Summary
Aims
Acid‐sensing ion channels (ASICs) are extracellular proton‐gated cation channels that have been implicated in multiple physiological and pathological processes, and peripheral ASIC3 prominently participate into the pathogenesis of chronic pain, itch, and neuroinflammation, which necessitates the need for discovery and development of novel modulators in a subtype‐specific manner.
Methods
Whole‐cell patch clamp recordings and behavioral assays were used to examine the effect of several natural compounds on the ASIC‐mediated currents and acid‐induced nocifensive behavior, respectively.
Results
We identified a natural flavonoid compound, (‐)‐epigallocatechin gallate (EGCG, compound 11), that acts as a potent inhibitor for the ASIC3 channel in an isoform‐specific way. The compound 11 inhibited ASIC3 currents with an apparent half maximal inhibitory concentration of 13.2 μmol/L when measured at pH 5.0. However, at the concentration up to 100 μmol/L, the compound 11 had no significant impacts on the homomeric ASIC1a, 1b, and 2a channels. In contrast to most of the known ASIC inhibitors that usually bear either basic or carboxylic groups, the compound 11 belongs to the polyphenolic family. In compound 11, both the chirality and the 3‐hydroxyl group of its pyrogallol part, in addition to the integrity of the gallate part, are crucial for the inhibitory efficacy. Finally, EGCG was found significantly to decrease the acid‐induced nocifensive behavior in mice.
Conclusion
Taken together, these results thus defined a novel backbone structure for small molecule drug design targeting ASIC3 channels to treat pain‐related diseases.
Keywords: (‐)‐epigallocatechin gallate, acid‐sensing ion channel 3, natural flavonoid compound, pain, subtype‐specific inhibitor
1. INTRODUCTION
Acid‐sensing ion channels (ASICs) are extracellular proton‐gated cation channels and belong to the epithelial sodium channel/degenerin (ENaC/DEG) superfamily.1, 2 ASICs comprise at least 6 isoforms (ASIC1a, 1b, 2a, 2b, 3, and 4) encoded by 4 genes.2 A functional ASIC is formed by 3 homologous or heterologous subunits,3 and different subunit combinations give rise to diverse subtypes of channels with variable biophysical and pharmacological characteristics.2 Together with the fact of differential expression and distribution for each ASIC subunit, the physiological and pathological functions of ASICs hence vary among different subtypes.4 Among all the isoforms, ASIC1a and ASIC3 have been studied extensively for their functional roles. ASIC1a has been physiologically implicated in synaptic transmission,5, 6 synaptic plasticity and learning and memory,5, 6, 7, 8 and also involved in pathological processes including ischemic neuronal death,9, 10 neurodegenerative diseases,11, 12, 13, 14 chronic pain,15, 16, 17 seizure termination,18 anxiety,19 and depression.20 By contrast, ASIC3 prominently participates into multimodal sensory perception,21 including nociception,22, 23, 24, 25, 26, 27 mechanosensation,28, 29, 30, 31 and chemosensation.32, 33, 34, 35, 36, 37, 38 As a result, targeting ASIC3 channel is beneficial for counteracting chronic hyperalgesia produced by the inflammation occurred on the muscle,39, 40 joint,41, 42 and skin.43, 44 Moreover, ASIC3 also contributes to dry skin‐induced scratching behavior and pathological changes under conditions with concomitant inflammation.45, 46 However, the peripheral roles played by ASIC1a and ASIC3, respectively, are not always consistent. Targeting different subtypes of ASICs with pharmacological agents likely produce pronounced but diverse effects for treatment of sensory disorders, raising a necessity for discovery and development of channel modulators in a subtype‐specific way.
Due to the important roles played by ASIC3 in diverse pain‐rated disease processes, some small molecules were reported to inhibit ASIC3 channels in the past few years. Those ASIC3 inhibitors (Figure 1) could be structurally divided into 2 classes based on acidity and basicity of their functional groups. Amiloride (1), A‐317567 (2), Nafamostat (3), benzothiophene methyl amines (4), and diminazene (5) are basic compounds bearing at least 1 basic group, such as amidino group, guanidino group, or amino group. Compound 1, the first nonspecific small‐molecule inhibitor against all subtypes of ASICs, even inhibits most of the DEG/ENaC family members.47 It blocks all kinds of ASIC currents with the IC50 value1, 47 to be 10‐20 μmol/L. Compound 2 which belongs to arylamidine family is the first nonamiloride inhibitor with relatively high ASIC3 selectivity (IC50: 2‐30 μmol/L).48 Arylamidine 3 reversibly blocks ASIC1a, ASIC2a, and ASIC3 channels (IC50: 2‐70 μmol/L).49 Structure 4 represents a series of amine chemotypes with potent inhibitory activities against ASIC3 channels (IC50 values are around 0.20‐50 μmol/L).50 Diamidine 5, which is used as an anti‐infective drug for animals, also inhibits the ASIC3 current51 by 74 ± 0.6% at concentration of 3 μmol/L. Besides those compounds that equipped with basic groups, compounds with carboxylic groups, like nonsteroidal anti‐inflammatory drugs (NSAIDs), including salicylic acid (6), diclofenac (7), and aspirin (8) also inhibit ASIC3 currents with the IC50 value from 90 to 260 μmol/L with no isoform selectivity.52 Chlorogenic acid (9) is a natural phenolic compound with 1 carboxylic group in the structure. And it inhibits ASIC‐like currents in sensory neurons53 with an IC50 around 0.2 μmol/L. Interestingly, puerarin (10), a natural flavonoid with neither basic groups nor carboxylic groups in the structure, but only several phenol groups, also inhibits ASIC‐like currents in hippocampal neurons54 with the IC50 at 38.4 μmol/L. Although the identity of channel targeted by the compound 10 was not established yet, its bioactivity prompted us to evaluate other flavonoids for their capabilities to antagonize ASIC3 channels. By electrophysiologically screening small molecules containing the phenol groups (Figure 2A), here we identify a natural flavonoid compound, (‐)‐epigallocatechin gallate (EGCG, compound 11), that acts as a potent inhibitor for ASIC3 channels in an isoform‐specific way, thus defining a novel backbone structure for small molecule drug design targeting ASIC3 channels to treat pain‐related diseases.
Figure 1.
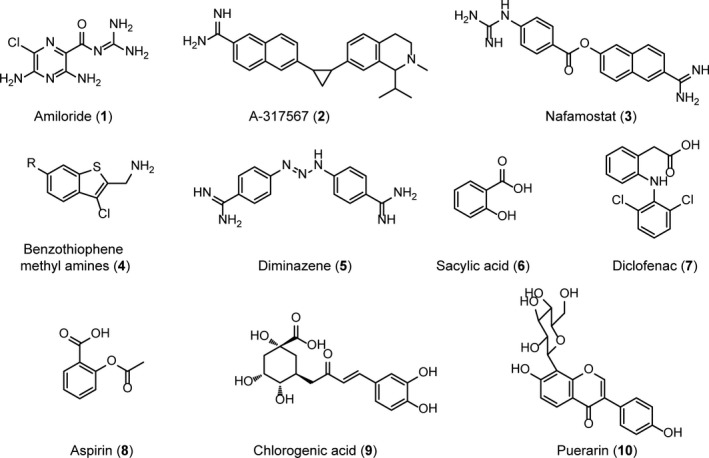
Reported small molecule antagonists targeting the ASIC channel with an emphasis on its capability on ASIC3 subtypes
Figure 2.
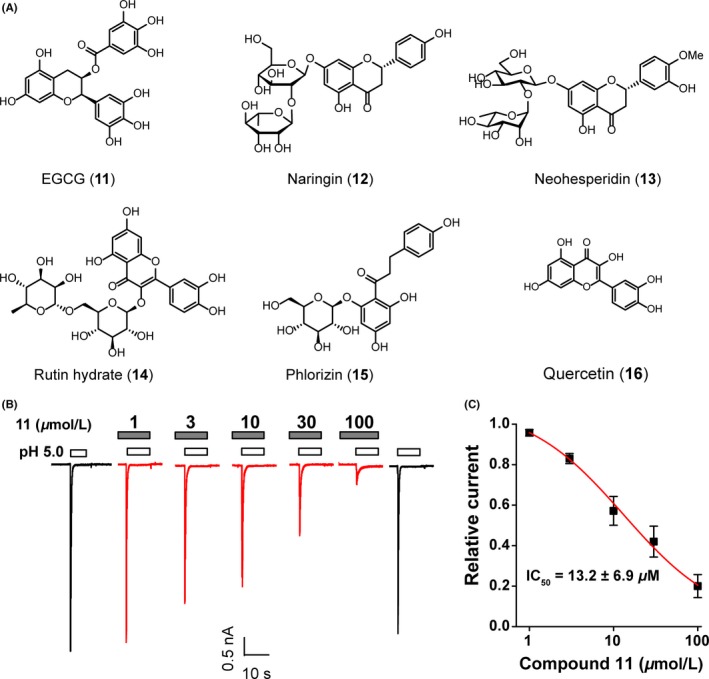
Effects of compound 11 on ASIC3 channels. (A) Chemical structures of natural compounds with phenol groups including (‐)‐epigallocatechin gallate (EGCG, compound 11). (B) Compound 11 dosedependently inhibits pH5.0‐evoked inward currents in ASIC3‐transfected CHO cells. (C) Concentrationresponse relationship of compound 11 on ASIC3 currents. Each point is the mean ± S.E.M. of 6 measurements normalized to the pH 5.0‐induced peak currents, and the solid line was the fit to the Hill equation
2. MATERIALS AND METHODS
2.1. Animal
Animal procedures were carried out in accordance with the guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University School of Medicine and approved by the Institutional Animal Care and Use Committee (Department of Laboratory Animal Science, Shanghai Jiao Tong University School of Medicine) (Policy Number DLAS‐MP‐ANIM. 01‐05). All efforts were made to minimize animal suffering and to reduce the number of animals used. Mice were kept in a standard 12‐hour light/dark cycle (light on at 07:00 am) at 21°C and 50%‐60% humidity and had access to food and water ad libitum except during tests. The male C57BL/6J mice (2‐3 months old) used in this study were obtained from Shanghai Slac Laboratory Animal Company Limited (Shanghai, China). The animals were housed 3 to 4 animals per cage. Animals were acclimatized to the testing room for at least 1 hour before all behavioral experiments, and we conducted each behavioral assessment once for each animal, in a randomized and blind order.
2.2. Cell culture and transfection
The cDNAs used for heterologous expression of ASIC channels were as follows: human ASIC1a (NM_001095.3); rat ASIC1b (AJ006519.1); rat ASIC2a (NM_001034014.1); rat ASIC2b (NM_012892.2); mouse ASIC3 (NM_183000.2). All constructs were expressed in Chinese hamster ovary (CHO) cells. Transient transfection of CHO cells was performed as reported previously.37 In brief, CHO cells were cultured in F‐12 Nutrient Mixture supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) gluta‐MAX™−1 (all from Thermo Fisher Scientific) at 37°C in a humidified atmosphere of 5% (v/v) CO2 and 95% O2 (v/v) and passaged twice a week. Transfection was performed using HilyMax liposome transfection reagent (Dojindo Laboratories). All ASIC plasmids used contained the coding sequence for enhanced green fluorescent protein (EGFP) to aid identification of transfected cells. Electrophysiological measurements were performed 24‐48 hours after transfection.
2.3. Chemicals
All compounds were purchased from Sigma‐Aldrich (St. Louis, MO) unless otherwise mentioned.
2.4. Electrophysiology
The compounds were tested in vitro using the standard electrophysiology‐based assay37 to identify their inhibitory activity against ASIC channels. Whole‐cell recordings were made using an Axon 700A patch clamp amplifier (Axon Instruments). Membrane currents were sampled and analyzed using a Digidata 1440 interface and a personal computer running Clampex and Clampfit software (Version 10, Axon Instruments). In most experiments, 70%‐90% of the series resistance was compensated. Voltage‐clamp (with the holding potential of ‐60 mV) recordings were performed as described previously.37
Compounds were dissolved in standard extracellular solution and acidic solution. The standard extracellular solution contains as follows (in mmol/L): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 N‐hydroxyethylpiperazine‐N‐2‐ethanesulfonic acid (HEPES), and 10 glucose, pH 7.4, adjusted with Tris‐base. The acidic solution contains as follows (in mmol/L): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 2‐(N‐morpholino)ethanesulfonic acid (MES), and 10 glucose, pH 5.0, adjusted with Tris‐base. The whole‐cell recordings were performed using the patch pipette solution with high Cl− concentration, which was composed of (in mmol/L): 150 KCl, 2 MgCl2, 1 CaCl2, 10 ethylene glycol tetraacetic acid (EGTA), 10 HEPES, pH 7.2, adjusted with KOH.
2.5. Pain‐related behavior assay
All behavioral measurements were performed using male C57BL/6J mice (2‐3 months old). Animals were acclimatized to the testing chamber for 30 minutes before all behavioral experiments. A total volume of 20 μL of solution (in saline) containing acetic acid (0.6%, pH 3.5‐4.0), or EGCG (100 μmol/L) plus acetic acid (0.6%) was injected intraplantarly using a 30 G needle, and paw‐licking behavior was quantified for 30 minutes.27, 37 Behavioral experiments were conducted and scored with the experimenter blinded to the treatment.
2.6. Data analysis
Results were expressed as the mean ± S.E.M. Statistical comparisons were performed using unpaired or paired Student's t tests. P < 0.05 represent statistically significant differences. The software Clampfit 10.5 was used for data analysis. The curve for the effect of EGCG on the pH 5.0‐induced currents was fitted by the following equation: I = I max(IC50)nH/[C nH + (IC50)nH], where I is the normalized value of the current, I max is the maximum whole cell current amplitude, C is the drug concentration, IC50 represents the antagonist concentration producing a half‐maximal inhibitory effect, and the others are the same as described above.
3. RESULTS
3.1. Chemistry and electrophysiological evaluation
To identify natural compounds with phenol groups targeting ASIC3 channels, a compound library containing several flavonoid polyphenols (Figure 2A) was set up to study their effects on ASIC3 current. The potency of compounds was evaluated on ASIC3 channels expressed in Chinese hamster ovary (CHO) cells using standard patch clamp techniques. In response to a pH drop from 7.4 to 5.0, the cells clamped at −60 mV gave rise to large inward ionic current ranging from 2 to 10 nA, which is mediated by homomeric ASIC3 channels. All the compounds were initially tested at the concentration of 100 μmol/L. Based on an initial screening on these compounds (data not shown), we picked up a flavonoid compound, (‐)‐epigallocatechin gallate (EGCG, Figure 2A, compound 11), the most potent compound, and performed additional mechanistic analyses and pharmacological studies. The compound 11 alone at the concentration up to 100 μmol/L induced no significant current in ASIC3‐expressing CHO cells, but its continuous pretreatment for 40 seconds and the following combination with pH 5.0 significantly inhibited the acid‐induced currents (Figure 2B).
Next, the IC50 value of compound 11 was measured. In the IC50 studies, for each concentration of the compound 11, the pH 5.0 solution was perfused for 20 seconds, followed by the pH 7.4 solution for 2 minutes for the cell to recovery. Then, the compound 11 dissolved in the pH 7.4 solution was perfused for 40 seconds followed by that dissolved in pH 5.0 solution for another 20 seconds, which gave the acid‐evoked current regulated by the different concentrations of compounds. When the stable inhibition was reached, the next dose was then added. As shown in Figure 2B, the compound 11 caused a concentration‐dependent inhibition of ASIC3 current. The current amplitude was reduced to around 96%, 83%, 57%, 42%, and 20% of the initial current at 1, 3, 10, 30, and 100 μmol/L (n = 6 each), respectively. The IC50 value of compound 11 against ASIC3 channels was then calculated to be 13.2 ± 6.9 μmol/L.
3.2. Identification of pharmacophore requirement of compound 11 for inhibition of ASIC3 channels
To understand the molecular basis of compound 11 interacting with ASIC3, a brief study on structure–activity relationship (SAR) was carried out using commercially available natural analogs of compound 11. The structure of compound 11 could be dissected into 2 fragments. One is flavane region, which is composed of benzopyran part and pyrogallol part, and the other is gallate region. To examine the role of the gallate region, the existence pattern of phenol groups and the configuration of chirality exhibited in the structure, 5 analogs of 11 (Figure 3, compounds 17−21) were chosen for the SAR study. The inhibitory activities of these 5 analogs were evaluated by electrophysiological assay. Compared with the activity of compound 11, all of these analogs at the concentration of 100 μmol/L displayed substantially reduced potency for inhibition of ASIC3 currents (Figure 3).
Figure 3.
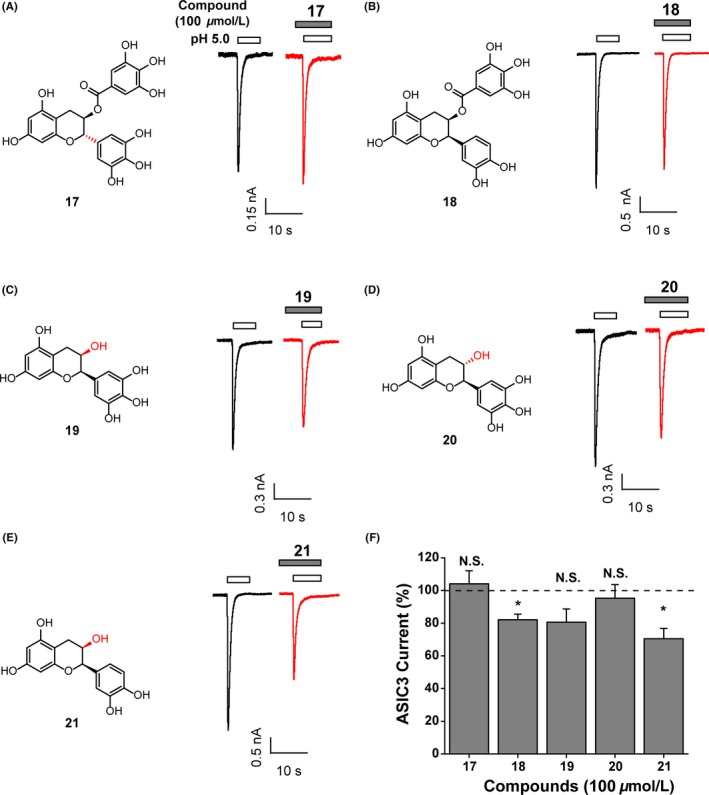
Structure–activity relationship of compound 11 derivatives. (A‐E) (Left) Chemical structures of compound 11 derivatives. (Right) Representative current traces showing the inhibition of pH 5.0‐activated currents from ASIC3 expressing CHO cells induced by compounds as indicated. Every compound was used at the concentration of 100 μmol/L. (F) Pooled data. Data points are means ± S.E.M. of 3‐6 measurements. N.S., not significant difference, *P < 0.05, compared with 100% (dashed line), paired Student's t test
Based on these observations, the following structural rules for polyphenol compounds to inhibit ASIC3 channels were summarized as follows (shown in Figure 4): First, the chirality of pyrogallol part is vital to keep the inhibitory activity (see compound 17 vs. compound 11); second, the 3‐hydroxyl group on the pyrogallol part is necessary for the inhibitory efficacy of compound 11 on ASIC3 (see compound 18 vs. compound 11); third, the absence of gallate part resulted in the complete loss of capability to antagonize ASIC3 (see compounds 19 and 20 vs compound 11). As expected, the compound 21 with the simultaneous lack of 3‐hydroxyl group on the pyrogallol part‐like compound 18 and the whole gallate part‐like compound 19 also lost the inhibitory potency on ASIC3 channels.
Figure 4.
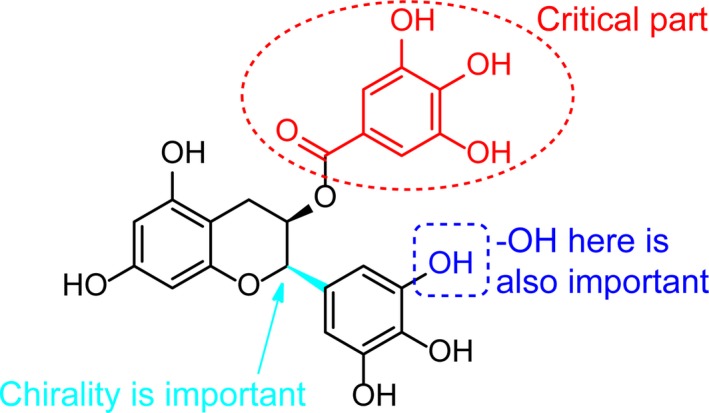
Schematic demonstration for structure‐activity relationship of the compound 11 on ASIC3 channels. Please see the text for more details
3.3. Subtype‐specificity of ASIC3 channel inhibition by compound 11
Further studies on CHO cells expressing different homomeric ASIC subunits revealed that compound 11 was a specific antagonist for ASIC3 but not ASIC1a, ASIC1b, nor ASIC2a homomers (Figure 5). This subunit specificity highlights the present finding as that no compound with acidic groups was reported to be the selective ASIC antagonist yet. Altogether, these results suggest that EGCG (compound 11) represents a novel class of isoform‐specific inhibitor for ASIC3, which pave the way for small‐molecule drug development targeting the channel‐dependent pain‐related diseases.
Figure 5.
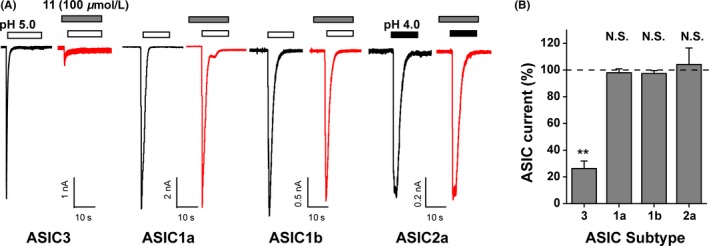
Subunit selectivity of compound 11 action on ASICs. (A) Representative current traces showing the inhibition of pH 5.0‐activated currents induced by compound 11 from CHO cells expressing different ASIC subunits as indicated. (B) Summary results. Data points are means ± S.E.M. of 3‐6 measurements. N.S., not significant difference, **P < 0.01, compared with 100% (dashed line), paired Student's t test
3.4. Compound 11 alleviates acid‐induced nocifensive behaviors
Finally, to gain insights into the in vivo consequence of inhibition of ASIC3 channels by EGCG (compound 11), we performed pain‐related behavioral tests27, 37 following the injection of acid with or without the compound 11 into the right‐hind paw of C57BL/6J mice, because previous studies have shown that ASIC3 channels mediate acid‐induced pain responses.22, 23, 24, 25, 26, 27 We measured the total time that the mice spent licking the injected paw during a 30‐minutes period. As shown in Figure 6, compared with the injection of saline, that of acetic acid (0.6%, pH 3.5~4.0) evoked a dramatic increase in paw‐licking time. Notably, inclusion of EGCG (compound 11, 100 μmol/L) significantly attenuated the acid‐induced pain‐like response. Thus, EGCG (compound 11) is able to alleviate pain‐related behaviors, likely through inhibition of ASIC3 channels, the aforementioned molecular mechanisms of subtype‐selective inhibition of ASIC3 channels by this natural flavonoid.
Figure 6.
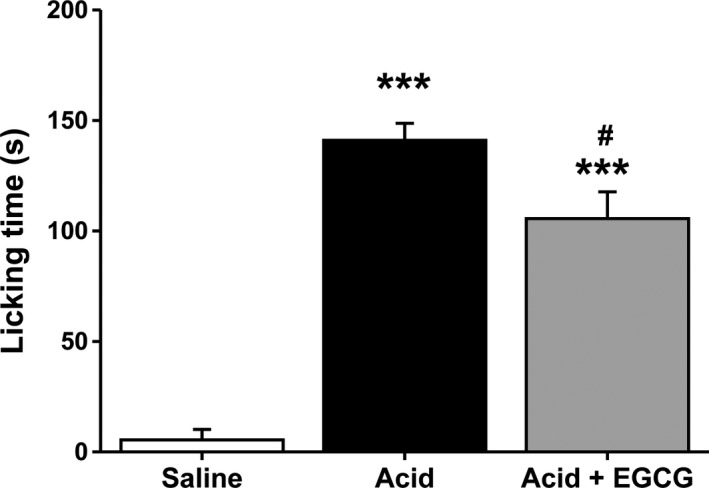
Compound 11 alleviates acid‐induced nocifensive behaviors. Pain‐related behaviors as determined by the time spent for following saline, acetic acid (0.6%, pH 3.5~4.0), or acetic acid plus EGCG (compound 11, 100 μmol/L). n = 7 each group. ***P < 0.001, compared with saline; #P < 0.05, compared with acetic acid; unpaired Student's t test
4. DISCUSSION
In the present study, we showed that the natural compound EGCG (compound 11) dose dependently suppressed the currents mediated by ASIC3 channels recombinantly expressing in CHO cells with an IC50 value of 13.2 ± 6.9 μmol/L (Figure 2). Profoundly, the inhibitory efficacy of compound 11 on ASICs was subtype specific, as it did not affect homeric ASIC1a, ASIC1b, and ASIC2a channels under tested concentration (Figure 5). These findings shed more light on the pharmacological modulation of ASICs and could guide the development of new drugs targeting this type of ion channels.
In line with the multifaceted physiological and pathophysiological roles of ASICs, the pharmacology and the drug development on these channels have advanced considerably in recent years. The small molecule inhibitors for ASICs can be largely classified into the compounds carrying basic groups (compounds 1–5)47, 48, 49, 50, 51 and the NSAIDs that contain a characteristic acidic carboxylic group (compounds 6–8).52 Recently, polyphenol compounds as the natural products isolated from plants, such as chlorogenic acid (compound 9)53 and puerarin (compound 10),54 were identified to exert significant inhibitory effects on acid‐induced ASIC‐like currents in hippocampal and sensory neurons, respectively. Due to the absence of functional ASIC3 expression in hippocampal neurons,2, 4, 21, 47 the inhibitory efficacy of compound 10 on the peripheral ASIC3 channel could not be deducted yet. By contrast, the compound 10 reduced the amplitude of homomeric ASIC1a channels in CHO cells.54 For the compound 9 targeting ASICs in sensory neurons,53 it is reasonable to speculate that this compound is likely capable of inhibiting ASIC3 channels due to the dominant expression of this channel subunit in sensory neurons.43 However, the subunit selectivity of compound 9 on different ASICs has not been examined yet.53 In the present study, we identified that compound 11 but not its structurally analogs (i.e compounds 17–21) subtype‐specifically antagonized ASIC3 channels, which indeed contains substantially new information on the mechanisms underlying the phenolic compound modulation on ASICs.
One may argue that, EGCG, as an amphiphilic molecule, would indiscriminately modulate the function of membrane protein without direct binding, but through its bilayer‐modifying effects.55 We appreciated the involvement of membrane perturbation mechanism for explanation of protein modulation by phytochemicals like EGCG.55 However, we believed that it was a different case here for identified inhibitory efficacy on ASIC3. By only altering the membrane property, EGCG would not be sufficient to cause the selective inhibition of ASIC3 channels. The fact that we found EGCG showed a higher selectivity on ASIC3 over other ASIC subtypes (Figure 5) suggested EGCG must interact with ASIC3 protein to cause the inhibition. Moreover, unlike EGCG, several structural analogs (i.e compounds 17–21, Figure 3) we tested did not exhibit the inhibition efficacy of ASIC3, restating a specific interaction between ligand and ASIC3 channels existed. As supporting evidence shown, several lipid agonists (i.e arachidonic acid and lysophosphatidylcholine, but not other types of lysophospholipids) for ASIC3 have been identified,56 again validating the specific manner for channel modulation by membrane‐associated chemical regents. Overall, we believed that phenolic EGCG inhibits ASIC3 most likely through specific binding sites in the channel, which remains an open question to be further characterized in the future.
Mechanistically, the following steps in the future studies may help advance the understanding on the molecular basis underpinning the inhibition of ASIC3 channels by phenolic compounds. First, the effects of EGCG on different ASIC subunits should be further considered and investigated. In our present study, only a single pH value (pH 5.0 for homomeric ASIC3, 1a, or 1b channels, pH 4.0 for homomeric ASIC2a channels) was used to examine the effect of EGCG on different ASIC subunits (Figure 5). Some compounds may cause a shift in dose–response curve but do not inhibit the maximal current. If that is the case, one would not see the effect at pH 5.0 but may see an inhibition at higher pH values. Nevertheless, the present observation of a strong inhibitory effects of EGCG on ASIC3 over other ASIC subunits supported a more preferential action manner on ASIC3 channels. Second, the systematic comparison study among differential pharmacological effects of compounds 9, 10, and 17–21 together with more unidentified analogs on ASIC3 over other ASIC subunits is necessary, which would shed more lights on the particular structural component for the ASIC3‐speficic modulation by phenolic compounds. Third, we reasoned that the compound 11 likely acts as an allosteric inhibitor of ASIC3 channels. There are basically 3 types of molecular mechanisms for ion channel inhibitors to exert an inhibitory action based on where they bind to the channel complexes. An open channel blocker binds to the channel pore to obstruct ion permeation, like amiloride (compound 1) on ASICs.2, 47 Due to the lack of positive charges in the compound 11 (Figure 2A), we believed that it unlikely acts as an open channel blocker. A competitive inhibitor usually binds to the site similar or close to the agonist binding domain (i.e proton sensor in ASICs) to compete with the agonist (i.e proton) for binding. It is not clear whether the known inhibitors for ASIC3 channels to employ this mechanism yet, but it has been strongly suggested that the compounds that inhibit ASIC1a channels at least should partially act as a competitive antagonist.57 The compound should not only shift the pH dependence of ASIC1a activation but also inhibit its maximal evoked response.57 The last type is the allosteric inhibitor, which binds to a distinct site from the channel pore or the agonist‐binding site to affect coupling of conformational changes to channel gating. Although it is not investigated to discern these possibilities using the compound 11 on ASIC3 channels, the polyphenol compound chlorogenic acid (compound 9) significantly shifted the proton concentration–response curve downward, with a decrease of 41.76 ± 8.65% in the maximum current response to protons but with no significant change in the pH50 value,53 suggesting an allosteric mechanism that likely confers the actions of phenolic compounds. Obviously, the binding sites for these compounds in ASIC3 channels together with the coupling mechanisms remain to be established. Nevertheless, the present identification of compound 11 selectively targeting ASIC3 over other ASIC subtypes lays the most important clue to search for structural basis for the channel modulation. Fourth, it is emergingly recognized that basic compounds such as amiloride (compound 1) and 2‐guanidine‐4‐methylquinazoline (GMQ) hold both inhibitory and paradoxically stimulatory effects on ASIC1a and ASIC3 channels.37, 58, 59, 60 It remains unclear whether here identified compound 11 and related phenolic analogs would also produce stimulatory effects. Altogether, the present identification would inspire more in depth studies using the compound 11 and its derivatives to explore the structure and function of ASIC3 channels and to treat pain‐related diseases.39, 40, 41, 42, 43, 44
In summary, natural polyphenolic flavonoid 11 was reported to inhibit ASIC3 channel at μmol/L level (IC50 = 13.2 ± 6.9 μmol/L) with a high selectivity against other ASICs isoforms herein. Unlike the amidine or other basic ASIC3 inhibitors, compound 11 belongs to polyphenol class. Therefore, it can be a valuable tool for further study of structure and function of ASIC3 channels. The preliminary SAR study showed that the gallate region and the absolute configuration of the chiral centers were important to maintain its inhibitory activity. Further study will aim at the development of compounds based on compound 11 and the extension of its pharmacological application based on the targeting on ASIC3 channel.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
This study was supported by grants from the National Basic Research Program of China (2014CB910300 to T.‐L. X. and J.‐H. L), the National Natural Science Foundation of China (81402775 to J.‐H. L., 81730095 and 91632304 to T.‐L. X., 81771214 to W.‐G. L.), and the Shanghai Committee of Science and Technology (18QA1402500 to W.‐G. L.).
Yan X‐G, Li W‐G, Qi X, et al. Subtype‐selective inhibition of acid‐sensing ion channel 3 by a natural flavonoid. CNS Neurosci Ther. 2019;25:47–56. 10.1111/cns.12979
The first two authors contributed equally to this work.
Contributor Information
Tian‐Le Xu, Email: xu-happiness@shsmu.edu.cn.
Jian‐Hua Liu, Email: jhliu7912@shsmu.edu.cn.
REFERENCES
- 1. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton‐gated cation channel involved in acid‐sensing. Nature. 1997;386:173‐177. [DOI] [PubMed] [Google Scholar]
- 2. Kellenberger S, Schild L. International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid‐sensing ion channels and the epithelial Na+ channel. Pharmacol Rev. 2015;67:1‐35. [DOI] [PubMed] [Google Scholar]
- 3. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid‐sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316‐323. [DOI] [PubMed] [Google Scholar]
- 4. Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477‐483. [DOI] [PubMed] [Google Scholar]
- 5. Du J, Reznikov LR, Price MP, et al. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci U S A. 2014;111:8961‐8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreple CJ, Lu Y, Taugher RJ, et al. Acid‐sensing ion channels contribute to synaptic transmission and inhibit cocaine‐evoked plasticity. Nat Neurosci. 2014;17:1083‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wemmie JA, Chen J, Askwith CC, et al. The acid‐activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463‐477. [DOI] [PubMed] [Google Scholar]
- 8. Li WG, Liu MG, Deng S, et al. ASIC1a regulates insular long‐term depression and is required for the extinction of conditioned taste aversion. Nat Commun. 2016;7:13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong ZG, Zhu XM, Chu XP, et al. Neuroprotection in ischemia: blocking calcium‐permeable acid‐sensing ion channels. Cell. 2004;118:687‐698. [DOI] [PubMed] [Google Scholar]
- 10. Gao J, Duan B, Wang DG, et al. Coupling between NMDA receptor and acid‐sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635‐646. [DOI] [PubMed] [Google Scholar]
- 11. Arias RL, Sung ML, Vasylyev D, et al. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis. 2008;31:334‐341. [DOI] [PubMed] [Google Scholar]
- 12. Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid‐sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong HK, Bauer PO, Kurosawa M, et al. Blocking acid‐sensing ion channel 1 alleviates Huntington's disease pathology via an ubiquitin‐proteasome system‐dependent mechanism. Hum Mol Genet. 2008;17:3223‐3235. [DOI] [PubMed] [Google Scholar]
- 14. Gonzales EB, Sumien N. Acidity and acid‐sensing ion channels in the normal and Alzheimer's disease brain. J Alzheimers Dis. 2017;57:1137‐1144. [DOI] [PubMed] [Google Scholar]
- 15. Duan B, Wu LJ, Yu YQ, et al. Upregulation of acid‐sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139‐11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mazzuca M, Heurteaux C, Alloui A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943‐945. [DOI] [PubMed] [Google Scholar]
- 17. Diochot S, Baron A, Salinas M, et al. Black mamba venom peptides target acid‐sensing ion channels to abolish pain. Nature. 2012;490:552‐555. [DOI] [PubMed] [Google Scholar]
- 18. Ziemann AE, Schnizler MK, Albert GW, et al. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziemann AE, Allen JE, Dahdaleh NS, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coryell MW, Wunsch AM, Haenfler JM, et al. Acid‐sensing ion channel‐1a in the amygdala, a novel therapeutic target in depression‐related behavior. J Neurosci. 2009;29:5381‐5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li WG, Xu TL. ASIC3 channels in multimodal sensory perception. ACS Chem Neurosci. 2011;2:26‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high‐intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992‐8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007;133:150‐160. [DOI] [PubMed] [Google Scholar]
- 24. Sluka KA, Winter OC, Wemmie JA. Acid‐sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693‐704. [PMC free article] [PubMed] [Google Scholar]
- 25. Li WG, Yu Y, Zhang ZD, Cao H, Xu TL. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain. 2010;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deval E, Noel J, Gasull X, et al. Acid‐sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059‐6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Li WG, Yu Y, et al. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci. 2013;33:4265‐4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price MP, McIlwrath SL, Xie J, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071‐1083. [DOI] [PubMed] [Google Scholar]
- 29. Lee CH, Sun SH, Lin SH, Chen CC. Role of the acid‐sensing ion channel 3 in blood volume control. Circ J. 2011;75:874‐883. [DOI] [PubMed] [Google Scholar]
- 30. Lin SH, Cheng YR, Banks RW, Min MY, Bewick GS, Chen CC. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun. 2016;7:11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fromy B, Lingueglia E, Sigaudo‐Roussel D, Saumet JL, Lazdunski M. Asic3 is a neuronal mechanosensor for pressure‐induced vasodilation that protects against pressure ulcers. Nat Med. 2012;18:1205‐1207. [DOI] [PubMed] [Google Scholar]
- 32. Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid‐sensing ion channel 3 matches the acid‐gated current in cardiac ischemia‐sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501‐509. [DOI] [PubMed] [Google Scholar]
- 34. Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid‐sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res. 2007;101:1009‐1019. [DOI] [PubMed] [Google Scholar]
- 35. Tan ZY, Lu Y, Whiteis CA, et al. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Immke DC, McCleskey EW. Lactate enhances the acid‐sensing Na+ channel on ischemia‐sensing neurons. Nat Neurosci. 2001;4:869‐870. [DOI] [PubMed] [Google Scholar]
- 37. Yu Y, Chen Z, Li WG, et al. A nonproton ligand sensor in the acid‐sensing ion channel. Neuron. 2010;68:61‐72. [DOI] [PubMed] [Google Scholar]
- 38. Birdsong WT, Fierro L, Williams FG, et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sluka KA, Radhakrishnan R, Benson CJ, et al. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292‐304. [DOI] [PubMed] [Google Scholar]
- 41. Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikeuchi M, Kolker SJ, Sluka KA. Acid‐sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan‐induced arthritis. J Pain. 2009;10:336‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deval E, Noel J, Lay N, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047‐3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid‐sensing ion channel 3 in sub‐acute‐phase inflammation. Mol Pain. 2009;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peng Z, Li WG, Huang C, et al. ASIC3 mediates itch sensation in response to coincident stimulation by acid and nonproton ligand. Cell Rep. 2015;13:387‐398. [DOI] [PubMed] [Google Scholar]
- 46. Jiang YM, Huang C, Peng Z, et al. Acidosis counteracts itch tachyphylaxis to consecutive pruritogen exposure dependent on acid‐sensing ion channel 3. Mol Pain. 2017;13:1744806917721114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735‐767. [DOI] [PubMed] [Google Scholar]
- 48. Kuduk SD, Di Marco CN, Bodmer‐Narkevitch V, et al. Synthesis, structure‐activity relationship, and pharmacological profile of analogs of the ASIC‐3 inhibitor A‐317567. ACS Che Neurosci. 2010;1:19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuduk SD, Chang RK, Wai JM, et al. Amidine derived inhibitors of acid‐sensing ion channel‐3 (ASIC3). Bioorg Med Chem Lett. 2009;19:4059‐4063. [DOI] [PubMed] [Google Scholar]
- 50. Kuduk SD, Chang RK, Di Marco CN, et al. Identification of non‐amidine inhibitors of acid‐sensing ion channel‐3 (ASIC3). Bioorg Med Chem Lett. 2011;21:4255‐4258. [DOI] [PubMed] [Google Scholar]
- 51. Chen X, Qiu L, Li M, et al. Diarylamidines: high potency inhibitors of acid‐sensing ion channels. Neuropharmacology. 2010;58:1045‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti‐inflammatory drugs inhibit both the activity and the inflammation‐induced expression of acid‐sensing ion channels in nociceptors. J Neurosci. 2001;21:8026‐8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qu ZW, Liu TT, Qiu CY, Li JD, Hu WP. Inhibition of acid‐sensing ion channels by chlorogenic acid in rat dorsal root ganglion neurons. Neurosci Lett. 2014;567:35‐39. [DOI] [PubMed] [Google Scholar]
- 54. Gu L, Yang Y, Sun Y, Zheng X. Puerarin inhibits acid‐sensing ion channels and protects against neuron death induced by acidosis. Planta Med. 2010;76:583‐588. [DOI] [PubMed] [Google Scholar]
- 55. Ingolfsson HI, Thakur P, Herold KF, et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem Biol. 2014;9:1788‐1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marra S, Ferru‐Clement R, Breuil V, et al. Non‐acidic activation of pain‐related Acid‐Sensing Ion Channel 3 by lipids. EMBO J. 2016;35:414‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buta A, Maximyuk O, Kovalskyy D, et al. Novel potent orthosteric antagonist of ASIC1a prevents NMDAR‐dependent LTP induction. J Med Chem. 2015;58:4449‐4461. [DOI] [PubMed] [Google Scholar]
- 58. Alijevic O, Kellenberger S. Subtype‐specific modulation of acid‐sensing ion channel (ASIC) function by 2‐guanidine‐4‐methylquinazoline. J Biol Chem. 2012;287:36059‐36070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li WG, Yu Y, Huang C, Cao H, Xu TL. Nonproton ligand sensing domain is required for paradoxical stimulation of acid‐sensing ion channel 3 (ASIC3) channels by amiloride. J Biol Chem. 2011;286:42635‐42646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Besson T, Lingueglia E, Salinas M. Pharmacological modulation of Acid‐Sensing Ion Channels 1a and 3 by amiloride and 2‐guanidine‐4‐methylquinazoline (GMQ). Neuropharmacology. 2017;125:429‐440. [DOI] [PubMed] [Google Scholar]


