Summary
Introduction
A possible target for stroke management is modulation of neuroinflammation. Evidence suggests that food components may exert anti‐inflammatory properties and thus may reduce stroke‐induced brain damage.
Aim
To investigate the efficacy of a diet, containing anti‐inflammatory ingredients, as treatment for focal ischemic brain damage induced by photothrombotic stroke in the somatosensory cortex of rats.
Results
Brain lesions were surrounded by strong astrogliosis on both day 7 and day 21 after stroke and were accompanied by a trend toward globally decreased glucose metabolism on day 7. The investigational diet applied 2 weeks before the ischemia did not affect astrocyte activation on day 7, but reduced it at day 21. The investigational diet applied immediately after the ischemia, increased astrocyte activation on day 7 and completely reversed this effect on day 21. Moreover, postischemic intervention increased glucose metabolism in somatosensory cortex ipsilateral to the lesion on day 7.
Conclusion
This study reveals potentially beneficial effects of a diet containing elevated amounts of anti‐inflammatory nutrients on the recovery from ischemic brain damage. Therefore, dietary intervention can be considered as an adjuvant therapy for recovery from this brain pathology.
Keywords: neuroinflammation, nutrition, PET imaging, photothrombotic stroke
1. INTRODUCTION
Ischemic stroke occurs when the blood supply to part of the brain is interrupted due to obstruction of a vessel by an embolism or thrombus. Current treatments need to be applied shortly after the onset of stroke, which is not always possible.1 Therefore, there is a need for additional treatment and prevention strategies that are more broadly applicable.
Several risk factors for stroke are potentially modifiable, including cigarette smoking, alcohol consumption, obesity, lack of physical activity and an unhealthy diet.2, 3 Diet may play an important role in the prevention of stroke. For example, high consumption of fruit, vegetables, olive oil and fish is associated with a lower incidence of stroke4 and can reduce its risk. Therefore, a proper diet could be a lifestyle intervention to prevent stroke or ameliorate its consequences.5
Many food components with potential preventive effects for stroke onset in humans, such as omega‐3 fatty acids, vitamins and fibers, have anti‐inflammatory properties.6, 7, 8, 9, 10 Indirect evidence suggests a positive impact of anti‐inflammatory diet on the survival following stroke in humans.11 Preclinical studies demonstrated a positive effect of anti‐inflammatory food components on neuroprotection.12, 13 It is known that stroke can trigger an inflammatory response and cause neuronal death.14 The inflammatory response is characterized by the activation and increased proliferation of microglia and astrocytes that produce inflammatory mediators. This process has an ambivalent role as it has been shown to be involved in both neurotoxicity and neuroprotection.15 It is currently believed that early neuroinflammatory response has beneficial effect by limiting tissue damage and promoting repair, but chronic and persistent neuroinflammation can lead to cell dystrophy and promote neurodegeneration.16 Since inflammation is under investigation as one of the potential targets for new therapeutic approaches in stroke,17, 18 we hypothesized that anti‐inflammatory food components may have a preventive and possibly a therapeutic effect on stroke and could possibly be considered for future adjuvant therapy.
The aim of this study was to investigate whether a diet containing elevated amounts of anti‐inflammatory nutrients can have beneficial effects on the recovery from stroke.19 The photothrombotic stroke model was selected for this proof‐of‐concept study, because it is minimally invasive and relatively moderate. The inflammatory response caused by the stroke is expected to be relatively mild, and therefore, ceiling effects that may obscure the effects of dietary intervention are less likely to occur. The investigational diet in this study was based on results from our20 and other studies6, 10, 21, 22 and was designed to maximize the effect by combining the anti‐inflammatory properties of its components. The dietary intervention was started either 2 weeks before, or directly after the induction of ischemia. We monitored astrocyte activation at day 7 and 21 following ischemia, since activated astrocytes are believed to play a protective role at the early stages of stroke,23 but may become detrimental if the activation persists. We also studied brain glucose metabolism as a functional parameter, using positron emission tomography (PET) with the glucose analogue [18F]FDG. This technique has already been successfully used to noninvasively monitor stroke progression in animals24 and in humans.25 Moreover, we investigated the effect of the diet on motor dysfunction with the cylinder test.26
2. MATERIALS AND METHODS
2.1. Experimental animals
Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Groningen (protocol DEC6971A) and performed in accordance with Dutch Regulations for Animal Welfare.
Male outbred Sprague Dawley rats (10 weeks of age, n = 48, 302 ± 3 g) were purchased from Harlan (Horst, The Netherlands) and housed in groups (2‐6 animals per cage) in thermo‐regulated (21 ± 2°C) and humidity‐controlled rooms under a 12‐12‐hour light‐dark cycle (lights on at 6 am). Food and water were available ad libitum, and paper rolls were used as cage enrichment. The rats were allowed to acclimatize for at least 7 days after arrival. All rats were fed with a standard laboratory chow (AIN93‐G) from the time of arrival until the start of the experiment.27 Rats were housed individually after surgery until the end of the experiment.
2.2. Diet
Two iso‐caloric diets were used in the study: a control diet (AIN93‐G) and an investigational diet based on AIN93‐G (Research Diet Services, Wijk bij Duurstede, The Netherlands). As shown in Table 1, the investigational diet contains low‐glycemic index carbohydrates and is supplemented with vitamins, specific dietary fibers, tryptophan, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Diets were stored at −20°C prior to use to prevent fatty acid oxidation.
Table 1.
The composition of the investigational diet
| Investigational diet components compared to control diet (per kg diet) | Supplier |
|---|---|
| Carbohydrates | |
| Dextrinized corn starch and sucrose substituted by: | |
| 41.5 wt% maltodextrin (DE6) | Roquette (Lestrem, France) |
| 15.0 wt% free galactose | Inalco (Milan, Italy) |
| 42.5 wt% isomaltulose | Beneo‐Palatinit (Mannheim, Germany) |
| 1 wt% fructose | Brenntag (Dordrecht, The Netherlands) |
| Fibers | |
| 2.8% cellulose substituted by: | |
| 2% rice fiber RemyLiVe200 | Beneo Orafti (Oreye, Belgium) |
| 0.72% GOS | Friesland Campina (Amersfoort, The Netherlands) |
| 0.08% Beneo Raftiline HP FOS | Beneo (Leuven, Belgium) |
| Proteins | |
| Soy protein isolate 770LN substituted by: | |
| 1:1 soy protein isolate 770LN | Solae company (St. Louis, MO, USA) |
| α‐lac enhanced whey | Arla Food ingredients (Wageningen, The Netherlands) |
| Addition of: 2.3 g tryptophan | |
| Lipids | |
| To obtain 0.53% DHA and 0.92% EPA, Part of lipid fraction substituted by: | |
| 27.5 g Nissui anchovy oil | Nippon Suisan Kaisha (Tokyo, Japan) |
| 6.5 g Biopure DHA IF tuna oil | Bioriginal (Den Bommel, The Netherlands) |
| 7.6 g soy lecithin Emulpur | Cargill (Mechelen, Belgium) |
| Vitamins | |
| Extra vitamins (reaching 200% value as compared to the control diet): vitamin A, B6, B12, D2, folic acid | |
2.3. Study design
Experimental procedures and data analysis were performed according to RIGOR criteria recommended for translational research.28 Rats were randomly divided into 8 groups (n = 6 per group, Figure 1). Photothrombotic stroke and sham‐surgeries were performed on experimental day 0, rats were sacrificed on day 7 (CC7, SC7, SI‐pre7, SI‐post7) or day 21 (CC21, SC21, SI‐pre21, SI‐post21). Six groups were subjected to the induction of focal cortical ischemia and to one of 3 different feeding protocols: Stroke control groups (SC7 and SC21) were fed with control diet during the entire experiment; The preventive stroke intervention groups (SI‐pre7 and SI‐pre21) were fed with the investigational diet starting from 2 weeks before ischemia (day‐14); The poststroke intervention groups (SI‐post7 and SI‐post21) were fed with the investigational diet starting from the day of the ischemia induction (day 0). The sham control groups (CC7 and CC21) were subjected to sham‐surgery and fed with the control diet during the entire experiment.
Figure 1.
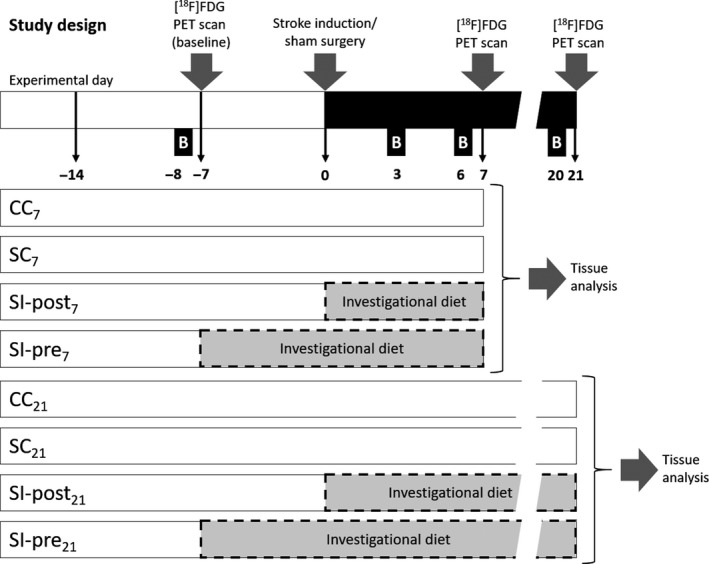
Study design. Eight experimental groups with 3 different dietary regimens were subjected to focal cortical ischemia or sham‐surgery at experimental day 0 and sacrificed on day 7 (CC 7, SC 7, SI‐post7, SI‐pre7) or day 21 (CC 21, SC 21, SI‐post21, SI‐pre21). The stroke control groups (SC 7 and SC 21) were fed with the control diet for the whole experiment; the stroke groups receiving preischemic treatment with the investigational diet (SI‐post7 and SI‐pre21) were fed with the investigational diet from the day of the ischemia (day 0) until the end of the experiment (day 7 or day 21, respectively); and the stroke groups receiving preischemic treatment with the investigational diet (SI‐pre7 and SI‐pre21) were fed with the investigational diet from 2 weeks before ischemia (day 14) until the end of the experiment (day 7 or day 21, respectively). The control groups (CC 7 and CC 21) were subjected to sham‐surgery at day 0 and fed with the control diet for the whole experiment. Behavioral tests were performed 8 days before (day 8, baseline) and 3, 6 and 21 days after ischemia or sham‐surgery. Positron emission tomography (PET) scans were performed 7 days before (day 7, baseline) stroke induction or sham‐surgery and on day 7 (groups CC 7, SC 7, SI‐post7 and SI‐pre7) or 21 (groups CC 21, SC 21, SI‐post21 and SI‐pre21) after stroke or sham‐surgery
Animals were weighted daily. Motor dysfunction was assessed with the cylinder test 8 days before surgery (baseline) and 3, 6 and 20 days after ischemia. [18F]FDG PET imaging was performed 7 days before the ischemia induction or sham‐surgery (baseline) and either 7 or and 21 days after the ischemia. The animals were terminated after last scan for tissue collection.
Due to complications with anesthesia, 2 rats did not survive the surgery on day 0 (from the CC21 group), and 2 rats did not survive the [18F]FDG PET scan on day 21 (from the SC21 and SI‐post21 groups). Thus, 4 animals were excluded from the study.
2.4. Stroke induction and sham‐surgery
Focal ischemic cortical lesions were induced by photothrombotic stroke as described previously.19 Briefly, rats were anesthetized with a mixture of ketamine (Ketalar®, 60 mg/kg; Pfizer, Brussels, Belgium) and medetomidine (Domitor®, 0.4 mg/kg intraperitoneal; Brussels, Belgium, Pfizer). The body temperature of the animals was maintained with heating pads; eye salve was applied onto the eyes to prevent dehydration. Before surgery, 100 μL of xylocaine with 2% adrenaline was applied on the skin as a local anesthetic and to reduce bleeding. The skull was exposed by a lateral incision of the skin. After intravenous injection of the photosensitizer Rose Bengal (20 mg/kg; Sigma‐Aldrich, St. Louis, MO), an area of the exposed intact skull was irradiated for 20 minutes with green light (wavelength 540 nm, bandwidth 80 nm) from a xenon lamp (model L‐4887; Hamamatsu Photonics, Hamamatsu City, Japan) with heat‐absorbing green filters. The radiation with an intensity of 0.68 W/cm2 was directed with a 3‐mm optical fiber placed on the skull above the right somatosensory cortex next to Bregma. Light‐induced oxidation of the photosensitizer causes endothelial damage, platelet activation and consequently vascular occlusion.29 At the end of surgery, anesthesia was reversed with atipamezole (Antisedan®, 1 mg/kg intraperitoneal; Orion Pharma, Newbury, UK). Finadyne (1 mg/kg) was given prior to and 24 hour after surgery to reduce pain. For sham‐surgery, the same procedure was performed, except for the application of radiation on the skull. Afterward, rats were housed individually until the end of the experiment.
2.5. Cylinder test
The cylinder test was used to quantify asymmetric forelimb use.26 Rats were placed in a 20‐cm‐wide clear glass cylinder located in front of 2 mirrors to facilitate the observation of the behavior from each side. A total of 20 contacts with the cylinder wall by either forepaw were scored from the video by an independent observer, who was blinded to the treatment of the animals. The number of contralateral forelimb (left forelimb) contacts was expressed as a percentage of total forelimb contacts. Normal rats should score 50% in this test.30 The test was performed in light phase and recorded on video.
2.6. PET imaging
[18F]FDG PET scans were performed using a dedicated small animal PET scanner (Focus 220, Siemens Medical Solutions USA, Malvern, PA). The body temperature of the rats was maintained with heating pads, eye salve was applied onto the eyes to prevent dehydration, and heart rate and blood oxygen levels were monitored with a BioVet system (M2M Imaging, Cleveland, OH). Two rats from different experimental groups were scanned simultaneously in each scanning session.
The rats were anesthetized with isoflurane mixed with oxygen (5% induction and 2% maintenance, 0.8 L/min), and a cannula was inserted into the tail vein for tracer injection. Before each PET acquisition, a transmission scan of 10 minutes with a 57Co point source was performed and used to correct for attenuation, scatter and random coincidences. Next, 18 ± 7 MBq [18F]FDG was injected with a pump for 1 minutes and immediately a 60‐minutes PET scan was started. There were no statistically significant differences in the injected tracer dose between the groups (F(7, 39) = 0.208, P = 0.98). After the baseline scan (day 7), rats were allowed to recover in their home cages. After the scans on day 7 or 21, rats were sacrificed and brains were collected.
2.7. PET image reconstruction and analysis
List‐mode data from the 60‐minutes [18F]FDG emission scan were reconstructed into 3 frames (2400 seconds, 2 x 600 seconds). Emission sinograms were normalized and corrected for attenuation and decay of radioactivity and iteratively reconstructed using OSEM2D (4 iterations and 16 subsets).31 PET data were not corrected for blood glucose levels, because previous reports indicate that glucose correction does not improve the inter‐subject variability32 and it may even introduce more noise.33
A 10‐minutes frame of the [18F]FDG PET scan, starting 50 minutes post injection, was used to explore the differences in brain glucose metabolism between the groups. The images were automatically coregistered with a tracer‐specific template,34 using Vinci 4.26 software (Max Planck Institute for Neurological Research, Cologne, Germany). Standardized uptake values (SUV) of the tracer in the brain were calculated according to the following equation: SUV = [tissue activity concentration [(MBq/mL) x bodyweight (g)]/[injected dose (MBq) x brain tissue density (g/mL)]. It is assumed that the brain tissue density is 1 g/mL. The data were analyzed using the SUV in the whole brain and in the somatosensory cortex ipsilateral to the lesion.
2.8. Immunohistochemistry
After the last PET scan, the rats were sacrificed under deep isoflurane anesthesia (5%) by transcardiac perfusion with saline. Brains were dissected and fixed in 4% paraformaldehyde, followed by cryopreservation in 30% sucrose and cut in a cryostat into 10‐μm‐thick sagittal sections.
For staining, brain sections were blocked with 5% bovine serum albumin (BSA, Sigma‐Aldrich) in PBS for 30 minutes at room temperature. As primary antibody, mouse anti Glial fibrillary acidic protein (GFAP) (Sigma‐Aldrich, G3893) was applied overnight (~16 hour) in a 1:400 dilution in PBS containing 1% BSA at 4°C. Next, the sections were washed 3 times with PBS and the secondary antibody, antimouse Cy3 (Life Technologies) in a 1:1000 dilution in PBS containing 1% BSA, was applied for 1 hour. The sections were washed 3 times with PBS, and the slides were covered with quick‐hardening mounting medium (Eukitt®, Sigma‐Aldrich) and a microscope coverslip. Digital images from areas of interest on the sections (identified based on a stereotaxic atlas35) were acquired with a TissueFAXS system (Tissue Gnostics).
The images were scored by an independent observer, who was blinded to the treatment of the animals. Expression of GFAP‐stained cells was determined in randomly selected 0.0432 mm2 sections of 3‐5 brain slices per rat using ImageJ software (NIH, USA). The area surrounding the infarct (located 350 μm and 700 μm from the lesion) and the contralateral side were analyzed by integrated density to assess the surface covered by the staining.
2.9. Statistical analysis
Statistical analysis of bodyweight, behavior and immunohistochemistry data was performed using IBM SPSS software Statistics 22 (SPSS Inc., United States). Results are presented as mean ± standard error of the mean (SEM). Differences in bodyweight between groups at the start of the experiment were analyzed by one‐way ANOVA. Bodyweight changes following ischemia induction or sham‐surgery were analyzed for differences between time points and between groups with the generalized estimating equations (GEE) model with a Bonferroni post hoc correction to account for multiple comparisons.36, 37 The exchangeable correlation matrix and the Wald test were used to calculate P‐values. Results from the behavioral test, immunohistochemistry and [18F]FDG SUV were analyzed with one‐way ANOVA followed by Bonferroni post hoc correction to account for multiple comparisons, unless stated otherwise in the results section. Differences in lesion size changes between groups were analyzed with an unpaired t‐test. Differences were considered statistically significant when P < 0.05.
3. RESULTS
3.1. Bodyweight
Results are summarized in Table 2. The average bodyweight on day 0, before ischemia induction or sham‐surgery, was not statistically different between groups (F(3) = 2.000, P = 0.112, Figure 2). The SI‐post21 group had a significantly lower bodyweight than all other groups (CC21, SC21, SI‐pre21; P < 0.05) at all time points between stroke induction and termination (day 1‐day 21).
Table 2.
The main effects of ischemia and its modulation by the investigational diet
| Parameter | The effects of ischemia | The effects of the investigational diet | |
|---|---|---|---|
| SC vs CC | SI‐pre vs SC | SI‐post vs SC | |
| Bodyweight (day 1‐day 21) | ≅ | ≅ | ↓ |
| Motor function (cylinder test, day 3) | ↓ | ≅ | ≅ |
| Infarct size growth (day 7 → day 21) | ↑ | ≅ | ≅ |
| Astrocyte activation (GFAP staining, day 7) | ↑ | ≅ | ≅ |
| Astrocyte activation (GFAP staining, day 21) | ↑ | ↓ | ↓↓ |
| Whole brain, day 7 | ≅ | ≅ | ≅ |
| Ipsilateral glucose metabolism (somatosensory cortex), day 7 | ≅ | ↑ | ≅ |
The table presents the main effects of ischemia and investigational diet on bodyweight, early motor function (on day 3), astrocyte expression close to the lesion (300 μm), glucose metabolism on day 7. Motor function on later time points (day 6, 21), Glial fibrillary acidic protein (GFAP) expression 700 μm from the lesion, glucose metabolism on day 21 are not included, since little effect on these parameters was observed.
Figure 2.
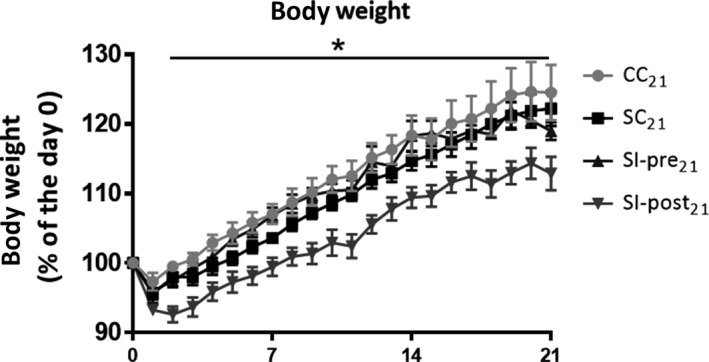
Changes in body weight in animals sacrificed on day 21 after ischemia induction or sham‐surgery: body weight is displayed as percentage of the body weight on the day of ischemia induction or sham‐surgery (day 0). CC 21 = control group (n = 4), SC 21 = stroke + control diet (n = 5), SI‐post21 = stroke + postischemic dietary intervention (n = 5), SI‐pre21 = stroke + preischemic dietary intervention (n = 6). The body weight of the stroke group treated with the investigational diet after stroke induction (SI‐post21) was significantly lower than all the other groups (CC 21, SC 21, SI‐pre21) at all time points after stroke induction (day 1‐day 21). No significant differences between the CC 21, SC 21 and SI‐pre21 groups were observed. *P < 0.05 for SI‐post21, when compared to CC 21, SC 21, SI‐pre21 (GEE model)
3.2. Infarct size
At day 7 and 21 after ischemia induction, the infarct size was assessed on the isolated brains by measuring the maximum length and width (alongside and perpendicular to Bregma, respectively) of the visible scar. No significant differences between the groups subjected to focal ischemia were observed on day 7 (F(2) = 0.043, P = 0.958) or 21 (F(2) = 1.006, P = 0.39, Figure 3). Although the scar was substantially smaller (−35%) in the SI‐post21 group, statistical significance was not reached due to the large between‐subject variation in initial lesion size (SC21: 5.8 ± 0.7 mm2 vs SI‐post21: 3.8 ± 0.2 mm, P = 0.31; SC21 vs SI‐pre21: 6.3 ± 0.5 mm, P = 0.83).
Figure 3.
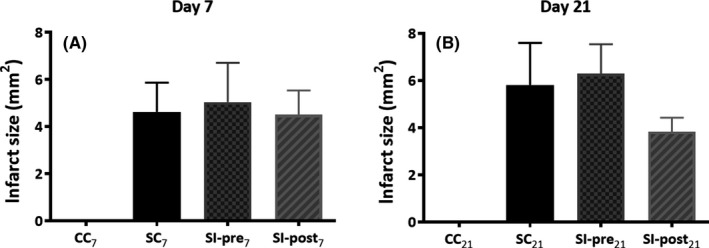
Infarct size on day 7 A, and day 21 B, after focal ischemia induction. The infarct size was assessed on the isolated brains by measuring the length (alongside Bregma) and width (perpendicularly to Bregma) of the visible scar. Data are displayed as length*width in millimeters for animals sacrificed at day 7 A, and day 21 B, following ischemia. SC 7, SC 21 = stroke + control diet (n = 6, n = 5), SI‐post7, SI‐post21 = stroke + postischemic dietary intervention (n = 6, n = 5), SI‐pre7, SI‐pre21 = stroke + preischemic dietary intervention (n = 6, n = 6)
3.3. Cylinder test
Baseline measurements before surgery (day 8) did not reveal any preference for the left or right paw, and consequently, no significant differences between groups (F(3) = 0.085, P = 0.97, Figure 4) were observed. The cylinder tests on day 3 post surgery demonstrated a significantly reduced use of the paw contralateral to the lesion (F(3) = 8.746, CC7 60.8 ± 2.7%, SC7 35.0 ± 4.1%, P < 0.01). Only a trend toward asymmetric forepaw use was still observed on day 6 (F(3) = 2.606, P = 0.067), whereas no asymmetry was observed anymore on day 20 (F(3) = 0.303, P = 0.82).
Figure 4.
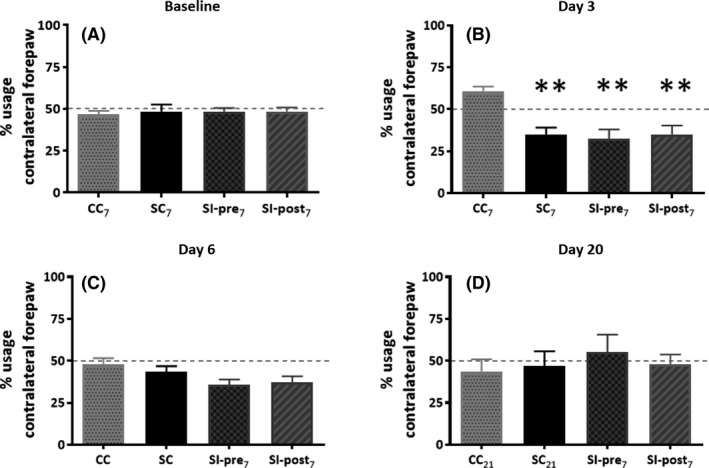
Cylinder test. To investigate the impact of ischemia and the investigational diet on asymmetric paw use, the cylinder test was performed. Rats were subjected to cylinder tests 1 week before the stroke induction or sham‐surgery (baseline) and on days 3, 6 and 20 following the ischemia or sham‐surgery. The graphs represent the percentage usage of the left paw, contralateral to the stroke lesion. CC 7, CC 21 = control group (n = 6, n = 4), SC 7, SC 21 = stroke + control diet (n = 6, n = 5), SI‐post7, SI‐post21 = stroke + postischemic dietary intervention (n = 6, n = 5), SI‐pre7, SI‐pre21 = stroke + preischemic dietary intervention (n = 6, n = 6). CC = all control animals (12), SC = all surgery + control diet (n = 11), SI‐pre = all stroke + preischemic dietary intervention (n = 12), SI‐post = all stroke + postischemic dietary intervention (n = 11),. Significant differences between experimental groups and the CC 7 group are indicated by **P < 0.01
The investigational diet did not reverse the stroke‐induce asymmetric paw use on day 3 (SC7 35.0 ± 4.1%, SI‐post7 35.0 ± 5.3%, SI‐pre 32.5 ± 5.4).
3.4. Astrocyte expression
Glial fibrillary acidic protein staining demonstrated significant differences between the groups in astrocyte expression close to the lesion site (350 μm, F(3) = 30.84, P < 0.0001, Figure 5A and B), with a significant increase in GFAP staining following ischemia, both on day 7 (SC7 2.5 ± 0.13, CC7 1.0 ± 0.17, P < 0.001) and on day 21 (SC21 1.94 ± 0.20, CC21 1.00 ± 0.08, P < 0.0001). Further away from the lesion site (700 μm), no significant effect of ischemia on GFAP staining was observed (Figure 6A and B).
Figure 5.
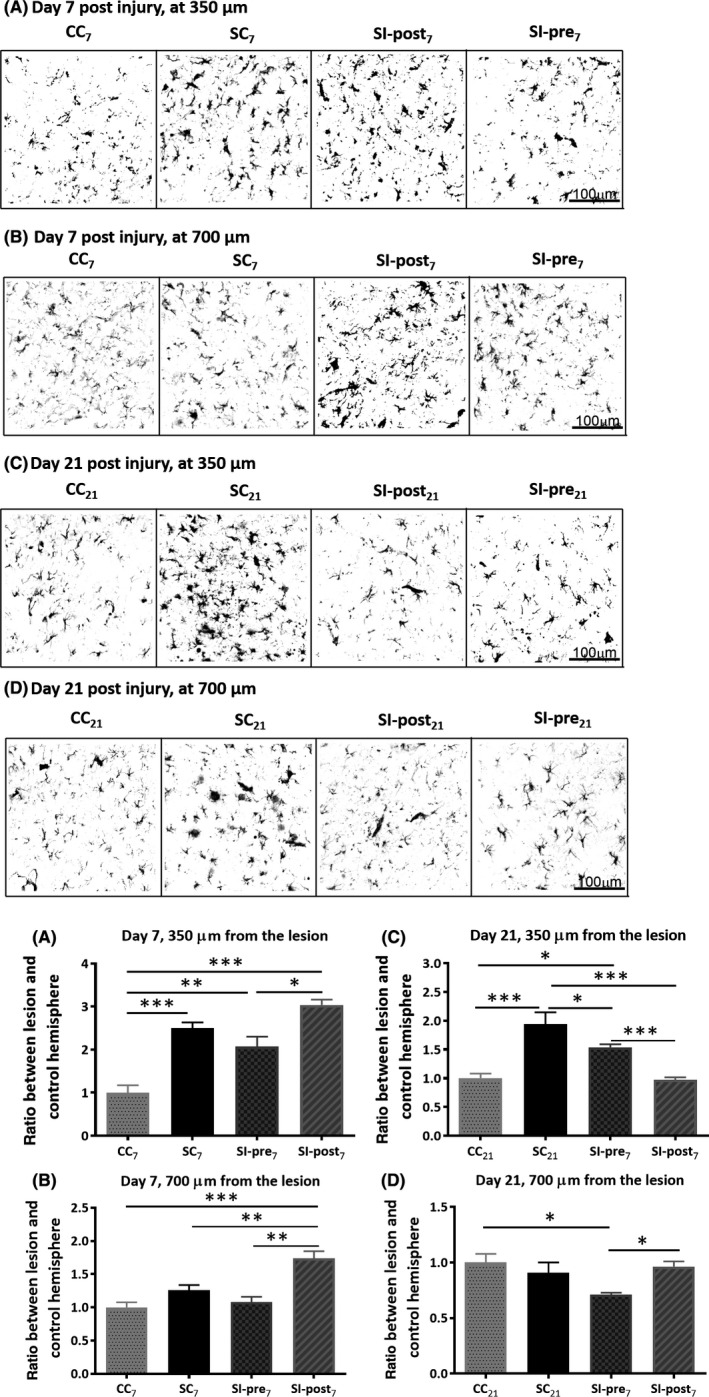
Astrocyte activation: Glial fibrillary acidic protein (GFAP) staining was performed to assess the effects of ischemia on astrocytes expression surrounding the lesion (SC vs CC) and the effect of the investigational diet thereon (SC vs SI‐post and SC vs SI‐pre). An example of GFAP staining and its quantification in the cortex surrounding the stroke lesion on day 7 (A) and at 700 μm away from the lesion at day 7 (B), surrounding the stroke lesion on day 21 (C) and at 700 μm away from the lesion at day 21 (D). Astrocyte activation was assessed by the area covered by GFAP staining in the region of interest. The data are displayed as the ratio between the lesion and the contralateral hemisphere, normalized to the values of the corresponding control group (CC 7 or CC 21). CC 7, CC 21 = control group (n = 6, n = 4), SC 7, SC 21 = stroke + control diet (n = 6, n = 5), SI‐post7, SI‐pre7, SI‐pre21 = stroke + preischemic dietary intervention (n = 6, n = 6), SI‐post21 = stroke + postischemic dietary intervention (n = 6, n = 5). Statistically significant differences are indicated as *P < 0.05, **P < 0.01, ***P < 0.001. For data analysis and illustration of the results, the colors on the white/black images have been inverted (white background)
Figure 6.
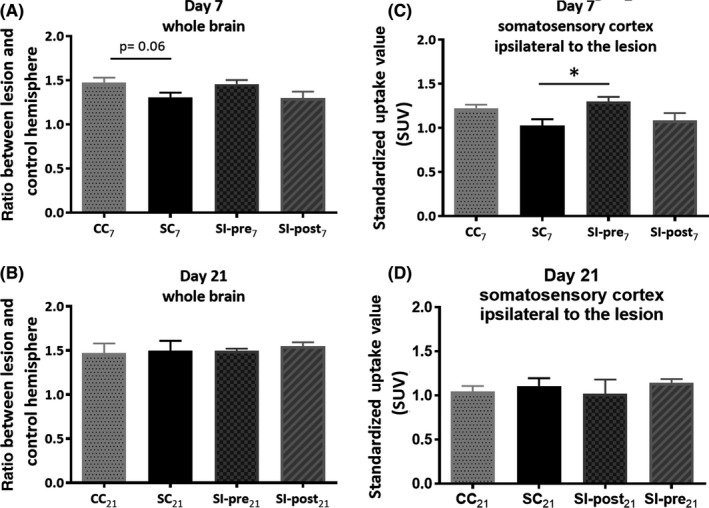
Brain glucose metabolism was measured with [18F]FDG positron emission tomography (PET) imaging to investigate whether stroke induction can lead to detectable changes in glucose metabolism (SC vs CC) and whether dietary intervention has an impact on glucose metabolism following stroke induction (SC vs SI‐post and SC vs SI‐pre). Differences in brain metabolism caused by ischemia and by the investigational diet in animals sacrificed on day 7. (A) [18F]FDG standardized uptake values (SUV) uptake in the whole brain (A, B) and somatosensory cortex ipsilateral to the lesion (C, D) on day 7 and day 21 following ischemia. CC 7, CC 21 = control group (n = 6, n = 4), SC 7, SC 21 = stroke + control diet (n = 6, n = 5), SI‐post7, SI‐pre7, SI‐pre21 = stroke + preischemic dietary intervention (n = 6, n = 6), SI‐post21 = stroke + postischemic dietary intervention (n = 6, n = 5)
At day 7, the investigational diet did not have a significant effect on the ischemia‐induced expression of astrocytes close to the lesion site (SI‐pre7: 3.03 ± 0.13, P = 0.39 and SI‐post7: 2.07 ± 0.23, vs SC7 P = 0.06). On day 21, the postischemic dietary intervention had completely reversed the effect of ischemia on GFAP staining (SI‐post21: 0.97 ± 0.04, SI‐post21 vs SC21 P < 0.0001, SI‐post21 vs CC21 P = 0.86). The preischemic dietary intervention caused partial reversal of astrocyte activation on day 21 (SI‐pre21: 1.54 ± 0.06, SI‐pre21 vs SC21 P < 0.05, SI‐pre21 vs CC21 P < 0.05, SI‐post21 vs SI‐pre21: P < 0.05).
3.5. Brain metabolism
[18F]FDG PET imaging demonstrated a trend toward a reduction in whole brain metabolism due to ischemia on day 7 (CC7 = 1.47 ± 0.02 vs SC7 = 1.31 ± 0.02, P = 0.06, Figure 6), but not anymore on day 21 (CC21 = 1.47 ± 0.05 vs SC21 = 1.5 ± 0.05, P = 0.88). In the somatosensory cortex, a trend toward lower glucose metabolism due to stroke was observed on day 7 (CC7 = 1.22 ± 0.02 vs SC7 = 1.03 ± 0.03, P = 0.08, Figure 6)
The whole brain glucose metabolism was not affected by the dietary intervention (SI‐post7 = 1.3 ± 0.03, SI‐pre7 = 1.46 ± 0.02; SC7 vs SI‐post7, P = 0.99; SC7 vs SI‐pre7, P = 0.19). The somatosensory cortex ipsilateral to the lesion revealed a significant increase in metabolism due to the dietary intervention (SC7 = 1.03 ± 0.03 vs SI‐pre7 = 1.3 ± 0.02; SC7 vs SI‐pre7, P = 0.02).
The diet by itself did not influence global brain glucose metabolism as shown by comparison of the baseline scans (day 7) of the SI‐pre groups vs the other groups (t‐test, P = 0.35, supplementary Figure 2).
4. DISCUSSION
Despite epidemiological,4, 11, 38, 39, 40, 41, 42 clinical43, 44 and preclinical45 evidence that food can contribute to the prevention of stroke and have a positive impact on survival of stroke patients, many questions around the impact of diets on recovery after stroke remain unanswered. The aim of this study was to assess the potential of an anti‐inflammatory dietary intervention to modulate the effects of cortical ischemia in a rat model of photothrombotic stroke. We demonstrated that anti‐inflammatory diet intervention can affect astrocyte activation and brain glucose metabolism following ischemic damage.
Photothrombotic stroke is a minimally invasive, reproducible method to create a chemically induced cortical lesion.46 The severity of the model is relatively low and can be modulated by changing the duration of irradiation. Both induction of ischemia and sham‐surgery temporarily caused up to 10% decrease in bodyweight. This suggests that not the ischemic lesion itself, but the whole surgical procedure caused a transient decrease in bodyweight. This effect could be ascribed to a reduction in food intake during and early after surgery (supplementary Figure 1). The fact that ischemia did not cause any further decrease in body weight agrees with the low severity of this stroke model.
Cylinder test data demonstrated that introduction of ischemia, but not sham‐surgery, led to transient lateral motor dysfunction, which was observed on day 3 after ischemia induction and was normalized again from day 6 onwards, indicating low severity of the model. This is consistent with previous studies demonstrating lateral motor dysfunction shortly after ischemia26 and suggests that the function of the affected area was quickly restored, possibly as a result of compensation by other brain regions. More persistent effects in brain were detected with [18F]FDG PET and GFAP staining.
To gain more insight in the effects of stroke on brain function, we applied [18F]FDG PET imaging to investigate the effect of cortical ischemia on brain metabolism. The global brain metabolism was not significantly affected by the cortical damage, indicating a low severity of the model. PET imaging at either day 7 or day 21 did not detect hypometabolic lesion at the location of the photothrombotic stroke. A trend toward a decrease in glucose metabolism was observed in the brain of animals subjected to ischemia and control diet as compared to sham‐operated animals. This lack of sensitivity could be due to the limited resolution of the PET camera (ca. 1.7 mm at 5 cm from the center of the field‐of‐view), which is in the same range as the size of the lesion, resulting in significant partial volume effects. Moreover, the location of the lesion might be slightly different between animals and consequently the effect of the small lesion on brain glucose metabolism will be averaged out over a larger region when group comparisons are made.
The main objective of this study was to investigate the effects of an investigational diet on symptoms observed following photothrombotic stroke. A disadvantage of the photothrombotic model is that it causes permanent occlusion of the vessel. However, several studies have shown that anti‐inflammatory treatment could cause a significant decrease in infarct size following photothrombotic stroke47 and improve behavioral outcome.48
The diet investigated in this study was designed to target neuroinflammation, as it contains elevated amounts of components, such as vitamins A and D, omega‐3 fatty acids and specific amino acids (tryptophan), which all have been described to exert anti‐inflammatory effects on immune cells in vitro and in vivo.6, 7, 20, 49, 50 The indigestible galacto‐oligosaccharides and fructooligosaccharides have been included in the investigational diet, because they have been shown to modulate the immune system via alteration of gut microbiota and by direct interaction with peripheral immune cells and thus could have an indirect effect on neuroinflammation via the gut‐immune‐brain axis.10
Body weight loss caused by the surgery (both sham and to induce stroke) was aggravated by the postischemic intervention with the investigational diet. This may be explained by the change of diet early directly after surgery in this group. Apparently, the animals need some time to get used to the new diet, as they hardly consumed little food in the first 2 days after the change of diet. Such an effect was also observed immediately after a change in diet in the groups subjected to preischemic diet intervention.
In the test performed in this investigation, we did not detect beneficial effects of the investigational diets on the motor dysfunction, possibly because motor dysfunction was too transient and had already disappeared at day 6; furthermore, our are test we might not be sensitive enough to pick up more subtle changes in motor function. Dietary intervention also showed no significant effect on lesion size, probably due to the high variability between the size of the lesion between animals. On average, postischemia dietary interventions seemed to reduce the growth of the ischemic lesion between day 7 and 21; however, this effect was not statistically significant.
Assessment of astrocyte expression revealed more persistent changes following stroke‐induced ischemia, and these changes were modifiable by the diet. The 2 treatment regimens either prophylactic of therapeutic seemed to have interesting distinct spatiotemporal effects on astrocyte activation. The robust increase in astrocyte activation seen at 7 days after ischemia induction was further increased by the investigational diet when started immediately after ischemia, but it was not affected by the investigational diet started 2 weeks before the ischemia. On day 21, however, the postischemic dietary intervention caused complete reversal of the ischemia‐induced astrocyte activation to control levels, while the preischemic dietary intervention caused only partial reversal of the astrocyte activation in the area close to the lesion. Astrocytes are believed to act as double‐edged sword, by being involved in both neurotoxic and neuroprotective mechanisms.51 It is believed that the early response of astrocytes has a positive impact on recovery from stroke, while astrogliosis is detrimental for regeneration of the brain in later stages.23 The enhancement of the beneficial effect of astrocyte activation in the early response to stroke and complete inhibition of detrimental effect at a later stage could indicate a positive effect of the postischemia diet.
[18F]FDG PET on day 7 demonstrated that preischemic dietary intervention did not affect the glucose metabolism changes following ischemia. The postischemic dietary intervention caused an increase in brain glucose metabolism in somatosensory cortex ipsilateral to the lesion following cortical ischemia. This could be an indication of lower damage or increased metabolism in the tissue surrounding the lesion (due to the activation of immune cells involved in the damage repair or compensation mechanisms).
In conclusion, we showed potential beneficial effects of a dietary intervention containing elevated amounts of specific anti‐inflammatory nutrients on neuroinflammation following cortical ischemia. Although the cylinder test was not sensitive enough to detect effects of the intervention after a mild stroke (due to the low severity of the model and consequently transient effect on motor function), glucose measurement, a subtler assessment of brain function than the cylinder test, indicated that there were deficits in brain function, and that these were affected by the postischemic diet. Both post‐ and preischemic diet intervention modulated astrocyte activation. Taken together, these results warrant further investigation of postischemic dietary intervention as new therapeutic option for stroke.
CONFLICT OF INTEREST
J.M. Verkuyl and L.M. Broersen are employees of Nutricia Research and therefore declare potential conflict of interest. All other authors report no financial interest or potential competing of interest.
Supporting information
ACKNOWLEDGMENTS
This study is part of the BrainMenu project and financially supported by the STW‐Danone Partnership Program (project number: 11650). Cindy Casteels is a postdoctoral fellow of the Flemish Fund for Scientific Research (FWO), Flanders, Belgium. Caroline Real is a postdoctoral fellow of the São Paulo Research Foundation (FAPESP), Brazil. The authors thank Bram Maas, Rolf Zijlma, Marianne Schepers, Chantal Kwizera and Hilde Dekens for tracer synthesis and Jurgen Sijbesma for his support with the PET procedures.
Kurtys E, Casteels C, Real CC, et al. Therapeutic effects of dietary intervention on neuroinflammation and brain metabolism in a rat model of photothrombotic stroke. CNS Neurosci Ther. 2019;25:36–46. 10.1111/cns.12976
REFERENCES
- 1. Li Y, Liu Z, Xin H, et al. The role of astrocytes in mediating exogenous cell‐based restorative therapy for stroke. Glia. 2014;62:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacco RL, Benjamin EJ, Broderick JP, et al. Risk factors. Stroke. 1997;28:1507‐1517. [DOI] [PubMed] [Google Scholar]
- 3. Di Legge S, Koch G, Diomedi M, et al. Stroke prevention: managing modifiable risk factors. Stroke Res Treat. 2012;2012:391538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a mediterranean‐style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matz K, Teuschl Y, Firlinger B, et al. Multidomain lifestyle interventions for the prevention of cognitive decline after ischemic stroke: randomized trial. Stroke. 2015;46:2874‐2880. [DOI] [PubMed] [Google Scholar]
- 6. Orr SK, Trépanier M‐O, Bazinet RP. n‐3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:97‐103. [DOI] [PubMed] [Google Scholar]
- 7. Mathew JS, Sharma RP. Effect of all‐trans‐retinoic acid on cytokine production in a murine macrophage cell line. Int J Immunopharmacol. 2000;22:693‐706. [DOI] [PubMed] [Google Scholar]
- 8. Jin Y, Yan E, Li X, et al. Neuroprotective effect of sodium ferulate and signal transduction mechanisms in the aged rat hippocampus. Acta Pharmacol Sin. 2008;29:1399‐1408. [DOI] [PubMed] [Google Scholar]
- 9. Del Angel‐Meza AR, Dávalos‐Marín AJ, Ontiveros‐Martinez LL, et al. Protective effects of tryptophan on neuro‐inflammation in rats after administering lipopolysaccharide. Biomed Pharmacother. 2011;65:215‐219. [DOI] [PubMed] [Google Scholar]
- 10. Jeurink PV, Van Esch BC, Rijnierse A, et al. Mechanisms underlying immune effects of dietary oligosaccharides. Am J Clin Nutr. 2013;98:572‐577. [DOI] [PubMed] [Google Scholar]
- 11. Tuttle KR, Shuler LA, Packard DP, et al. Comparison of low‐fat versus Mediterranean‐style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). Am J Cardiol. 2008;101:1523‐1530. [DOI] [PubMed] [Google Scholar]
- 12. Yasuhara T, Hara K, Maki M, et al. Dietary supplementation exerts neuroprotective effects in ischemic stroke model. Rejuvenation Res. 2008;11:201‐214. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q, Dai P, Bao H, et al. Anti‐inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp Ther Med. 2017;13:263‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ceulemans A‐G, Zgavc T, Kooijman R, et al. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2016;17:49‐59. [DOI] [PubMed] [Google Scholar]
- 17. Andresen L, Theodorou K, Grünewald S, et al. Evaluation of the therapeutic potential of Anti‐TLR4‐Antibody MTS510 in experimental stroke and significance of different routes of application. PLoS One. 2016;11:e0148428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y‐H, Fu H‐L, Tian M‐L, et al. Neuron‐derived FGF10 ameliorates cerebral ischemia injury via inhibiting NF‐κB‐dependent neuroinflammation and activating PI3K/Akt survival signaling pathway in mice. Sci Rep. 2016;6:19869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vandeputte C, Casteels C, Struys T, et al. Small‐animal PET imaging of the type 1 and type 2 cannabinoid receptors in a photothrombotic stroke model. Eur J Nucl Med Mol Imaging. 2012;39:1796‐1806. [DOI] [PubMed] [Google Scholar]
- 20. Kurtys E, Eisel UL, Verkuyl JM, et al. The combination of vitamins and omega‐3 fatty acids has an enhanced anti‐inflammatory effect on microglia. Neurochem Int. 2016;99:206‐214. [DOI] [PubMed] [Google Scholar]
- 21. Labrousse VF, Nadjar A, Joffre C, et al. Short‐term long chain Omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One. 2012;7:e36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis M, Ghassemi P, Hibbeln J. Therapeutic use of omega‐3 fatty acids in severe head trauma. Am J Emerg Med. 2013;31:273. e5‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chouchane M, Costa MR. Cell therapy for stroke: use of local astrocytes. Front Cell Neurosci. 2012;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim H‐S, Kim D, Kim RG, et al. A rat model of photothrombotic capsular infarct with a marked motor deficit: a behavioral, histologic, and microPET study. J Cereb Blood Flow Metab. 2014;34:683‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasu S, Hata T, Nakajima T, et al. Evaluation of 18F‐FDG PET in acute ischemic stroke: assessment of hyper accumulation around the lesion. Kaku Igaku. 2002;39:103‐110. [PubMed] [Google Scholar]
- 26. Vandeputte C, Taymans J‐M, Casteels C, et al. Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 2010;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeves PG, Nielsen FH, Fahey GC. AIN‐93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN‐76A rodent diet. J Nutr. 1993;123:1939‐1951. [DOI] [PubMed] [Google Scholar]
- 28. Lapchak PA, Zhang JH, Noble‐Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4:279‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vercammen L, Van der Perren A, Vaudano E, et al. Parkin protects against neurotoxicity in the 6‐hydroxydopamine rat model for Parkinson’s disease. Mol Ther. 2006;14:716‐723. [DOI] [PubMed] [Google Scholar]
- 31. Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601‐609. [DOI] [PubMed] [Google Scholar]
- 32. Claeys J, Mertens K, D’Asseler Y, et al. Normoglycemic plasma glucose levels affect F‐18 FDG uptake in the brain. Ann Nucl Med. 2010;24:501‐505. [DOI] [PubMed] [Google Scholar]
- 33. Williams S‐P, Flores‐Mercado JE, Baudy AR, et al. The power of FDG‐PET to detect treatment effects is increased by glucose correction using a Michaelis constant. EJNMMI Res. 2012;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vállez Garcia D, Casteels C, Schwarz AJ, et al. A standardized method for the construction of tracer specific PET and SPECT rat brain templates: validation and implementation of a toolbox. PLoS One. 2015;10:e0122363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Academic Press; 2006. [Google Scholar]
- 36. Hardin JW, Hilbe JM. Generalized Estimating Equations, 2nd ed London, UK: Chapman & Hall; 2013. [Google Scholar]
- 37. Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated‐measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med. 2012;37:99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu D, Huang J, Wang Y, et al. Fruits and vegetables consumption and risk of stroke: a meta‐analysis of prospective cohort studies. Stroke. 2014;45:1613‐1619. [DOI] [PubMed] [Google Scholar]
- 39. Larsson SC, Wolk A. Dietary fiber intake is inversely associated with stroke incidence in healthy Swedish adults. J Nutr. 2014;144:1952‐1955. [DOI] [PubMed] [Google Scholar]
- 40. Dong J‐Y, Iso H, Kitamura A, et al. Multivitamin use and risk of stroke mortality: the Japan collaborative cohort study. Stroke. 2015;46:1167‐1172. [DOI] [PubMed] [Google Scholar]
- 41. Larsson SC. Dietary fats and other nutrients on stroke. Curr Opin Lipidol. 2013;24:41‐48. [DOI] [PubMed] [Google Scholar]
- 42. Larsson SC, Håkansson N, Wolk A. Dietary cysteine and other amino acids and stroke incidence in women. Stroke. 2015;46:922‐926. [DOI] [PubMed] [Google Scholar]
- 43. Aquilani R, Scocchi M, Iadarola P, et al. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin Rehabil. 2008;22:1042‐1050. [DOI] [PubMed] [Google Scholar]
- 44. Aquilani R, Scocchi M, Boschi F, et al. Effect of calorie‐protein supplementation on the cognitive recovery of patients with subacute stroke. Nutr Neurosci. 2008;11:235‐240. [DOI] [PubMed] [Google Scholar]
- 45. Wiesmann M, Zinnhardt B, Reinhardt D, et al. A specific dietary intervention to restore brain structure and function after ischemic stroke. Theranostics. 2017;7:493‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Labat‐gest V, Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp. 2013;9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liebigt S, Schlegel N, Oberland J, et al. Effects of rehabilitative training and anti‐inflammatory treatment on functional recovery and cellular reorganization following stroke. Exp Neurol. 2012;233:776‐782. [DOI] [PubMed] [Google Scholar]
- 48. Liu Y, Sun Q, Chen X, et al. Linolenic acid provides multi‐cellular protective effects after Photothrombotic cerebral ischemia in rats. Neurochem Res. 2014;39:1797‐1808. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Leung DYM, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase‐1. J Immunol. 2012;188:2127‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim CJ, Kovacs‐Nolan J, Yang C, et al. L‐cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta. 2009;1790:1161‐1169. [DOI] [PubMed] [Google Scholar]
- 51. Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


