Summary
Aim
Substance P (SP) causes vasodilation and blood pressure (BP) reduction. However, the involvement of tachykinin receptors (NKRs) within baroreflex afferent pathway in SP‐mediated BP regulation is largely unknown.
Methods
Under control and hypertensive condition, NKRs’ expressions were evaluated in nodose (NG) and nucleus of tractus solitary (NTS) of male, female, and ovariectomized (OVX) rats; BP was recorded after microinjection of SP and NKRs agonists into NG; Baroreceptor sensitivity (BRS) was tested as well.
Results
Immunostaining and immunoblotting data showed that NK1R and NK2R were estrogen‐dependently expressed on myelinated and unmyelinated afferents in NG. A functional study showed that BP was reduced dose‐dependently by SP microinjection, which was more dramatic in males and can be mimicked by NK1R and NK2R agonists. Notably, further BP elevation and BRS dysfunction were confirmed in desoxycorticosterone acetate (DOCA)‐salt model in OVX compared with DOCA‐salt model in intact female rats. Additionally, similar changes in NKRs’ expression in NG were also detected using DOCA‐salt and SHR. Compared with NG, inversed expression profiles of NKRs were also found in NTS with either gender.
Conclusion
The estrogen‐dependent NKRs’ expression in baroreflex afferent pathway participates at least partially in sexual‐dimorphic and SP‐mediated BP regulation under physiological and hypertensive conditions.
Keywords: baroreflex afferent function, blood pressure, Substance P, tachykinin receptor
1. INTRODUCTION
Substance P (SP) is an undecapeptide (a peptide composed of a chain of 11 amino acid residues) member of the tachykinin neuropeptide family. It is a neuropeptide, acting as a neurotransmitter and neuromodulator.1, 2 SP can be released from the terminals of specific sensory nerves associated with inflammatory processes and pain. It has been reported that SP is a potent vasodilator released from the endothelium through the nerukinin‐1 receptor (NK1R) activation.3 Early observation has demonstrated that SP is involved in the regulation of heart frequency, blood pressure (BP), and stretching of vessels.4 SP also plays an important role in ischemia and reperfusion and cardiovascular response to stress.5, 6, 7 SP is synthesized and released from baroreceptor afferent neurons. Excitatory NK1R could be activated by baroreceptive input at the aortic arch (baroreceptor terminal) to the cell body of the first‐order neurons within the nodose (NG) through aortic depressor nerve (ADN), and then relay to the second‐order neurons within the nucleus of tractus solitary (NTS). The modulating roles of SP in baroreflex have been well reviewed.8 Recent observations have shown that SP may play a major role in the secondary injury process following traumatic brain injury, particularly with respect to neuro‐inflammation, increased blood‐brain‐barrier permeability, and tissue edema 9, 10 due mainly to SP release‐mediated vasodilation and fluid extravasation into the tissues.11 This secondary pathophysiological process may subsequently change the blood pressure to noxious level. So, the questions remain about the specific role and significance of SP in baroreflex afferent function and BP regulation under physiological and hypertensive conditions. A large body of evidence from our group has demonstrated sexual dimorphism in baroreflex afferent function12, 13, 14, 15 and female hormone‐dependent BP regulation in rodents.16, 17, 18, 19 However, the question regarding if estrogen plays a central role in unusual changes of BP associated with tachykinin receptors’ (NKRs) expression in baroreflex afferent pathway needs to be answered. The current observation provides the first line of evidence that estrogen‐dependent NKRs’ expression participates significantly in BP regulation under both physiological and pathophysiological situations via baroreflex afferent function, in which NG and NTS play exactly opposite roles by inverse expression of NKRs.
2. MATERIALS AND METHODS
2.1. Animals
All animal use protocols were preapproved by Institutional Animal Care and Use Committees of School of Medical Science, Harbin Medical University, China. Adult age‐matched male, intact female, and ovariectomized (OVX) Sprague‐Dawley (SD) rats (12‐14 weeks weighing 220‐260 g) obtained from the animal center of Harbin Medical University were used in this study for molecular experiments and functional investigation. Spontaneously hypertensive rats (SHR) and Wistar rats (10‐12 weeks) were directly purchased from Wei Tong Li Hua Experimental Animal Technology Co, Ltd, Beijing, China, with SPF grade and licensed under SCXK (Beijing) 2012‐0001. All animal use protocols were preapproved by Institutional Animal Care and Use Committees of School of Medical Science, Harbin Medical University, China, which are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication “Guide for the Care and Use of Laboratory Animals”.
2.2. Chemicals
Substance P (SP) was purchased from LKT Labs (St. Paul, Minnesota, 55130 USA). [Sar9, Met (O2)11]‐Substance P ([Sar9] SP, a selective agonist of NK1 receptor) was purchased from TOCRIS (Ellisville, MO, USA). [β‐Ala8] Neurokinin A ([βAla8] NKA, a selective agonist of NK2 receptor) was purchased from Zi Qi Bio‐tech Company (Shanghai, China). Phenylephrine (PE), sodium nitroprusside (SNP), and DMSO for dissolving SP were ordered directly from Sigma (St. Louis, MO, USA), and all other chemical agents for molecular experiments were ordered from the routine commercial source.
2.3. Hypertension models
All SD rats received food and water ad libitum and a 13‐hour: 11‐hour light: dark cycle in 1 week of acclimation. Hypertension was induced by DOCA‐salt treatment as previously described.20 DOCA was injected (25 mg/kg of body weight in 0.4 mL of dimethylformamide subcutaneously) twice weekly for 4 weeks and tap water for drinking was replaced by 1% sodium chloride (NaCl) during the experiments. The systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and heart rate (HR) were monitored during an entire observation using tail‐cuff method21 with noninvasive blood pressure system (BP‐2010 Series, Blood Pressure Meter, Softron). The average value of SBP for each rat was obtained from 5 SBP readings after the rats were acclimated to the environment.
2.4. Nodose ganglion microinjection and blood pressure monitoring
As described in our previous work,16 the baseline arterial blood pressure was recorded by the physiological pressure transducer (AD Instruments MLT844, Norway) before the neck surgery. Rats were placed in a supine position and the neck was disinfected with 75% ethanol. Then with a 3.0‐cm longitudinal midline cut in the middle of the neck, we carefully blunt dissected the left side of nodose ganglion under stereomicroscopy (x40) to make sure the complete separation of the nodose from the arteria. The connective tissue surrounding nodose was open to reduce the resistance while microinjecting. After surgery and the baseline MABP became a stable, slight tension was applied on the vagus nerve by a tweezer, and then SP or its agonists was administrated into the nodose ganglion using a precision glass micro‐syringe (HAMILTON) affixed with a 30G half‐inch stainless steel syringe needle with a 35° beveled tip with 2‐μL drug or DMSO as the vehicle control.
2.5. ELISA measurements of serum substance P concentration
After animals were anaesthetized, the blood of the three groups of rats was collected immediately and allowed to clot at 4°C overnight before centrifugation for 20 minutes at 1000 g. Then the supernatant was collected to measure substance P (SP) concentration using SP Enzyme‐Linked Immunosorbent Assay Kit (Cloud‐clone Corp., Houston, USA) according to the manufacturer's instructions.
2.6. Surgical ovariectomy
The surgery was performed following the protocols described previously.22 Briefly, anesthetized animals (combination of xylazine 10 mg/kg and ketamine 75 mg/kg) were placed in a lateral position, and both flanks were shaved and cleaned using chlorhexidine scrub and disinfected with 70% ethanol and povidone‐iodine (7.5%). A 2.0‐cm incision was made on the left lateral side along a line spanning from the 2nd to the 5th lumbar vertebra, using a scalpel blade. The left ovary and associated fat were located and externalized by gentle retraction. After removal of the ovary, the peritoneal cavity, muscle layers, and skin were closed successively with 4.0 absorbable sutures and then penicillin (80 000 Units) was given via intramuscular injection. The same procedure was repeated for removal of the right ovary. After recovering from anesthesia, the animals were monitored for at least 30 minutes to ensure that there was no bleeding from the surgery, and then were returned to the animal facility.
2.7. Echocardiographic measurements
Trans‐thoracic echocardiography with an ultrasound machine (Vevo 2100 imaging system, Visual Sonics, Toronto, Canada) was used to test the heart functions of rats. Left ventricular systolic/diastolic internal diameter (LVIDs/LVIDd, mm), interventricular septum systolic/diastolic thickness (IVSs/IVSd, mm), and left ventricular systolic/diastolic posterior wall (LVPWs/LVPWd, mm) were measured, and ejection fraction (EF, %) and fractional shortening (FS, %) were calculated from the short axis (SAX) or parasternal long axis (PSLAX)‐mode recording.
2.8. Measurement of baroreceptor sensitivity
By following the protocol,23, 24 the mean arterial pressure (MAP), heart rate (HR), and baroreflex sensitivity (BRS) of anesthetized (3% amobarbital sodium, 25 mg/kg, i.p) rats were tested by cannulas of arteria (left, for artery pressure detection) and vena (right, for drug administration) femoralis and electrocardiographic (ECG) recording (LabChart 7 Pro software, AD instruments, Australia) with body temperature maintained at approximately 35°C. BRS was established by intravenous bolus injections of PE or SNP at an incremental dose (1, 3, and 10 μg/kg), respectively, and 15‐20 minutes were given before the next injection. The maximum changes in HR and the associated MAP were calculated as BRS (ΔHR/ΔMAP).
2.9. Tissue preparation of NG and NTS
The nodose ganglia and liver tissue were isolated from each group of rats as previously described by our laboratory method.17, 18 Briefly, upon lack of reflex response to tail pinch after 3% Pentobarbital Sodium intraperitoneal administration, the animals were immediately sectioned at the mid‐auxiliary region in order to preserve enough length of the vagus nerve, by which one easily finds out the NG along the vagus nerve toward the distal end. The entire NG with the attached nerve trunk was carefully excised under stereomicroscopy (×40, Olympus, Japan) and immediately transferred into a Petri dish containing chilled (4°C) normal saline or extracellular solution and then the surrounding connective tissue was gently removed and stored in liquid nitrogen for further molecular investigation. For NTS tissue collection, the hindbrain was removed and placed in ice‐cold artificial cerebrospinal fluid (ACSF). The bilateral medulla was trimmed to a 1‐cm block (rostral‐caudal) centered on the obex under a microscope.25 The trimmed tissue block containing NTS was then stored at −80°C for further molecular examination.
2.10. Real‐time PCR detection for mRNA in tissue levels of nodose ganglion and NTS
One mRNA sample of NG or NTS tissue was extracted from 4‐5 rats in the same group (control or HFD). All primers used can be seen in the Table S1. The mRNA expression was determined using SYBR Green reagent in ABI 7500 Real‐Time PCR System (Applied Biosystems). Data of relative gene expression were analyzed with 2−ΔΔCT method.26
2.11. Western blot
Total protein was prepared by homogenizing the isolated NTS, NG, or liver tissue for 1 hour at 4°C in RIPA buffer containing Protease Inhibitor Cocktail. One protein sample of NG or NTS tissue was extracted from 4‐5 rats in the same group. Protein extracts (100 μg/sample, accessed through a BCA protein assay) were subjected to 10% SDS‐Tris glycine gel electrophoresis and then transferred (Bio‐Red Laboratories, USA) to a nitrocellulose (NC) membrane. The membranes were blocked in 5% nonfat dry milk/PBS buffer for 2 hours, and then incubated at 4°C overnight with primary antibodies (1:200‐1: 500): anti‐GAPDH (internal control, Sigma, USA), anti‐NK1R (Cat#: ATR‐001, Source: Rabbit, Type: Polyclonal; Alomone Labs, Jerusalem, Israel), anti‐NK2R (Cat#: ATR‐002, Source: Rabbit, Type: Polyclonal; Alomone, Labs, Jerusalem, Israel), and anti‐NK3R (Cat#: ab49201, Source: Rabbit, Type: Polyclonal, Abcam, USA), then the appropriate secondary antibodies (1:8000; LI‐COR Biosciences, Lincoln, NE) were used at room temperature for 1 hour. The results were detected and analyzed via odyssey system (LI‐COR Biosciences, Lincoln, NE).
2.12. Immunohistochemical analysis
The immunohistochemistry protocol for NG was described in our previous reports17, 27: Horizontal sections of 7‐μm thickness were collected for later NKRs protein staining. For the whole section visualization of NTS,21, 28 briefly, rats were anesthetized with 3% amobarbital sodium (25 mg/kg, i.p), then transcardially perfused for 10 minutes with 4% paraformaldehyde (PFA). Harvested brains were placed in 4% PFA overnight before being transferred to 30% sucrose and rotated for 24‐48 hours at 4°C. Coronal brainstem sections (35 μm thickness, Bregma −12.6 mm) were cut on a freezing microtome (Leica Biosystem, Germany) and stored in −80°C. Brainstem sections were washed in PBS for 10 minutes each before immunostaining, and blocked in 4% normal goat serum/0.3% Triton X‐100/PBS for 1 hour at room temperature. For double‐labeling, sections were incubated with a mixture of primary antibodies overnight at 4°C: anti‐NK1R or NK2R (Alomone, USA) at a dilution of 1:100 in blocking solution, respectively. Sections were washed three times with PBST for 10 minutes each, and then incubated in appropriate secondary antibodies mixture both diluted to 1:5000 in PBS: anti‐rabbit IRDye 800CW for NK1R and anti‐rabbit IRDye 800CW for NK2R (LI‐COR Biosciences, Lincoln, NE) at room temperature for 1 hour. All sections were washed five times in PBST for 10 minutes each. And then fluorescent‐imaging was detected using a LI‐COR Odyssey infrared imager (21 μm resolution, 1 mm offset at the highest quality).
2.13. Data analysis
Statistical differences between expression levels were determined by either Student's t test or an ANOVA. A paired t test was used to compare the difference before and after treatment and average data were presented as mean ± SD. Labchart 7 (AdInstrument, Norway) was used for initial data readings. Excel (Microsoft, Northampton, USA) was used for statistical analysis. Origin (Microsoft, Northampton, USA) was used for trace filtering and data reduction processes for data graphing. The value equal or less than 0.05 was considered as marked difference compared with before test.
3. RESULTS
3.1. Serum level of substance P
Early study has shown that SP can be synthesized within the NG and bidirectionally transported toward the CNS and thoracic and abdominal viscera.29 Gender difference regarding SP released from primary afferents evoked by formalin has been reported recently.30, 31 However, the resting level of SP in NG tissue and estrogen sensitivity have not been evaluated. In this regard, ELISA assay was performed and the result showed no difference in serum level SP (Figure S1) among adult male, age‐matched female, and OVX female rats.
3.2. Distribution of tachykinin receptors’ (NKRs) expression in NG and NTS
To further understand if the effect of SP on BP is mediated via baroreflex afferent pathway, the distribution and quantification of estrogen‐dependent expression of NKRs were verified. Immunohistochemical analysis indicated that NK1R and NK2R were expressed in both myelinated (HCN1‐positive) and unmyelinated (HCN1‐negative) NG neurons and also the region of NTS (Figure 1). Immunoblotting evidence showed that the expression levels of NK1R and NK2R were significantly higher in the NG tissue of adult females (P < 0.01 or P < 0.05) compared with age‐matched male rats, and OVX procedure completely reversed the expression to the level of males (Figure 1A and B). No similar expression profile for NK3R was found Figure (S2). Apparently, the expression profiles of NK1R and NK2R in NG were exactly opposite to that in NTS (Figure 1C and D). These results suggest that SP might influence BP by estrogen‐dependent expression of NKRs, which may modulate baroreflex afferent function and offer inversed outcome due to an opposite expression pattern between NG and NTS.
Figure 1.
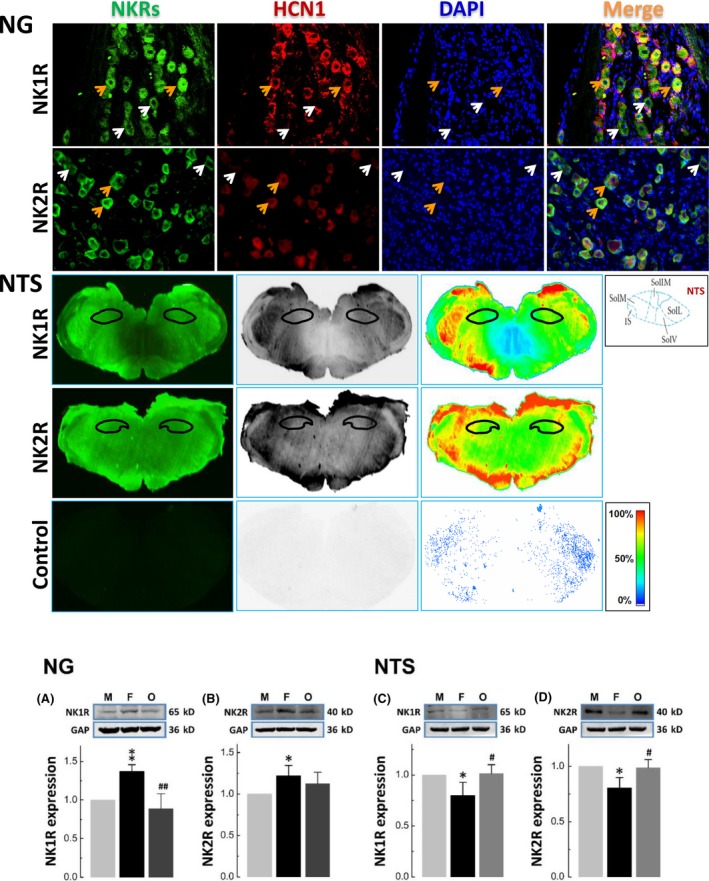
Identification of NKRs’ expression using immunostaining and western blot in both nodose (NG) and nucleus of tractus solitary (NTS). The upper panel: Immunohistochemical identification of positive expression of NK1R and NK2R in myelinated (HCN1‐positive, orange arrowheads) and unmyelinated (HCN1‐negative, white arrowheads) afferent neurons of NG in adult male rats. Similar results were also observed in age‐matched female and ovariectomized rats (data not shown). The middle panel: Immunohistochemical identification of positive expression of NK1R and NK2R in NTS of adult male rats. Similar results were also observed in age‐matched female and ovariectomized rats (data not shown). The lower panel: Expression profiles of NK1R and NK2R in NG (A and B) and NTS (C and D) compared with internal control (GAPDH). The ganglion tissue was collected from male (M), age‐matched female (F), and ovariectomized (O) rats. Averaged data were expressed as mean ± SD, n = 4 from 12 rats. *P < 0.05 and **P < 0.01 vs male; # P < 0.05 and ## P < 0.01 vs female
3.3. Substance P‐mediated blood pressure reduction through NG microinjection
The key feature of SP is to induce vasodilation that would benefit its inflammatory processes on the one hand and to promote inflammatory cells’ migration to the sites through endothelium on the other.32 Vasodilation may also modulate BP by competing with sympathetic nerve activity33 and cause a local circulation disturbance9 after brain injury. Since NKRs’ expression in the NG is closely associated with estrogen, whether direct activation of these NKRs by microinjection of SP into NG can induce paralleled changes in BP became our immediate focus. Interestingly, mean arterial blood pressure was obviously reduced by SP microinjection (0.5, 1, 2, and 5 μg/kg in 2.0 μL) in a dose‐dependent manner in both adult male and age‐matched female rats. However, BP reduction in females was significantly less potent (P < 0.01) than that in males, whereas the difference of the recovery time from the peak of BP reduction back to the control level was not observed between groups (Figure 2A‐C). The EC50 for BP reduction in the presence of SP was nearly 0.74 μg and 0.92 μg for males and females, respectively (Figure S3). These data highly suggest that a relatively less potent BP reduction in the presence of SP is presumably attributed to estrogen‐dependent up‐regulation of NKRs’ expression under physiological conditions conjugated with reduced sensitivity of NKRs to SP.
Figure 2.
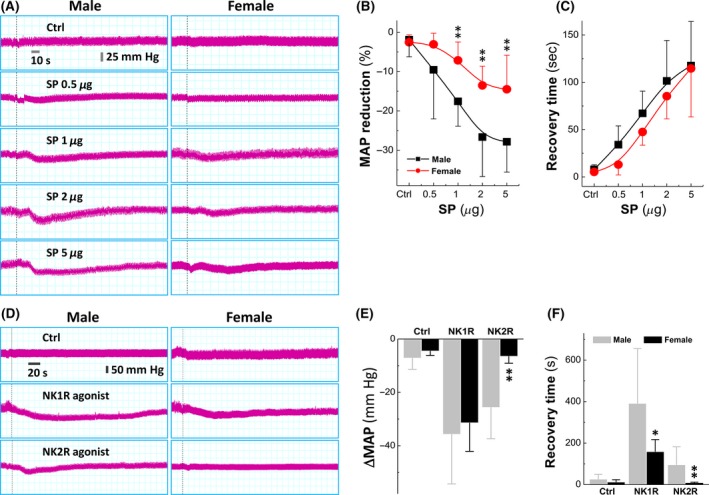
Sexual difference in substance P (SP)‐mediated blood pressure (BP) reduction and mimicking effect of NKRs’ agonists via microinjection into nodose (NG). A, BP reduction in the presence of a series of dosage of SP in adult male and age‐matched female rats. Dot lines represent the points of microinjections. B, Averaged reduction of mean arterial pressure (MAP). Averaged data were presented as mean ± SD, n = 6 rats, **P < 0.01 vs male. C, Averaged data of the recovery from the point of maximal reduction. D, The representative recordings of BP in the presence of 5 μg NK1R ([Sar, Met(O2)11‐SP]) or 5 μg NK2R agonists or ([(β‐Ala8) Neurokinin A]) agonist. E and F, Summarized data of the changes in MAP and the recovery time. Averaged data were presented as mean ± SD, n = 6 rats, *P < 0.05 and **P < 0.01 vs male
3.4. Mimicking effect of tachykinin receptor agonists on blood pressure reduction
Before verifying the potential effects of estrogen, NKRs involved in SP‐mediated BP reduction need to be clarified. To this regard, the agonists for NK1R, [Sar, Met(O2)11‐SP] or NK2R, [(β‐Ala8) Neurokinin A], were microinjected into the NG. The results showed that the agonist for NK1R caused a significant long‐lasting BP reduction, while the agonist for NK2R induced detectable and brief transient BP reduction in male and female rats, even though NK2R mediated equally potent BP reduction as NK1R (Figure 2D). Beyond that, the recovery time from the peak of BP reduction was significantly longer in males (P < 0.05 vs females, Figure 2E and F), suggesting that NK1R may be a key player in mediating SP‐induced BP reduction by modulating sexually dimorphic baroreflex afferent function.
3.5. Blood pressure reduction in DOCA‐salt hypertension models with intact and ovariectomized female rats
In order to test the protective effects of estrogen on SP‐mediated BP regulation under pathophysiological situations, the development of hypertension and the parallel change in BRS were evaluated in DOCA‐salt hypertension rat model established using intact and OVX female rats (Figure S4). The results demonstrated that during the consecutive 6 weeks observation (Figure 3A), BP stayed at a low level in intact females (F‐Ctrl, black) compared with OVX (OVX‐Ctrl, green) after adaptive phase of the first 2 weeks (P < 0.05). BP observed in intact female DOCA‐model (F‐DOCA model, red) significantly and continuously raised (P < 0.05 or P < 0.01 vs F‐Ctrl) since the 2nd week after DOCA procedure. Surprisingly, elevated BP found in F‐DOCA model was even further increased in OVX‐DOCA model (blue, P < 0.01). These observations imply that estrogen may help buffer BP elevation in salt‐sensitive hypertensive conditions.
Figure 3.
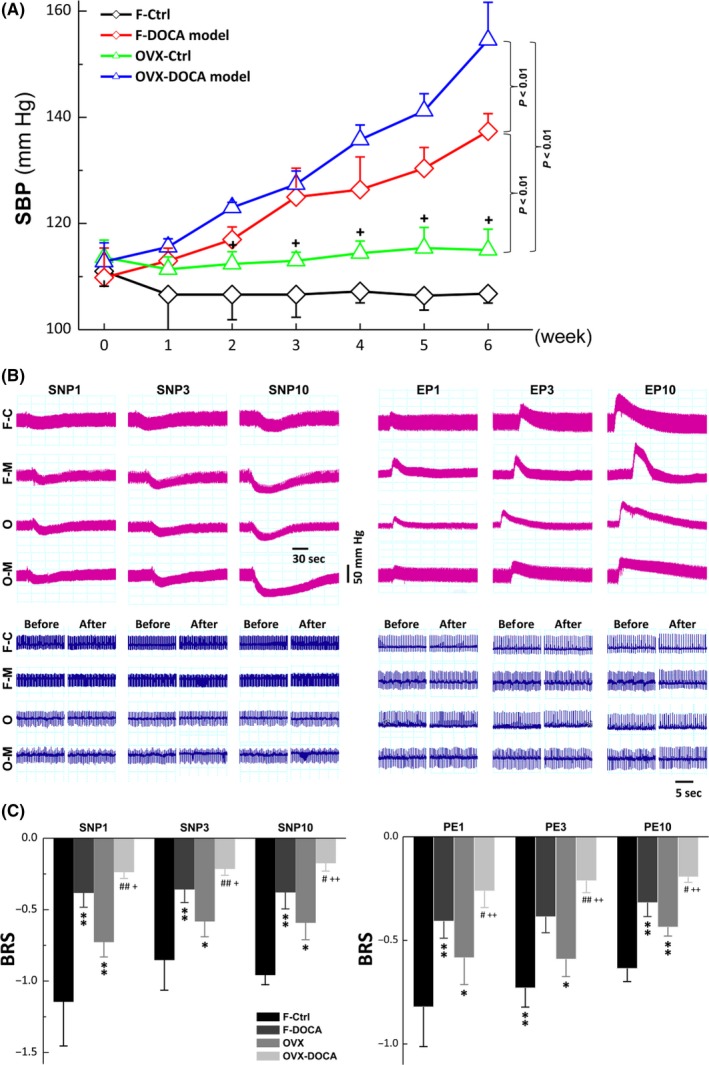
The changes in systolic blood pressure (SBP) and baroreceptor sensitivity (BRS) in intact adult female (F‐Ctrl), intact female DOCA‐model (F‐M), ovariectomized (O‐Ctrl), and ovariectomized DOCA‐model (O‐M). A, The DOCA‐salt hypertension rat models were established using adult female and OVX female rats and SBP was monitored during six consecutive weeks. Significant rise in SBP was observed at the 2nd or 3rd week in female (F) DOCA and ovariectomized DOCA model rats, respectively, after successful DOCA model. Averaged data were presented as mean ± SD, n = 5 rats for each group. P < 0.01 between groups as indicated; + P < 0.05 vs F‐Ctrl. B, The representative changes in blood pressure (BP) and heart rate were monitored in the presence of 1, 3, and 10 μg/kg sodium nitroprusside (SNP) or phenylephrine (PE). C, The BRS (ΔHR/ΔMSAP) was calculated before and after SNP and PE. Averaged data were expressed by mean ± SD, n = 5 rats for each group. *P < 0.05 and **P < 0.01 vs F‐Ctrl, # P < 0.05 and ## P < 0.01 vs F‐M, and + P < 0.05 and ++ P < 0.01 vs ovariectomized (OVX)
3.6. Dysfunction of baroreceptor sensitivity measured in intact and ovariectomized DOCA‐salt hypertensive rats
In our previous reports, estrogen plays a greater role in neurocontrol of circulation through baroreflex afferent function12 and restores neuro‐excitability of sexually dimorphic subset of myelinated vagal afferents in OVX rats.18 These observations give us a clue that the baroreflex afferent function is likely to be modulated and contributes significantly to the protective effect of estrogen on BP elevation. To this end, the baroreceptor sensitivity (BRS: ΔHR/ΔMABP), a particular parameter for baroreflex function, was introduced in the following observation (Figure 3B). The averaged data clearly indicated that the BRS calculated in the presence of 1, 3, 10 μg/kg of SNP or PE was significantly down‐regulated in intact female DOCA model rats (F‐DOCA, P < 0.01 vs intact female, F‐Ctrl). The reduced BRS observed in intact female DOCA‐model rats was, as expected, further and significantly reduced in OVX DOCA‐model rats (OVX‐DOCA, P < 0.01, Figure 3C), suggesting that the buffering of estrogen against BP elevation disappeared under salt‐sensitive hypertensive conditions due at least partially to the dysfunction of baroreflex afferent function.
3.7. Expression changes in tachykinin receptors in DOCA‐salt hypertensive rats
Based upon elevated BP, reduced BRS, and potential buffering of estrogen, down‐regulation of NKRs in the NG in DOCA‐salt hypertensive rats is speculated. As expected, significantly lower expressions of NK1R and NK2R in the NG were detected in both male (Figure 4A) and female (Figure 4C) DOCA model rats, which explained well the notions of BP elevation and BRS dysfunction in DOCA model rats. It is worth mentioning that the degree of down‐regulation for both NK1R and NK2R in female‐DOCA model rats was about 50% of those seen in male‐DOCA model rats, which is likely to explain the buffering (protective effect) of estrogen against BP elevation (Figure 3A) and BRS dysfunction (Figure 3B‐C). Consistently, this molecular modification of NK1R and NK2R in the NG was confirmed by immunohistochemical analysis, with which the mean fluorescent density of NK1R and NK2R detected in DOCA model was reduced significantly in the NG (P < 0.01) of males. This reduction of mean fluorescent density was not only observed in myelinated (HCN1‐positive) but also in unmyelinated (HCN1‐negative) afferents (Figure 5), again supporting the possibility of all fiber types’ involvement in SP‐NKRs‐mediated BP regulation. A similar result of immunostaining was also seen in female DOCA models (data not shown). The NTS is the terminus of baroreflex receiving the afferent input from NG; the expression of NK1R and NK2R was tested under a similar experimental condition. The data showed that the expression pattern of NKRs was completely opposite in the NTS of both males (Figure 4B) and females (Figure 4D) compared with that of NG, which supports an inverse role in mediating afferent function of blood pressure regulation of NG and NTS in the process of baroreflex.
Figure 4.
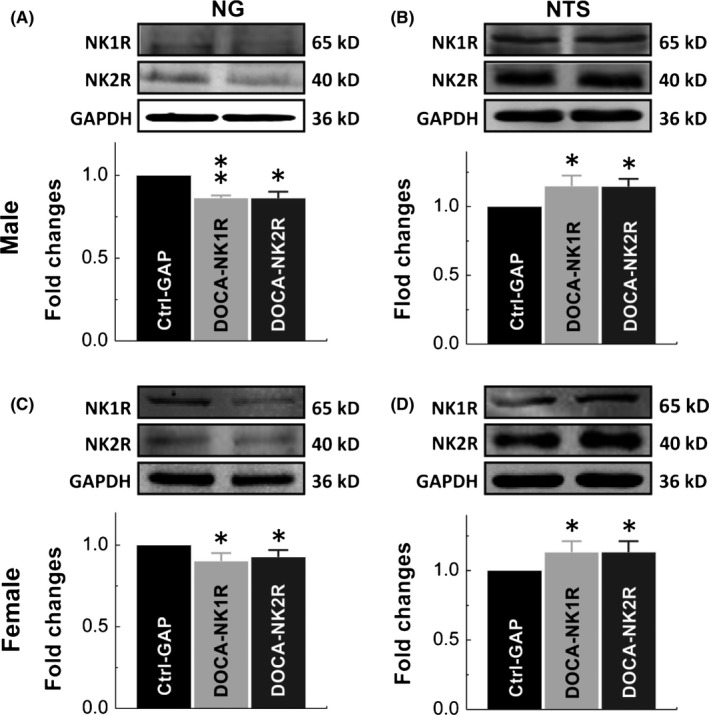
Molecular identification (protein bands) and summarized result of expression changes. Expression changes in NK1R and NK2R were verified in nodose (NG) and nucleus of tractus solitary (NTS) of DOCA‐salt hypertension models of adult male (A and C) and age‐matched female (B and D) rats. GAPDH means the internal control (Ctrl‐GAPDH). Averaged data were presented as mean ± SD, n = 4 from 12 rats. *P < 0.05 and **P < 0.01 vs Ctrl
Figure 5.
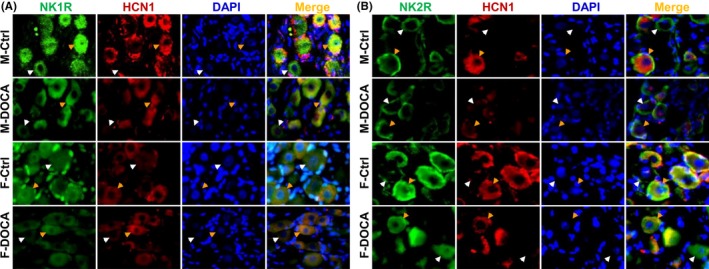
Immunohistochemical quantification of the expression changes in NK1R and NK2R in control (Ctrl) and male DOCA‐model rats of either sex. White and orange arrowheads represented the HCN1‐negative (unmyelinated) and HCN1‐positive (myelinated) afferent neurons within nodose (NG) of adult male and female rats. The mean fluorescence intensity was calculated by measuring the fluorescence from each individual HCN1‐positive or HCN1‐negative neuron using Image‐J software
3.8. Expression changes in tachykinin receptors in spontaneous hypertension rats (SHR)
DOCA‐salt hypertension rat model is established for representing salt‐sensitivity and secondary hypertension in clinics. So, the question remains: Would similar molecular changes be found in primary hypertension? To answer this question, the tissue was collected from the NG and NTS of adult male and age‐match female SHR and exactly similar expression profiles of NK1R and NK2R were confirmed (Figure 6A‐D). These data once again support our hypothesis that NKRs play an important role in SP‐mediated BP regulation and estrogen takes a central place in protective action against unusual elevation of BP under certain physiological and pathophysiological conditions.
Figure 6.
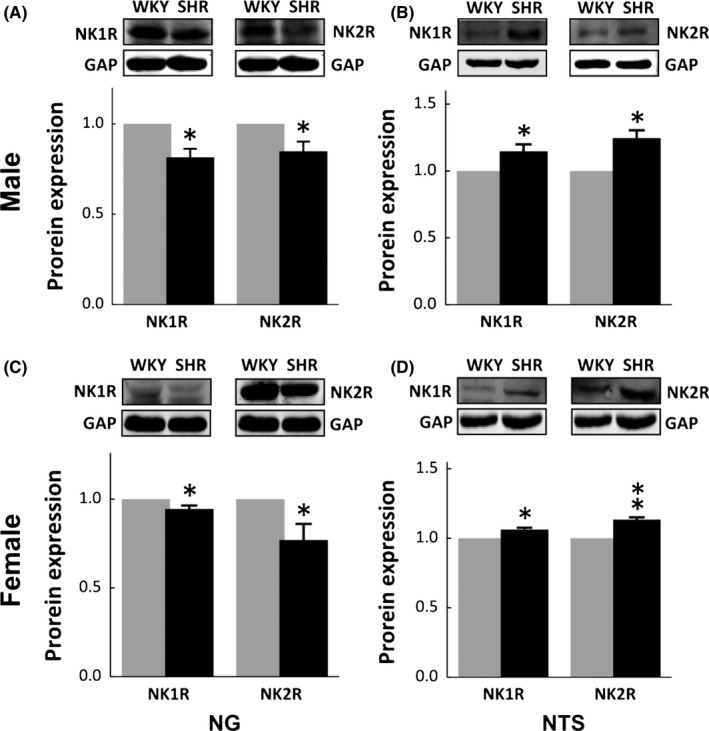
Immunoblotting analysis of expression changes in NK1R and NK2R in nodose (NG) and nucleus of tractus solitary (NTS) from adult male and age‐matched female spontaneous hypertension rats (SHR). A and C, representative protein bands and summarized results of NK1R and NK2R in the NG; B and D, representative protein bands and summarized results of NK1R and NK2R in the NTS. Summarized data were presented as mean ± SD, n = 3 rats for WKY and n = 4 rats for SHR, respectively, *P < 0.01 and **P < 0.01 vs WKY. GAP means the internal control
4. DISCUSSION
4.1. Major findings
The major contributions of the current investigation are summarized as follows: (i) NK1R, NK2R, and NK3R were distributed in both NG and NTS with relatively lower expression levels as revealed by PCR cycle number. Importantly, the expression levels were dramatically higher in females and this expression profile in NG was in stark contrast with NTS; (ii) Microinjection of SP into NG caused BP reduction dose‐dependently with significantly less response in females; additionally, SP‐induced BP reduction was mimicked well by NK1R and NK2R agonists with equal efficacy but NK1R mediated a significant long‐lasting action; (iii) Under hypertensive conditions (DOCA‐salt hypertension model), BP was significantly elevated in intact female DOCA rats and this rise in BP was further enhanced in OVX‐DOCA rats; interestingly, using the same DOCA model, the BRS underwent an exact change; (iv) Consistent with functional data, NK1R and NK2R expressions were down‐regulated in DOCA‐salt hypertensive rats in NG, while the expression patterns were completely opposite in NTS vs NG; (v) Similar molecular observations regarding NKRs’ expression were also confirmed in spontaneous hypertensive rats.
4.2. Remaining questions
Can SP be released34, 35, 36, 37 by the visceral sensory neurons (the first‐order neurons housed in NG) including baroreceptor afferent neurons that terminate their projections to the NTS (the 2nd‐order neurons)? Can SP‐immunoreactivity (SP‐ir) be detected in both NG and NTS38? The sexual dimorphic release of SP from the NG31 and in other brain regions39 has also been reported. The release of SP as a neurotransmitter and neuromodulator has been involved in neurogenic inflammation, which is a local inflammatory response to certain types of injury9, 40, 41, 42 and pain.43 SP is a key first responder to most noxious stimuli (stressors), by which to compromise potentially any biological integrity. Therefore, SP is regarded as part of an immediate defense and survival system. As an inflammatory mediator, SP plays a crucial role in chemotaxis32 that attracts inflammatory cells to the sites of injury and this cellular process is assisted by the vasodilation property of SP itself.3, 44 Apparently, SP release and expression of its receptor may not naturally subside in diseases marked by chronic inflammation and this phenomenon definitely benefits the inflammatory process but may cause disturbances in BP regulation by means of SP‐mediated vasodilation33 over the course of a pathological condition. The specific role and significance of SP and its receptors’, NKRs, expression in BP regulation under physiological and hypertensive disease conditions might be currently a source of frustration and confusion. The association of SP‐NKRs and baroreflex function has been well reviewed8 and previous evidence demonstrated that SP induced hypertension in conscious rats by microinjection of SP into the NTS and this pressor response could be further enhanced by nodose ganglionectomy,45 highly suggesting that the modulatory role of SP in the NG differs from that in the NTS. From the clinical point of view, gender‐based differences in SP release31 and SP‐related pathophysiological conditions30, 46 are extraordinarily brought into focus.
4.3. NG plays an opposite role in BP regulation compared with that of NTS
A previous report showed that the microinjection of SP increased the BP and this effect was further enhanced by ganglionectomy,45 highly indicating that NG may play a distinct role compared with NTS. As expected, SP‐mediated BP reduction was observed by microinjection of SP into NG dose‐dependently and this BP reduction was more dramatic in adult male rats with relatively less EC50 compared with age‐matched females. Intriguingly, SP‐induced BP reduction was also mimicked by microinjection of NK1R and NK2R agonists with equal efficacy, but a long‐lasting action was only observed in the presence of NK1R. These data imply that the NG plays an exact opposite role in BP regulation vs NTS and the protective role of estrogen‐dependent NKRs expression in the NG and the sensitivity NKRs to SP47. Intriguing observations from our recent experiments and other cardiovascular literature have demonstrated that the difference in central and peripheral activation of neuropeptide Y mediates hypotensive48 and hypertensive action.17, 49 Importantly, inversed hemodynamic or autonomic response after peripheral and central activation varies upon the neurotransmitter and is not always the fact. In case of histamine, type‐1 histamine receptor activation at both peripheral and central locations causes blood pressure up‐regulation.50, 51 So far, the cellular mechanism and neural circuitry underlying NTS information processing in autonomic networks are poorly understood, while the neurotransmitters delivered by sensory terminals, the corresponding ligand‐specific receptors located at presynaptic and postsynaptic membranes, and the coupling of such receptors with a number of cellular effector systems in the respective neurons, as well as the subsequent transduction of given sensory signals via enzymatic cascades and ion channels, may all be associated with the complexity of autonomic activation at peripheral and central locations.
4.4. Estrogen‐dependent NKRs’ expression and SP‐mediated BP reduction under physiological condition
Firstly, the NKRs’ expression was found in NG and NTS by means of immunohistochemical analysis. The fluorescence was detected not only in myelinated (HCN1‐positive) but also in unmyelinated (HCN1‐negative) afferent neurons of NG, suggesting that all fiber types of NG neurons are likely to be involved in SP‐mediated function. Secondly, immunoblotting data showed that NK1R and NK2R expressed estrogen‐dependently in NG with significantly higher expression levels in females and this observation provided further evidence to support that estrogen stimulates SP gene expression in sensory afferents.52, 53 Surprisingly, this higher level of expression of NKRs could be completely reversed by surgically removing bilateral ovaries. These molecular findings further explained the beneficial role of estrogen‐dependent NKRs’ expression in SP‐induced BP regulation. Apparently, the estrogen‐related expression of NKRs was definitely observed in the NTS but the expression patterns were exactly opposite compared with the NG, which further supports the notion of distinct BP regulation between NG and NTS.
4.5. Down‐regulation of estrogen‐dependent NKRs’ expression and BP elevation under hypertensive condition
To further confirm the protective role of estrogen in hypertensive conditions, DOCA‐salt rat models were developed using intact and OVX female rats. These results clearly demonstrated that DOCA procedure alone significantly and gradually increased BP in intact females, which was further aggravated by OVX, suggesting that estrogen protects against BP elevation not only in physiological (vs OVX) but also hypertensive disease conditions (vs OVX‐DOCA). In order to verify the involvement of baroreflex afferent function in DOCA condition with or without estrogen, the BRS gain was evaluated in this regard. Impressively, decreased BRS gain in DOCA of intact females (with estrogen) was reduced even further in DOCA of OVX rats (without estrogen) and this synergistic effect was highly speculated in the aspect of estrogen‐dependent down‐regulation of NKRs’ expressions in baroreflex afferent pathway (Figure S5). Taking functional and molecular findings together, the protective effect of estrogen‐specific expression of NKRs in baroreflex afferent pathway could be pinpointed. Consistently, recent animal study showing that intact female mice have a significant lesser damage after traumatic brain injury compared with male and OVX mice54 and the outcomes of OVX mice recover dramatically with estrogen treatment,55 suggesting a potential key role of estrogen‐dependent NKRs expression in the protection against neuroinflammation by down‐regulation of SP‐mediated cardiovascular responses, which further explains why the sensitivity to traumatic brain injury and caused brain damage are far less in females.
4.6. Potential mechanism of an opposite action of SP‐NKRs between NG and NTS
SP‐mediated BP regulation and the involvement of baroreflex remain a debate. The most reliable evidence using conscious rats has shown that microinjection of SP into NTS up‐regulates the BP45 and this pressor response could be buffered by lacking estrogen, which points out that the potential role of the NG differs from the NTS. In stark contrast, the current observation has demonstrated for the first time that the depressor response could be elicited by microinjection of SP or its receptor agonists into the NG, strongly indicating that direct activation of NG produces depressor responses due largely to its character as an excitatory neurotransmitter56, 57 to relay the neuroexcitability58 and eventually initiate the depressor response. Another line of evidence has also noticed that the activation of pressure‐sensitive afferents by stretch baroreceptor terminal endings would increase glutamate release at presynaptic terminals to relay the baroreflex and compromise the elevated BP.36 However, activation of ascending spinal neurons from the cervical dorsal horn by contraction‐sensitive skeletal muscle afferents induces pressor response by selectively inhibiting arterial baroreceptor signaling in the NTS via SP‐mediated activation of a GABAergic mechanism.35, 59 Controversial findings of a depressor response evoked by the direct activation of NTS with SP exist.60, 61
4.7. Clinical relevance
Substance P is a neuropeptide acting as a neurotransmitter and neuromodulator associated with neuro‐inflammation and pain after its release from the terminals of specific sensory nerves. The vasodilation property of substance P not only promotes inflammatory cells’ migration but also increases fluid extravasation during brain injury. The vasodilation‐mediated blood pressure reduction of substance P is likely to be synergistically enhanced by its direct baroreceptor activation, causing an unusual blood pressure reduction. The current, novel observations have confirmed that substance P clearly modifies the baroreflex afferent function through estrogen‐specific expression of NKRs in both nodose and NTS under physiological and hypertensive disease conditions. Therefore, modifications of substance P release from sensory terminals including baroreceptor afferents and protein expression of NKRs in baroreflex afferent pathway will have important implications in the clinical management of hypertension in those patients with neuro‐inflammation and brain injury. To gain a detailed picture into the ion channel mechanism of SP‐NKRs underlying neuroexcitability, an electrophysiological study needs to be conducted at both the levels of baroreceptor neurons in the NG and baroreceptive neurons in the NTS.
5. CONCLUSION
Our findings suggest that estrogen‐dependent NKRs’ expression, particularly NK1R in the NG, contributes significantly to SP‐mediated BP reduction under both physiological and hypertensive conditions.
CONFLICT OF INTEREST
These authors declare no conflict of interest.
Supporting information
Yuan M, Ma M‐N, Wang T‐Y, et al. Direct activation of tachykinin receptors within baroreflex afferent pathway and neurocontrol of blood pressure regulation. CNS Neurosci Ther. 2019;25:123–135. 10.1111/cns.12993
Funding information
This project was fully supported by the National Natural Science Foundation of China (31171122 and 81573431 for B.‐Y. L., 81173051 and 81773731 for G.‐F. Q.)
The first two authors contributed equally to this work.
Contributor Information
Guo‐Fen Qiao, Email: Qiaogf88@163.com.
Bai‐Yan Li, Email: liby@ems.hrbmu.edu.cn.
REFERENCES
- 1. Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555‐576. [DOI] [PubMed] [Google Scholar]
- 2. Datar P, Srivastava S, Coutinho E, Govil G. Substance P: structure, function, and therapeutics. Curr Top Med Chem. 2004;4:75‐103. [DOI] [PubMed] [Google Scholar]
- 3. Bossaller C, Reither K, Hehlert‐Friedrich C, et al. In vivo measurement of endothelium‐dependent vasodilation with substance P in man. Herz. 1992;17:284‐290. [PubMed] [Google Scholar]
- 4. Mistrova E, Kruzliak P, Chottova Dvorakova M. Role of substance P in the cardiovascular system. Neuropeptides. 2016;58:41‐51. [DOI] [PubMed] [Google Scholar]
- 5. Levick SP, Melendez GC. Targeting substance P and relaxin: a future combination therapy approach for heart failure? Int J Cardiol. 2016;204:154‐155. [DOI] [PubMed] [Google Scholar]
- 6. Dehlin HM, Levick SP. Substance P in heart failure: the good and the bad. Int J Cardiol. 2014;170:270‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dehlin HM, Manteufel EJ, Monroe AL, et al. Substance P acting via the neurokinin‐1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int J Cardiol. 2013;168:4643‐4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helke CJ, Seagard JL. Substance P in the baroreceptor reflex: 25 years. Peptides. 2004;25:413‐423. [DOI] [PubMed] [Google Scholar]
- 9. Vink R, Gabrielian L, Thornton E. The role of Substance P in secondary pathophysiology after traumatic brain injury. Front Neurol. 2017;8:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alhelal MA, Palaska I, Panagiotidou S, et al. Trigeminal nerve stimulation triggers oral mast cell activation and vascular permeability. Ann Allergy Asthma Immunol. 2014;112:40‐45. [DOI] [PubMed] [Google Scholar]
- 11. Brown T, Gonzalez J, Monteleone C. Angiotensin‐converting enzyme inhibitor‐induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19:1377‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santa Cruz Chavez GC, Li BY, Glazebrook PA, et al. An afferent explanation for sexual dimorphism in the aortic baroreflex of rat. Am J Physiol Heart Circ Physiol. 2014;307:H910‐H921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang YY, Yan ZY, Qu MY, et al. KCa1.1 is potential marker for distinguishing Ah‐type baroreceptor neurons in NTS and contributes to sex‐specific presynaptic neurotransmission in baroreflex afferent pathway. Neurosci Lett. 2015;604:1‐6. [DOI] [PubMed] [Google Scholar]
- 14. Wang LQ, Liu SZ, Wen X, et al. Ketamine‐mediated afferent‐specific presynaptic transmission blocks in low‐threshold and sex‐specific subpopulation of myelinated Ah‐type baroreceptor neurons of rats. Oncotarget. 2015;6:44108‐44122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qian Z, Liu DJ, Liu Y, et al. Increase in neuroexcitability of unmyelinated C‐type vagal ganglion neurons during initial postnatal development of visceral afferent reflex functions. CNS Neurosci Ther. 2013;19:954‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Wu D, Qu MY, et al. Neuropeptide Y‐mediated sex‐ and afferent‐specific neurotransmissions contribute to sexual dimorphism of baroreflex afferent function. Oncotarget. 2016;7:66135‐66148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Zhou JY, Zhou YH, et al. Unique expression of angiotensin type‐2 receptor in sex‐specific distribution of Myelinated Ah‐Type baroreceptor neuron contributing to sex‐dimorphic neurocontrol of circulation. Hypertension. 2016;67:783‐791. [DOI] [PubMed] [Google Scholar]
- 18. Qiao GF, Li BY, Lu YJ, et al. 17Beta‐estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol. 2009;297:C654‐C664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He JL, Li JN, Zuo CM, et al. Potentiation of 17beta‐estradiol on neuroexcitability by HCN‐mediated neuromodulation of fast‐afterhyperpolarization and late‐afterdepolarization in low‐threshold and sex‐specific myelinated Ah‐type baroreceptor neurons via GPR30 in female rats. Int J Cardiol. 2015;182:174‐178. [DOI] [PubMed] [Google Scholar]
- 20. Bhatia J, Tabassum F, Sharma AK, et al. Emblica officinalis exerts antihypertensive effect in a rat model of DOCA‐salt‐induced hypertension: role of (p) eNOS, NO and oxidative stress. Cardiovasc Toxicol. 2011;11:272‐279. [DOI] [PubMed] [Google Scholar]
- 21. He JL, Zhao M, Xia JJ, et al. FGF21 ameliorates the neurocontrol of blood pressure in the high fructose‐drinking rats. Sci Rep. 2016;6:29582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiao GF, Qian Z, Sun HL, et al. Remodeling of hyperpolarization‐activated current, Ih, in Ah‐type visceral ganglion neurons following ovariectomy in adult rats. PLoS ONE. 2013;8:e71184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold AC, Shaltout HA, Gallagher PE, et al. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension. 2009;54:1001‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang D, Liu J, Tu H, et al. In vivo transfection of manganese superoxide dismutase gene or nuclear factor B shRNA in nodose Ganglia improves aortic baroreceptor function in heart failure rats. Hypertension. 2013;63:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng WH, Lu PJ, Ho WY, et al. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2‐RSK signaling pathway to modulate central control of blood pressure. Circ Res. 2010;106:788‐795. [DOI] [PubMed] [Google Scholar]
- 26. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101‐1108. [DOI] [PubMed] [Google Scholar]
- 27. Li J, Li X, He J, et al. Sex‐and afferent‐specific differences in histamine receptor expression in vagal afferents of rats: a potential mechanism for sexual dimorphism in prevalence and severity of asthma. Neuroscience. 2015;303:166‐177. [DOI] [PubMed] [Google Scholar]
- 28. Covington HE, Maze I, LaPlant QC, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451‐11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLean DB. Substance P synthesis and transport in explants of nodose ganglion/vagus nerve: effects of double ligation, 2‐deoxyglucose, veratridine, and ouabain. J Neurochem. 1987;48:1794‐1803. [DOI] [PubMed] [Google Scholar]
- 30. Bellucci F, Bueno L, Bugianesi R, et al. Gender‐related differential effect of tachykinin NK2 receptor‐mediated visceral hyperalgesia in guinea pig colon. Br J Pharmacol. 2016;173:1329‐1338. Epub 2016/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nazarian A, Tenayuca JM, Almasarweh F, et al. Sex differences in formalin‐evoked primary afferent release of substance P. Eur J Pain. 2014;18:39‐46. [DOI] [PubMed] [Google Scholar]
- 32. Sloniecka M, Le Roux S, Zhou Q, et al. Substance P enhances keratocyte migration and neutrophil recruitment through interleukin‐8. Mol Pharmacol. 2016;89:215‐225. [DOI] [PubMed] [Google Scholar]
- 33. Supowit SC, Ethridge RT, Zhao H, et al. Calcitonin gene‐related peptide and substance P contribute to reduced blood pressure in sympathectomized rats. Am J Physiol Heart Circ Physiol. 2005;289:H1169‐H1175. [DOI] [PubMed] [Google Scholar]
- 34. Morilak DA, Morris M, Chalmers J. Release of substance P in the nucleus tractus solitarius measured by in vivo microdialysis: response to stimulation of the aortic depressor nerves in rabbit. Neurosci Lett. 1988;94:131‐137. [DOI] [PubMed] [Google Scholar]
- 35. Potts JT, Fuchs IE, Li J, et al. Skeletal muscle afferent fibres release substance P in the nucleus tractus solitarii of anaesthetized cats. J Physiol. 1999;514(Pt 3):829‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Potts JT, Fuchs IE. Naturalistic activation of barosensitive afferents release substance P in the nucleus tractus solitarius of the cat. Brain Res. 2001;893:155‐164. [DOI] [PubMed] [Google Scholar]
- 37. Zhong W, Chebolu S, Darmani NA. Thapsigargin‐induced activation of Ca(2+)‐CaMKII‐ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology. 2016;103:195‐210. [DOI] [PubMed] [Google Scholar]
- 38. Gillis RA, Helke CJ, Hamilton BL, et al. Evidence that substance P is a neurotransmitter of baro‐ and chemoreceptor afferents in nucleus tractus solitarius. Brain Res. 1980;181:476‐481. [DOI] [PubMed] [Google Scholar]
- 39. DePalatis LR, Khorram O, McCann SM. Age‐, sex‐, and gonadal steroid‐related changes in immunoreactive substance P in the rat anterior pituitary gland. Endocrinology. 1985;117:1368‐1373. [DOI] [PubMed] [Google Scholar]
- 40. Corrigan F, Leonard A, Ghabriel M, et al. A substance P antagonist improves outcome in female Sprague Dawley rats following diffuse traumatic brain injury. CNS Neurosci Ther. 2012;18:513‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Donkin JJ, Cernak I, Blumbergs PC, et al. A substance P antagonist reduces axonal injury and improves neurologic outcome when administered up to 12 hours after traumatic brain injury. J Neurotrauma. 2011;28:217‐224. [DOI] [PubMed] [Google Scholar]
- 42. Donkin JJ, Nimmo AJ, Cernak I, et al. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1388‐1398. [DOI] [PubMed] [Google Scholar]
- 43. De Felipe C, Herrero JF, O'Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394‐397. [DOI] [PubMed] [Google Scholar]
- 44. Karabucak B, Walsch H, Jou YT, et al. The role of endothelial nitric oxide in the Substance P induced vasodilation in bovine dental pulp. J Endod. 2005;31:733‐736. [DOI] [PubMed] [Google Scholar]
- 45. Abdala AP, Haibara AS, Colombari E. Cardiovascular responses to substance P in the nucleus tractus solitarii: microinjection study in conscious rats. Am J Physiol Heart Circ Physiol. 2003;285:H891‐H898. [DOI] [PubMed] [Google Scholar]
- 46. Yamaura K, Tomono A, Suwa E, et al. Sex‐related differences in SLIGRL‐induced pruritus in mice. Life Sci. 2014;94:54‐57. [DOI] [PubMed] [Google Scholar]
- 47. Pisera D, Theas S, De Laurentiis A, et al. The hormonal status modulates the effect of neurokinin A on prolactin secretion in female rats. J Endocrinol. 1998;159:389‐395. [DOI] [PubMed] [Google Scholar]
- 48. Cheng PW, Wu AT, Lu PJ, et al. Central hypotensive effects of neuropeptide Y are modulated by endothelial nitric oxide synthase after activation by ribosomal protein S6 kinase. Br J Pharmacol. 2012;167:1148‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malmstrom RE, Lundberg JM. Neuropeptide Y accounts for sympathetic vasoconstriction in guinea‐pig vena cava: evidence using BIBP 3226 and 3435. Eur J Pharmacol. 1995;294:661‐668. [DOI] [PubMed] [Google Scholar]
- 50. Bhuiyan ME, Waki H, Gouraud SS, et al. Histamine receptor H1 in the nucleus tractus solitarii regulates arterial pressure and heart rate in rats. Am J Physiol Heart Circ Physiol. 2011;301:H523‐H529. [DOI] [PubMed] [Google Scholar]
- 51. Chen YY, Lv J, Xue XY, et al. Effects of sympathetic histamine on vasomotor responses of blood vessels in rabbit ear to electrical stimulation. Neurosci Bull. 2010;26:219‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villablanca AC, Hanley MR. 17beta‐estradiol stimulates substance P receptor gene expression. Mol Cell Endocrinol. 1997;135:109‐117. [DOI] [PubMed] [Google Scholar]
- 53. Mowa CN, Usip S, Storey‐Workley M, et al. Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis. Peptides. 2003;24:761‐771. [DOI] [PubMed] [Google Scholar]
- 54. Clevenger AC, Kim H, Salcedo E, et al. Endogenous sex steroids Dampen Neuroinflammation and improve outcome of traumatic brain injury in mice. J Mol Neurosci. 2018;64:410‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu H, Ma K, Jin L, et al. 17beta‐estradiol rescues damages following traumatic brain injury from molecule to behavior in mice. J Cell Physiol. 2018;233:1712‐1722. [DOI] [PubMed] [Google Scholar]
- 56. Nicoll RA, Schenker C, Leeman SE. Substance P as a transmitter candidate. Annu Rev Neurosci. 1980;3:227‐268. [DOI] [PubMed] [Google Scholar]
- 57. Yeh SY, Huang WH, Wang W, et al. Respiratory network stability and modulatory response to substance P require Nalcn. Neuron. 2017;94:294‐303. e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498‐501. [DOI] [PubMed] [Google Scholar]
- 59. Potts JT, Paton JF, Mitchell JH, et al. Contraction‐sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience. 2003;119:201‐214. [DOI] [PubMed] [Google Scholar]
- 60. Chan JY, Barnes CD, Chan SH. Tonic enhancement of the sensitivity of baroreceptor reflex response by endogenous substance P in the rat. Regul Pept. 1990;29:199‐213. [DOI] [PubMed] [Google Scholar]
- 61. Abdala AP, Schoorlemmer GH, Colombari E. Ablation of NK1 receptor bearing neurons in the nucleus of the solitary tract blunts cardiovascular reflexes in awake rats. Brain Res. 2006;1119:165‐173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


