Abstract
Purpose
Given the increasing number and diversity of cancer survivors in the USA and persistent racial/ethnic disparities in breast cancer care, we sought to examine the role of acculturation in adherence to recommended surgical treatment and survivorship care recommendations.
Methods
Study participants included 343 Mexican American women with stage I to III breast cancer who participated in the Ella Binational Breast Cancer Study and were treated at The University of Texas MD Anderson Cancer Center in Houston, Texas, between March 2007 and June 2011. Participants completed a questionnaire measuring acculturation, and clinical and demographic variables were obtained from an institutional database. Multivariable logistic regression models were constructed to examine differences in surgical procedures received and adherence to long-term survivorship care by acculturation level.
Results
Bilingual (odds ratio [OR] = 1.85; 95% confidence interval [CI] = 0.85–4.02, P = .11) and English-dominant women (OR = 2.39; 95% CI = 1.02–5.61, P = .04) were more likely to receive breast-conserving surgery (versus mastectomy) than were Spanish-dominant women. Among all patients, adherence to surveillance mammography and clinic visits decreased over time; the decline in clinic visit adherence was statistically significant (P = .005). Although no statistically significant association was found between acculturation and adherence to long-term survivorship care, receipt of breast-conserving surgery (versus mastectomy) was significantly associated with higher adherence to surveillance mammograms.
Conclusion
Acculturation may play a role in decision-making about surgical management of breast cancer, and further studies with larger samples are needed to explore its role in adherence to survivorship care recommendations. Findings from this study may help identify patients requiring additional support while making decisions pertaining to their cancer treatment and survivorship care.
Keywords: Breast cancer, Treatment, Acculturation, Survivorship, Mexican American
Introduction
Hispanics are the second largest racial/ethnic group and the largest racial/ethnic minority group in the USA, accounting for 17.6% (56.6 million of 318 million) of the total US population in 2016 [1, 2]. Cancer is the leading cause of death among Hispanics, and breast cancer is the most commonly diagnosed cancer and leading cause of cancer death among Hispanic women [1, 3]. Given their rapidly increasing numbers, substantial differences in cancer incidence and mortality when compared to non-Hispanic whites (NHWs), and vulnerability to cancer inequalities, it is imperative to study patterns of cancer care and associated behaviors in the US Hispanic population [1, 3, 4].
Hispanics are diverse in terms of country of origin, immigrant status, degree of acculturation, ancestral history, and demographic characteristics [1]. However, studies often report cancer data in aggregate, thereby masking significant differences among various Hispanic subpopulations [1]. The few studies that examine sub-population level disparities reported sizable differences in cancer incidence and death rates among Hispanic subpopulations. Therefore, it is important to study factors potentially contributing towards such diversity in cancer care within Hispanic subpopulations [5]. Although Mexican Americans have lower breast cancer incidence rates relative to non-Hispanic Whites and other Hispanic subpopulations (such as Puerto Ricans or Cubans) [6], they have the highest proportion of deaths from breast cancer relative to all other Hispanic subpopulations [5] and they form the largest subgroup (64.3%) among US Hispanics [1].
Disparities in receipt of cancer treatment and adherence to surveillance care contribute to differences in the health outcomes of breast cancer survivors. The type of treatment received for primary breast cancer is an important determinant of a patient’s long-term health status [7]. Similarly, breast cancer survivorship care, which includes continued disease surveillance with mammograms and clinic visits, offers an opportunity to reduce complications and detect metastases or recurrences at an early stage [8, 9]. Established disparities in these cancer care behaviors among Hispanics often stem from their disproportionately lower educational and socioeconomic levels, younger age at diagnosis, immigrant status, and higher barriers to health care [1]. Understanding the causes of these disparities provides opportunities to improve our understanding of cancer behaviors among this population.
Additionally, given that immigration may exacerbate racial/ethnic disparities in adherence to breast cancer care, it is important to study these behaviors in immigrant populations using a framework that goes beyond the traditional racial/ethnic categories, especially among immigrant populations [8, 10]. “Culturally appropriate care” is treatment that is consistent with the patient’s basic values and cultural structure [11]. As immigrants adapt to new sociocultural environments, they often tend to modify their behavior and attitudes to conform to those of the host culture, a process known as acculturation [12]. Interest in studying the impact of acculturation on immigrant health behaviors has increased as the Hispanic population in the USA has grown [13, 14]. Examining whether and how acculturation affects the type of cancer treatment patients receive and how they adhere to breast cancer surveillance care will contribute to our understanding of the sources of disparities in cancer outcomes.
Breast-conserving surgery (BCS) followed by adjuvant radiation or mastectomy is a recommended surgical procedure for patients with a confirmed diagnosis of early-stage breast cancer [15]. Randomized trials have reported no significant difference in overall and relapse-free survival rates in patients receiving BCS plus radiation and those receiving mastectomy [16–18]. Although use of BCS is accompanied by higher treatment satisfaction, better body image, and improved quality of life [19, 20], studies have shown a constant rise in use of mastectomy since the early 2000 among patients eligible to receive a BCS and among populations including the Hispanic community [21]. Studies investigating the decision-making process related to breast surgery have attributed this change to patient misperceptions of subsequent health outcomes from adjuvant radiation after BCS [16, 22–27], and perceived concerns around survival and recurrence in the intact breast [16, 22, 24–27]. Among minority populations, breast cancer treatment decisions may also be influenced by the patient’s belief that they lack the knowledge and expertise to decide for themselves and being in agreement with their physician’s recommendation may guarantee receipt of good treatment [24, 25]. The role that acculturation plays in choice of breast surgery among Mexican American women has not been well studied. Additionally, despite the reported value of surveillance mammograms and follow-up clinic visits [28], previous studies reported inadequate adherence to these survivorship care recommendations among minority populations, including Hispanic breast cancer survivors [8].
The purpose of this study was to (a) examine whether the level of acculturation was associated with receipt of BCS (compared to mastectomy) as definitive local treatment for breast cancer among Mexican American women in Texas and (b) examine the role of acculturation and other demographic factors in patients’ adherence to breast cancer survivorship care.
Methods
Data source and participants
The study used data from the Ella Binational Breast Cancer Study (Ella Study). A description of the study design and recruitment strategy has been previously published [3]. Briefly, the Ella Study is a case series of women of self-reported Mexican descent who were 18 years of age or older and had been diagnosed with invasive breast cancer 24 months prior to enrollment. The study’s aim was to compare risk factor patterns, disease phenotypes, and clinical characteristics among Mexican Americans in the USA and those living in Mexico [3]. Participants were recruited from three study sites in Mexico and two in the USA, one of which was The University of Texas MD Anderson Cancer Center in Houston, Texas [29]. Recruitment at all sites took place from March 2007 through June 2011 and used a predominantly clinic-based recruitment strategy and the same procedures, recruiters, and data collection instruments [30]. For this analysis, we included Mexican American women enrolled at MD Anderson who had a diagnosis of unilateral stages I–III breast cancer (as defined by the American Joint Committee on Cancer Staging Manual, 6th edition) [31]. The institutional review board at MD Anderson approved the study, and all participants provided written informed consent [29].
Measuring covariates
Trained bilingual interviewers administered the Ella Risk Factor Questionnaire (RFQ) in English or Spanish, depending upon the participant’s preference [3, 30]. The RFQ was used to collect information on sociodemographic characteristics such as age at diagnosis, education, insurance status, receipt of screening mammograms prior to diagnosis, and other breast cancer risk factors; it also included questions on acculturation. Information about clinical variables, e.g., cancer stage, type and date of breast surgery (BCS or mastectomy), and clinical outcomes (recurrence, death) were obtained from the Breast Cancer Management System database at MD Anderson, which captures clinical data from all breast cancer patients seen at the institution.
Measuring acculturation
The measure of acculturation used in the Ella Study was Marin and Gamba’s [30, 32] Bidimensional Acculturation Scale (BAS), which has been validated in Hispanic populations [33–35]. To measure the level of acculturation, the Ella RFQ included two orthogonal, four-item measures of cultural orientation. One scale assessed the degree of English language use and exposure, and a second scale assessed the degree of Spanish language use and exposure [30]. Each of the eight items was scored on a five-point scale ranging from 1 (never) to 5 (always). Both English (Cronbach’s α = 0.94) and Spanish (Cronbach’s α = 0.94) acculturation scales had high internal consistency and reliability, and the scales were moderately inversely correlated (ρ = − 0.33, P < .001) [30]. Participants who completed both scales were placed into one of three acculturation groups using the recommended mean cutoff score of 2.99 for both scales: (1) bilingual (mean score ≥ 3.0 on both the English and Spanish scales), (2) Spanish-dominant (mean score ≥ 3.0 on the Spanish scale only), and (3) English-dominant (mean score ≥ 3.0 on the English scale only) [30]. The Spanish-dominant group was considered the least acculturated and served as the reference group in our analysis. This method has been previously employed to compare levels of acculturation among Hispanics of varying levels of acculturation [29, 30].
Metrics for adherence to survivorship care guidelines
We used the MD Anderson Enterprise Institutional Warehouse for Current Procedural Terminology professional and technical charge codes to determine the frequency of surveillance mammograms and clinic visits after the completion of active treatment for each study participant. We obtained data for at least 4 initial years of survivorship care. The start of follow-up in survivorship care was defined as the time of the first clinic visit or mammogram that occurred between 7 and 20 months after the start of first treatment for breast cancer (surgery or systemic therapy). Patients were excluded if they did not have a mammogram or clinic visit during this period or experienced a new breast cancer event (new primary breast cancer, locoregional reoccurrence, metastasis, or death) [8]. These criteria were applied to ensure that patients included in the analysis had completed their initial primary treatment for breast cancer and that the goal of mammography and clinic visits was breast cancer survivorship care. Once a patient was entered into the analysis, she was observed for mammogram use and clinic visits until the end of surveillance (i.e., 4 years from the first mammogram performed between 7 and 20 months after the start of treatment) or until she experienced a new breast event. Patients included in the analysis of the association between acculturation and receipt of surveillance mammograms could not have undergone bilateral mastectomies before the end of the surveillance year. We considered clinic visits to the Breast Medical Oncology, Breast Surgery, and Radiation Oncology departments and the Cancer Prevention Center as survivorship care visits. Patients were considered adherent to mammogram guidelines if they had at least one mammogram per year during the survivorship follow-up period. Full adherence to clinic visit guidelines was conservatively defined as a mean of two or more clinic visits per year during the survivorship follow-up period.
Statistical analyses
Descriptive statistics included frequencies and percentages for all categorical variables. Chi-square tests were used to examine associations between acculturation levels and other patient characteristics. Multivariable logistic regression models were constructed to examine the association between acculturation and three separate outcomes: (1) receipt of BCS versus mastectomy; (2) adherence to surveillance mammography; and (3) adherence to surveillance clinic visits. Variables with P values < .05 for Wald statistics of maximum likelihood estimates in the univariable analysis were retained in the final multivariable model for that outcome. We also examined potential interactions between education, area of residence, and insurance status with acculturation in all analyses [36]; any interaction term with P < .05 was retained in the final model for that analysis. All P values were reported at a two-sided significance level of .05. Data were processed and analyzed with Stata software version 13.1 (StataCorp, College Station, Texas).
Results
Patient characteristics
Of the 384 Ella Study participants recruited at MD Anderson, 343 were included in the initial analysis (Fig. 1). At the time of enrollment, most participants were ≤ 65 years of age and had an education level of high school or less (55%). Fifty-two percent resided within the Houston metropolitan area and had private health insurance (61%). Most participants (75%) had stage I–II breast cancer at diagnosis. Forty percent received BCS and 60% received mastectomy. Bilingual and English-dominant women were more likely to have greater than high school education (P < .001) and private health insurance (P < .001) than were Spanish-dominant women (Table 1). Spanish- and English-dominant women were more likely to reside within the Houston metropolitan area (P < .001) than were bilingual women.
Fig. 1.
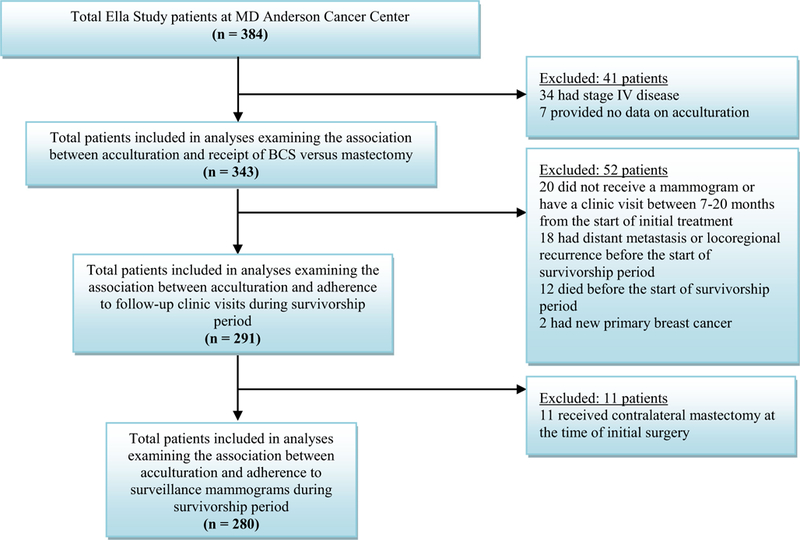
Patient eligibility and exclusion criteria for each analysis
Table 1.
Characteristics of Mexican American Ella Study participants by acculturation subgroups (N = 343)
| Characteristic | Spanish-dominant MA (n = 71) N (%) | Bilingual MA (n = 187) N (%) | English-dominant MA (n = 85) N (%) | Pa |
|---|---|---|---|---|
| Age, years (n = 343) | ||||
| ≤ 50 | 34 (47.9) | 105 (56.1) | 55 (64.7) | 0.27 |
| 51–65 | 28 (39.4) | 67 (35.8) | 23 (27.1) | |
| > 65 | 9 (12.7) | 15 (8.0) | 7 (8.2) | |
| Education (n = 343) | ||||
| < high school | 52 (73.2) | 27 (14.4) | 11 (12.9) | < 0.001b |
| = high school | 16 (22.5) | 55 (29.4) | 29 (34.1) | |
| > high school | 3 (4.2) | 105 (56.2) | 45 (52.9) | |
| Health insurance (n = 322) | ||||
| Public (Medicaid/Medicare) | 49 (74.2) | 53 (30.3) | 25 (30.9) | < 0.001 |
| Private | 17 (25.8) | 122 (69.7) | 56 (69.1) | |
| Area of residence (n = 330) | ||||
| Within Houston metropolitan area | 46 (68.7) | 77 (42.5) | 48 (58.5) | < 0.001 |
| Outside Houston metropolitan area | 21 (31.3) | 104 (57.5) | 34 (41.5) | |
| Cancer stagec (n = 343) | ||||
| I | 18 (25.4) | 44 (23.5) | 18 (21.2) | 0.93 |
| II | 38 (53.5) | 95 (50.8) | 46 (54.1) | |
| III | 15 (21.1) | 48 (25.7) | 21 (24.7) | |
| Adherence to screening mammogram prior to diagnosis (n = 343) | ||||
| Non-adherent | 25 (35.2) | 50 (26.7) | 18 (21.2) | 0.14 |
| Adherent | 46 (64.8) | 137 (73.3) | 67 (78.8) | |
| Surgery (n = 337) | ||||
| Mastectomy | 43 (60.6) | 110 (60.1) | 48 (57.8) | 0.93 |
| BCS | 28 (39.4) | 73 (39.9) | 35 (42.2) | |
| Adherence to surveillance mammogram (n = 280) | ||||
| Non-adherent | 19 (30.2) | 49 (31.6) | 22 (35.5) | 0.79 |
| Adherent | 44 (69.8) | 106 (68.4) | 40 (64.5) | |
| Adherence to clinic visit guidelines (n = 291) | ||||
| Non-adherent | 18 (28.1) | 50 (30.9) | 17 (26.2) | 0.76 |
| Adherent | 46 (71.9) | 112 (69.1) | 48 (73.9) | |
Italicized P values were statistically significant at P<0.05
MA Mexican American, BCS breast-conserving surgery
Chi-square test for differences between the patients in each acculturation group
F-test was used for this analysis because the sample size was < 5 for one subgroup
As defined by the American Joint Committee on Cancer Staging Manual, 6th edition
Acculturation and receipt of BCS versus mastectomy
A total of 343 participants were included in this analysis. In the univariable analysis, patient characteristics that were significantly associated with receipt of BCS (compared to mastectomy) included age (P = .003), education (P = .005), and stage at diagnosis (P < .001). Although insurance status and area of residence were not significant in the univariate analyses, we retained these variables in the final model because they can be associated with the type of surgery patients receive [37, 38]. In the adjusted multivariable analysis, bilingual women (odds ratio [OR] = 1.85; 95% confidence interval [CI] = 0.85–4.02; P = .11) and English-dominant women (OR = 2.39; 95% CI = 1.02–5.61; P = .04) had higher odds of receiving BCS than did Spanish-dominant women (Table 2). Additionally, women between 51 and 65 years of age (P = .01) were significantly more likely to receive BCS than those ≤ 50 years. Women with education beyond high school (P = .01) and more advanced disease stage at diagnosis (stages II and III; P = .001 and P < .001, respectively) were less likely to receive BCS.
Table 2.
Multivariable analysis for receipt of breast-conserving surgery vs. mastectomy by level of acculturation and other factors among Mexican American women (N = 343)
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Acculturation | ||
| Spanish-dominant | Ref | – |
| Bilingual | 1.85 (0.85–4.02) | 0.11 |
| English-dominant | 2.39 (1.02–5.61) | 0.04 |
| Age (years) | ||
| ≤ 50 | Ref | – |
| 51–65 | 2.04 (1.18–3.53) | 0.01 |
| > 65 | 1.69 (0.63–4.53) | 0.29 |
| Education | ||
| < high school | Ref | – |
| = high school | 0.84 (0.40–1.77) | 0.64 |
| > high school | 0.36 (0.16–0.78) | 0.01 |
| Health insurance | ||
| Public (Medicaid/Medicare) | Ref | – |
| Private | 1.23 (0.68–2.22) | 0.50 |
| County of residence | ||
| Within Houston metro area | Ref | – |
| Outside Houston metro area | 0.89 (0.52–1.51) | 0.67 |
| Cancer stage | ||
| I | Ref | – |
| II | 0.37 (0.20–0.67) | 0.001 |
| III | 0.09 (0.04–0.20) | < 0.001 |
Italicized P values were statistically significant at P<0.05
OR odds ratio, CI confidence interval
Acculturation and adherence to breast cancer survivorship care
Although the percentage of participants who adhered to both recommended surveillance mammography and clinic visits decreased as the length of survivorship increased, the decline was statistically significant only for clinic visits (P = .005) (Fig. 2). Overall, the study population was adherent, with 67% adhering to the recommended yearly surveillance mammograms and 71% adhering to recommended follow-up clinic visits. A moderately positive correlation was observed between adherence to surveillance mammography and adherence to clinic visit guidelines (Pearson’s correlation = 0.3838; P < .0001).
Fig. 2.
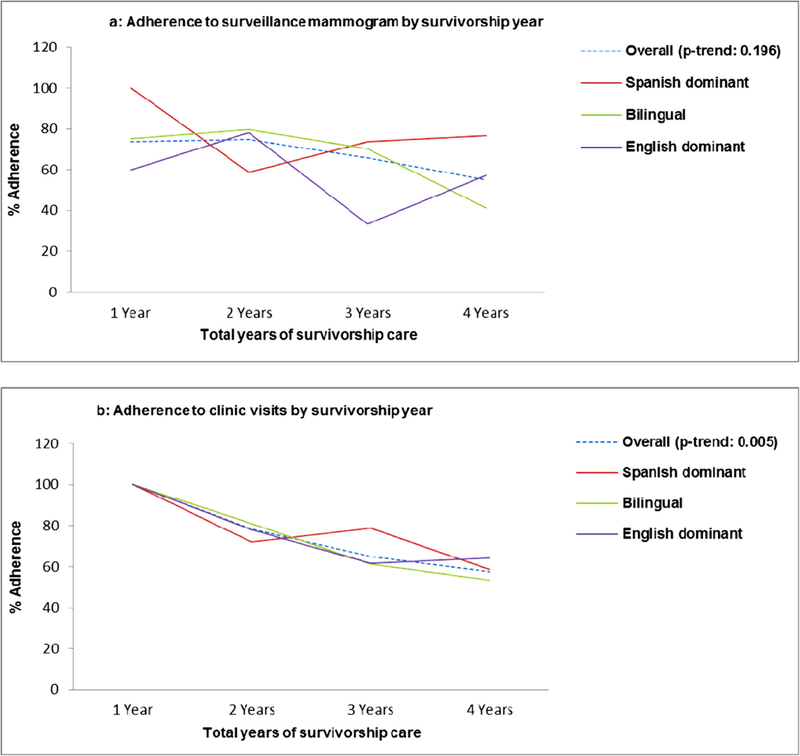
Trend in adherence to breast cancer survivorship care
The analysis of associations between acculturation and adherence to surveillance mammograms included 280 patients. In the univariable analysis, age (P = .01), education (P = .01), area of residence (P = .02), and receipt of screening mammogram prior to breast cancer diagnosis (P = .04) were significantly associated with adherence to surveillance mammography. Additionally, type of surgery received (BCS compared to mastectomy) was significantly associated with adherence to surveillance mammography (P < .001).
In the adjusted multivariable model, we found no significant association between acculturation and adherence to surveillance mammogram (Table 3). Although bilingual women (OR = 1.54; 95% CI = 0.64–3.73) had higher odds of undergoing yearly surveillance mammograms than Spanish-dominant women, this relationship was not statistically significant. Women who received BCS had significantly higher odds (OR = 3.55; 95% CI = 1.81–6.96) of being adherent to recommended yearly surveillance mammograms compared to women who received unilateral mastectomy. Additionally, women with >high school education (OR = 0.35; 95% CI = 0.13–0.91; P = .03) and those living outside the Houston metropolitan area (OR = 0.53; 95% CI = 0.29–0.98; P = .04) were significantly less likely to adhere to the surveillance mammograms.
Table 3.
Multivariable analysis for adherence to surveillance mammogram and follow-up clinic visits by level of acculturation and other factors among Mexican American women
| Adherence to surveillance mammogram (N = 280) |
Adherence to follow-up clinic visits (N = 291) |
|||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | P | OR (95% CI) | P |
| Acculturation | ||||
| Spanish-dominant | Ref | – | Ref | – |
| Bilingual | 1.54 (0.64–3.73) | 0.33 | 1.01 (0.43–2.35) | 0.98 |
| English-dominant | 0.90 (0.35–2.37) | 0.83 | 1.01 (0.38–2.65) | 0.99 |
| Age (years) | ||||
| ≤ 50 | Ref | – | Ref | – |
| 51–65 | 1.37 (0.70–2.67) | 0.36 | 1.29 (0.67–2.48) | 0.44 |
| > 65 | 1.21 (0.38–3.81) | 0.74 | 1.04 (0.35–3.05) | 0.94 |
| Education | ||||
| < High school | Ref | – | Ref | – |
| = High school | 0.54 (0.22–1.31) | 0.17 | 0.80 (0.34–1.86) | 0.60 |
| > High school | 0.35 (0.13–0.91) | 0.03 | 0.53 (0.22–1.28) | 0.15 |
| Health insurance | ||||
| Public (Medicaid/Medicare) | Ref | – | Ref | – |
| Private | 1.84 (0.90–3.73) | 0.09 | 2.40 (1.22–4.71) | 0.01 |
| County of residence | ||||
| Within Houston metro area | Ref | – | Ref | – |
| Outside Houston metro area | 0.53 (0.29–0.98) | 0.04 | 0.41 (0.23–0.74) | 0.003 |
| Cancer stage | ||||
| I | Ref | – | Ref | – |
| II | 1.19 (0.57–2.50) | 0.64 | 2.06 (1.05–4.05) | 0.03 |
| III | 1.91 (0.78–4.68) | 0.15 | 1.85 (0.80–4.24) | 0.14 |
| Type of surgery | ||||
| Mastectomy | Ref | – | Ref | – |
| Breast conserving surgery | 3.55 (1.81–6.96) | < 0.001 | 0.84 (0.45–1.56) | 0.57 |
Italicized P values were statistically significant at P<0.05
OR odds ratio, CI confidence interval
The analysis of associations between acculturation and adherence to clinic visits included 291 patients. In the univariable analysis, area of residence (P = .003) and stage at diagnosis (P = .04) were significantly associated with adherence to follow-up clinic visits. In the adjusted multivariable analysis, patients with private compared to public health insurance (OR = 2.40; 95% CI = 1.22–4.71; P = .01) and with stage II compared to stage I disease at diagnosis (OR = 2.06; 95% CI = 1.05–4.05; P = .03) were significantly more likely to adhere to the clinic visits. Those living outside the Houston metropolitan area (P = .003) were significantly less likely to adhere to these recommendations. There was no significant association between the level of acculturation or type of surgery received with adherence to clinic visits.
Discussion
Treatment decisions for patients with early-stage breast cancer are complex and often are associated with various clinical, institutional, and physician-related factors. These decisions may also vary according to the patient’s demographic characteristics, including age, race/ethnicity, or, in the case of immigrants, level of acculturation into the USA. Our study examined the role of acculturation and other demographic and clinical factors in decisions related to surgical management of breast cancer and adherence to long-term survivorship care among Mexican American women residing in the state of Texas. We found that women with a higher level of acculturation (English-dominant women) were significantly more likely to receive BCS (compared to mastectomy) than were less-acculturated women; however, level of acculturation did not predict adherence to long-term survivorship care. These findings suggest that acculturation plays a role in surgical decision-making related to breast cancer and future studies with larger samples are needed to explore its role in adherence to survivorship care recommendations.
To our knowledge, only three studies have previously explored the association between acculturation and receipt of BCS or mastectomy; none were focused on Mexican American women. The findings by Gomez et al. [39] were consistent with our results, suggesting that patients with a higher level of acculturation tend to prefer BCS over mastectomy. However, the primary goal of that study was to examine ethnic differences among patients receiving mastectomy, BCS, or no surgery, and the authors determined that only a small part of the difference in receipt of any of these surgical procedures was explained by acculturation [39]. Additionally, they used time since immigration and language use as proxy measures of acculturation, a method that failed to capture other key elements of acculturation. Hawley et al. [22] found no statistically significant difference in the likelihood of undergoing mastectomy or BCS among women with different levels of acculturation. They used a unidimensional scale to measure acculturation, such that losses in one cultural orientation were considered to be simultaneous gains in the other, reflecting an oversimplified assumption that acculturation is a linear process. More recently, in 2011, Kaplan et al. [40] used language use as another proxy measure for acculturation and found no difference in receipt of mastectomy and BCS by ethnicity or language preference. Our study used a bidimensional instrument (BAS) that categorized participants into three mutually exclusive acculturation groups (Spanish-dominant, bilingual, and English-dominant) and captured multiple elements surrounding language preference (including general language use, language proficiency, and language use in media) towards measuring this concept, thereby improving the validity of our findings. Methodology regarding the measure used to assess level of acculturation in our study, including reliability of the measure, has been previously published [30]. Our results reinforce the need to focus on disparities in breast cancer treatment decisions among women with low levels of acculturation.
We found no significant direct association between a patient’s level of acculturation and adherence to survivorship care. However, more acculturated women had significantly higher odds of receiving BCS and women who received BCS were in turn significantly more likely to adhere to the recommended surveillance mammograms. Previous research suggested that more acculturated individuals feel less stress from the demands of adjustment to breast cancer and exhibit better health status and health behaviors [41]. Similarly, acculturation has been shown to positively influence women’s trust in Western medicine, suggesting that acculturation spurs the adoption of healthy care practices [41]. This evidence suggests that acculturated women may be more inclined to adhere to physician recommendations for long-term survivorship care.
Besides acculturation, other factors were found to be associated with receipt of recommended breast cancer care. Mexican American women who were 51 to 65 years old were more likely to receive BCS compared to mastectomy than were younger women. This finding contrasts with those of previous studies demonstrating that older women are less likely to have BCS [42–44]; however, these studies were primarily focused on white women or had samples composed mostly of white women. Gomez et al. evaluated the relationship between socioeconomic status and trend in mastectomy rates among four ethnic groups, including Hispanic women using the California Cancer Registry [39]. In the period 1998–2001, there was a higher proportion of mastectomy that occurred among women in the lowest compared to the highest socio-economic quartile [45]. BCS is often followed by adjuvant radiation therapy and the additional time and out-of-pocket cost associated with BCS may also influence the decision to undergo mastectomy among lower socio-economic groups [46–48].
In our study, patients with higher education (>high school) were significantly less likely to receive BCS and to adhere to recommended surveillance mammograms. We conducted an exploratory interaction analysis between acculturation and level of education on type of breast surgery received and found no significant interaction. A possible explanation for the finding is that patients with higher education were more likely to reside outside of the Houston metropolitan area, and the increased distance to the treatment facility may have influenced their decision to decline BCS which requires daily travel for adjuvant radiation therapy. Future studies with a larger sample of Mexican American women are needed to assess other unmeasured factors such as transportation or childcare resources that may influence the associations between acculturation, education level, and the receipt of BCS versus mastectomy.
We acknowledge that this study had several limitations. The biggest limitation was our sample size, which may have hampered the study’s statistical power to determine significant differences. Second, because the analyses were restricted to the information obtained from the Ella Study and MD Anderson databases, we were unable to evaluate the role of other factors that are known to affect treatment decisions and survivorship care, such as income, employment, psychosocial variables, and presence of comorbidities. Third, we used patient self-report data and not ancestral hereditary markers to identify women of Mexican descent which is another potential limitation [5]. Fourth, it is possible that after completion of initial treatment, some patients sought follow-up care (e.g., surveillance mammograms or follow-up clinic visits) from their local health care providers and not at MD Anderson. In order to minimize the bias of considering such a patient as non-adherent, we limited our inclusion criteria to Texas residents who opted to receive their first surveillance mammogram and follow-up clinic visit at MD Anderson. Lastly, although we used a reliable and previously validated bidimensional scale to measure acculturation, we acknowledge that multidimensional acculturation scales [49] may better capture the complexity of acculturation and should be used in future studies investigating the association between acculturation and receipt of breast cancer care.
Despite these limitations, our findings suggest that acculturation may play a significant role in the receipt of initial surgical treatment for breast cancer and that disparities in cancer care may involve cultural factors that extend beyond traditional categorizations of race/ethnicity. The findings are clinically relevant and may inform the development of health interventions focused on eliminating health disparities in cancer care. For example, the use of decision support tools that tailor information to the linguistic and cultural needs of less acculturated Mexican American women may lead to enhanced informed decision-making for breast cancer treatment and survivorship care. Considering the increasing diversity of the US population and the established intra-ethnic heterogeneity in cancer control behaviors among the Hispanic community [5], it is imperative to understand the influence of cultural values on the overall health of Americans, particularly how they receive cancer care [35]. Such investigations would represent an essential step towards eliminating health disparities.
Acknowledgements
Department of Scientific Publications at the University of Texas, M D Anderson Cancer Center.
Funding source Dr. Advani was supported by a grant from the Susan G. Komen Foundation, Graduate Training Program in Breast Cancer Disparities (GTDR14300827). Additionally, the study was supported by the M.D. Anderson Cancer Center Specialized Program of Research Excellence in Breast Cancer (P50 CA116199–02S1—Dr. Melissa Bondy, Dr. María Elena Martínez).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015;65(6):457–80. [DOI] [PubMed] [Google Scholar]
- 2.Profile America: Facts for features - Hispanic heritage, U.C. Bureau, Editor. 2016: Washington DC. [Google Scholar]
- 3.Martínez ME, Gutiérrez-Millan LE, Bondy M, Daneri-Navarro A, Meza-Montenegro MM, Anduro-Corona I, et al. Comparative study of breast cancer in Mexican and Mexican-American women. Health 2010;2(09):1040–8. [Google Scholar]
- 4.Grant SR, Walker GV, Guadagnolo BA, Koshy M, Allen PK, Mahmood U. Variation in insurance status by patient demographics and tumor site among nonelderly adult patients with cancer. Cancer 2015;121(12):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Tyson D, Barnett Pathak E, Soler-Vila H, Flores AM. Looking under the hispanic umbrella: Cancer mortality among Cubans, Mexicans, Puerto Ricans and other hispanics in Florida. J Immigr Minor Health 2009;11(4):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomark Prev 2009;18(8):2162–9. [DOI] [PubMed] [Google Scholar]
- 7.Group, E.B.C.T.C. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087–106. [DOI] [PubMed] [Google Scholar]
- 8.Advani PS, et al. Ethnic disparities in adherence to breast cancer survivorship surveillance care. Cancer 2013. [DOI] [PMC free article] [PubMed]
- 9.Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31(7):961–5. [DOI] [PubMed] [Google Scholar]
- 10.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94(5):334–57. [DOI] [PubMed] [Google Scholar]
- 11.Szapocznik J, Scopetta MA, King OE. Theory and practice in matching treatment to the special characteristics and problems of Cuban immigrants. J Community Psychol 1978;6(2):112–22. [DOI] [PubMed] [Google Scholar]
- 12.Rogler LH, Cortes DE, Malgady RG. Acculturation and mental health status among Hispanics: convergence and new directions for research. Am Psychol 1991;46(6):585–97. [DOI] [PubMed] [Google Scholar]
- 13.Abraído-Lanza AF, Armbrister AN, Flórez KR, Aguirre AN. Toward a theory-driven model of acculturation in public health research. Am J Public Health 2006;96(8):1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro FG. Is acculturation really detrimental to health? Am J Public Health 2007;97(7):1162. [Google Scholar]
- 15.Network, N.C.C., NCCN Clinical Practice Guidelines in Oncology, Breast Cancer 2013, National Comprehensive Cancer Network. [DOI] [PubMed]
- 16.Nold RJ, Beamer RL, Helmer SD, McBoyle MF. Factors influencing a woman’s choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg 2000;180(6):413–8. [DOI] [PubMed] [Google Scholar]
- 17.van Dongen JA, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92(14):1143–50. [DOI] [PubMed] [Google Scholar]
- 18.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347(16):1227–32. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ghazal S, Fallowfield L, Blamey R. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36(15):1938–43. [DOI] [PubMed] [Google Scholar]
- 20.Deng H, et al. Breast-conserving surgery versus modified radical mastectomy for early breast cancer: clinical efficacy and quality of life [J]. Chinese Journal of General Surgery 2012;9:018. [Google Scholar]
- 21.Kummerow KL, du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surgery 2015;150(1):9–16. [DOI] [PubMed] [Google Scholar]
- 22.Hawley ST, Griggs JJ, Hamilton AS, Graff JJ, Janz NK, Morrow M, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst 2009;101(19):1337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins ED, Moore CP, Clay KF, Kearing SA, O’Connor AM, Llewellyn-Thomas HA, et al. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol 2008;27(4):519–25. [DOI] [PubMed] [Google Scholar]
- 24.Kotwall CA, Maxwell JG, Covington DL, Churchill P, Smith SE, Covan EK. Clinicopathologic factors and patient perceptions associated with surgical breast-conserving treatment. Ann Surg Oncol 1996;3(2):169–75. [DOI] [PubMed] [Google Scholar]
- 25.Molenaar S, Oort F, Sprangers M, Rutgers E, Luiten E, Mulder J, et al. Predictors of patients’ choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer 2004;90(11): 2123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol 2012;19(10):3246–50. [DOI] [PubMed] [Google Scholar]
- 27.Stafford D, Szczys R, Becker R, Anderson J, Bushfield S. How breast cancer treatment decisions are made by women in North Dakota. Am J Surg 1998;176(6):515–9. [DOI] [PubMed] [Google Scholar]
- 28.Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, et al. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast 2002;11(3): 228–35. [DOI] [PubMed] [Google Scholar]
- 29.Nodora JN, et al. Reproductive and hormonal risk profile according to language acculturation and country of residence in the ella binational breast cancer study. J Women’s Health 2014. [DOI] [PMC free article] [PubMed]
- 30.Garcia RZ, Carvajal SC, Wilkinson AV, Thompson PA, Nodora JN, Komenaka IK, et al. Factors that influence mammography use and breast cancer detection among Mexican-American and African-American women. Cancer Causes Control 2012;23(1):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene FL, et al. AJCC cancer staging handbook: TNM classification of malignant tumors 2002: Springer Science & Business Media. [Google Scholar]
- 32.Marín G, Gamba RJ. A new measurement of acculturation for Hispanics: the Bidimensional acculturation scale for Hispanics (BAS). Hisp J Behav Sci 1996;18(3):297–316. [Google Scholar]
- 33.Hamilton AS, Hofer TP, Hawley ST, Morrell D, Leventhal M, Deapen D, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomark Prev 2009;18(7):2022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mainous AG, Diaz VA, Geesey ME. Acculturation and healthy lifestyle among Latinos with diabetes. Ann Fam Med 2008;6(2): 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson MD, Hoffman-Goetz L. Defining and measuring acculturation: a systematic review of public health studies with Hispanic populations in the United States. Soc Sci Med 2009;69(7):983–91. [DOI] [PubMed] [Google Scholar]
- 36.Balcazar H, Castro FG, Krull JL. Cancer risk reduction in Mexican American women: the role of acculturation, education, and health risk factors. Health Educ Behav 1995;22(1):61–84. [DOI] [PubMed] [Google Scholar]
- 37.Akinyemiju T, Sakhuja S, Vin-Raviv N. Racial and socio-economic disparities in breast cancer hospitalization outcomes by insurance status. Cancer Epidemiol 2016;43:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esnaola NF, Knott K, Finney C, Gebregziabher M, Ford ME. Urban/rural residence moderates effect of race on receipt of surgery in patients with nonmetastatic breast cancer: a report from the South Carolina central cancer registry. Ann Surg Oncol 2008;15(7): 1828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez SL, France A-M, Lee MM. Socioeconomic status, immigration/acculturation, and ethnic variations in breast conserving surgery, San Francisco Bay area. Ethn Dis 2004;14(1):134–40. [PubMed] [Google Scholar]
- 40.Kaplan CP, Nápoles AM, Hwang ES, Bloom J, Stewart S, Nickleach D, et al. Selection of treatment among Latina and non-Latina white women with ductal carcinoma in situ. J Women’s Health 2011;20(2):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim J-W, Gonzalez P, Wang-Letzkus MF, Ashing-Giwa KT. Understanding the cultural health belief model influencing health behaviors and health-related quality of life between Latina and Asian-American breast cancer survivors. Support Care Cancer 2009;17(9):1137–47. [DOI] [PubMed] [Google Scholar]
- 42.Morris CR, Cohen R, Schlag R, Wright WE. Increasing trends in the use of breast-conserving surgery in California. Am J Public Health 2000;90(2):281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilligan MA, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care 2002;40(3):181–9. [DOI] [PubMed] [Google Scholar]
- 44.Polednak AP. Trends in, and predictors of, breast-conserving surgery and radiotherapy for breast cancer in Connecticut, 1988–1997. Int J Radiat Oncol Biol Phys 2002;53(1):157–63. [DOI] [PubMed] [Google Scholar]
- 45.Gomez SL, Lichtensztajn D, Kurian AW, Telli ML, Chang ET, Keegan THM, et al. Increasing mastectomy rates for early-stage breast cancer? Population-based trends from California. J Clin Oncol 2010;28(10):e155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barlow WE, Taplin SH, Yoshida CK, Buist DS, Seger D, Brown M. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst 2001;93(6):447–55. [DOI] [PubMed] [Google Scholar]
- 47.Liu J-J, Zhang S, Hao X, Xie J, Zhao J, Wang J, et al. Breast-conserving therapy versus modified radical mastectomy: socioeconomic status determines who receives what—results from case-control study in Tianjin, China. Cancer Epidemiol 2012;36(1): 89–93. [DOI] [PubMed] [Google Scholar]
- 48.Hadley J, Mitchell JM. Breast cancer treatment choice and mastectomy length of stay: a comparison of HMO and other privately insured women. Inquiry 1997;34(4):288–301. [PubMed] [Google Scholar]
- 49.Suarez L, Pulley L. Comparing acculturation scales and their relationship to cancer screening among older Mexican-American women. J Natl Cancer Inst Monogr 1995;18:41–7. [PubMed] [Google Scholar]


