Abstract
Activation of transforming growth factor-β (TGF-β) signaling has been used to enhance healing of meniscal degeneration in several models. However, the exact role and molecular mechanism of TGF-β signaling in meniscus maintenance and degeneration are still not understood due to the absence of in vivo evidence. In this study, we found that the expression of activin receptor-like kinases 5 (ALK5) in the meniscus was decreased with the progression of age and/or osteoarthritis induced meniscal degeneration. Col2α1 positive cells were found to be specifically distributed in the superficial and inner zones of the anterior horn, as well as the inner zone of the posterior horn in mice, indicating that Col2α1-CreERT2 mice can be a used for studying gene function in menisci. Furthermore, we deleted Alk5 in Col2α1 positive cells in meniscus by administering tamoxifen. Alterations in the menisci structure were evaluated histologically. The expression levels of genes and proteins associated with meniscus homeostasis and TGF-β signaling were analyzed by quantitative real-time PCR analysis (qRT-PCR) and immunohistochemistry (IHC). Our results revealed severe and progressive meniscal degeneration phenotype in 3- and 6-month-old Alk5 cKO mice compared with Cre-negative control, including aberrantly increased hypertrophic meniscal cells, severe fibrillation, and structure disruption of meniscus. qRT-PCR and IHC results showed that disruption of anabolic and catabolic homeostasis of chondrocytes may contribute to the meniscal degeneration phenotype observed in Alk5 cKO mice. Thus, TGF-β/ALK5 signaling plays a chondro-protective role in menisci homeostasis, in part, by inhibiting matrix degradation and maintaining extracellular matrix proteins levels in meniscal tissues.
Keywords: activing receptor-like kinases 5 (ALK5), matrix degradation, meniscal degeneration, transforming growth factor-β (TGF-β) signaling
1 ∣. INTRODUCTION
Meniscal degeneration is a common knee disease, which leads to knee joint pain and dysfunction. Furthermore, meniscal degeneration may cause degenerative joint changes such as osteophyte formation, articular cartilage degeneration, knee dysfunction, and symptomatic osteoarthritis (OA; Fox, Bedi,& Rodeo, 2012; Howell, Kumar, Patel, & Tom, 2014). The exact pathophysiology and mechanisms underlying meniscal degeneration are not well understood. Currently, there is no effective therapeutic intervention to decelerate the progress of meniscal degeneration.
The menisci of mammals are crescent-shaped fibrocartilaginous tissues located between the femoral condyles and the tibial plateau in the knee. The menisci are essential for joint stability, shock absorption, distribution of contact forces, joint lubrication, and proprioception (Fox et al., 2012; Fox, Wanivenhaus, Burge, Warren, & Rodeo, 2015; Howell et al., 2014). The meniscus is an inhomogeneous structure, and the local cellular and extracellular content of the menisci varies depending on the distance from the peripheral edge. The outer zone of the meniscus contains an abundance of type I collagen, which is similar to ligaments, tendons and other fibrous connective tissues. The cells in this region are akin to fibroblasts with a fusiform shape. In contrast, the superficial and inner zone of the meniscus contains an abundance of type II collagen and higher aggrecan content, which is more similar to hyaline articular cartilage, and fibrochondrocyte is the predominant cell type in this region (Beaufils, Becker, Kopf, Matthieu, & Pujol, 2017; McNulty, Moutos, Weinberg, & Guilak, 2007; Wilusz, Weinberg, Guilak, & McNulty, 2008). Meniscal cells are responsible for maintaining the balance between the anabolic and catabolic processes in menisci by synthesizing and degrading extracellular matrix (ECM) in response to environmental cues. Loss of the function of meniscal cells to maintain a balance between the anabolic and catabolic processes in meniscus leads to meniscal degradation (Howell et al., 2014; Meister, Indelicato, Spanier, Franklin, & Batts, 2004; Mesiha et al., 2007).
In recent years, a variety of molecules and signaling pathways, such as insulin-like growth factors 1 and 2 (Webber, Zitaglio, & Hough, 1988), fibroblast growth factor 2 (Adesida, Grady, Khan, & Hardingham, 2006), platelet-derived growth factor (Bhargava et al., 1999; Webber, Harris, & Hough, 1985), hepatocyte growth factor (Bhargava et al., 1999), and bone morphogenetic proteins (Bhargava et al., 1999) have been found to play critical roles in regulating the biological activities and maintenance of meniscal cells. However, the molecular mechanisms underlying the pathogenesis of meniscal degeneration are still not clearly understood.
Recently, the role of transforming growth factor-β (TGF-β) signaling in the regulation of meniscal cells has received increasing and specific attention. In canonical TGF-β-Smad signaling, the TGF-β ligands initiate signaling cascades by binding to the TGF-β type II receptor (TGFβRII) first, and then TGFβRII and TGF-β type I receptor (also called activin receptor-like kinases 5 [ALK5]) assemble to form a heteromeric complex on the cell membranes and phosphorylate ALK5. The activated (phosphorylated) ALK5 subsequently recruits and phosphorylates Smad2 and Smad3, which then form a heteromeric complex with Smad4. The Smad complexes are subsequently translocated into the nucleus to regulate the transcriptions of the target genes in cooperation with other cofactors. In addition, TGF-β can also activate non-Smad signaling pathways such as mitogen-activating protein kinases, phosphatidylinositol 3 kinase/Akt and small GTPases in a context-dependent manner (Hata & Chen, 2016; Massague, 2012).
During aging and meniscal degeneration, the canonical TGF-β-Smad signaling molecule is reduced in the meniscal tissues (Katsuragawa et al., 2010; Muhammad et al., 2014). TGF-β1 has been studied as a therapeutical molecule for meniscal degeneration by increasing the proliferation and matrix synthesis of meniscal cells in several in vitro models (Collier & Ghosh, 1995; Cucchiarini, Schmidt, Frisch, Kohn, & Madry, 2015; de Mulder, Hannink, Giele, Verdonschot, & Buma, 2013; Gruber et al., 2008; Gunja, Uthamanthil, & Athanasiou, 2009; Pangborn & Athanasiou, 2005a, 2005b; Riera et al., 2011; Stewart et al., 2007). However, due to the lack of in vivo evidence, the accurate role and molecular mechanism of TGF-β signaling in menisci maintenance and degeneration remain largely unknown.
To determine the roles of TGF-β signaling in menisci homeostasis in vivo, we generated mice with inducible cartilage-specific deletion of Alk5 by breeding Alk5flox/flox (Larsson et al., 2001) with Col2a1-CreERT2 mice (Chen et al., 2007). By injection of tamoxifen into 2-week-old mice, we confirmed the deletion of Alk5 gene in meniscal superficial and inner zones and found that it led to pathological changes in menisci at the age of 3 and 6 months. Our data demonstrate that TGF-β signaling plays an essential role in maintaining menisci homeostasis, and inhibition of TGF-β signaling in meniscal superficial and inner zones at the postnatal stage accelerate age-dependent meniscal degeneration in mice.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Animals
Alk5flox/flox mice (Larsson et al., 2001) and Col2α1-CreERT2 transgenic mice (Chen et al., 2007) were kindly provided by Professor Yang Chai and Professor Di Chen, respectively. ROSAmT/mG reporter mice (Muzumdar, Tasic, Miyamichi, Li, & Luo, 2007) were purchased from Jackson Laboratory (Stock Number: 007676; USA). Col2α1-CreERT2 mice were crossed with ROSAmT/mG reporter mice to obtain Col2α1-CreERT2; ROSAmT/mG mice. The resulting Cre-negative and Col2α1-CreERT2; ROSAmT/mG mice were intraperitoneally injected with tamoxifen (1mg/10 g body weight, daily for 5 days) at the age of 2 weeks (from postnatal Day 14 to postnatal Day 18) and were killed at the age of 2 months. Alk5flox/flox mice were crossed with Col2a1-CreERT2 mice to obtain Alk5flox/flox; Col2α1-CreERT2 (hereafter referred to as Alk5Col2ERT2). The resulting Alk5Col2ERT2 and Cre-negative mice were intraperitoneally injected with tamoxifen (1mg/10 g body weight, daily for 5 days) at 2 weeks to specifically inactivate ALK5 signaling in a tamoxifen-inducible and chondrocyte-specific manner (hereafter referred to as Alk5 cKO mice). The Cre-negative and Alk5 cKO mice aged Day 21 (3 days after the last injection) were used for immunohistochemistry (IHC), and mice aged 3 months and 6 months were used for histologic assessment. For the surgical OA model, wild-type mice at the age of 10 weeks were used to induced OA by destabilization of the medial meniscus (DMM) surgery as previously described (Glasson, Blanchet, & Morris, 2007). The mice were killed 4 and 8 weeks after surgery and the knee joints were used for IHC. For aging studies, wild-type mice aged 3, 6, 12, and 18 months were killed and the knee joints were sampled for IHC. All mice were maintained in a C57BL/6J background. The animal experiments were performed according to the protocols approved by the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University (Chongqing, China).
2.2 ∣. Isolation and culture of knee menisci
Hind knee menisci were harvested from 21 days old (3 days after the last injection) Cre-negative and Alk5 cKO mice under a microscope (at least ten were pooled as one sample). Freshly dissected meniscus samples were washed in sterile phosphate buffer saline (PBS) and rest for 48 hr in Dulbecco’s Modified Eagle’s Medium/F12 (1:1) supplemented with 1% penicillin/streptomycin in a CO2 incubator at 37°C.
2.3 ∣. Cryostat sections and fluorescence detection
For cryosection preparation, the knee joints were isolated from anesthetized mice perfused with cold 4% paraformaldehyde (PFA), fixed in 4% PFA at 4°C for 24 hr, decalcified in 14% EDTA (pH 7.4) for 3 days, incubated in 30% sucrose at 4°C for 24 hr and then embedded in optimal cutting temperature (OCT). Frozen sections were cut at 10 μm thickness with Leica cryostat until the anterior and posterior horns appeared as triangles between the femoral condyle and the tibial plateau.
For fluorescence detection, frozen sections were washed three times with PBS and imaged with a fluorescence microscope.
2.4 ∣. Histologic assessment
The knee joints were fixed in 4% PFA overnight, decalcified in 20% formic acid for 7 days, and embedded in paraffin. Serial sagittal sections were cut at 5 μm thickness until the anterior and posterior horns appeared as triangles between the femoral condyle and the tibial plateau. The sections were then stained with Safranin O/Fast Green to assess meniscus destruction by three independent investigators using a histological scoring system. The sum scores for the anterior and posterior horns were calculated separately based on three criteria: (1) tissue surface structures, (2) cellularity, and (3) Safranin O staining distribution and intensity as previously described (Kwok et al., 2016).
2.5 ∣. Immunohistochemistry
IHC was performed with the SP-9000 Histostain-Plus kits (ZSGB-BIO) according to the manufacturer’s instructions as previously described (Wang et al., 2017). Briefly, sections were deparaffinized in xylene, rehydrated in a graded series of alcohol washes and washed twice in PBS for 5 min. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min followed by antigen retrieval using 0.1% trypsin for 15 min. The sections were then blocked with normal goat serum for 30 min and incubated with different antibodies at 4°C overnight. On the second day, the sections were rinsed with PBS after incubation with horseradish peroxidase conjugated secondary antibodies for 30 min at 37°C. Finally, the sections were stained with a diaminobenzidine kit. Primary antibodies against the following proteins were used: ALK5 (1:100, Santa Cruz Biotechnology, Cat# sc-398, RRID: AB_632493), runt-related transcription factor 2 (RUNX2; 1:200, Santa Cruz Biotechnology, Cat# sc-390715, RRID: AB_2637033), Collagen II (Col II; 1:200, Millipore, Cat# MAB8887, RRID: AB_2260779), and matrix metalloproteinase 13 (MMP13; 1:200, Proteintech, Cat# 18165-1-AP, RRID: AB_2144858).
2.6 ∣. Apoptosis assay
Apoptotic cells in the meniscus were detected by TdT mediated dUTP-X nick end labeling (TUNEL) assay using an in situ cell death detection kit (peroxidase) of Roche according to the manual.
2.7 ∣. RNA isolation and quantitative real-time PCR analysis
Menisci tissues were flash-frozen for RNA extraction using TRIzol reagent (Invitrogen). Quantitative real-time PCR (qRT-PCR) were run in triplicate and independently repeated three times using Mx3000P PCR machine (Stratagene) and SYBR Premix Ex TaqTM kit. The primers for target genes are listed in Table 1. The results were calculated as the relative quantification compared to the control group, which was set at 1. Cyclophilin A was used as the internal control.
TABLE 1.
Primers and sequences for real-time PCR analysis
| Forward primer | Reverse primer | |
|---|---|---|
| Alk5 | 5′-CATCAGGGTCTGGATCAGGTT-3′ | 5′-GTAACACAATGGTCCTGGCAA-3′ |
| Runx2 | 5′-AGAGTCAGATTACAGATCCCAGG-3′ | 5′-TGGCTCTTCTTACTGAGAGAGG-3′ |
| Mmp13 | 5′-CTTCTTCTTGTTGAGCTGGACTC-3′ | 5′-CTGTGGAGGTCACTGTAGACT-3′ |
| Adamts5 | 5′-GGAGCGAGGCCATTTACAAC-3′ | 5′-CGTAGACAAGGTAGCCCACTTT-3′ |
| Aggrecan | 5′-CCTGCTACTTCATCGACCCC-3′ | 5′-AGATGCTGTTGACTCGAACCT-3′ |
| Col2 | 5′-CTGGTGGAGCAGCAAGAGCAA-3′ | 5′-CAGTGGACAGTAGACGGAGGAAAG-3′ |
| Col10 | 5′-ACCCCAAGGACCTAAAGGAA-3′ | 5′-CCCCAGGATACCCTGTTTTT-3′ |
| Prg4 | 5′-GAAAATACTTCCCGTCTGCTTGT-3′ | 5′-ACTCCATGTAGTGCTGACAGTTA-3′ |
| Cyclophilin A | 5′-CGAGCTCTGAGCACTGGAGA-3′ | 5′-TGGCGTGTAAAGTCACCACC-3′ |
Note. Alk5, activing receptor-like kinases 5; Mmp13, matrix metalloproteinase 13; Prg4, Proteoglycan 4.
2.8 ∣. Statistical analysis
Data were presented as the Mean ± 95% confidence intervals. Differences between two groups were assessed by the Student unpaired t test. Differences among multiple groups were assessed by one-way analysis of variance followed by Tukey’s posthoc test. All data were analyzed using GraphPad Prism v.6.01 software. P < 0.05 was considered to be statistically significant.
3 ∣. RESULTS
3.1 ∣. Decreased expression of ALK5 is associated with age-related and OA induced meniscal degeneration
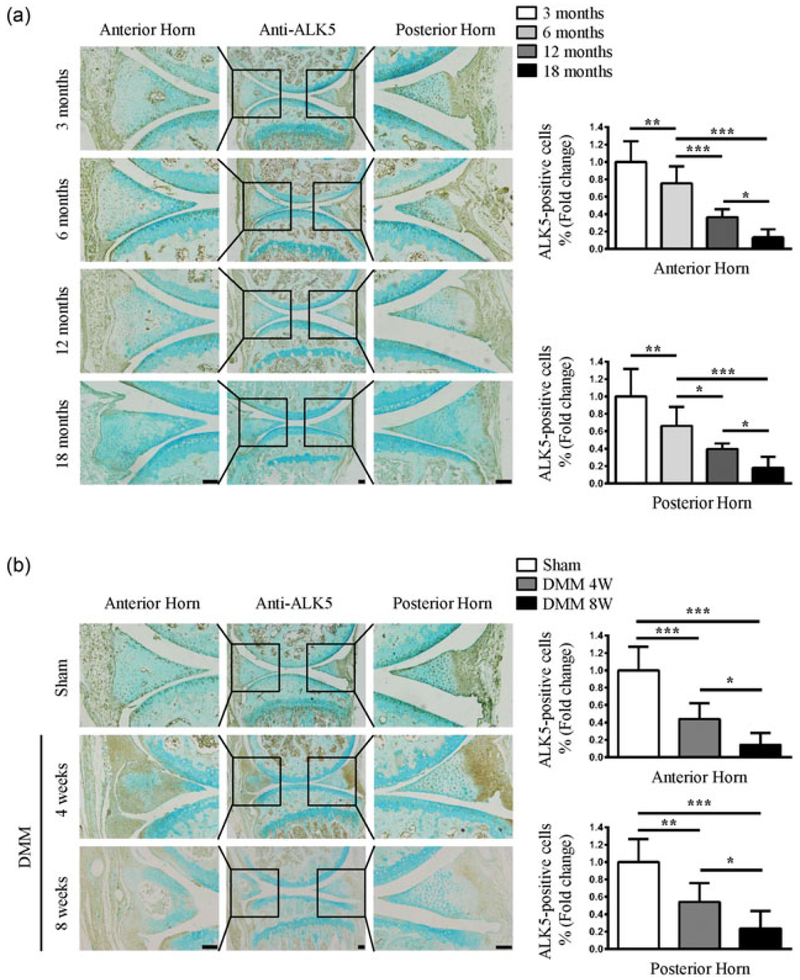
It has been shown that the expressions of TGF-β1 TGF-β3, and Smad2/3 were reduced with meniscal injury and degeneration (Katsuragawa et al., 2010; Muhammad et al., 2014). We questioned whether the expression of ALK5 is altered during meniscal injury and degeneration. Aging is a major risk factor for meniscal degeneration (Kwok et al., 2016). In this study, we first used IHC to detect the expression of ALK5 in meniscal tissues in young and aged mice. The results showed that ALK5 was abundantly expressed in the anterior and posterior horns in 3-month-old mice, which was gradually decreased in the meniscal tissues from aged mice (6–24 months; Figure 1a). Furthermore, we evaluated the ALK5 protein level in menisci of mice with post-traumatic OA induced by DMM surgery. The results showed that ALK5 was abundantly expressed in the meniscal anterior and posterior horns in the sham group. With OA progression, the expression of ALK5 in meniscal anterior and posterior horns was progressively decreased in mice of 4 and 8 weeks after DMM surgery (Figure 1b). These findings suggest a potential involvement of dysregulated TGF-β/ALK5 signaling in age-related and OA induced meniscal degeneration.
FIGURE 1.
Decreased expression of ALK5 in age-related and OA induced degenerative meniscus anterior and posterior horns. (a) Representative images of IHC staining for ALK5 showed that the number of positive cells in the anterior and posterior horns were strongly reduced with aging. Quantitative data are shown in the right panel (the percentage of positive cells in Cre-negative mice was defined as 1, n = 3 mice per group). (b) Representative images of IHC staining for ALK5 showed that the number of positive cells was gradually reduced in the anterior and posterior horns of mice at 4 and 8 weeks after DMM surgery. Quantitative data are shown in the right panel (the percentage of positive cells in Cre-negative mice was defined as 1, n = 3 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. *P < 0.05; **P < 0.01; ***P < 0.001. ALK5, activin receptor-like kinases 5; DMM, destabilization of the medial meniscus; IHC, immunohistochemistry; OA, osteoarthritis [Color figure can be viewed at wileyonlinelibrary.com]
3.2 ∣. Col2α1-CreERT2 drives Cre recombination in meniscal cells
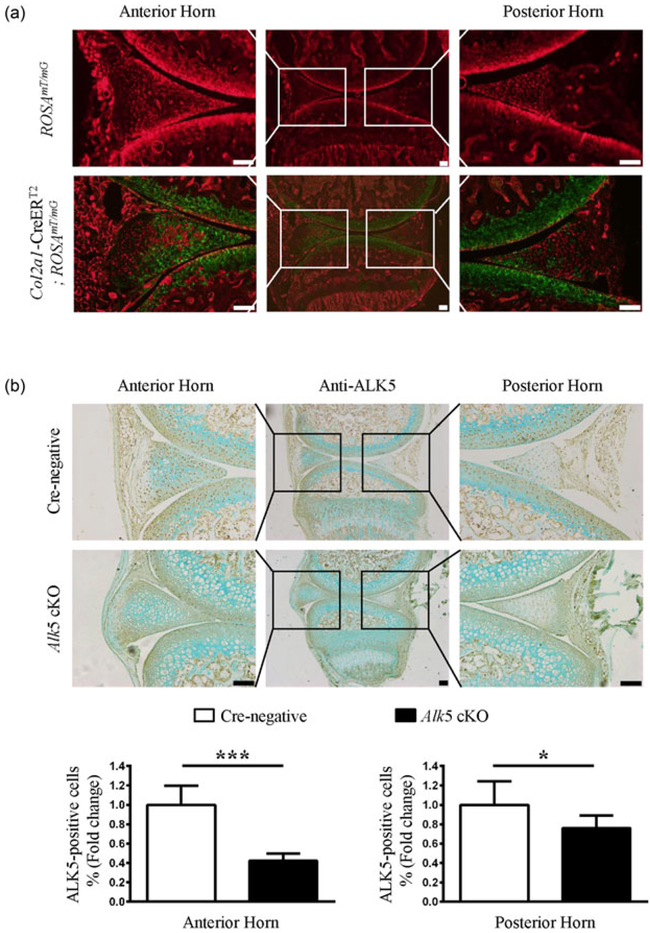
After observing the decreased ALK5 expression during meniscal degeneration, we further asked whether the dysregulation of TGF-β/ALK5 signaling is associated with meniscal injury and degeneration. We used mice with inducible cartilage-specific deletion of Alk5 to elucidate the effect of TGF-β/ALK5 signaling in the pathophysiology of meniscal degeneration. To evaluate the specificity of the tamoxifen-induced Col2α1-CreERT2-driven recombination in the meniscal tissues of postnatal mice, we first bred Col2α1-CreERT2 transgenic mice with ROSAmT/mG reporter mice. Analysis of frozen sections from 2-month-old mice using fluorescence microscopy showed that Col2α1-CreERT2 targeting cells (GFP-labelled cells) were located in the superficial and inner zones of the anterior horns, as well as the inner zone of the posterior horn in Col2α1-CreERT2; ROSAmT/mG mice (Figure 2a lower panel). Furthermore, we generated Alk5Col2ERT2 mice by crossing Alk5flox/flox with Col2a1-CreERT2 mice. IHC analysis demonstrated that ALK5 protein levels were decreased in the superficial and inner zones of anterior horns, as well as the inner zone of the posterior horn in Alk5 cKO mice compared with those in Cre-negative mice (Figure 2b). Taken together, these results demonstrated that Col2α1-CreERT2 mouse strain is a useful tool for studying gene function in the superficial and inner zones of menisci, and Alk5 gene could be effectively deleted in these regions of menisci in a tamoxifen-dependent manner.
FIGURE 2.
Directed Cre recombination in meniscus from Col2a1-CreERT2 mice. (a) Col2a1-CreERT2; ROSAmT/mG mice were generated by breeding Col2a1-CreERT2 transgenic mice with ROSAmT/mG reporter mice). Frozen histologic sections were harvested from 2-month-old Cre-negative and Col2a1-CreERT2; ROSAmT/mG mice after they were injected with tamoxifen at the age of 2 weeks (1 mg/10 g body weight, i.p. injection, daily for 5 days) and analyzed by fluorescence microscopy. The representative images showed that Col2a1-CreERT2 targeting GFP-positive cells are located in the meniscus anterior and posterior horns. (b) The resulting Alk5Col2ERT2 and aged matched Cre-negative mice were intraperitoneally injected with tamoxifen from postnatal Day 14 to postnatal Day 18. The mice were sacrificed on postnatal Day 21, 3 days after the last injection. Representative images of IHC staining for ALK5 showed that the number of positive cells in anterior and posterior horns is strongly reduced in 21-day-old Alk5 cKO mice compared with Cre-negative mice. Quantitative data are shown in the lower panel (the percentage of positive cells in Cre-negative mice was defined as 1, n = 3 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals, *P < 0.05; **P < 0.01; ***P < 0.001. ALK5, activing receptor-like kinases 5; GFP, green fluorescent protein; IHC, immunohistochemistry; i.p., intraperitoneal [Color figure can be viewed at wileyonlinelibrary.com]
3.3 ∣. Postnatal cartilage-specific deletion of Alk5 accelerates age-dependent meniscal degeneration
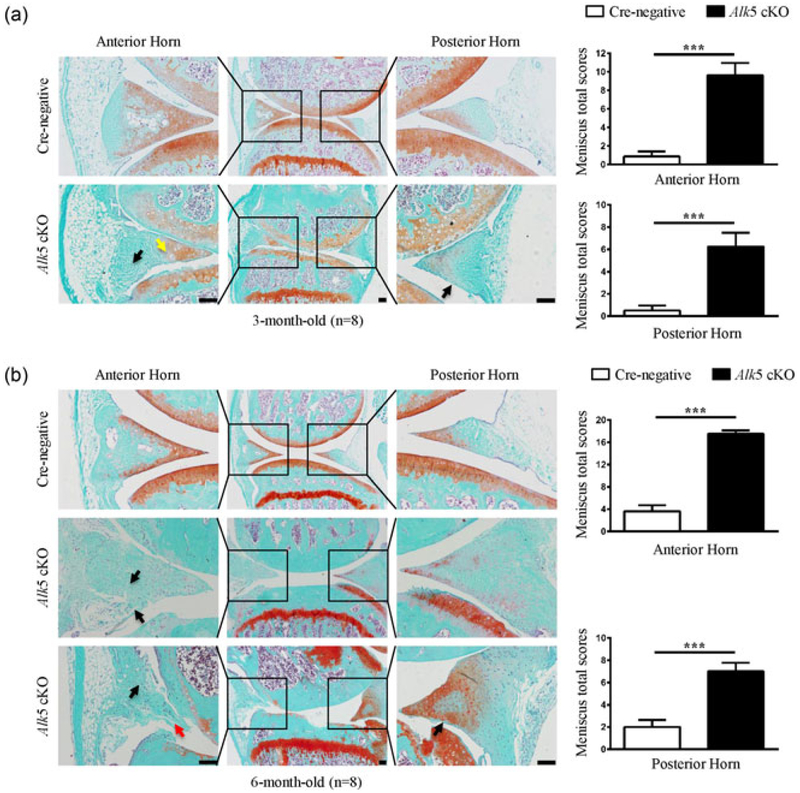
To investigate the role of TGF-β/ALK5 signaling in meniscal maintenance and degeneration at the postnatal stage, the above mentioned Cre-negative and Alk5Col2ERT2 mice were then subjected to a time-course experiment. Three-month-old Alk5 cKO mice showed fibrillation in the superficial, inner and outer zones with faint or no Safranin O staining, and aberrant hypertrophic chondrocytes in the superficial and inner zones (Figure 3a), indicating the degeneration of menisci. The meniscal degeneration phenotype became more profound in 6-month-old Alk5 cKO mice, which exhibited more severe fibrillation and structure disruption of meniscal tissues (Figure 3b). Accordingly, the meniscal histological grading system scores were significantly increased in 3- and 6-month-old Alk5 cKO mice compared with aged matched Cre-negative mice (Figure 3a,b, right panels). Taken together, these results revealed that cartilage-specific deletion of Alk5 accelerates age-dependent meniscal degeneration in mice, which demonstrates that postnatal chondrocytic TGF-β signaling plays an essential role in maintaining meniscal homeostasis and preventing meniscal degeneration.
FIGURE 3.
Histological analysis of structural damage in the meniscus of Alk5 cKO mice. (a) Knee joint samples were dissected from 3-month-old mice, and Safranin O/Fast green staining was performed. Representative images showed fibrillation in the superficial zone, inner and outer regions with faint or no Safranin O staining (black arrow) and aberrantly increased hypertrophic chondrocytes (yellow arrow) in menisci of Alk5 cKO mice. Quantitative data are shown in the right panel (n = 8 mice per group). (b) Knee joint samples were dissected from 6-month-old mice, and Safranin O/Fast green staining was performed. Representative images showed more severe fibrillation (black arrow) and disruption of meniscal tissue (red arrow) in meniscus of Alk5 cKO mice. Quantitative data are shown in the right panel (n = 8 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. *P < 0.05; **P < 0.01; ***P < 0.001. Alk5, activing receptor-like kinases 5 [Color figure can be viewed at wileyonlinelibrary.com]
3.4 ∣. Postnatal cartilage-specific deletion of Alk5 leads to increased expression levels of catabolic and hypertrophic marker genes and decreased expressions of anabolic marker genes in menisci
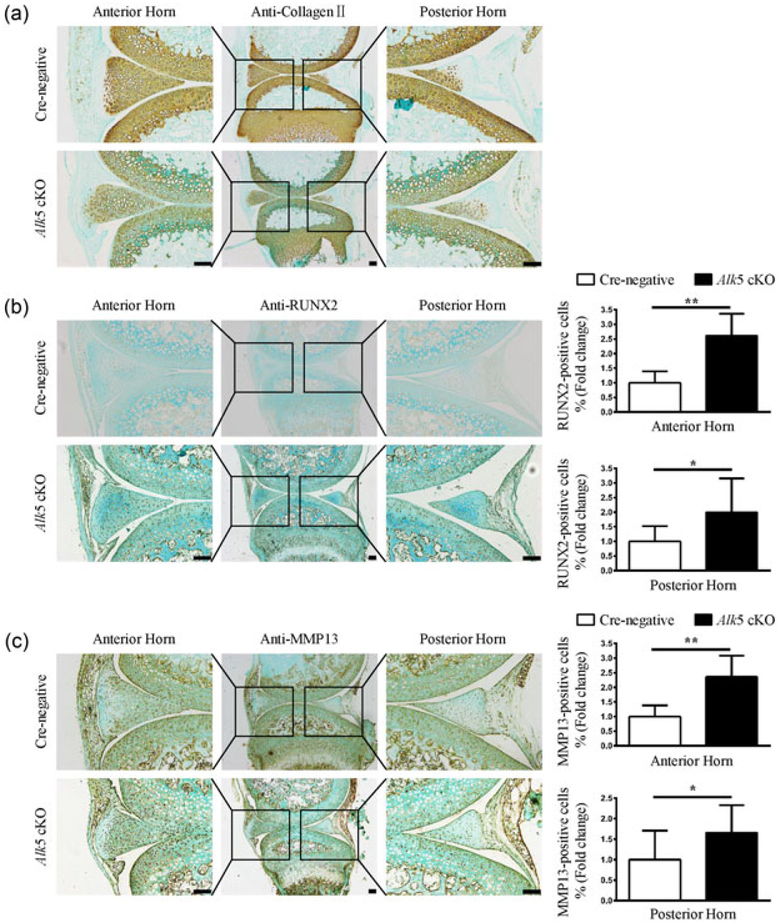
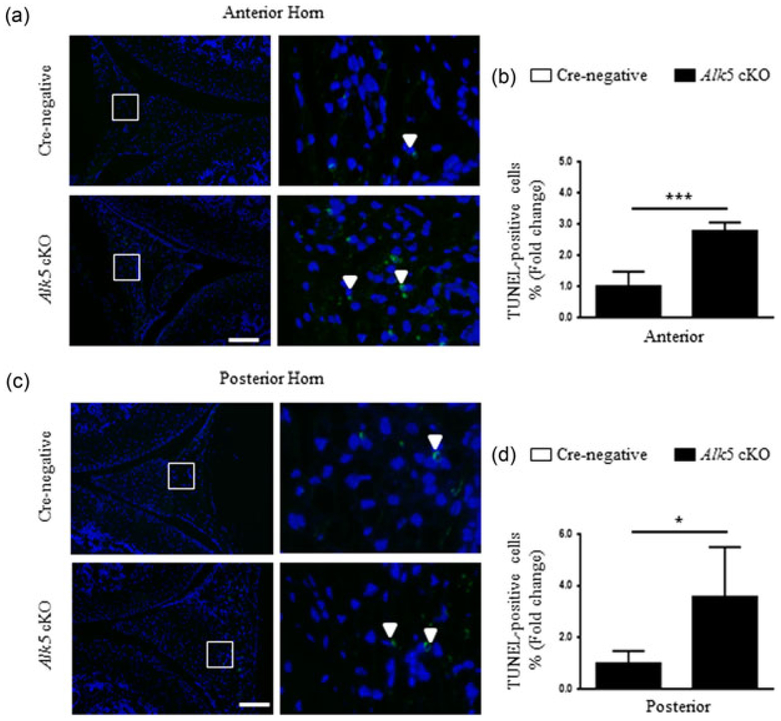
To explore the mechanisms underlying the accelerated meniscal degeneration in Alk5 cKO mice, we extracted RNA from the knee menisci tissues of Cre-negative and Alk5 cKO mice at 21-days-old (3 days after the last injection), and found that Alk5 gene was effectively deleted in the Alk5 cKO menisci tissue (Figure 4a). Next, the expressions of genes associated with cartilage homeostasis were detected. The mRNA expressions of proteoglycan 4 (Prg4), Aggrecan, and Col2α1 (anabolic marker genes) were decreased in the Alk5-deficient menisci tissue (Figure 4b–d). Meanwhile, the expressions of Runx2, Mmp13, a disintegrin and metalloproteinase with thrombospondin motif 5 (Adamts5) and Col10α1 (catabolic and hypertrophic marker genes) were significantly increased in Alk5-deficient menisci tissues (Figure 4e–h). Consistently, the IHC results showed that the Col II protein level (Figure 5a) was significantly decreased whereas the RUNX2 (Figure 5b) and MMP13 (Figure 5c) protein levels were remarkably increased in Alk5 cKO mice compared with the Cre-negative mice. Furthermore, we performed TUNEL assay to analyze the apoptosis of cells in meniscal cartilage. Our results showed that the percentage of positive cells in the anterior and posterior horns was significantly increased in Alk5 cKO mice compared with the Cre-negative mice at 21-days-old (Figure 6). Together, these results indicated that deletion of Alk5 in meniscal cartilage led to decreased synthesis and increased degradation of ECM and promoted meniscal fibrochondrocyte hypertrophic differentiation and meniscal cartilage cell apoptosis, which may partly disrupt menisci homeostasis and accelerate meniscal degeneration.
FIGURE 4.
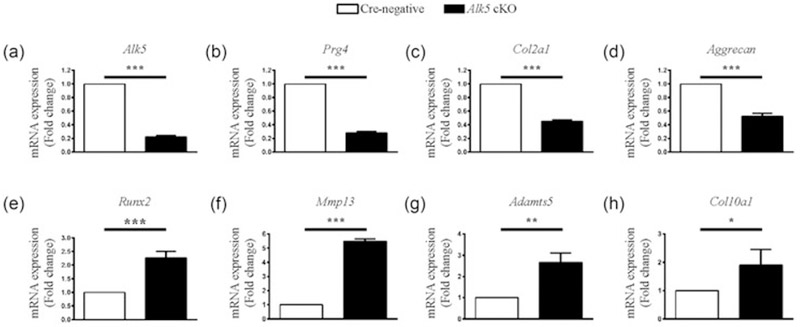
Loss of TGF-β/ALK5 signaling in meniscal cells decreased mRNA level of anabolic marker genes and increased mRNA level of catabolic and hypertrophic marker genes. qRT-PCR analysis showed effectively deletion of Alk5 gene (a), a significant decrease in the gene expressions of Prg4 (b), Col2a1 (c) and Aggrecan (d), and a significant increase in the gene expressions of Runx2 (e), Mmp13 (f), Adamts5 (g), and Col10a (h) in 21-day-old Alk5 cKO mice meniscl cells compared with Cre-negative mice meniscus cells. The analysis was repeated for three times. Data were expressed as the mean ± 95% confidence intervals. *P < 0.05; **P < 0.01; ***P < 0.001. ALK5, activing receptor-like kinases 5; Mmp13, matrix metalloproteinase 13; Prg4, proteoglycan 4; qRT-PCR, quantitative real-time PCR analysis; Runx2, runt-related transcription factor 2; TGF-β, transforming growth factor-β
FIGURE 5.
Loss of TGF-β/ALK5 signaling in meniscal cells decreased protein levels of anabolic marker genes and increased protein levels of catabolic and hypertrophic marker genes. (a) Representative images of IHC staining for Col II showed that the expression of Col II in anterior and posterior horns were significantly reduced in 21-day-old Alk5 cKO mice compared with Cre-negative mice (n = 3 mice per group). (b,c) Representative images of IHC staining for RUNX2 (b) and MMP13 (c) showed that the number of positive cells in anterior and posterior horns were significantly increased in 21-day-old Alk5 cKO mice compared with Cre-negative mice. Quantitative data were shown in the right panel (the percentage of positive cells in Cre-negative mice was defined as 1, n = 3 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. *P < 0.05; **P < 0.01; ***P < 0.001. ALK5, activing receptor-like kinases 5; Col II, Collagen II; IHC, immunohistochemistry; MMP13, matrix metalloproteinase 13; RUNX2, runt-related transcription factor 2; TGF-β, transforming growth factor-β [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 6.
Loss of TGF-β/ALK5 signaling in meniscal increased cell apoptosis. Representative images of TUNEL staining for cell apoptosis showed that the apoptotic cells in anterior (a) and posterior horns (c) were significantly increased in 21-day-old Alk5 cKO mice compared with Cre-negative mice (high magnification of the region occupied by white squares in the right panel, the positive cells are shown in white arrows). Quantitative data are shown in (b,d) (the percentage of positive cells in Cre-negative mice was defined as 1, n = 3 mice per group). Scale bar: 100 μm. Data were expressed as the mean ± 95% confidence intervals. *P < 0.05; **P < 0.01; ***P < 0.001. ALK5, activing receptor-like kinases 5; TGF-β, transforming growth factor-β; TUNEL, TdT mediated dUTP-X nick end labeling [Color figure can be viewed at wileyonlinelibrary.com]
4 ∣. DISCUSSION
In recent years, mouse models, especially genetic mouse models, have been widely used to investigate the cellular and molecular mechanisms of bone and joint diseases (Blaker, Clarke, & Little, 2017; Fang & Beier, 2014; Moon & Beier, 2015; Watanabe, Oue, & Ikegawa, 2011). Using the Col2α1-CreERT2 transgenic mouse model, a variety of interested genes have been deleted in Col2α1-expressing cells to dissect their role in bone and joint diseases in an inducible manner (Chen, Li, Xie, Wang, & Chen, 2014). In our study, using this transgenic mouse strain, we evaluated the role and underlying molecular mechanisms of TGF-β signaling in menisci homeostasis by specifically deleting the Alk5 gene to inhibit TGF-β signaling in Col2α1-expressing meniscal cells at the postnatal stage.
To date, few studies have focused on the gene functions of genetically modulated genes in specific compartments of the menisci to explore their roles in menisci development, homeostasis, and disease. In the current study, for the first time we evaluated the specificity of the Cre recombination activity in meniscal tissues at the postnatal stage using Col2α1-CreERT2; ROSAmT/mG. The results showed that Col2α1-CreERT2 targeting cells were mainly located in the superficial and inner zones of the anterior horn, as well as the inner zones of the posterior horn meniscal cells, which was consistent with IHC staining of Col II in wild-type mouse menisci (Gamer, Xiang, & Rosen, 2017). Thus Col2α1-CreERT2 mice can be used as a valuable tool for identifying the role of interested genes in regulating menisci homeostasis. In this study, we only evaluated the Col2α1-CreERT2-driven recombination at 2 months after birth; further assessment of the potential utility of Col2α1-CreERT2 mice will require a more detailed analysis of the spatio-temporal activity of the Col2α1 promoter activity in these mice because of the dynamic expression of type II collagen during menisci development, homeostasis, and aging.
Our results demonstrated that the Alk5 gene was effectively deleted in meniscal tissues, including the superficial and inner zones of the anterior horn, as well as the inner zone of the posterior horn. Importantly, we found that deletion of the Alk5 gene in these meniscal cells at the postnatal stage accelerated age-dependent meniscal degeneration in mice, including increased hypertrophic chondrocytes in the superficial and inner zones, severe fibrillation, and structure disruption of meniscal tissues. It should be noted that the degeneration of the anterior horn was more severe than the posterior horn according to histological staining and meniscal histological grading system scores. The possible reason is that TGF-β signaling was more efficiently inhibited in the anterior horn than the posterior horn due to the higher Col2α1 expression in the anterior horn as revealed by GFP fluorescence and ALK5 IHC results. To our knowledge, this is the first study showing the protective role of TGF-β signaling on meniscal tissue maintenance in vivo.
The ECM of menisci mainly contains type I and type II collagens (Col I and Col II) and Aggrecan, which could be degraded by specific collagenases such as MMP13, MMP9, and MMP3 and ADAMTS5 under pathological conditions, including meniscal degeneration (Fuller, Smith, Little, & Melrose, 2012; Kreinest et al., 2016; Ling, Lai, Wong, & Levenston, 2016; Upton, Chen, & Setton, 2006). Previous studies showed that TGF-β signaling pathway components exerted both proanabolic and anticatabolic effects on menisci during the repair and healing process of degenerative menisci (Collier & Ghosh, 1995; Cucchiarini et al., 2015; de Mulder et al., 2013; Gruber et al., 2008; Gunja et al., 2009; Pangborn & Athanasiou, 2005a, 2005b; Riera et al., 2011; Stewart et al., 2007). In this study, we found that the anabolic marker genes such as Prg4, Aggrecan, and Col2α1 were decreased, whereas the catabolic and hypertrophic marker genes such as Runx2, Mmp13, Adamts5, and Col10αl were significantly increased in Alk5-deficient meniscal tissues. Consistently, the IHC results showed that Col II level was decreased, whereas the RUNX2 and MMP13 protein levels were increased in the meniscal tissues of Alk5 cKO mice. The above changes in anabolism and catabolism related molecules in the meniscal tissues eventually tilt the balance away from matrix maintenance toward degradation, resulting in meniscal degeneration over time observed in Alk5 cKO mice, together with the accelerated hypertrophic differentiation and increased cell apoptosis.
It has been shown that cartilage-specific deletion of Alk5 (Wang et al., 2017) or Tgfbr2 (Jin et al., 2011; Shen et al., 2013) in knee cartilage and intervertebral disc cartilage induces a progressive OA-like phenotype and intervertebral disc degeneration. Further studies showed that inhibition of TGF-β signaling in the knee cartilage and intervertebral disc cartilage up-regulates the expressions of downstream target genes, such as Runx2, Mmp13, and Adamts5 in cartilage, leading to the development of knee OA and intervertebral disc degeneration (Jin et al., 2011; Shen et al., 2013; Wang et al., 2017). Our current study is the first to demonstrate that a similar set of molecules is also responsible for meniscal degradation. Further genetical deletion of Runx2, Mmp13, or Adamts5 under the Alk5 deficiency background could be helpful in dissecting the relationship between TGF-β signaling and the molecules in menisci homeostasis genetically. A previous study showed that broad-spectrum MMP inhibitors promote the integrative repair of meniscus in vitro (McNulty, Weinberg, & Guilak, 2009). Observing whether MMP13 inhibitors could rescue the meniscal degeneration phenotypes observed in Alk5 cKO mice will further justify the application of MMP13 inhibitor as a treatment for meniscal degeneration.
In summary, in this experiment, we found that cartilage-specific deletion of Alk5 gene in meniscus leads to meniscus degeneration by dysregulating the ECM metabolism and enhancing the hypertrophic differentiation and apoptosis of meniscal chondrocytes. Our study provides experimental data suggesting that modulating TGF-β signaling could be a potential therapy for delaying meniscal degeneration.
ACKNOWLEDGMENTS
We thank Professor Yang Chai (University of Southern California, USA) and Professor Di Chen (Rush University Medical Center, USA) for providing Alk5flox/flox mice and Col2α1-CreERT2 mice, respectively.
FUNDING
Contract grant sponsor: Special Funds for Major State Basic Research Program of China (973 program); Contract grant number: 2014CB942904. Contract grant sponsor: International (Regional) Cooperation and Exchange; Contract grant number: 81220108020). Contract grant sponsor: National Natural Science Foundation of China; Contract grant number: 81472074).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- Adesida A, Grady L, Khan W, & Hardingham T (2006). The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Research & Therapy, 8(3), R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufils P, Becker R, Kopf S, Matthieu O, & Pujol N (2017). The knee meniscus: Management of traumatic tears and degenerative lesions. EFORT Open Reviews, 2(5), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava MM, Attia ET, Murrell GAC, Dolan MM, Warren RF, & Hannafin JA (1999). The effect of cytokines on the proliferation and migration of bovine meniscal cells. The American Journal of Sports Medicine, 27(5), 636–643. [DOI] [PubMed] [Google Scholar]
- Blaker CL, Clarke EC, & Little CB (2017). Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 35(3), 424–439. [DOI] [PubMed] [Google Scholar]
- Chen M, Li S, Xie W, Wang B, & Chen D (2014). Col2CreER(T2), a mouse model for a chondrocyte-specific and inducible gene deletion. European Cells & Materials, 28, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O'Keefe RJ, & Chen D (2007). Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis, 45(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S, & Ghosh P (1995). Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis and Cartilage, 3(2), 127–138. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Schmidt K, Frisch J, Kohn D, & Madry H (2015). Overexpression of TGF-beta via rAAV-mediated gene transfer promotes the healing of human meniscal lesions ex vivo on explanted menisci. The American Journal of Sports Medicine, 43(5), 1197–1205. [DOI] [PubMed] [Google Scholar]
- de Mulder EL, Hannink G, Giele M, Verdonschot N, & Buma P (2013). Proliferation of meniscal fibrochondrocytes cultured on a new polyurethane scaffold is stimulated by TGF-beta. Journal of Biomaterials Applications, 27(5), 617–626. [DOI] [PubMed] [Google Scholar]
- Fang H, & Beier F (2014). Mouse models of osteoarthritis: Modelling risk factors and assessing outcomes. Nature Reviews Rheumatology, 10(7), 413–421. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Bedi A, & Rodeo SA (2012). The basic science of human knee menisci: Structure, composition, and function. Sports Health, 4(4), 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, & Rodeo SA (2015). The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clinical Anatomy, 28(2), 269–287. [DOI] [PubMed] [Google Scholar]
- Fuller ES, Smith MM, Little CB, & Melrose J (2012). Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthritis and Cartilage, 20(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Xiang L, & Rosen V (2017). Formation and maturation of the murine meniscus. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 35(8), 1683–1689. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Blanchet TJ, & Morris EA (2007). The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage, 15(9), 1061–1069. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Mauerhan D, Chow Y, Ingram JA, Norton HJ, Hanley EN, & Sun Y (2008). Three-dimensional culture of human meniscal cells: Extracellular matrix and proteoglycan production. BMC Biotechnology, 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja NJ, Uthamanthil RK, & Athanasiou KA (2009). Effects of TGF-beta1 and hydrostatic pressure on meniscus cell-seeded scaffolds. Biomaterials, 30(4), 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, & Chen YG (2016). TGF-beta signaling from receptors to smads. Cold Spring Harbor Perspectives in Biology, 8(9), a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell R, Kumar NS, Patel N, & Tom J (2014). Degenerative meniscus: Pathogenesis, diagnosis, and treatment options. World Journal of Orthopedics, 5(5), 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Shen J, Wang B, Wang M, Shu B, & Chen D (2011). TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Letters, 585(8), 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragawa Y, Saitoh K, Tanaka N, Wake M, Ikeda Y, Furukawa H, … Fukui N (2010). Changes of human menisci in osteoarthritic knee joints. Osteoarthritis and Cartilage, 18(9), 1133–1143. [DOI] [PubMed] [Google Scholar]
- Kreinest M, Reisig G, Ströbel P, Fickert S, Brade J, Wennemuth G,… Schwarz ML (2016). Analysis of gene expression and ultrastructure of stifle menisci from juvenile and adult pigs. Comparative Medicine, 66 (1), 30–40. [PMC free article] [PubMed] [Google Scholar]
- Kwok J, Onuma H, Olmer M, Lotz MK, Grogan SP, & D'Lima DD (2016). Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthritis and Cartilage, 24(4), 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Levéen P, … Karlsson S (2001). Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. The EMBO Journal, 20, 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling CHY, Lai JH, Wong IJ, & Levenston ME (2016). Bovine meniscal tissue exhibits age- and interleukin-1 dose-dependent degradation patterns and composition-function relationships. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 34(5), 801–811. [DOI] [PubMed] [Google Scholar]
- Massagué J (2012). TGFbeta signalling in context. Nature Reviews Molecular Cell Biology, 13(10), 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Moutos FT, Weinberg JB, & Guilak F (2007). Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis and Rheumatism, 56(9), 3033–3042. [DOI] [PubMed] [Google Scholar]
- McNulty AL, Weinberg JB, & Guilak F (2009). Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clinical Orthopaedics and Related Research, 467(6), 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister K, Indelicato PA, Spanier S, Franklin J, & Batts J (2004). Histology of the torn meniscus: A comparison of histologic differences in meniscal tissue between tears in anterior cruciate ligament-intact and anterior cruciate ligament-deficient knees. The American Journal of Sports Medicine, 32(6), 1479–1483. [DOI] [PubMed] [Google Scholar]
- Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, & Murray MM (2007). Pathologic characteristics of the torn human meniscus. The American Journal of Sports Medicine, 35(1), 103–112. [DOI] [PubMed] [Google Scholar]
- Moon PM, & Beier F (2015). Novel Insights into osteoarthritis joint pathology from studies in mice. Current Rheumatology Reports, 17(8), 50. [DOI] [PubMed] [Google Scholar]
- Muhammad H, Schminke B, Bode C, Roth M, Albert J, von der Heyde S,. Miosge N (2014). Human migratory meniscus progenitor cells are controlled via the TGF-beta pathway. Stem Cell Reports, 3(5), 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, & Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis, 45(9), 593–605. [DOI] [PubMed] [Google Scholar]
- Pangborn CA, & Athanasiou KA (2005a). Effects of growth factors on meniscal fibrochondrocytes. Tissue Engineering, 11(7–8), 1141–1148. [DOI] [PubMed] [Google Scholar]
- Pangborn CA, & Athanasiou KA (2005b). Growth factors and fibrochondrocytes in scaffolds. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 23(5), 1184–1190. [DOI] [PubMed] [Google Scholar]
- Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, & McNulty AL (2011). Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: Influences on meniscal cell proliferation and migration. Arthritis Research & Therapy, 13(6), R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, … Chen D (2013). Deletion of the transforming growth factor beta receptor type ii gene in articular chondrocytes leads to a progressive osteoarthritislike phenotype in mice. Arthritis and Rheumatism, 65(12), 3107–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K, Pabbruwe M, Dickinson S, Sims T, Hollander AP, & Chaudhuri JB (2007). The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds. Tissue Engineering, 13(2), 271–280. [DOI] [PubMed] [Google Scholar]
- Upton ML, Chen J, & Setton LA (2006). Region-specific constitutive gene expression in the adult porcine meniscus. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 24(7), 1562–1570. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tan QY, Xu W, Qi HB, Chen D, Zhou S,… Chen L (2017). Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthritis and Cartilage, 25(11), 1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Oue Y, & Ikegawa S (2011). Animal models for bone and joint disease. Mouse models develop spontaneous osteoarthritis. Clinical Calcium, 21(2), 286–293. [PubMed] [Google Scholar]
- Webber RJ, Zitaglio T, & Hough AJ (1988). Serum-free culture of rabbit meniscal fibrochondrocytes: Proliferative response. Journal of Orthopaedic Research, 6(1), 13–23. [DOI] [PubMed] [Google Scholar]
- Webber RJ, Harris MG, & Hough AJ Jr. (1985). Cell culture of rabbit meniscal fibrochondrocytes: Proliferative and synthetic response to growth factors and ascorbate. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 3(1), 36–42. [DOI] [PubMed] [Google Scholar]
- Wilusz RE, Weinberg JB, Guilak F, & McNulty AL (2008). Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 26(4), 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]