐tubulin βIII‐TUB (1:500, Biolegend, 801202), anti‐growth associated protein GAP43 (1:100, Millipore, AB5220) for growth cones and regenerating nervous fibers; anti‐SMI31 (1:1000, Covance, SMI31R) to detect phosphorylated isoforms of the heavy subunit of neurofilament; anti‐neurofilament NF200 (1:400, Sigma‐Aldrich, N0142) for axons; anti‐glial fibrillary acidic protein GFAP (1:500, Dako, Z0334) for astrocytes, anti‐neural/glial antigen 2 NG2 (1:400, Dako, AB5320) for core proteins of GAGs chains and anti‐ionized calcium binding adaptor molecule 1 IBA1 (1:1000, Wako, 019‐19741) to identify microglia/macrophages. Primary antibodies were then probed with Alexa 488‐Mouse (1:1000, Life Technologies, A11001) or Cy3‐Rabbit (1:1000, Jackson, 111166045) conjugated secondary antibodies, counterstained with Hoechst (1:500, Invitrogen, H1399) and mounted with FluorSave reagent (Calbiochem). Fluorescence images were collected with Zeiss Axioplan 2 microscope. Morphometric quantification of gliosis size, axonal sprouting/regeneration, and IBA1 + reactivity area in injured spinal cords were performed on longitudinal sections using ImageJ software as previously described 28 Briefly, spinal cords sections, previously classified in dorsal (from 0 μm to 1120 μm in depth) and ventral (from 1136 μm to 2240 μm in depth) regions, were divided in rostral (500 μm rostral to the lesion edge, 3 images for area), lateral (350 μm lateral to lesion edge, 3 images for area), and caudal (500 μm caudal to lesion edge, 3 images for area) areas. Longitudinal sections were stained with GFAP and NG2 markers and images were captured at 10× magnification, and then merged. Gliosis were quantified on 54 images for immunohistochemical marker (3 dorsal and 3 ventral whole longitudinal sections per animal) per marker. Images were then processed with Image J software. Pixel area was converted to percentage of reactivity area and measurements of all sections were analyzed to produce an average reactivity in each animal. Similarly, IBA1 and nerve markers were captured at 10× magnification and processed with Image J software: the color image of positive cells localized into the lesion was converted into binary images (black and white, 8‐bit), then the average (among 54 images per animal) percentage (over the injured area) of positive pixels was measured using the automated threshold algorithm (Thr values within 0 and 255) to quantify the percentage of pixels positive for nervous markers.
2.10. Overall Statistical analysis
Data were processed using GraphPad Prism 7 software. The obtained data are presented as means values ± standard error of the mean (mean ± SEM). All in vitro results were analyzed via one‐way ANOVA followed by the Tukey multiple comparison test, with statistical significance set at P < .05. In vivo data results of the BBB scores among 3 groups were analyzed using two‐way ANOVA with repeated measures (including the C4S degradation assay), and histological data and C4S degradation were analyzed by one‐way ANOVA with random intercept. *P < .05, ** P < .01, and *** P < .001 were considered statistically significant.
3. RESULTS
3.1. ChABC in storage condition
Like others, we tested different stabilizers of ChABC in aqueous solutions: short‐term parallel experiments were pursued to directly compare different possible substances at different concentrations such as glycerol, glucose, maltose, and sucrose.29
Using the absorbance technique to assess the thermal stability of ChABC (see methods for details), best results were obtained using ChABC (final concentration 0.15 U/mL) in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L solutions at 24 hours at 37°C. Indeed in Figure S1 can be seen, after 4 hours of incubation at 37°C, that enzymatic activity in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L showed comparable values to enzymatic activity of ChABC in Glucose 2 mol/L, Glucose 2.5 mol/L, Glycerol 2 mol/L and mixtures of Glycerol 2 mol/L + Sucr 1 mol/L, Glycerol 2 mol/L + Sucr 1.5 mol/L. On the other hand, after 24 hours, ChABC in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L was significantly better preserved than in maltose, glucose, and glycerol aqueous solutions (P***<.001).
We then assessed in more detail the activity of ChABC in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L solutions vs saline solution (SAL) for up to 2 weeks (n = 5).
Over time, the activity in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L was better preserved when compared to SAL (Figure 1B).
At day 0, enzymatic activity gave the following values: 0.1052 ± 0.006, 0.1029 ± 0.011, 0.1100 ± 0.012, 0.1013 ± 0.001 U/mL, respectively, for ChABC in Sal, in Sucr 2 mol/L, in Sucr 2.5 mol/L, and in Sucr 3 mol/L (Figure 1B). Statistical analysis showed no significant difference between obtained results of Sal vs Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L.
At 1 day, ChABC in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L exhibited 0.0453 ± 0.013, 0.080 ± 0.017, and 0.0702 ± 0.018 U/mL, respectively, while in Sal gave 0.0017 ± 0.001 U/mL. Interestingly, ChABC in Sal has retained just 1.69% of the initial enzymatic activity, while in the case of Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L it was 44%, 73%, and 69%, respectively (Figure 1C). Results yield significant differences between enzymatic activity of Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L vs Sal (P***<.001).
On the other hand, after 2 days, the retained activity of ChABC in Sucr 2 mol/L decreased to 29.84%, with a value of enzymatic activity amounting to 0.0307 ± 0.002 U/mL, while the enzymatic activity in Sucr 2.5 mol/L and Sucr 3 mol/L were 53% and 65% (0.0519 ± 0.009, 0.0654 ± 0.006 U/mL, respectively), maintaining higher values compared with Sal (Figure 1B‐C). In this regard, the activity in Sucr 2.5 mol/L and Sucr 3 mol/L was very high, showing significant differences in respect to Sal (P***<.001).
At day 3, ChABC activity in Sucr 2 mol/L decreased to 28%, while the retained activity in Sucr 2.5 mol/L was similar to day 2 (26%): lastly, activity value in Sucr 3 mol/L was 51%, 33 times higher than in Sal. In this case, statistical analysis showed significant differences between Sucr 2.5 mol/L vs Sal (P**<.01) and Sucr 3 mol/L vs Sal (P***<.001).
After 1 week, ChABC retained 40% and 32% of its initial activity in Sucr 2.5 mol/L and in Sucr 3 mol/L, respectively (Figure 1C). Furthermore, values of activity in Sucr 2.5 mol/L were significantly higher than in Sal (P***<.001) and Sucr 2 mol/L (P ♦♦<.01): same thing for Sucr 3 mol/L vs Sal (P**<.01).
The activity in Sucr 2.5 mol/L and in Sucr 3 mol/L after 14 days was 13.83% and 7.65%, respectively, while they were almost equal to 0 in Sal.
Taken together, these results suggest that the thermostability of ChABC was intriguingly improved in both Sucr 2.5 mol/L and Sucr 3 mol/L solutions.
3.2. ChABC in culture medium
To verify the stabilizing properties of the proposed solutions once injected in vivo, we “mimicked” (i) the expected dilution of ChABC solution after injection, (ii) the body temperature, and (iii) the presence of other cytokines, ions and sugars of the interstitial fluid. Namely, we diluted 10 times the previously prepared Sucr 2.5 mol/L and Sucr 3 ChABC solutions into a medium standardly used for culturing neural stem cells (Figure 1D, see methods for details). Again, the enzymatic activity was assessed for up to 2 weeks (n = 5). Although 88% and 80%, respectively, of activity in Sucr 0.25 mol/L (former 2.5 mol/L) and in Sucr 0.3 mol/L (former 3 mol/L) was lost after 1 day of incubation (Figure 1E), statistical analysis showed significantly better values than in Sal (P***<.001).
After 2 days, the retained activity in Sucr 0.25 mol/L was reduced to 8%, while in Sucr 0.3 mol/L was similar to value obtained at 1 day, maintaining higher value compared with Sal (Sucr 0.3 mol/L vs Sal, P**<.01).
During the following days, the remained ChABC activity in Sucr 0.25 mol/L and Sucr 0.3 mol/L was constantly higher than in Sal. Including time as a factor, the residual activity of ChABC in Sucr 0.3 mol/L after 14 days was significantly lower than Sucr 0.3 mol/L incubated for 1 day (P••<.01 Sucr 0.3 mol/L 1 day vs Sucr 0.3 mol/L 14 days), while the remaining activity of ChABC in Sucr 0.25 mol/L showed no significant differences between Sucr 0.25 mol/L 1 day vs Sucr 0.25 mol/L 14 days (Figure 1F).
3.3. Neural stem cells culture
We next conducted in vitro tests to evaluate the possible toxicity of high concentration of sucrose solutions on NSCs. These analyses were performed in 2 ways: assessment of (i) viable proliferating NSCs in floating cultures via automated fluorescence plate readings and of (ii) viable differentiating NSCs in differentiation medium (adhering cultures) via fluorescence microscope (see Live/Dead section in methods for details).
Previously cultured NSCs in neural proliferation media, grown into neurospheres, were mechanically dissociated and incubated for 1 day in fresh proliferation media supplemented with sucrose (final concentration of Sucr was 0.25 mol/L and 0.3 mol/L; Figure 2A).
Figure 2.
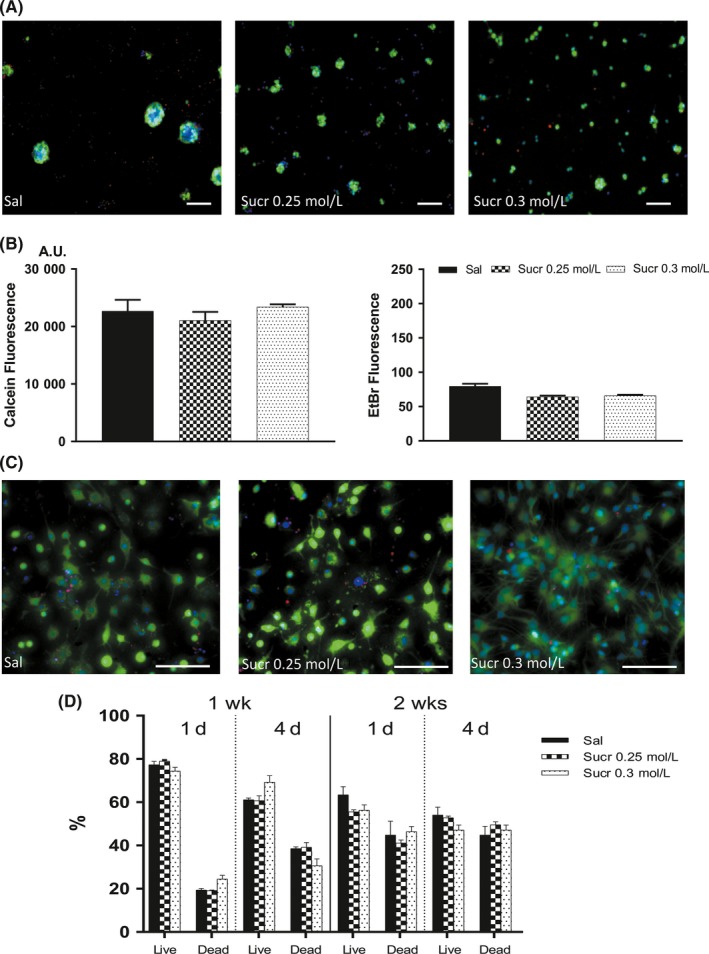
Cytotoxicity effect of sucrose solutions on NSCs in proliferation and differentiation conditions. A, Images show similar mNSC morphologies in cultures with media containing Sal (left), Sucr 0.25 mol/L (middle), and Sucr 0.3 mol/L (right). Live cells (green), dead cells (red‐orange). Scale bars represent 100 μm. B, At 1 d of incubation, values ascribable to viable cells (left) in medium containing Sucr 0.25 mol/L and Sucr 0.3 mol/L were not statistically different to cells cultured in medium containing Sal. In the same way, dead cells (right) in medium containing Sucr 0.25 mol/L and Sucr 0.3 mol/L showed no significant difference compared to medium with Sal. C, Effect of sucrose solutions on mouse differentiated NSCs, qualitative images (1 d incubation) show no density difference in differentiating cultures. Live cells fluorescent bright green, whereas dead cells with compromised membranes are in red‐orange. Scale bars represent 100 μm. D, Histograms represent the percentages of live/dead differentiated NSCs. Cellular quantification reveals a comparable viability between Sal and Sucrose solutions, both at day 1 and day 4
Through the Live/Dead kit, cultured cells grown in Sucr 0.25 mol/L and Sucr 0.3 mol/L showed fluorescence signals, ascribable to the number of viable/dead cells, similar to standard conditions (Sal; Figure 2B).
We next investigated the viability/cytotoxicity of mNSCs previously differentiated in vitro for 7 and 14 days (Figure 2C).
After medium replacement, at 1 day of incubation in differentiation media supplemented with sucrose and saline, the percentage of live 7 days differentiated NSCs in Sal was 77.06 ± 1.713%, while in Sucr 0.25 mol/L and Sucr 0.3 mol/L they were 79.20 ± 0.863% and 73.14 ± 1.790%, respectively; Also, the percentage of live differentiated NSCs at 14 days in Sal was 63.44%, while in Sucr 0.25 mol/L and Sucr 0.3 mol/L they were 55.64% and 56.4%, respectively. in the same way, percentages of dead 7 and 14 days differentiated cells were comparable among all 3 conditions (19.89 ± 1.490% and 44.9% for Sal; 19.03 ± 0.742% and 41.23% for Sucr 0.25 mol/L; 25.56 ± 1.861% and 46.48% for Sucr 0.3 mol/L; Figure 2D).
In the same way, after 4 days, morphology and cell viability were not affected. These results have shown comparable data indicating that sucrose solutions may be suited for in vivo applications (Figure 2D).
3.4. In vivo results
In the present study, we tested the ability of ChABC in 2.5 mol/L Sucr to break up the gliotic scar in a contusive model of chronic SCI. Four weeks after contusion, we re‐exposed the gliotic scar and injected 3 μL of ChABC (2 U/mL) in Sucr 2.5 mol/L per injection site (Figure 3A; see methods for details). For the ChABC in saline group, we injected 3 μL of ChABC (2 U/mL) in saline solution while control group received only saline injections.
Figure 3.
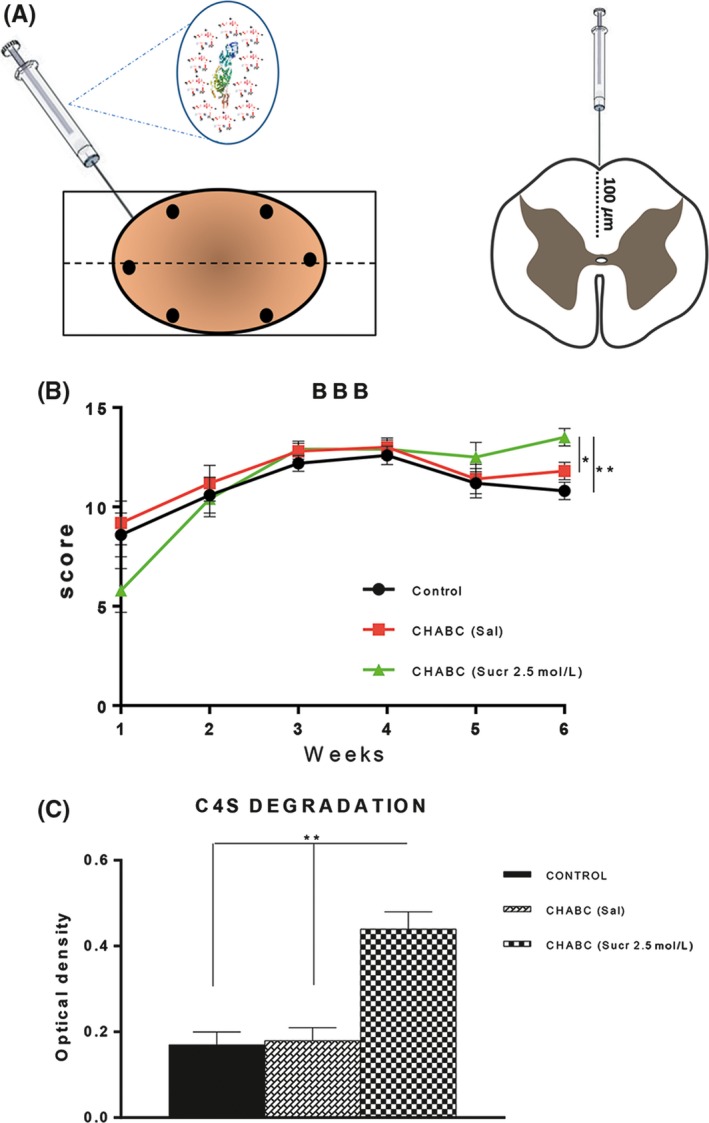
ChABC in Sucr 2.5 mol/L improved hindlimbs locomotor function (BBB scale) and C4S degradation. A, Experimental design showing intraspinal injections into the gliotic scar of ChABC in Sucr 2.5 mol/L at the depth of 100 μm (see methods for details). B, Each animal was subjected to treadmill training. Results point out that at 6 wk after contusion, 2 wk after ChABC treatment, treated animals showed significantly higher BBB score compared to control group (P**<.01 control vs treated) and compared to ChABC (Sal) group (P*<.05 ChABC (Sal) vs ChABC (Sucr 2.5 mol/L) C, quantification of C4S degradation reveals a significant digestion at the epicenter of injury following ChABC (Sucr 2.5 mol/L) treatment (** indicate significant difference compared with both Control and ChABC (Sal); P < .01, one‐way repeated‐measures ANOVA, Tukey's post hoc)
3.5. Locomotor functional recovery
The progressive hindlimbs recovery of each rat were assessed using BBB Locomotor Rating Scale.26
The results of BBB scoring at each time point are depicted in detail in Figure 3B.
Starting from 1 week post‐injury, every 7 days, the movement of hindlimbs of all rats was observed in an open field. This behavioral test happened after daily rehabilitation (see methods for details) by treadmill designed for rats.
All animals demonstrated normal motor function prior to the injury (BBB score of 21), with a significant drop after contusion, where animals only showed plantar placement of the paw with no weight support for control saline group; plantar placement of the paw with weight support in stance only stationary for ChABC in saline group; and slight movement of 2 joints and extensive movement of the third for ChABC in sucrose group.
After treatment, for the ChABC in sucrose group, the rats represented higher BBB scores and better performance of hindlimb locomotor function. The score of treated group after 6 weeks of injury corresponds to frequent‐to‐consistent weight supported by plantar steps and frequent FL‐HL coordination.
A similar effect was observed for ChABC in saline group as compared with saline group. As shown in Figure 3B, the treatment with ChABC in saline leads to an amelioration of hindlimb motor functions, after 6 weeks, the average BBB scale score reached 11.8. At this time, the animals showed frequent weight supported plantar stepping and occasional forelimbs‐hind limbs coordination. Instead for control group reached the maximum averaged score around the 4th week. In the BBB test, the score reached by control group corresponds to frequent to consistent weight supported plantar steps but no forelimbs‐hindlimbs (FL‐HL) coordination.
3.5.1. C4S degradation
ChABC is known to degrade the sugar chains of the CSPGs that form the glial scar. We therefore assessed the extent of CSPG degradation achieved in vivo in the chosen experimental groups (Figure 3C). Extent of C4S was quantified with absorbance analyses of tissue sonicated from the lesion epicenter and from caudal (lumbar cord) and rostral (thoracic cord) regions at 6 weeks following contusion and intraspinal injections.
This test revealed abundant values of digested sugar chains in treated group (Figure 3C) in comparison with lower levels detected in ChABC (Sal) and in Saline control group. CSPG digestion in ChABC (Sucr 2.5 mol/L) was also supported by sections immunostained for GFAP and NG2 markers.
3.6. Gliosis
At 2 weeks after treatment, presence of glial cells was quantified by ImageJ software (see methods for details) to assess the effect of ChABC sucrose solution administration over gliosis (Figure 4).
Figure 4.
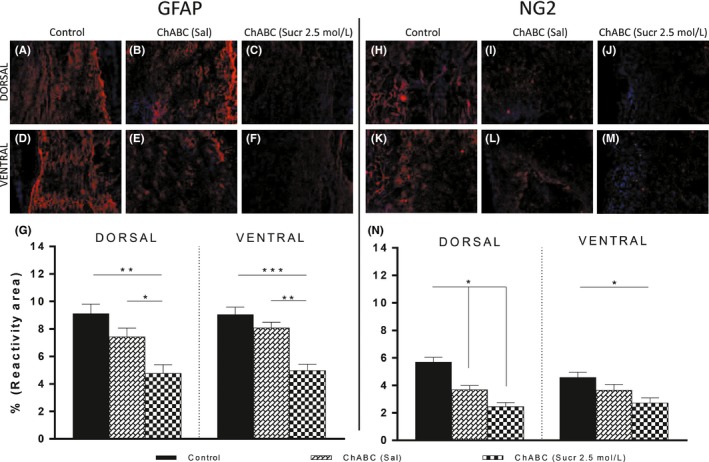
Morphological analysis of gliosis. GFAP and NG2 reactivities were decreased in treated group. Images showing immunofluorescence sections of injured spinal cord stained for GFAP (A, F blue = DAPI, red = GFAP) and NG2 (H, M blue = DAPI, red = NG2). These are representative images taken from longitudinal sections. A‐J, Images showing dorsal sections; D‐M, Images showing ventral sections. Scale bars represent 100 μm. N, Results suggest gliosis reactive to GFAP and NG2 was significantly decreased in ChABC (Sucr 2.5 mol/L) if compared to control and ChABC (Sal) groups. (P**<.01 dorsal control vs dorsal treated and P*<.05 dorsal ChABC (Sal) vs dorsal treated; P***<.001 GFAP ventral control vs ventral treated and P**<.01 ventral ChABC (Sal) vs ChABC (Sucr 2.5 mol/L); P*<.05 NG2 dorsal control and dorsal ChABC (Sal) vs NG2 dorsal treated; P*<.05 ventral control vs ventral treated)
GFAP immunoreactivity analysis showed reactive astrocytosis surrounding the lesion: GFAP was present at the borders of the cavity area, forming an intense glial border (Figures 4A and 5F). GFAP was 9.12 ± 0.69%, 7.44 ± 0.61%, and 4.77 ± 0.63% (Figure 4G) in dorsal sections of, respectively, control (Figure 4A), ChABC in saline group (Figure 4B) and treated (Figure 4C) animals. Statistical analysis showed significant differences between treated and control animals (P**<.01), and between treated and ChABC (Sal) groups (P*<.05). Similarly, ventral GFAP reactivity was 9.05 ± 0.53%, 8.08 ± 0.41%, and 4.98 ± 0.45% (Figure 4G) in control (Figure 4D), ChABC in saline group (Figure 4E) and treated (Figure 4F) animals (P***<.001 ventral control group vs ventral‐treated group; P**<.01 ventral ChABC (Sal) group vs ventral ChABC (Sucr 2.5 mol/L) group).
Figure 5.
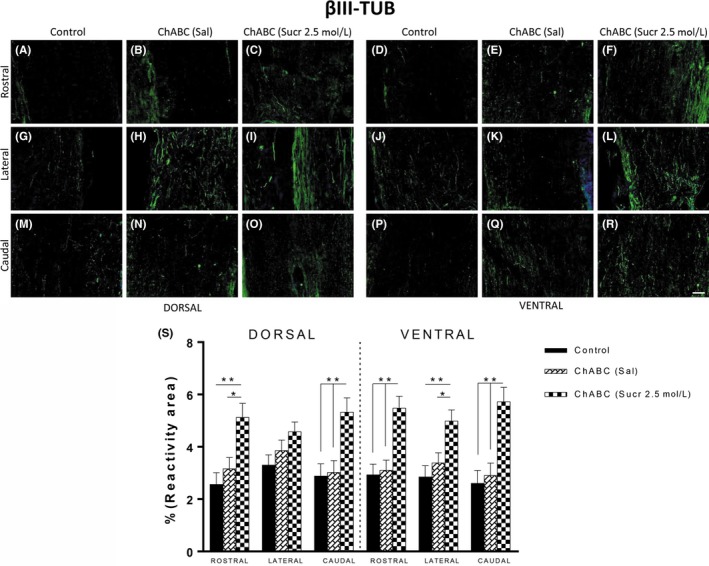
Increased reactivity of neural marker in ChABC (Sucr 2.5 mol/L) animals vs control and ChABC (Sal) groups. A‐R, Immunofluorescence images of areas used for quantification of βIII‐TUB (blue = DAPI, green = βIII‐TUB), subdivided into dorsal and ventral zones. Histological sections have been further regionalized into rostral, lateral, and caudal areas. Scale bars represent 100 μm. Y, Histological analysis points out the ChABC with sucrose injections significantly increased βIII‐TUB immunoreactivity. (P**<.01 control vs treated; P*<.05 control vs ChABC (Sal))
We also tested in dorsal and ventral sections the reactivity against NG2, one of the main components of the gliotic scar. NG2 formed an intense reactive glial border near the cavity area of control group (Figure 4H, K). Percentages of NG2 positive area in dorsal sections (Figure 4H) of control and ChABC (Sal) group (Figure 4I) were 5.70 ± 0.34% and 3.69 ± 0.29%, while in ventral (Figure 4K, L) sections were 4.58 ± 0.37% and 3.66 ± 0.41% (Figure 4N). Instead NG2 was lower in all treated animals (Figure 4J, M), giving 2.45 ± 0.30% in dorsal (Figure 4J) and 2.72 ± 0.37% in ventral (Figure 4M) sections. Statistical analysis showed significance difference between groups (P*<.05 dorsal control vs dorsal‐treated and dorsal control vs dorsal ChABC (Sal); P*<.05 ventral control vs ventral‐treated).
3.7. Axonal regeneration
To assess whether the detected reduction of the gliotic scar at the lesion site may enhance neuroplasticity, we assessed the presence of fibers positive to βIII‐TUB (Figure 5) and GAP43 (Figure 6) and SMI31, NF200 markers (Figures S6 and S7) in longitudinal sections of all 3 groups. In control animals, the reactivity for SMI31, neuronal marker of phosphorylated neurofilament H, showed similar values in rostral, lateral, and caudal sections (Figure S6S). In ChABC (Sal) and treated groups, SMI31 showed comparable values to control group.
Figure 6.
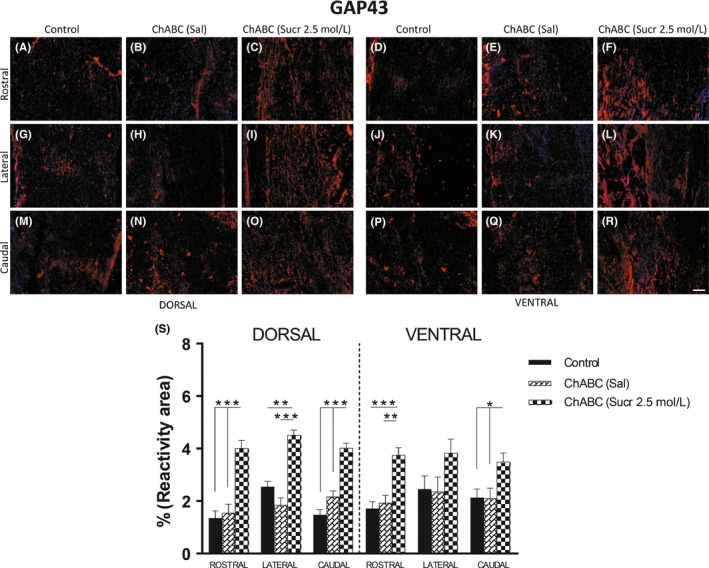
A‐R, Immunofluorescence images of areas used for quantification of GAP43 (blue = DAPI, red = GAP43), subdivided into dorsal and ventral zones. Histological sections have been further regionalized into rostral, lateral, and caudal areas. Scale bars represent 100 μm. S, Histological analysis points out the ChABC with sucrose injections significantly increased GAP43 immunoreactivity. (dorsal: P***<.001 rostral, lateral, and caudal control and rostral, caudal ChABC (Sal) vs dorsal treated; P**<.01 lateral ChABC (Sal) vs ChABC (Sucr 2.5 mol/L); ventral: P***<.001 rostral control vs treated; P**<.01 rostral ChABC (Sal) vs treated and P*<.05 caudal control and ChABC (Sal) vs caudal treated)
Similarly, fibers positive to NF200 were observed both in all 3 spinal cord groups with no significant difference (Figure S7).
βIII‐TUB marker was used for regenerating axons (Figure 5). Two weeks after ChABC treatment, βIII‐TUB+ axons (Figure 5S) were significantly increased in dorsal and ventral regions of all examined rostral, lateral, and caudal areas of ChABC (Sucr. 2.5 mol/L) group (Figure 5C,O,F,L,R) compared with control (5, A, G, M, O, J, P) and ChABC (Sal) (5 G, H, N, E, K,Q) groups. In particular, βIII‐TUB reactivity in dorsal sections of ChABC (Sucr 2.5 mol/L) was 5.13 ± 0.53% and 5.33 ± 0.54% in rostral and caudal areas, respectively (rostral: P**<.01 control vs treated; P*<.05 ChABC (Sal) vs treated; caudal: P**<.01 control and ChABC vs treated). Similarly, reactivity in ventral sections was 5.48 ± 0.45%, 4.99 ± 0.42%, and 5.73 ± 0.54%, respectively, for rostral, lateral, and caudal areas (rostral: P**<.01 control and ChABC (Sal) vs treated; lateral: P**<.01 control vs treated, P*<.05 ChABC (Sal) vs treated; caudal: P**<.01 control and ChABC (Sal) vs treated).
On the other hand, fibers positive to GAP43 (Figure 6), a marker of actively extending neuronal fibers, were found at rostral, lateral, and caudal edges of the lesion site (Figure 6A, R). GAP43 was 4.01 ± 0.30%, and 3.75 ± 0.28% (Figure 6S), respectively, in rostral area of dorsal and ventral sections of treated animals (Figure 6C, F), while the obtained results in control and ChABC in saline groups (6A, 6B, 6D, 6E) values were 1.35 ± 0.28% and 1.55 ± 0.33% for dorsal rostral area and 1.71 ± 0.26%, 1.93 ± 0.29%, respectively, for ventral rostral area (Figure 6S). Statistical analysis showed significant differences, both in dorsal and ventral area in favor of treated animals compared with control and ChABC in saline groups (dorsal area of treated vs control P***<.001 and ventral area of treated vs control P***<.001; dorsal area of treated vs ChABC (Sal) P***<.001 and ventral area of treated vs ChABC in saline P**<.01). In lateral area, GAP43 was 4.51 ± 0.19% in dorsal sections of treated animals (Figure 6I), while it was 2.54 ± 0.21% in control group (Figure 6G) and 1.85 ± 0.27% in ChABC (Sal; Figure 6H), showing significant differences in favor of treated group (P**<.01 control vs treated; P***<.001 ChABC in saline vs treated). Finally, GAP43 values in treated animals were 4.02 ± 0.19% and 3.49 ± 0.34% in caudal area of dorsal and ventral sections (Figures 6O, R), respectively, whereas it measured 1.47 ± 0.20% and 2.13 ± 0.33% in control group (Figure 6M, P) and 2.16 ± 0.23 of dorsal and 2.11 ± 0.38% of ventral caudal area of ChABC in saline group (Figure 6N, Q). Again, statistical analysis showed a significant increase in GAP43 reactivity in caudal area of treated animals (dorsal treated vs dorsal control P***<.001 and dorsal treated vs dorsal ChABC (Sal); ventral treated vs ventral control and ventral ChABC in saline P*<.05).
4. DISCUSSION
The present research proposes an improvement in the treatment of chronic SCI using sucrose‐stabilized ChABC. Over the last decade, various animal studies showed that ChABC could be a good candidate for the treatment of injured spinal cords; however, its enzymatic activity declines rapidly at body temperature. Several methods were used to solve this problem, such as mutating specific amino acids of the enzyme.30 Our aim was to restore a proper biological microenvironment at the boundary of the scar spontaneously formed after chronic contusive injury: we combined ChABC and sucrose (used to increase enzyme catalytic thermal stability) delivery with daily rehabilitation via treadmill.
In biotechnology, sucrose solutions have been increasingly used to stabilize proteins and their physical properties are being thoroughly studied.31, 32 Our results demonstrate that the thermal stability of ChABC was significantly improved, that is, the rate of thermal inactivation was significantly slowed in the presence of sucrose, indicating that the sucrose features the best (among the tested stabilizers) protective and stabilizing effect against thermal inactivation.
In recent work, by Shahaboddin,30 the activity of their modified ChABC, stabilized via an aromatic mutation, against C4S substrate was higher than wild‐type ChABC up to 20 days (at 25°C).
On the other hand, others reported the diverse stability of ChABC vs temperature.33 In our work, we showed that the activity of wild‐type ChABC was higher than in standard conditions (controls) for 7 days at 37°C (Figure 1B) in sucrose solutions, thus suggesting that exposure of ChABC to sucrose could enhance its thermostability and prolong its activity for days.19
In particular, after 1 day, the ChABC in Sal lost more than 98% of enzyme activity, confirming the instability of the enzyme at body temperature. Instead the ChABC in Sucr 2 mol/L, Sucr 2.5 mol/L, and Sucr 3 mol/L showed a good stability in all samples, for up to 1 week: others used sucrose for thermodynamic stabilization of biological macromolecule in solution 31, 32 and our results confirmed the effectiveness of the sucrose as protein stabilizer. Indeed, ChABC activity in the tested solutions was detected for up to 2 weeks. Sucrose is known to increase the surface tension of water and to create a protective shell in aqueous solutions surrounding the protein.32 Above 5% (w/w), the surface tension of sucrose solutions increases well above the surface tension of water.34
In the light of the stabilization results, we simulated the in vivo protocol of usage of ChABC. To the best of our knowledge, this is the first time that the enzymatic activity of ChABC was evaluated in an in vivo “simulation” in vitro. We injected (and diluted) in cell culture medium the same concentration of ChABC (2 U/mL) and Sucrose (Sucr 2.5 mol/L) to be used for animals (Figure 1D). In this case, supposing a dilution of the original injection solution in vivo, the final concentration of ChABC was 0.1 U/mL, 33% less than the final concentration of ChABC in storage condition while the final concentrations of sucrose were 0.25 mol/L and 0.3 mol/L (see methods for details). After 48 hours, although the sucrose concentration was diluted in culture medium of 10 times, the remaining activities were significantly higher than results obtained with standard ChABC. It is well known from the literature 35 that the effect stabilizers are strictly dependent on concentration and are usually impaired at low concentrations. Remarkably, after 10 days, the activity was still present: 2 and 4 times higher than in 1 day of incubation with saline solution. Moreover, presence of metal ions, proteins, and other sugars may all interfere with the positive outcome obtained in storage conditions36: indeed, ChABC activity was reduced but sucrose solutions still gave significant improvements.
The present study demonstrates that the relationship between sucrose concentration and this thermolabile enzyme could be a major determinant of the improvement of a future therapy of ChABC.
Biological response to high concentration of sucrose was evaluated by culturing NSCs in cell culture medium supplemented with Sucr 2.5 mol/L and Sucr 3 mol/L. We verified the possible toxicity of high sucrose concentration against mouse NSCs in proliferation state and in differentiation condition. After 1 day of incubation, results showed comparable values to cells cultured in proliferative standard medium, demonstrating negligible toxicity of high concentration of sucrose (Figure 2A,B).
In the same way, at 1 week and 2 weeks of NSC differentiation, we added Sucr 2.5 mol/L and Sucr 3 mol/L in fresh culture medium. After 1 and 4 days, the morphological analysis of cells cultured on Sucr 2.5 mol/L and Sucr 3 mol/L showed a spread and branched morphology comparable to cells cultured in standard differentiation culture medium (Figure 2C). Moreover, we observed no differences in percentage of Nestin+, βIII‐Tub + and GFAP + between all 3 groups, highlighting that different concentrations of sucrose in culture medium has not changed the normal expression of these markers (Figure S3).
Similar to the proliferative cell viability assay, quantitative data accounting for the percentages of live and dead cells showed no significant differences in Sucr 0.25 mol/L and Sucr 0.3 mol/L vs Sal. Additionally, MTS assay results too (Figure S4) corroborated the idea of a negligible harmful effect of modestly high concentrations of sucrose.
As we successfully improved enzymatic activity of ChABC while maintaining low cytotoxicity of the tested solutions, we assessed the in vivo performance of stabilized ChABC in an animal model of chronic SCI.
As often the case in humans, SCI is given by an initial contusive insult; therefore, we used a weight‐drop contusion animal model.37 At 1 month after contusion, chronic injury yields to the formation of a gliotic scar, at this time point we injected ChABC (Sucr 2.5 mol/L), the best performing solution in vitro, in the glial scar and at the depth of 100 μm.38
The presence of glial cells and axons was quantified. After lesion, extent of CSPGs and immunoreactivity dramatically increases around the lesion site: indeed, CSPG has been detected by absorbance concomitantly to GFAP‐positive astrocytes inside the lesion area and in the cystic cavity border.39 In our data, 1 session of intraspinal injections of ChABC in Sucr 2.5 mol/L fostered a significant value of digested sugar chains concurrently with a reduction of GFAP reactivity. In the same way, proteoglycan NG2 reactivity, that is associated with axon growth inhibition not necessarily related to CS GAG chains,40 was significantly reduced in the treated group.
Additionally, the macrophage response following SCI is known to be neurotoxic and inhibitory to axon regeneration.5 In this study, we showed that stabilized ChABC induced expression of IBA1, a well‐characterized marker of microglia, in macrophages in the lesion epicenter at 2 weeks posttreatment obtaining results comparable to values obtained for saline group (Figure S5).
Moreover, our results showed that the intraspinal injections of ChABC in Sucr 2.5 mol/L increased the reactivity of βIII‐TUB‐positive fibers into the gliotic scar. Like GAP43, the outgrown axons were present in the lesion site at least 2 weeks after treatment, suggesting that intraspinal injections promoted βIII‐TUB expression in chronic SCI. Although βIII‐TUB significance was achieved in rostral and dorsal caudal areas only, a clear beneficial trend was present in all processed areas of animals belonging to treated group. In contrast to reactivity for βIII‐TUB, values for NF200 were lower in regenerating neurons: while tubulin is known to participate directly in the mechanism of axonal elongation, neurofilaments are major intrinsic determinants of axonal caliber in myelinated nerve fibers and mainly present in more mature fibers.41 Therefore, this discrepancy may arise by the sort observational timeframe we adopted for in vivo experiments, but we cannot exclude it regenerating nervous fibers may stabilize and become more mature for timeframes beyond 2 weeks.
Present findings also show degradation of NG2 in favor of increased GAP43 labeled axons. It is known that after SCI, axons located rostrally to the lesion site may undergo incomplete regeneration/plasticity, particularly by producing short axonal projections that are unable to penetrate and bridge the central lesion. On the contrary, the axons positioned below the lesion site retract from postsynaptic neurons and undergo irreversible Wallerian degeneration.42
Our data indicate degradation of the inhibitory extracellular matrix (ECM) to a more permissive environment extending and facilitating early axonal regenerative processes at 2 weeks after treatment. NG2 reduction testifies for ECM reorganization, an ongoing process (at time of sacrifice) allowing for growth cone extensions protruding within the ChABC‐treated gliotic scar. In this study, 6 weeks after the initial contusive injury, ChABC in Sucr 2.5 mol/L increased the plasticity of injured tissue by providing a more favorable microenvironment contributing to spontaneous collateral sprouting and increased penetration of GAP43 nerve fibers within central lesion. Others previously showed that high‐dose (20 U/mL) intraspinal injections of ChABC produced extensive CSPG digestion in the injured rat spinal cord,43 indicating that injecting ChABC is a very effective and efficient method for delivery, but repeated injections to different days 43 or intrathecal catheter to distribute the enzyme 11, 42, 44, 45 may produce additional damage to the spinal cord.46 Therefore, our results highlight the need for sugar solutions to overcome ChABC (3 μL, 2 U/mL per injection) thermal inactivation, showing that treatment to the injury site leads to digestion of CSPG in perineuronal nets, structures associated with the restriction of neuronal plasticity. All these findings well correlate with the motor recovery of animals assessed via BBB test. In treated animals, a statistically significant improvement of motor functions was induced in comparison to control groups. Therefore, data suggest that a single session of delivery of ChABC previously stabilized in sucrose solutions, in combination with treadmill training, may have fostered locomotor recovery and early axonal regeneration/sprouting.
It is known that promoting adaptive structural and functional plasticity with rehabilitation can help the formation of appropriate connections in the spinal cord, increase neurotrophic factors production,47 modify electrical properties of motoneurons through their neuronal energy balance,48 and enhance the formation of new neuronal circuits.49 We can reasonably hypothesize that spared lateral spinal tracts, showing increased sprouting following ChABC treatment, may have been further helped in their reinnervation by our general rehabilitation protocol, sufficient enough to improve rats performance (BBB score) in the short term.
Nevertheless, this study has some potential limitations: (i) better insights about the stabilization mechanism exerted by sucrose (and evaluation for a potential protocol improvement) may be given by molecular dynamics and, in particular, protein docking analyses; (ii) a deep characterization of sucrose effects on the tissues surrounding injection site is mandatory before bringing this approach to clinics; and (iii) longer observational timeframes may be required to asses any potential incremental functional recovery and nervous regeneration.
Indeed, we foresee extra room for functional improvement if experimental timeframes are extended beyond 2 weeks: however, as no additional ChABC is added, from 1 month on it will likely be completely eluted/degraded and any additional improvement (if present) may mainly come from protracted rehab sessions. It is in our commitment to prolong our experimental timeframes to better assess this point.
It could also be a feasible strategy to provide a bigger reservoir of stabilized ChABC by implanting a biodegradable scaffold preserving the storage condition of ChABC we tested: this could be achieved, for example, by co‐injecting nanoparticles, previously loaded with stabilized ChABC, with self‐assembling peptide scaffolds that already showed promising results in SCI regeneration in vivo.50, 51, 52 In another interesting approach, Bartus et al53. showed that lentiviral vector local production of ChABC reduced injury cavitation and fostered preservation of spared nervous tissue, improving sensorimotor function in a clinically relevant model of a contusive spinal contusion.53 Nonetheless, the proposed “delivery” method of ChABC may arise safety issues like insertional mutagenesis given by lentiviral vectors 54 that will slow its translation to clinics. Our study, using a very simple stabilizing approach for ChABC brought interesting results and supposedly did not lead to significant harmful stimuli up to 2 weeks posttreatment.
In summary, SCI is one of the most devastating human pathologies, having a significant impact on life quality, bringing considerable costs to primary care and the overall society. Every new step favoring the translation of potential therapies for SCI may significantly improve the life of many. We demonstrated that intraspinal injections of sucrose‐stabilized ChABC, corroborated by daily rehabilitation sessions, are a feasible protocol causing reduction of gliosis in chronic SCI and improved behavioral recovery: they locally modified the scar extracellular matrix producing a favorable environment for nervous regeneration (Figure 3C). Limiting the number of subsequent deliveries may bring ChABC closer to a clinical treatment for chronic SCI.
CONFLICT OF INTEREST
The authors declare no competing interests.
Supporting information
ACKNOWLEDGMENTS
We thank Drs. M.Copetti and A. Fontana for their helpful assistance in statistical analysis. This work was supported in part by Fondazione Cariplo (No. 2011‐0352), by the “Ricerca Corrente 2017” funding granted by the Italian Ministry of Health, by Revert Onlus, by the “5 × 1000” voluntary contributions and by Fondazione Banca Del Monte di Lombardia.
Raspa A, Bolla E, Cuscona C, Gelain F.Feasible stabilization of chondroitinase abc enables reduced astrogliosis in a chronic model of spinal cord injury. CNS Neurosci Ther. 2019;25:86–100. 10.1111/cns.12984
REFERENCES
- 1. Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci USA. 2010;107:3340‐3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsui F, Oohira A. Proteoglycans and injury of the central nervous system. Congenit Anom (Kyoto). 2004;44:181‐188. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. Bellamkonda, CS‐4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545‐558. [DOI] [PubMed] [Google Scholar]
- 4. Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146‐156. [DOI] [PubMed] [Google Scholar]
- 6. Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116‐120. [DOI] [PubMed] [Google Scholar]
- 7. McKeon RJ, Hoke A, Silver J. Injury‐induced proteoglycans inhibit the potential for laminin‐mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32‐43. [DOI] [PubMed] [Google Scholar]
- 8. Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite‐promoting potential of spinal cord tissue. Exp Neurol. 1998;154:654‐662. [DOI] [PubMed] [Google Scholar]
- 9. Yick LW, Wu W, So KF, Yip HK, Shum DK. Chondroitinase ABC promotes axonal regeneration of Clarke's neurons after spinal cord injury. NeuroReport. 2000;11:1063‐1067. [DOI] [PubMed] [Google Scholar]
- 10. Yick LW, Cheung PT, So K, Wu W. Axonal regeneration of Clarke's neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol. 2003;182:160‐168. [DOI] [PubMed] [Google Scholar]
- 11. Bradbury EJ, Moon LDF, Popat RJ. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636‐640. [DOI] [PubMed] [Google Scholar]
- 12. Tester NJ, Howland DR. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol. 2008;209:483‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soheila Karimi‐Abdolrezaee EE, Jian W, Desiree S, Michael G. Fehlings, synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liebscher T, Schnell L, Schnell D, et al. Nogo‐A antibody improves regeneration and locomotion of spinal cord‐injured rats. Ann Neurol. 2005;58:706‐719. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332‐9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia‐Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task‐specific rehabilitation. Nat Neurosci. 2009;12:1145‐1151. [DOI] [PubMed] [Google Scholar]
- 17. Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter LM, McMahon SB, Bradbury EJ. Delayed treatment with chondroitinase ABC reverses chronic atrophy of rubrospinal neurons following spinal cord injury. Exp Neurol. 2011;228:149‐156. [DOI] [PubMed] [Google Scholar]
- 19. Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC's ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res. 2007;85:1110‐1118. [DOI] [PubMed] [Google Scholar]
- 20. Pakulska MM, Vulic K, Shoichet MS. Affinity‐based release of chondroitinase ABC from a modified methylcellulose hydrogel. J Control Release. 2013;171:11‐16. [DOI] [PubMed] [Google Scholar]
- 21. Cordone L, Cottone G, Cupane A, Emanuele A, Giuffrida S, Levantino M. Proteins in saccharides matrices and the trehalose peculiarity: biochemical and biophysical properties. Curr Org Chem. 2015;19:1684‐1706. [Google Scholar]
- 22. Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523‐1535. [PubMed] [Google Scholar]
- 23. Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF‐generated CNS progenitor cells. Neuron. 1993;11:951‐966. [DOI] [PubMed] [Google Scholar]
- 24. Gelain F, Lomander A, Vescovi AL, Zhang S. Systematic studies of a self‐assembling peptide nanofiber scaffold with other scaffolds. J Nanosci Nanotechnol. 2007;7:424‐434. [DOI] [PubMed] [Google Scholar]
- 25. Gelain F, Bottai D, Vescovi A, Zhang S. Designer self‐assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3‐dimensional cultures. PLoS ONE. 2006;1:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1‐21. [DOI] [PubMed] [Google Scholar]
- 27. Battistuzzo CR, Rank MM, Flynn JR, et al. Effects of treadmill training on hindlimb muscles of spinal cord‐injured mice. Muscle Nerve. 2017;55:232‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raspa A, Saracino GA, Pugliese R, et al. Complementary Co‐assembling peptides: from in silico studies to in vivo application. Adv Func Mater. 2014;24:6317‐6328. [Google Scholar]
- 29. Lakshmi TS, Nandi PK. Effects of sugar solutions on the activity coefficients of aromatic amino acids and their N‐acetyl esters. J Phys Chem. 1976;80:249‐252. [Google Scholar]
- 30. Shahaboddin ME, Khajeh K, Maleki M, Golestani A. Improvement of activity and stability of Chondroitinase ABC I by introducing an aromatic cluster at the surface of protein. Enzyme Microb Technol. 2017;105:38‐44. [DOI] [PubMed] [Google Scholar]
- 31. Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979;18:5191‐5196. [DOI] [PubMed] [Google Scholar]
- 32. Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256:7193‐7201. [PubMed] [Google Scholar]
- 33. Chen Z, Li Y, Feng Y, Chen, Qipeng Yuan L, Yuan Q. Enzyme activity enhancement of chondroitinase ABC I from Proteus vulgaris by site‐directed mutagenesis. RSC Adv. 2015;5:76040‐76047. [Google Scholar]
- 34. Timasheff SN. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67‐97. [DOI] [PubMed] [Google Scholar]
- 35. Kita Y, Arakawa T, Lin T‐Y, Timasheff SN. Contribution of the surface free energy perturbation to protein‐solvent interactions. Biochemistry. 1994;33:15178‐15189. [DOI] [PubMed] [Google Scholar]
- 36. Bisswanger H. Enzyme assays. Perspectives. Science. 2014;1:41‐55. [Google Scholar]
- 37. Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231‐255. [DOI] [PubMed] [Google Scholar]
- 38. Hu R, Zhou J, Luo C, et al. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. Laboratory investigation. J Neurol Neurosurg Spine. 2010;13:169‐180. [DOI] [PubMed] [Google Scholar]
- 39. Giles W, Bates ML, Bunge MB. Mary bartlett bunge, inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral schwann cell graft‐transected spinal cord interface. Mol Cell Neurosci. 2001;17:471‐487. [DOI] [PubMed] [Google Scholar]
- 40. Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616‐7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci USA. 1987;84:3472‐3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barritt AW, Davies M, Marchand F, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856‐10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tom VJ, Houle JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226‐239. [DOI] [PubMed] [Google Scholar]
- 45. Kim BG, Dai HN, Lynskey JV, McAtee M, Bregman BS. Degradation of chondroitin sulfate proteoglycans potentiates transplant‐mediated axonal remodeling and functional recovery after spinal cord injury in adult rats. J Comp Neurol. 2006;497:182‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Tech. 2001;54:317‐324. [DOI] [PubMed] [Google Scholar]
- 47. Ying Z, Roy RR, Edgerton VR, Gómez‐Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193):411‐419. [DOI] [PubMed] [Google Scholar]
- 48. Plunet WT, Streijger F, Lam CK, Lee JH, Liu J, Tetzlaff W. Dietary restriction started after spinal cord injury improves functional recovery. Exp Neurol. 2008;213:28‐35. [DOI] [PubMed] [Google Scholar]
- 49. Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993‐3003. [DOI] [PubMed] [Google Scholar]
- 50. Pugliese R, Gelain F. Peptidic biomaterials: from self‐assembling to regenerative medicine. Trends Biotechnol. 2017;35:145‐158. [DOI] [PubMed] [Google Scholar]
- 51. Raspa A, Pugliese R, Maleki M, Gelain F. Recent therapeutic approaches for spinal cord injury. Biotechnol Bioeng. 2016;113:253‐259. [DOI] [PubMed] [Google Scholar]
- 52. Cigognini D, Silva D, Paloppi S, Gelain F. Evaluation of mechanical properties and therapeutic effect of injectable self‐assembling hydrogels for spinal cord injury. J Biomed Nanotechnol. 2014;10:309‐323. [DOI] [PubMed] [Google Scholar]
- 53. Bartus K, James ND, Didangelos A, et al. Large‐scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822‐4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hacein‐Bey‐Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X‐linked severe combined immunodeficiency. N Engl J Med. 2003;348:255‐256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


