Abstract
Background.
Patients with glioma exhibit a great variability in clinical symptoms apart from variations in response to therapy and survival. Many patients present with epileptic seizures at disease onset, especially in case of low-grade gliomas, but not all have seizures. A large proportion of patients develop refractory seizures. It is likely that the variability in epileptic symptoms cannot exclusively be explained by tumor-related factors, but rather reflects complex interaction between tumor-related, environmental and hereditary factors.
Material and methods.
No data exist on susceptibility genes associated with epileptic symptoms in patients with glioma. However, an increasing number of candidate genes have been proposed for other focal epilepsies such as temporal lobe epilepsy. Some of the susceptibility candidate genes associated with focal epilepsy may contribute to epileptic symptoms also in patients with glioma.
Results.
This review presents an update on studies on genetic polymorphisms and focal epilepsy and brings forward putative candidate genes for tumor-associated epilepsy, based on the assumption that common etiological pathways may exist for glioma development and glioma-associated seizures.
Conclusion.
Genes involved in the immune response, in synaptic transmission and in cell cycle control are discussed that may play a role in the pathogenesis of tumor growth as well as epileptic symptoms in patients with gliomas.
Gliomas
Gliomas of astrocytic, oligodendrocytic and ependymal origin comprise about 40% of all adult primary brain tumors [1,2]. The most frequent and most malignant histological subtype is the glioblastoma, with an annual incidence of 4–5 per 100 000 and a median age at diagnosis of around 60 years [3]. The annual incidence of low-grade gliomas is estimated to 1–2 per 100 000 inhabitants [3]. Despite multimodal treatment approaches consisting of surgery, radiotherapy and chemotherapy, the prognosis of patients with glioblastoma is still poor [4]. The median survival of adults with low-grade gliomas is around 5–10 years, but for most if not all patients the disease has a fatal outcome. Gliomas account for as many as 26 000 USA and European deaths each year.
Apart from high-dose ionizing radiation as an established risk factor and a consistent inverse relationship of glioma with allergies and asthma, the etiology of gliomas is largely unknown [2]. Only 5% of all glioma cases constitute familiar forms, leaving the vast majority of patients with sporadic gliomas. Evidence strongly suggests that inherited susceptibility plays a role with two-fold increased risk of glioma among first-degree relatives of glioma cases [5]. Apart from rare Mendelian cancer pre-disposition syndromes, the genetic basis of glioma susceptibility has not been fully elucidated.
Genetic susceptibility and glioma
A recent report on the genetic basis of susceptibility to gliomas showed several candidate genes associated with increased overall risk of glioma, although presently few associations have been confirmed in several independent data sets [6]. The most commonly studied candidate genes for glioma risk are genes involved in cell cycle control, DNA repair and immune response. Table I shows examples of genes and their specific polymorphisms that have shown to be associated with an altered risk for glioma development [7–17]. CHAF1A and P53 have been associated in single studies for glioma and glioblastoma respectively [18]. In addition, a recent pooled analysis of four US data sets showed associations with DNA repair genes and glioblastoma in 1 000 glioblastoma cases and 2 000 controls [19]. Several studies have associated infection and immune function with a decreased risk of glioma [7]. Allergies have shown to be inversely correlated to gliomas and consistent associations were reported for an IL13 polymorphism and an IL4R haplotype, although it remains unclear whether allergies protect against tumors or whether immunosuppressive gliomas inhibit allergies [20,21].
Table I.
Some examples of case-control studies of gene polymorphisms showing associations with altered glioma risk.
| First Author | Gene and specific polymorphism | Tumor type | Number of cases and controls |
|---|---|---|---|
| Brenner [7] | Cytokines | Glioma | 756 cases, 1190 controls |
| IL-4 (rs224348) | |||
| IL-6 (rs1800795) | |||
| Rajaraman [8] | Cell cycle | Glioma | 388 cases, 533 controls |
| CCND1 (rs603965) | |||
| CCNH (rs2266690) | |||
| MDM2 (rs769412) | |||
| Liu [9] | DNA repair | Glioma | 373 cases, 365 controls |
| ERCC1 (3’UTR) | |||
| XRCC1 (R399Q) | |||
| APEX1 (E148D) | |||
| PARP1 (A762V) | |||
| MGMT (F84L) | |||
| LIG1 (5Ú TR) | |||
| Carpentier [10] | Telomerase | Glioblastoma (GB) & Anaplastic astrocytoma (AA) | 205 GB, 147 AA, 305 controls |
| hTERT (MNS16A) | |||
| Costa [11] | EGF (EGF+61) | Glioma | 197 cases, 570 controls |
| Lu [12] | Promoter Matrix metalloproteinase | Astrocytoma | 236 cases, 366 controls |
| MMP-1–1607 1G/1G | |||
| MMP-1 1G-MMP-3 6A | |||
| Parhar [13] | P53 codon 72 | Glioma (including pediatric cases) | 92 adult cases, 43 pediatric cases, 71 controls |
| P53 Arg72Pro | |||
| Bhowmick [14] | EGF-receptor (EGFR 5Ú TR, G/A & G/G genotype) | Glioblastoma (GB) | 31 primary GB, 11 secondary GB 78 controls |
| Wrensch [15] | Glutathione-S-transferase | GB, astrocytoma (A), oligodendro-glioma (OD), oli-goastrocytoma (OA) | 179 GB, 62A, 94 OD or OA, 32 others, 428 controls |
| GSTP I105V | |||
| Wang [16] | DNA repair | Glioma | 309 cases, 342 controls |
| XRCC/ G6721T (G/T & T/T genotype) | |||
| Frigerio [17] | Tumour necrosis factor | Glioblastoma | 58 patients, 95 controls |
| TNFb4 |
Epilepsy and gliomas
Focal epileptic seizures are among the most common symptoms at disease onset in patients with gliomas. Seizures can frequently predate other symptoms or diagnosis by many years [22]. There is an inverse relationship between tumor growth rate and seizure risk, and symptomatic seizures are significantly more common in low-grade than high-grade gliomas [23]. Up to 80–90% of all patients with low-grade gliomas experience seizures or epilepsy, but not all have seizures in spite of similar tumor localization and histology (Figure 1). Epilepsy may be the only symptom for months or years in the non-progressive phase of the disease. In a recent study, approximately half of the patients with low-grade gliomas who presented with seizures were pharmacoresistant before surgery [24].
Figure 1.
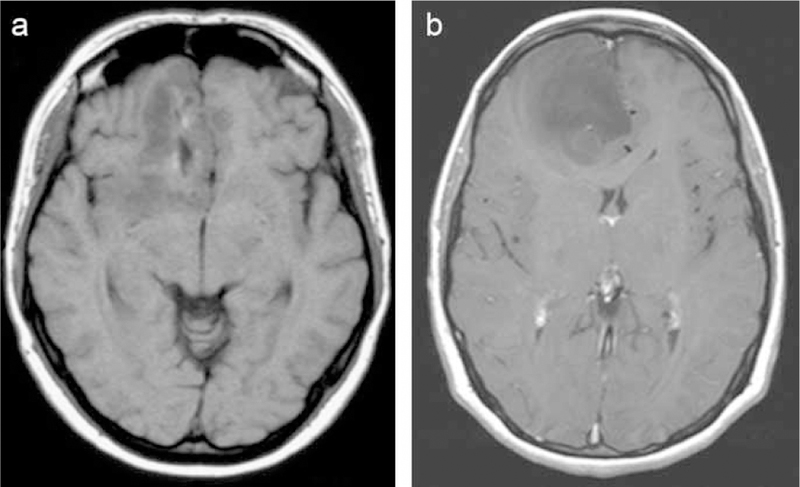
MRI (T1-weighted images) of two patients with an oligodendroglioma (grade II) in the right frontal lobe, both presenting with focal epileptic seizures as the first symptoms.
a) This patient became seizure-free on antiepileptic drugs after an initial seizure.
b) This patient developed pharmacoresistent seizures in spite of multiple antiepileptic drugs.
Other important factors underlying the development of epilepsy in patients with glioma besides the growth rate of the tumor are the localization in the brain and the proximity with the cortical gray matter [23]. Tumors localized in the vicinity of the primary motor cortex and tumors with limbic and perilimbic cortical localization are highly epileptogenic, whereas occipital tumors are less likely to manifest with seizures. Epileptic seizures in high-grade gliomas are less frequent but may be more difficult to control. The pathogenesis of seizure development is likely to occur by different mechanisms for high- and low-grade gliomas [22,25]. In fast growing high-grade gliomas the focal peri-tumoral ischaemia and deafferentiation of cortical areas due to mass effect may be causative factors, whereas gliosis and chronic inflammatory changes in peri-tumoral regions of slowly growing gliomas may predispose for epileptic seizures (Figure 2). Increased levels of Fe3+ ions in intra- or peri-tumoral areas, due to small bleedings from pathological blood vessels, may also contribute to the development of tumor-associated seizures and are more likely to occur in high-grade gliomas [22]. Our knowledge of tumor-associated epileptogenicity, however, is limited and current therapy is far from perfect [26].
Figure 2.
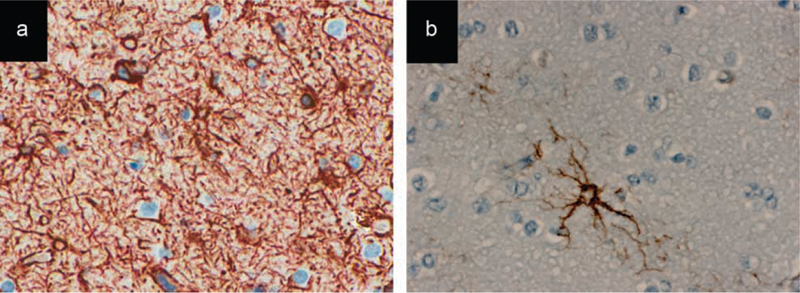
Immunohistochemical stainings with antibodies for the astrocytic marker GFAP (glial fibrillary acidic protein) of the peritumoural cortices of two different samples of diffuse astrocytomas (WHO grade II).
a) A significant increase of reactive astrocytes is demonstrated in the peri-tumoural cortex of this patient with chronic epileptic seizures, compared to. b) the peri-tumoural cortex of a patient who did not have any epileptic seizures.
Epileptic seizures and survival in patients with glioma
A deeper understanding of the correlation between symptoms and course of disease for patients with gliomas is of importance in clinical neuro-oncology. The recurrence of epileptic seizures after a long seizure-free period, for example, may be the first sign of progressive disease before radiological progression is visible, but this is not always the case. Little is known on the impact of seizures on survival for patients with high-grade glioma, but for low-grade gliomas a number of studies have established an association between a more favourable prognosis and epileptic seizures at disease onset [27]. As stated previously, epileptic seizures as initial symptoms in low-grade gliomas are also strongly correlated to a more cortical tumor localization, in contrast to patient with centrally localized tumors who do not frequently present with seizures and have a poor prognosis [27]. We have recently found that patients with low-grade glioma who presented with seizures as initial symptoms but became seizure-free during the early stage of disease had a longer survival than those with recurrent seizures [28]. These findings suggest that the specific symptoms of disease may reflect not only the localization of the tumor in the brain but also the biological behavior of the tumor, and warrant further studies of putative common pathogenetic pathways for course and symptoms of disease.
Genetic variability and focal epilepsy
The genetic background for tumor-associated epilepsy is unknown and no data are available on the genetic variability associated with the presence of epileptic seizures and the outcome in terms of response to antiepileptic drugs in patients with gliomas. More is known though on the genetics of other non tumor-associated focal epilepsies. A few inherited syndromes for focal epilepsies have been described [29–31], and an increasing number of candidate genes have been proposed for focal epilepsies during recent years [32]. Since the focus of this review is on genetic association studies, we performed a literature search on susceptibility genes for all types of focal epilepsies. We searched for studies published in PubMed in the English language during the last ten years, with a final search performed in September 2008. Tables II–IV present a systematic overview of these studies, demonstrating positive as well as negative associations for the different candidate genes [33–48].
Table II.
Case-control studies on polymorphisms of interleukins (IL) and focal epilepsy.
| First Author | Specific polymorphism | Epilepsy type | Nr of patients and controls | Outcome statistics and p-value |
|---|---|---|---|---|
| Kanemoto [33] | IL- 1β- 511 | TLE-HS+ | 50 cases and 112 controls |
χ2=9.55 p<0.017 |
| Kanemoto [34] | IL- 1β- 511 | TLE-HS+ and PFC | 66 cases, 133 controls | TLE-HS+: χ2=9.38 p=0.0022 |
| PFC: χ2=9.90 P=0.0016 |
||||
| Ozkara [35] | IL-1β-511; IL-1β+3953; IL-1α-889 | TLE-HS+ | 47 cases, 99 controls | NS |
| Heils [36] | IL-1β-511 | 86 cases, 133 controls |
χ2=0.436 p=0.804 |
|
| Buono [37] | IL-1β −511 | TLE-HS+ | 61 cases, 119 controls |
χ2=5.22 p=0.09 |
| Jin [38] | IL-1β- 511 | TLE-HS+ and TLE-HS− | 112 cases, 115 controls | NS |
TLE: temporal lobe epilepsy.
TLE-HS+: temporal lobe epilepsy with hippocampal sclerosis.
TLE-HS−: temporal lobe epilepsy without hippocampal sclerosis.
PFC: prolonged febrile convulsions.
Table IV.
Studies on polymorphisms of apolipoprotein E (ApoE) and brain-derived neurotrophic factor (BDNF) and focal epilepsy.
| First Author | Gene, specific polymorphism | Epilepsy type | Type of study | Number of patients and control group | Statistics and P-value |
|---|---|---|---|---|---|
| Blumcke [43] | Apo E | TLE with or without Ammons horn sclerosis (AHS) | Genetic linkage study | 125 patients (65 TLE-AHS+, 53 TLE-AHS−) | NS |
| Gambardella [44] | Apo E −491 A/T |
Non-lesional TLE | Case- control | 63 patients, 220 controls | NS |
| Yeni [45] | Apo E4 | Mesial TLE–HS | Case- control | 47 cases, 62 controls | OR=1.06 CI=0.38–2.95 p>0.05 |
| Briellmann [46] | ApoE4 | Chronic TLE | No controls | 43 patients, 31 HS+ | ApoE4 may have impact on earlier onset of seizures p=0.004 |
| Kanemoto [47] | ProBDNF 240T |
Focal epilepsy | Case- control | 219 cases, 311 controls | X2=8.59 p=0.0034 |
| Lohoff [48] | BDNF C240T Val66Met |
TLE | Case- control | 151 cases, 189 controls | NS |
A number of single studies have reported positive associations between polymorphisms and adult focal epilepsies that have not yet been confirmed by others and therefore have not been included in the tables. One such example is functional polymorphisms of the prodynorphin gene promoter (PDYN) that were found in familial cases of idiopathic generalized epilepsy. Endogenous dynorphin is an opioid with several physiological effects including a role in the regulation of hippocampal excitability, indicating a probable anticonvulsant effect [49]. Patients with temporal lobe epilepsy carrying the low frequency PDYN allele showed a higher risk of developing secondary generalized seizures and status epilepticus [50]. PDYN may therefore be a general risk factor for epilepsy, but further studies are needed to confirm this hypothesis. In another single study, an allele variant of the cellular prion protein gene was identified at codon 171 (Asn171Ser) in a Brazilian cohort of patients with refractory temporal lobe epilepsy [51].
Putative common pathways for glioma and tumor-associated epilepsy
Based on the assumptions that 1) some of the susceptibility genes associated with focal epilepsy may be involved in tumor-associated seizures, and 2) the biological activity of the tumor may to some extent be related to the epileptic symptoms of the patient, we discuss here some putative common pathways for glioma development and tumor-associated epilepsy.
Immune response
Several studies have associated infection and immune function with a decreased risk of glioma [7,20,21]. Immunological factors are likely to play a role also in tumor-associated epilepsy, and proinflammatory cytokines and their receptors have been suggested to be involved in the pathogenesis of epilepsy. Cytokines have modulating effects on neurotoxic neurotransmitters that are discharged during excitation or inflammation in the central nervous system. For many patients with gliomas the epileptic focus is not contiguous with the tumor, and seizure etiology involves peri-tumoral brain regions in which immune mediated neurochemical changes by microglial components are known to occur [22,52,53]. An immune-mediated neuronal damage of the peri-tumoral brain area, coupled to the balance between stimulatory and inhibitory cytokines, may contribute to the development of tumor-related epilepsy [53].
Synaptic transmission by GABA
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter of the central nervous system. GABA acts mainly on two receptor types, type A and type B, which control neurotransmitter release and postsynaptic silencing of excitatory neurotransmission. Although the exact role of GABA in the development of epilepsy is not clear, there is evidence that dysfunction of both pre- and postsynaptic GABA-B receptor mediated processes contributes to temporal lobe epilepsy [54–56]. No association studies so far have coupled polymorphisms of GABAR subunits to an altered glioma risk, but several reports support a possible role of GABA in glioma development. Both astrocytes and microglia in the brain express the peripheral-type benzodiazepine receptor (PBR), which are multiprotein complexes located mainly at the outer mitochondrial membrane with GABAergic properties. PBR are widely expressed on different types of tumor cells [57]. The binding density of PBR is thought to correlate with the proliferative activity of the tumor, and high levels of PBR ligands were correlated with the tumorigenicity of glioma cells in vitro [58]. Consistently, PBR protein expression in astrocytoma samples strongly correlated with the histological malignancy grade of the tumor and with patient survival, with highest levels found in glioblastoma [59]. Interestingly, GABA may also play a direct immunomodulatory role in the brain, shown by the formation of functional extrasynaptic-like GABA channels on pathogenic T lymphocytes entering the brain [60]. Such immunomodulation by GABA may cause neurochemical changes in intra- and peri-tumoral regions, thereby affecting tumor growth as well as tumor-associated seizures.
Synaptic transmission by serotonin
Serotonin is released by presynaptic neurons and its action is terminated by re-uptake via the serotonin transporter protein. Variations in serotonergic activity are linked to both the development of epileptic foci and the severity of seizures [61]. A number of association studies have shown functional polymorphisms of the serotonin transporter promoter region in various psychiatric disorders, but so far only one study confirmed two previously identified polymorphisms of the serotonin transporter gene in patients with focal epilepsy [42]. The role of serotonin in gliomas is unclear, and no polymorphisms of serotonin-related genes have been associated with the disease. However, glioblastoma cells express the serotonin receptor 5-HT7, and stimulation of this receptor couples to multiple second messenger systems that amongst others can induce the expression of neurotrophic factors [62]. Upregulation of glial cell line-derived neurotrophic factor (GDNF) through serotonin receptors is one of the gene-gene interactions that has been demonstrated in patients with depression treated by antidepressants, but may occur in a wider variety of brain disorders including gliomas [63].
Synaptic transmission by glutamate
Although not confirmed by any association studies, a role for glutamate in both tumor-associated seizures and in glioma is widely accepted [64]. Glioma cells release glutamate, which causes excitotoxic death of surrounding neurons as one of the mechanisms for their destructive and invasive growth in the brain [65]. The release of glutamate occurs primarily via a Na+-independent cystine-glutamate exchanger, and may also contribute to seizures that start in the peri-tumoral regions. Animal studies in which human gliomas were xenographed into mice showed that chronic inhibition of glutamate release leads to smaller and less invasive tumors compared to controls [66]. Thus, future studies of genetic variability of genes involved in glutamate release, including GluR1, the most abundant AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) receptor subunit in gliomas, and the Na+-independent cystine-glutamate exchanger, are of great interest in patients with glioma-associated seizures.
Neurotrophic factors
Brain-derived neurotrophic factor (BDNF) regulates neuronal morphology and synaptogenesis and is known to exhibit neuroprotective effects in diverse areas of the central nervous system during development [67]. BDNF promotes neuronal survival and differentiation, and modulates synaptic transmission by increasing NMDA (N-methyl-D-aspartic) receptor activity [68]. Expression of BDNF was shown in the neuronal component of gangliogliomas and co-localized with NMDA receptors in these tumors [68]. Thus, BDNF and other neurotrophic factors in the brain may be involved in the growth regulation and epileptogenesis of tumors of glioneuronal origin.
Cell cycle control and DNA repair
Polymorphisms in a number of cell cycle control and DNA repair genes have been associated with glioma risk [19]. It is not known whether these genes may have a role also in the development of focal epilepsy. Interestingly, a recent gene expression profile analysis of epilepsy-associated gangliogliomas revealed altered expression levels of genes involved in the immune system and synaptic transmission, as well as in cell cycle control [69]. Increased expression levels of cyclin D1 and cyclin-dependent kinases (CDK) were found compared to normal control tissues, suggesting a role for these genes in the pathogenesis and possibly also the epileptogenesis of these lesions.
Apolipoprotein E
The apolipoprotein E (ApoE) ε4 allele is by far the most important genetic determinant of susceptibility to Alzheimer disease. ApoE promotes the deposition of β-amyloid (Aβ) in the brain parenchyma [70]. Mackenzie and Miller showed the occurrence of senile plaques in temporal lobe epilepsy [71], an observation which was later confirmed by the finding of increased levels of Ab precursor protein in surgically resected human temporal lobe tissue [72]. In accordance, ApoE was shown a susceptibility gene for temporal lobe epilepsy by several studies, although negative findings have also been reported [43–46]. A role for apoE in glioma through delivery of lipids to tumor cells has been proposed [73]. Tau-associated neurodegenerative changes were found in gangliogliomas in an age-dependent quantity, but the distribution of ApoE genotypes was similar among those with tumors that contained tauassociated neuropathology and those that did not [74].
Conclusions
Studies of genetic variants as a causal factor to focal epilepsy have brought forward an increasing number of candidate genes. In this review, we describe several examples of association studies on this issue and hypothesize that some of the identified candidate genes, such as genes involved in the immune response, cell cycle control and synaptic transmission, may be of importance also for tumor-associated epilepsy. We also bring forward some highly interesting candidates, such as genes involved in the glutamate system, for which no association studies have been reported yet.
Unfortunately many studies have used inadequate sample size with limited statistical power to detect the low odds ratios that low-penetrance alleles are likely to confer. In addition, we found many examples of follow-up studies that have not been able to confirm initial positive association studies. This could be due to several reasons such as lack of statistical power, non-associations, chance findings, differences in study design and studies of different populations.
Large multi centre studies including well characterized cases of low- and high-grade gliomas with and without symptomatic seizures are required to identify the underlying genetic variability and to increase our insight into the pathogenesis of tumor-related epilepsy and the possible overlapping pathways with glioma development. This is best studied by a first agnostic genome wide approach to identify candidate genes, subsequently needing independent confirmation in separate data sets. Such an approach may provide an important tool for the clinical management of patients with glioma suffering from epileptic seizures in the future.
Table III.
Case-control studies on polymorphisms of GABA receptors and serotonin transporters and focal epilepsy.
| First Author | Gene, specific poly-morphisms | Epilepsy type | Number of patients and controls | P-value, odds ratio and confidence interval |
|---|---|---|---|---|
| Gambardella [39] | GABA (B) receptor G1465A | Non-lesional TLE | 141 cases and 372 controls | OR=6.47; CI=2.02–20.75 p=0.003 |
| Ma [40] | G1465A | TLE preceded by febrile seizures (FS) | 120 cases, 218 controls | NS |
| Ma [41] | GABRA1, GABRA5, GABRG2, GABRD | Familial focal epilepsy preceded by FS | 74 cases, 118 controls | NS |
| Manna [42] | Serotonin transporter (5-HTT) | TLE | 276 cases, 309 controls | 5-HTTLPR: p=0.0086; |
| 5-HTTLPR 5-HTTVNTR | 5-HTTVNTR: NS |
Acknowledgements
The authors confirm that they have read the Journals position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This work has been supported by funds from the Uppsala University Hospital (AS), the Lions Cancer Foundation at the Uppsala University Hospital (AS) and the King Gustav V Jubilee Fund, Karolinska Institutet (MQ, AS).
Footnotes
Declaration of interest: No financial or other competing interests exist that could be perceived as biasing this study for any of the authors.
References
- [1].Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005;109:93–108. [DOI] [PubMed] [Google Scholar]
- [3].Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro Oncol 2002;4:278–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [5].Malmer B, Adatto P, Armstrong G, Barnholtz-Sloan J, Bernstein JL, Claus E, et al. GLIOGENE an International Consortium to Understand Familial Glioma. Cancer Epidemiol Biomarkers Prev 2007;16:1730–4. [DOI] [PubMed] [Google Scholar]
- [6].Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer 2008;113:1953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brenner AV, Butler MA, Wang SS, Ruder AM, Rothman N, Schulte PA, et al. Single-nucleotide polymorphisms in selected cytokine genes and risk of adult glioma. Carcinogenesis 2007;28:2543–7. [DOI] [PubMed] [Google Scholar]
- [8].Rajaraman P, Wang SS, Rothman N, Brown MM, Black PM, Fine HA, et al. Polymorphisms in apoptosis and cell cycle control genes and risk of brain tumors in adults. Cancer Epidemiol Biomarkers Prev 2007;16:1655–61. [DOI] [PubMed] [Google Scholar]
- [9].Liu Y, Scheurer ME, El-Zein R, Cao Y, Do KA, Gilbert M, et al. Association and interactions between DNA repair gene polymorphisms and adult glioma. Cancer Epidemiol Bio-markers Prev 2009;18:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carpentier C, Lejeune J, Gros F, Everhard S, Marie Y, Kaloshi G, et al. Association of telomerase gene hTERT polymorphism and malignant gliomas. J Neurooncol 2007; 84:249–53. [DOI] [PubMed] [Google Scholar]
- [11].Costa BM, Ferreira P, Costa S, Canedo P, Oliveira P, Silva A, et al. Association between functional EGF+61 polymorphism and glioma risk. Clin Cancer Res 2007;13: 2621–6. [DOI] [PubMed] [Google Scholar]
- [12].Lu Z, Cao Y, Wang Y, Zhang Q, Zhang X, Wang S, et al. Polymorphisms in the matrix metalloproteinase-1, 3, and 9 promoters and susceptibility to adult astrocytoma in northern China. J Neurooncol 2007;85:65–73. [DOI] [PubMed] [Google Scholar]
- [13].Parhar P, Ezer R, Shao Y, Allen JC, Miller DC, Newcomb EW. Possible association of p53 codon 72 polymorphism with susceptibility to adult and pediatric high-grade astrocytomas. Mol Brain Res 2005. 13;137:98–103. [DOI] [PubMed] [Google Scholar]
- [14].Bhowmick DA, Zhuang Z, Wait SD, Weil RJ. A functional polymorphism in the EGF gene is found with increased frequency in glioblastoma multiforme patients and is associated with more aggressive disease. Cancer Res 2004;64: 1220–3. [DOI] [PubMed] [Google Scholar]
- [15].Wrensch M, Kelsey KT, Liu M, Miike R, Moghadassi M, Aldape K, et al. Glutathione-S-transferase variants and adult glioma. Cancer Epidemiol Biomarkers Prev 2004;13:461–7. [PubMed] [Google Scholar]
- [16].Wang LE, Bondy ML, Shen H, El-Zein R, Aldape K, Cao Y, et al. Polymorphisms of DNA repair genes and risk of glioma. Cancer Res 2004;64:5560–3. [DOI] [PubMed] [Google Scholar]
- [17].Frigerio S, Ciusani E, Pozzi A, Silvani A, Salmaggi A, Boiardi A. Tumor necrosis factor microsatellite polymorphisms in Italian glioblastoma patients. Cancer Genet Cytogenet 1999;109:172–4. [DOI] [PubMed] [Google Scholar]
- [18].Bethke L, Webb E, Murray A, Schoemaker M, Johansen C, Christensen HC, et al. Comprehensive analysis of the role of DNA repair gene polymorphisms on risk of glioma. Hum Mol Genet 2008;17:800–5. [DOI] [PubMed] [Google Scholar]
- [19].McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, Ruder AM, Butler M, Rajaraman P, et al. Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev 2009;18:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wiemels JL, Wiencke JK, Kelsey KT, Moghadassi M, Rice T, Urayama KY, et al. Allergy-related polymorphisms influence glioma status and serum IgE levels. Cancer Epidemiol Biomarkers Prev 2007;16:1229–35. [DOI] [PubMed] [Google Scholar]
- [21].Scheurer ME, Amirian E, Cao Y, Gilbert MR, Aldape KD, Kornguth DG, et al. Polymorphisms in the interleukin-4 receptor gene are associated with better survival in patients with glioblastoma. Clin Cancer Res 2008;14:6640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochir (Wien) 2000;142:1–15. [DOI] [PubMed] [Google Scholar]
- [23].Schaller B, Ruegg SJ. Brain tumor and seizures: Pathophysiology and its implications for treatment revisited. Epilepsia 2003;44:1223–32. [DOI] [PubMed] [Google Scholar]
- [24].Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg 2008;108:227–35. [DOI] [PubMed] [Google Scholar]
- [25].Rosati A, Tomassini A, Pollo B, Ambrosi C, Schwarz A, Padovani A, et al. Epilepsy in cerebral glioma: Timing of appearance and histological correlations. J Neurooncol 2009. January 29 [Epub ahead of print] [DOI] [PubMed]
- [26].van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol 2007;6:421–30. [DOI] [PubMed] [Google Scholar]
- [27].Piepmeier J, Christopher S, Spencer D, Byrne T, Kim J, Knisel JP, et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery 1996;38:872–8. [DOI] [PubMed] [Google Scholar]
- [28].Danfors T, Ribom R, Ghaderi Berntsson S, Smits A. Epileptic seizures and survival during early disease in grade 2 gliomas. Eur J Neurol 2009. (in press). [DOI] [PubMed]
- [29].Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet 2002;30:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hayman M, Scheffer IE, Chinvarun Y, Berlangieri SU, Berkovic SF. Autosomal dominant nocturnal frontal lobe epilepsy: Demonstration of focal frontal onset and intrafamilial variation. Neurology 1997;49:969–75. [DOI] [PubMed] [Google Scholar]
- [31].Phillips HA, Scheffer IE, Berkovic SF, Hollway GE, Sutherland GR, Mulley JC. Localization of a gene for autosomal dominant nocturnal frontal lobe epilepsy to chromosome 20q 13.2. Nat Genet 1995;10:117–8. [DOI] [PubMed] [Google Scholar]
- [32].Cavalleri GL, Lynch JM, Depondt C, Burley MW, Wood NW, Sisodiya SM, et al. Failure to replicate previously reported genetic associations with sporadic temporal lobe epilepsy: Where to from here? Brain 2005;128:1832–40. [DOI] [PubMed] [Google Scholar]
- [33].Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol 2000;47:571–4. [PubMed] [Google Scholar]
- [34].Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, et al. Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 2003; 44:796–9. [DOI] [PubMed] [Google Scholar]
- [35].Ozkara C, Uzan M, Tanriverdi T, Baykara O, Ekinci B, Yeni N, et al. Lack of association between IL-1beta/alpha gene polymorphisms and temporal lobe epilepsy with hippocampal sclerosis. Seizure 2006;15:288–91. [DOI] [PubMed] [Google Scholar]
- [36].Heils A, Haug K, Kunz WS, Fernandez G, Horvath S, Rebstock J, et al. Interleukin-1beta gene polymorphism and susceptibility to temporal lobe epilepsy with hippocampal sclerosis. Ann Neurol 2000;48:948–50. [PubMed] [Google Scholar]
- [37].Buono RJ, Ferraro TN, O’Connor MJ, Sperling MR, Ryan SG, Scattergood T, et al. Lack of association between an interleukin 1 beta (IL-1beta) gene variation and refractory temporal lobe epilepsy. Epilepsia 2001;42:782–4. [DOI] [PubMed] [Google Scholar]
- [38].Jin L, Jia Y, Zhang B, Xu Q, Fan Y, Wu L, Shen Y. Association analysis of a polymorphism of interleukin 1 beta (IL-1 beta) gene with temporal lobe epilepsy in a Chinese population. Epilepsia 2003;44:1306–9. [DOI] [PubMed] [Google Scholar]
- [39].Gambardella A, Manna I, Labate A, Chifari R, La Russa A, Serra P, et al. GABA(B) receptor 1 polymorphism (G1465A) is associated with temporal lobe epilepsy. Neurology 2003;60:560–3. [DOI] [PubMed] [Google Scholar]
- [40].Ma S, Abou-Khalil B, Sutcliffe JS, Haines JL, Hedera P. The GABBR1 locus and the G1465A variant is not associated with temporal lobe epilepsy preceded by febrile seizures. BMC Med Genet 2005;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ma S, Abou-Khalil B, Blair MA, Sutcliffe JS, Haines JL, Hedera P. Mutations in GABRA1, GABRA5, GABRG2 and GABRD receptor genes are not a major factor in the pathogenesis of familial focal epilepsy preceded by febrile seizures. Neurosci Lett 2006;394:74–8. [DOI] [PubMed] [Google Scholar]
- [42].Manna I, Labate A, Gambardella A, Forabosco P, La Russa A, Le Piane E, et al. Serotonin transporter gene (5-Htt): Association analysis with temporal lobe epilepsy. Neurosci Lett 2007;421:52–6. [DOI] [PubMed] [Google Scholar]
- [43].Blumcke I, Brockhaus A, Scheiwe C, Rollbrocker B, Wolf HK, Elger CE, et al. The apolipoprotein E epsilon 4 allele is not associated with early onset temporal lobe epilepsy. Neuroreport 1997;8:1235–7. [DOI] [PubMed] [Google Scholar]
- [44].Gambardella A, Aguglia U, Cittadella R, Romeo N, Sibilia G, LePiane E, et al. Apolipoprotein E polymorphisms and the risk of nonlesional temporal lobe epilepsy. Epilepsia 1999;40:1804–7. [DOI] [PubMed] [Google Scholar]
- [45].Yeni SN, Ozkara C, Buyru N, Baykara O, Hanoglu L, Karaagac N, et al. Association between APOE polymorphisms and mesial temporal lobe epilepsy with hippocampal sclerosis. Eur J Neurol 2005;12:103–7. [DOI] [PubMed] [Google Scholar]
- [46].Briellmann RS, Torn-Broers Y, Busuttil BE, Major BJ, Kalnins RM, Olsen M, et al. APOE epsilon4 genotype is associated with an earlier onset of chronic temporal lobe epilepsy. Neurology 2000;55:435–7. [DOI] [PubMed] [Google Scholar]
- [47].Kanemoto K, Kawasaki J, Tarao Y, Kumaki T, Oshima T, Kaji R, et al. Association of partial epilepsy with brain-derived neurotrophic factor (BDNF) gene polymorphisms. Epilepsy Res 2003;53:255–8. [DOI] [PubMed] [Google Scholar]
- [48].Lohoff FW, Ferraro TN, Dahl JP, Hildebrandt MA, Scattergood TM, O’Connor MJ, et al. Lack of association between variations in the brain-derived neurotrophic factor (BDNF) gene and temporal lobe epilepsy. Epilepsy Res 2005;66:59–62. [DOI] [PubMed] [Google Scholar]
- [49].Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: Anticonvulsant net effect via kappa opioid receptors. Brain 2007;130:1017–28. [DOI] [PubMed] [Google Scholar]
- [50].Stogmann E, Zimprich A, Baumgartner C, Aull-Watschinger S, Hollt V, Zimprich F. A functional polymorphism in the prodynorphin gene promotor is associated with temporal lobe epilepsy. Ann Neurol 2002;51:260–3. [DOI] [PubMed] [Google Scholar]
- [51].Walz R, Castro RM, Velasco TR, Alexandre V Jr., Lopes MH, Leite JP, et al. Surgical outcome in mesial temporal sclerosis correlates with prion protein gene variant. Neurology 2003;61:1204–10. [DOI] [PubMed] [Google Scholar]
- [52].Whittle IR, Sellar R, Ironside JW. Epileptogenic anaplastic astrocytoma imaged only by T2-weighted magnetic resonance studies: Clinical and surgical implications. Br J Neurosurg 1992;6:537–42. [DOI] [PubMed] [Google Scholar]
- [53].Chao CC, Molitor TW, Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol 1993;151:1473–81. [PubMed] [Google Scholar]
- [54].Scanziani M, Debanne D, Muller M, Gahwiler BH, Thompson SM. Role of excitatory amino acid and GABAB receptors in the generation of epileptiform activity in disinhibited hippocampal slice cultures. Neuroscience 1994; 61:823–32. [DOI] [PubMed] [Google Scholar]
- [55].Mangan PS, Lothman EW. Profound disturbances of pre- and postsynaptic GABAB-receptor-mediated processes in region CA1 in a chronic model of temporal lobe epilepsy. J Neurophysiol 1996;76:1282–96. [DOI] [PubMed] [Google Scholar]
- [56].Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 2001;31:47–58. [DOI] [PubMed] [Google Scholar]
- [57].Han Z, Slack RS, Li W, Papadopoulos V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: Relationship to breast, colorectal, and prostate tumor progression. J Recept Signal Transduct Res 2003;23:225–38. [DOI] [PubMed] [Google Scholar]
- [58].Veenman L, Levin E, Weisinger G, Leschiner S, Spanier I, Snyder SH, et al. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem Pharmacol 2004;68:689–98. [DOI] [PubMed] [Google Scholar]
- [59].Vlodavsky E, Soustiel JF. Immunohistochemical expression of peripheral benzodiazepine receptors in human astrocytomas and its correlation with grade of malignancy, proliferation, apoptosis and survival. J Neurooncol 2007;81:1–7. [DOI] [PubMed] [Google Scholar]
- [60].Bjurstö m H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol 2008;205:44–50. [DOI] [PubMed] [Google Scholar]
- [61].Jobe PC, Dailey JW, Wernicke JF. A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol 1999;13:317–56. [DOI] [PubMed] [Google Scholar]
- [62].Noda M, Higashida H, Aoki S, Wada K. Multiple signal transduction pathways mediated by 5-HT receptors. Mol Neurobiol 2004;29:31–9. [DOI] [PubMed] [Google Scholar]
- [63].Hisaoka K, Nishida A, Takebayashi M, Koda T, Yamawaki S, Nakata Y. Serotonin increases glial cell line-derived neurotrophic factor release in rat C6 glioblastoma cells. Brain Res 2004;1002:167–70. [DOI] [PubMed] [Google Scholar]
- [64].Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia 2006;54:358–68. [DOI] [PubMed] [Google Scholar]
- [65].Sontheimer H A role for glutamate in growth and invasion of primary brain tumors. J Neurochem 2008;105:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 2007;67:9463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Barde YA. Trophic factors and neuronal survival. Neuron 1989;2:1525–34. [DOI] [PubMed] [Google Scholar]
- [68].Aronica E, Leenstra S, Jansen GH, van Veelen CW, Yankaya B, Troost D. Expression of brain-derived neurotrophic factor and tyrosine kinase B receptor proteins in glioneuronal tumors from patients with intractable epilepsy: Colocalization with N-methyl-D-aspartic acid receptor. Acta Neuropathol 2001;101:383–92. [DOI] [PubMed] [Google Scholar]
- [69].Aronica E, Boer K, Becker A, Redeker S, Spliet WG, van Rijen PC, et al. Gene expression profile analysis of epilepsy-associated gangliogliomas. Neuroscience 2008;151:272–92. [DOI] [PubMed] [Google Scholar]
- [70].Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993;90:9649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol 1994;87:504–10. [DOI] [PubMed] [Google Scholar]
- [72].Sheng JG, Boop FA, Mrak RE, Griffin WS. Increased neuronal beta-amyloid precursor protein expression in human temporal lobe epilepsy: Association with interleukin-1 alpha immunoreactivity. J Neurochem 1994;63:1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nicoll JA, Zunarelli E, Rampling R, Murray LS, Papanastassiou V, Stewart J. Involvement of apolipoprotein E in glioblastoma: Immunohistochemistry and clinical outcome. Neuroreport 2003;14:1923–6. [DOI] [PubMed] [Google Scholar]
- [74].Brat DJ, Gearing M, Goldthwaite PT, Wainer BH, Burger PC. Tau-associated neuropathology in ganglion cell tumours increases with patient age but appears unrelated to ApoE genotype. Neuropathol Appl Neurobiol 2001;27:197–205. [DOI] [PubMed] [Google Scholar]


