Abstract
We developed a rapid-test to screen for effects of biochar on seed germina- tion and soils. Crop seeds were placed in containers and covered with 15 g of soil with 1% biochar by weight. Two agricultural soils from South Carolina USA were used. Eighteen biochars were produced from six primary feedstocks [pine chips (PC), poultry litter (PL), swine solids (SS), switchgrass (SG); and two blends of PC and PL, 50% PC/50% PL (55), and 80% PC/20% PL (82)]. Each feedstock was pyrolyzed at 350, 500 and 700°C. There were few biochar effects on seed germination. Shoot dry weight was increased for carrot, cucumber, lettuce, oat, and tomato; primarily with biochars containing PL. Soil pH, electrical conductivity and extractable phosphorus primarily increased with PL, SS, 55, and 82 treatments for both soil types and across species. This method can be an early indicator of biochar effects on seed germination and soil health.
Keywords: Soil, feedstock, temperature, crop
Introduction
Biochar is a carbon-rich material produced by heating biomass in the absence of, or limited oxygen (pyrolysis), which has been proposed as an amendment to address a wide range of soil environ- mental problems (Lehmann and Joseph 2009). Biochar can affect soil properties critical for seed germination and plant growth including nutrients including phosphorus (P) (Chan and Xu 2009; DeLuca, MacKenzie, and Gundale. 2009; Gong et al. 2001); pH (Deska et al. 2011) and electrical conductivity (EC) (Niu et al. 2010); as well as soil microbial activity (Thies and Rillig 2009), and soil moisture storage (Novak et al. 2012).
Successful seed germination is essential for subsequent plant growth and development; thus, seed germination characteristics can be an early indicator of the effects of biochar quality on plant performance (Rogovska et al. 2011). A variety of methods have been used to assess effects of biochar on seed germination depending on different study objectives. In terms of biochar, the International Biochar Initiative has issued a technical bulletin #101 which describes a germination test (http://www.biochar-international.org/sites/default/files/Technical_bulletin_101_english.pdf, downloaded 10 February 2016). The test uses simple plates to determine general germination rates and is not detailed enough for research experimental purposes. On the other hand, there are commercially available seed germination and early plant growth assays (e.g. PHYTOTOXKIT http://www.microbiotests.be/slideshows/04.%20Phytotoxkit.pdf ), which have been used on a limited basis to screen for phytotoxicity of biochar (Oleszczuk, Jośko, and Kuśmiez 2013). This presents a conundrum since there are very few tests for biochar suitability in soils, and biochar’s chemistry are variable due to feedstock selection, pyrolysis temperature, and material size differences (Novak et al. 2014). A rapid screening test would be a useful tool to determine the suitability of biochar as a soil amendment considering the high number of different feedstocks available and the diversity in temperatures used to produce biochars (Lehmann and Joseph 2009).
The simplest test system is by placing seeds in petri dishes, which have been used by several researchers to determine effects of biochar on seed germination. For example, petri dishes were used in the soil-less bioassay used by Solaiman, Murphy, and Abbott (2012) to determine effects of biochar on wheat (Triticum aestivum) germination and growth and by Soudek et al. (2017) to study the relationship between biochar and toxicity of heavy metals on sorghum (Sorghum bicolor) seeds. Gascó et al. (2016) used petri dishes to evaluate the effects of phytotoxic compounds on seed germination for 5 crop species. Rosende et al. (2016) determined effects of biochar on the impact of mine soils on Lolium perenne seeds grown in petri dishes, and compared those responses to the impact of the biochar on readily accessible pools of heavy metals from the biochar using automatic flow-through dynamic extraction. Several studies used modifications of the standard rolled germina- tion paper methods (e.g., ISTA 2016) using either soil (Free et al. 2010) or leached aqueous extracts from biochar (Rogovska et al. 2011). Germination trays with individual wells were used to determine the effects of biochar on 3 crop species grown with two soils (Van Zwieten et al. 2010). Buss and Mašek (2014) devised specialized setups to assess the effects of volatile organic compounds from biochar on seed germination where (1) seeds were exposed only to volatile compounds or (2) seeds were exposed only to volatile compounds, were exposed to volatile compounds and leachate; or were in direct contact with volatile compounds, leachate and biochar in the same system.
In addition to tests used for biochar studies, there are standardized seed germination tests for use with toxic chemicals and pesticides. Both the US EPA Seedling Emergence and Seedling Growth Test (OCSPP 850.4100, US EPA 2012) and Organisation for Economic Co-operation and Development (OECD) Seedling Emergence and Seedling Growth Test No. 208 (OECD 2006), though flexible, normally require larger amounts of soil and multiple replicate containers with few (e.g., 1–10) seeds per containers.
Though these germination systems were useful for specific purposes, they were not adequate for use with large numbers of biochars under replicated, standardized conditions. We were especially interested in a semi-automated system for uniform placement of a large number of seeds in a regular pattern using a minimum amount of biochar and soil.
Thus, we decided to develop a seed germination system based on procedures from the Oregon State Seed Testing Laboratory (http://seedlab.oregonstate.edu/home), and ISTA (2016). The objective of our study was to utilize this standardized rapid test to determine if seed germination and early seedling growth as well as basic soil characteristics are affected by biochar, and to determine effects of 18 biochars on eight crop species commonly used in phytotoxicity testing, and key associated soil characteristics, pH, EC, and EP concentration.
Materials and Methods
Soils and biochar
Two sandy Coastal Plain soils from South Carolina were used (Norfolk and Coxville) in this study. A detailed description of their parent materials, soil characteristics, and past tillage and cropping management activities are outlined in Novak et al. (2014) and Sigua et al. (2014). The general characteristics of each are shown in Table 1.
Table 1.
Selected soil chemical and mineralogical properties for the Coxville and Norfolk soils (Sigua et al. 2014; Novak et al. 2014; Novak Personal Communication 2017).
| Soil | pH (H2O) | Organic C | Total N | Sand | Silt | Clay | K | P |
|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (%) | (%) | (%) | (mg/kg) | (mg/kg) | ||
| Coxville | 5.1 | 26.3 | 1.8 | 42.1 | 43.4 | 14.5 | 36a | 39a |
| Norfolk | 5.9 | 3.9 | - b | 80.7 | 16.7 | 2.6 | 19c | 27c |
Abbreviations: H2O = water, C = carbon, N = nitrogen, K = potassium, EP = extractable phosphorus.
Novak, J. personal communication (2017).
Below detection limit of 0.1 g/100 g.
Mehlich I extractable K and P (Novak et al. 2014).
There were six biochar feedstocks for each soil type: pine chips (PC), poultry litter (PL), swine solids (SS), switchgrass (SG), and two blends of pine chips and poultry litter- 50% of each, herein referred to as (55), and 80% PC and 20% PL, herein referred to as (82). Procedures to produce the biochar feedstocks are described in Novak, Cantrell, and Watts (2013) and occurred in South Carolina. In summary, all feedstocks were processed before pyrolysis, including air-drying, grinding, and sieving to pass a 6-mm sieve. Each feedstock was pyrolyzed at a low (350°C), medium (500°C) and high (700°C) temperature using furnace-retort system (Lindberg/MPH, Riverside, MI) for 1–2h depending on sample size (Novak, Cantrell, and Watts 2013). All chars then were ground to pass a 0.25-mm sieve, followed by storage in a desiccator until shipment to Corvallis. For each experimental container, 1% biochar (0.15 g dry weight basis) from one of 18 feedstock × temperature combination of biochars was used.
Preparation of seed and soil plates
Seeds used in this study were for eight species recommended for EPA Seed Germination Tests (US EPA, 2012): cabbage [Brassica oleracea L. variety (var.) capitata L. cultivar (cv.) Derby Day], carrot (Daucus carota subsp. sativus (Hoffm.) Schübl. & G. Martens cv. Tendersweet), cucumber (Cucumis sativus L. cv. Straight Eight), lettuce (Lactuca sativa L. cv. Black-seeded Simpson), oat (Avena sativa L. cv. WW4414E), onion (Allium cepa L. cv. Hybrid Yellow Sweet Spanish), perennial ryegrass (Lolium perenne L. cv. Pinnacle) and tomato (Solanum lycopersicon L. cv. Siletz). Seeds were germinated in 11.0 cm square × 3.5 cm deep clear acrylic plastic containers (germination boxes) fitted with blotter paper and acrylic snap on plastic lids. Figure 1 is a flow-chart of the standardized seed germination procedure. The paper was premoistened with reverse-osmosis (R/O) water, followed by placement of seeds on the blotter paper (usually 25 in a uniform 5 × 5 pattern) using a vacuum-assisted seed counting head (Hoffman Manufacturing: VPWS ¾ HP 115V/60 HZ vacuum work station, Jefferson OR), to provide for rapid placement of many seeds in a regular pattern. The seeds then were covered with 15 g of the soil-biochar mixture. A measured amount (~ 5 mL) of reverse osmosis (R/O) water was added to each germination box to moisten the soil and blotter paper. After establishment, if the soil appeared to be drying out, 1 or 2 mL of R/O water was added as needed to the soil to maintain saturation.
Figure 1.
Generalized procedure for screening the effects of biochar on seed germination.
Plant growth conditions
Seeds were germinated in a Conviron Model ATC26 growth chamber set with an 8 hr light/16 hr dark photoperiod and approximately 70 μmol m−2 s−1 photosynthetically active radiation supplied by incandescent and fluorescent lamps. The temperature was 25°/15° light/dark for ryegrass, 20°/20° for onions, lettuce and oats, and 30°/20° cabbage, carrot, cucumber, and tomato. Relative humidity was approximately 70%.
Plant measurements
Seedling germination (green hypocotyl visible) was observed and counted beginning the day after planting, and continued daily for approximately one week after maximum germination, when biomass was collected. Maximum germination was the date after which there was no or only a few more germinated plants. When biomass was collected a final count of live germinating seedlings was made, and live and dead shoots were cut off at the hypocotyl root junction and pooled per plate and dried at 60°C for at least three days. Data for analysis were on a per germination box basis. The % germinating seedlings was determined as: (live seedlings at harvest/seedlings planted) * 100.
Soil properties
A general description of the chemical characteristics of the parent biochar types is shown in Table 2. The pH, EC, extractable P (EP) and total phosphorus (TP) were measured for each biochar used in this study, while the other parameters are based on the literature and from unpublished data (personal communication Dr. J. Novak). Because biochar properties and, to a lesser extent, seed germination could affect soil pH, EC, and EP; these also were measured on air-dry soil collected following seedling harvest. After drying, soils were carefully scraped off the blotter paper. There were 4 replicate samples for pH, EC, and EP for each parent biochar type and each soil plus biochar type treatment.
Table 2.
Selected chemical properties of biochar pyrolyzed from different feedstocks and different temperatures used in this study. All % are on a % dry-weight basis except for pH (as H2O), EC and EP.
| Feedstock | °C | pH | EC | Ash | C | H | O | N | S | TP | EP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mS/cm | % | % | % | % | % | % | % | mg/L | |||
| Poultry litter | 350 | 8.73 | 16.45 | 35.9 a | 46.1 a | 3.7 a | 8.6 a | 5.0 a | 0.98 | 2.5 | 81.3 |
| Poultry litter | 500 | 9.76 | 18.94 | 40.9 c | 48.3 c | 1.5 c | 4.6 c | 3.9 c | 1.16 | 3.4 | 61.0 |
| Poultry litter | 700 | 10.30 | 20.39 | 52.4 a | 44.0 a | 0.3 a | 0.01 a | 2.8 a | 0.98 | 3.5 | 16.4 |
| PC:PL 55 | 350 | 7.68 | 8.59 | 18.5 b | 63.7 b | 3.8 c | 10.3 c | 3.4 b | 0.50 | 1.3 | 201.4 |
| PC:PL 55 | 500 | 9.99 | 8.99 | 22.2c | 69.4 c | 1.8 c | 3.7 c | 2.4 c | 0.48 | 1.8 | 100.1 |
| PC:PL 55 | 700 | 10.44 | 9.92 | 24.8 c | 71.2 c | 0.7 c | 1 c | 1.7 c | 0.30 | 1.8 | 67.4 |
| PC:PL 82 | 350 | 7.69 | 2.54 | 7.3 b | 75.8 b | 4.6 c | 11 c | 1.3 b | 0.15 | 0.5 | 195.0 |
| PC:PL 82 | 500 | 9.66 | 3.04 | 9.2 c | 83.6 c | 2.7 c | 3.2 c | 2.4 c | 0.14 | 0.6 | 104.2 |
| PC:PL 82 | 700 | 10.08 | 3.78 | 10.1 c | 88.6 c | 1 c | < 0.01c | 1.7 c | <0.01 | 0.5 | 60.3 |
| Swine solids | 350 | 6.94 | 3.14 | 33.3 c | 50.4 c | 4.3 c | 4.4 c | 6.6 c | 1.08 | 5.0 | 181.4 |
| Swine solids | 500 | 7.81 | 2.98 | 43.8 c | 45.1 c | 1.5 c | 3 c | 5.5 c | 1.01 | >5.0 | 195.2 |
| Swine solids | 700 | 8.74 | 1.64 | 48.8 c | 45.4 c | 0.5 c | 0.5 c | 3.6 c | 0.72 | >5.0 | 136.8 |
| Pine chip | 350 | 5.74 | 0.37 | 1.5 a | 74.7 a | 5.0 a | 18.4 a | 0.5 a | <0.01 | 0.03 | 7.2 |
| Pine chip | 500 | 7.57 | 0.42 | 2.6 c | 88.8 c | 3.1 c | 5 c | 0.5 c | <0.01 | 0.06 | 3.6 |
| Pine chip | 700 | 8.92 | 0.51 | 2.3 a | 87.2 a | 3.6 a | 6.5 a | 0.4 a | <0.01 | 0.02 | 0.04 |
| Switchgrass | 350 | 5.76 | 0.33 | 3.3 c | 75.4 c | 4.5 c | 16.2 c | 0.6 c | 0.07 | 0.08 | 13.6 |
| Switchgrass | 500 | 8.38 | 0.79 | 7.8 a | 84.4 a | 2.4 a | 4.3 a | 1.1 a | 0.07 | 0.15 | 44.7 |
| Switchgrass | 700 | 9.56 | 0.80 | 5.5 c | 94.1 c | 1.2 c | <0.01 c | 0.5 c | <0.01 | 0.06 | 30.2 |
Abbreviations: PC = pine chips, PL = poultry litter, 82 = 80% PC and 20% PL, 55 = 50% PC and 50% PL, EC = electrical conductivity, C = carbon, H = hydrogen, O = oxygen, N = nitrogen, S = sulfur, TP = total phosphorus and EP = extractable phosphorus. The pH, EC, and EP data are averages of four measurements from this study, the S and TP data are based on analysis of one sample by a commercial laboratory, and other data are based on Novak, Cantrell, and Watts (2013) a, Novak et al. (2014)b and J. Novak personal communication (2017)c.
The biochar pH and EC values were measured with a 2:1 water to biochar or soil ratio (v/v) using MilliQ water. The EC measurements were made prior to the pH measurements as pH electrodes change the EC of samples. The EC was measured with an Amber Science Electrical Conductivity Meter, Model 4083. The pH measurements were made with a Sartorius Economy hand-held pH/mV/ Temperature Meter, Model PT-15. The EP was measured spectrophotometrically using a following the procedure described by Olsen and Sommers (1982) Air-dried soil or biochar samples were extracted with a solution of 0.03 M ammonium fluoride (NH4F) and 0.025 M hydrochloric acid (HCl), shaken vigorously for 1 minute, centrifuged for 5 minutes at 4000 rpm, and filtered through Whatman#42 filter paper (5.5 cm diameter). The filtrate was measured by the ascorbic acid colorimetric method using a HACH DR6000 spectrophotometer at 882 nm. The EP concentration was determined by comparing sample absorbance to a P standard curve. Biochar S and total phosphorus (TP) was measured by a commercial laboratory (Bureau Veritas Minerals) for one sample for each biochar by Aqua Regia digestion and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analysis. For the digestion, a sample was cold leached with nitric acid, followed by a hot water digestion. After cooling, a modified Aqua Regia solution [(HCl), nitric acid (HNO3) and deionized water (DI H2O)] was added to each in a heating block of a hot water bath. Each sample was brought to volume with dilute HCl and filtered prior to analysis. Analysis was using a Perkin Elmer Inc. NexION 300 ICP-MS (Perkin Elmer, Waltham, MA).
Experimental design and statistical analysis
There were 19 soil × biochar combinations for each of the two soil types: nil biochar control, and soil with biochar (1% w/w) from one of the 18 biochars. There were four replicate acrylic containers for each of the 38 soil-biochar types, or 152 plates per species. Containers with each different soil and biochar were blocked by the two levels in the growth chamber, with two replicates of each soil and treatment combination in each block. The soil health parameters (pH, EC and EP) containers were not true replicates since all soil and biochar samples for all species were from the same batch of soil, and were not based on soil samples from various locations in the field. Thus, while the treatment means were accurate the variance was not true standard errors. However, because it would have been difficult to obtain the soil samples for true replication while maintaining uniform soils for the seed germination tests, and because there was some evidence that species could affect the soil health parameters, the statistical analysis for the soil health parameters using a form of pseudoreplication is included in this paper.
The % germination data were arcsine transformed and the shoot dry weight, EC, and EP data were log10 transformed prior to statistical analysis. A weighted analysis of variance (ANOVA) was used due to heterogeneity of variance, with weights proportional to the inverse of the variances for the transformed data for each soil and treatment combination. For an individual species, if the variance was 0 for a soil and treatment combination, a value 5 times the maximum weight (5*max) was used as the weight. For % germination across all species, if the variance was 0, the largest 5*max value of the species with 0 variance was used. A full ANOVA was conducted without the nil biochar control plants for each parameter considering species, block, feedstock, pyrolysis temperature, and soil type as main effects; and interactions among species, soil, feed- stock, and temperature using the PROC GLM procedure in SAS (SAS Inc, Cary, NC) (Table 3). Dunnett’s test (p < 0.05) considering blocks, was used to compare the individual feedstock × temperature treatments to the nil biochar control plants; using mixed model to account for repeated treatment using SAS (PROC MIXED, with REML estimation method). The analysis results were presented by soil if there was a significant treatment × soil interaction, and across soils if there was no significant interaction. The least squares mean and standard error data were back transformed for the figures for all parameters except pH. Version 9.4 of the SAS® System (SAS Inc., Cary, NC) for Windows®7 Enterprise operating systems for the personal computer (PC) was used for these analyses.
Table 3.
Results of across species analysis of variance for plants [% germination (% Germ) and shoot dry weight (SHDW) per plant], and soil [pH, electrical conductivity (EC), and extractable phosphorus (EP)].
| Parameter | BK | SP | FS | SP*FS | T | SP*T | FS*T | SP*FS | SO | SP*SO | SO*FS | SP*SO | SO*T | SP*SO | SO*FS | SP*SO | repa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *T | *FS | *T | *T | *FS*T | |||||||||||||
| ANOVA P VALUES | |||||||||||||||||
| % Germ | 0.390 | <0.001 | 0.198 | 0.032 | 0.040 | 0.492 | 0.563 | 0.116 | <0.001 | <0.001 | 0.874 | 0.001 | 0.711 | 0.382 | 0.110 | 0.042 | 0.758 |
| SHDW | 0.019 | <0.001 | <0.001 | 0.001 | 0.583 | 0.235 | 0.004 | 0.026 | <0.001 | <0.001 | 0.038 | 0.005 | 0.049 | 0.569 | 0.311 | 0.157 | 0.926 |
| pH | 0.175 | <0.001 | <0.001 | 0.002 | <0.001 | 0.471 | <0.001 | 0.862 | <0.001 | <0.001 | <0.001 | 0.674 | 0.029 | 0.789 | <0.001 | 0.925 | 0.067 |
| EC | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | 0.599 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | 0.097 | 0.004 | 0.459 | <0.001 | 0.121 | 0.832 |
| EP | 0.045 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.662 |
| ANOVA F VALUES | |||||||||||||||||
| % Germ | 0.7 | 336.5 | 1.5 | 1.5 | 3.2 | 1.0 | 0.9 | 1.2 | 41.9 | 11.1 | 0.4 | 1.9 | 0.3 | 1.1 | 1.6 | 1.3 | 0.1 |
| SHDW | 5.5 | 24064.6 | 23.4 | 1.9 | 0.5 | 1.3 | 2.6 | 1.4 | 231.0 | 35.7 | 2.4 | 1.8 | 3.0 | 0.9 | 1.2 | 1.2 | 0.0 |
| pH | 1.8 | 253.8 | 505.0 | 1.9 | 91.6 | 1.0 | 10.1 | 0.8 | 7349.4 | 24.6 | 44.0 | 0.9 | 3.5 | 0.7 | 3.3 | 0.8 | 3.4 |
| EC | 7.9 | 114.6 | 850.4 | 19.9 | 91.2 | 0.9 | 15.5 | 1.6 | 1645.5 | 10.6 | 40.7 | 1.3 | 5.6 | 1.0 | 3.4 | 1.2 | .00 |
| EP | 4.0 | 27.2 | 25914.3 | 22.0 | 121.2 | 7.0 | 45.9 | 6.4 | 21447.6 | 15.3 | 2168.2 | 11.3 | 18.5 | 7.4 | 3.6 | 6.1 | 0.2 |
Significant p values at 0.05 are in bold. Treatment abbreviations: BK = block, SP = species, FS = feedstock, T = temperature, SO = soil; other abbreviations: repa = replicates within a block. Note that if an F value was very large in comparison to others (in bold), that factor would be considered as more important than others, even if they all had significant p values.
Results and Discussion
Seed germination and shoot growth
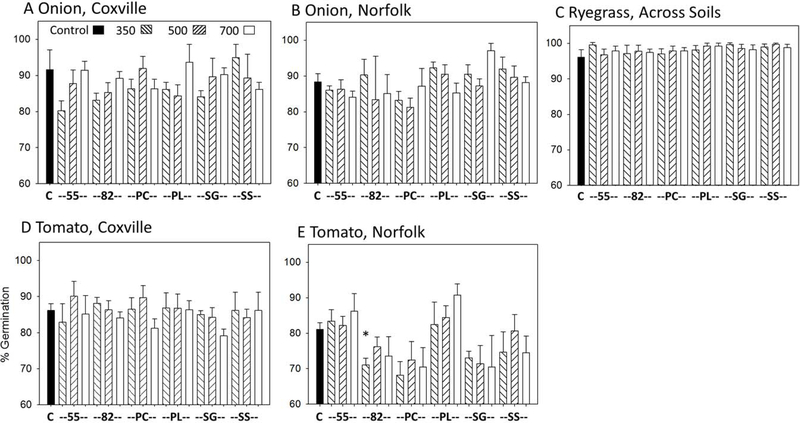
Overall, the test was successful in providing a tool to assess germination and early shoot growth for a variety of crop species, even though there were few significant responses due to biochar. The results from the overall ANOVA (Table 3) across species indicated that seed germination was affected primarily by species and soil type, with % germination lower across all treatments with the Norfolk than with Coxville soil carrot, cucumber, and tomato (Figures 2 and 3). The % germination also was slightly higher with the Coxville than with the Norfolk soil for cabbage across treatments (data not shown). The ANOVA indicated only a few feedstock or feedstock × temperature interactions, which were likely inconsequential across species as the F values were so much lower than for species and soil. However, even though the F values were small for any species × treatment interaction, the Dunnett’s test for germination was by species because there were a few significant responses, which could not be observed if the analysis was across species. Figures 2 and 3 illustrate the results of the Dunnett’s test for each species and soil, with results across soils if there was no significant soil × treatment effect. Based on Dunnett’s test, the only individual treatments that were significantly different from the nil biochar controls were an increased germination with PL at 500° C for carrot and the Coxville soil (Figure 2B), and with the 55 mixture at 350° C and PC at 700° C for lettuce and the Coxville soil (Figure 2F); and a decreased germination with the 82 mixture at 350° C for tomato and the Norfolk soil (Figure 3G). In these cases, the significant germination response primarily may be related to tighter error bars compared to other treatments, even though the decreased germina- tion for tomato and the Norfolk soil with the 82 mixture was consistent with the response for PC biochars.
Figure 2.
Effects of biochar on seed germination for cabbage across soils (A), carrot with Coxville (B) and Norfolk (C) soil, cucumber with Coxville (D) and Norfolk (E) soil, lettuce with Coxville (F) and Norfolk (G) soil, and oat across soils (H). Note different y-axis scale for carrot. Bars are means plus upper bound standard error for four replicate trays; except for three for Oat, Norfolk, SG-700. An “*” indicates a significant difference vs. the controls at p < 0.05 using Dunnett’s test. There were no significant differences between biochar treatments and controls for cabbage, cucumber, and oat. Biochar treatment abbreviations are: C = control (no biochar), PC = pine chips, PL = poultry litter, 55 = 50% PC and 50% PL, 82 = 80% PC and 20% PL, SG = switchgrass, and SS = swine solids. The boxes in panel “A” indicate no biochar controls, and biochars pyrolyzed at 350°, 500° and 700°C.
Figure 3.
Effects of biochar on seed germination for onion with the Coxville (A) and Norfolk (B) soil, ryegrass across soils (C), and tomato with the Coxville (D) and Norfolk (E) soil. Bars are means plus upper bound standard error for four replicate trays. An “*” indicates a significant difference vs. the controls at p < 0.05 using Dunnett’s test. There were no significant differences between biochar treatments and controls for onion and ryegrass. Biochar treatment abbreviations are: C = control (no biochar), PC = pine chips, PL = poultry litter, 55 = 50% PC and 50% PL, 82 = 80% PC and 20% PL, SG = switchgrass, and SS = swine solids. The boxes in panel “A” indicate no biochar controls, and biochars pyrolyzed at 350°, 500° and 700°C.
In a germination test with similar species, Gascó et al. (2016) reported generally decreased germination and early seedling growth with biochars. For tomato, Gascó et al. (2016) reported a decreased germination with a cellulosic (wood chip) biochar; whereas we found a trend for decreased germination for the Norfolk soil with the cellulosic pine chip and switchgrass biochars, and a significant decrease in germination only for the predominantly cellulosic 82 biochar at a 350°C pyrolysis temperature. Both Gascó et al. (2016) and our study found no change in germination for cucumber with both cellulosic and manure-type biochars. However, while Gascó et al. (2016) found germination inhibition for lettuce with both cellulosic and manure-type biochars, we found slightly increased germination for lettuce primarily with mature-type biochars. The reason for the differ- ences between our study and Gascó et al. (2016) is unknown.
The species and soil affected early seedling shoot dry weight across species, and there were a few feedstock temperature or soil interactions based on the ANOVA (Table 3). Even though the interactions would not be considered to be meaningful due to the small F values; as for germination, the Dunnett’s test for germination was by species because there were a few significant responses that could not be observed across species. Based on the Dunnett’s test a few species had significant increases in shoot dry weight for individual feedstock by temperature treatments vs. the controls. Shoot dry weight increased for carrot across both soils with PL at 500°C (Figure 4C); lettuce planted in Coxville soil with PL or SS at 500°C (Figure 4F); and lettuce planted in Norfolk soil with the 55 mixture or PL at 350° C, planted in the 82 mixture, PL or SS at 500°C, and planted in the 55 mixture, PL or SS at 700° C (Figure 4G). There were also increases in shoot dry weight for oats planted in the Coxville soil with the 55 mixture at 350° and 500°C and at SG at 700°C (Figure 5A), and in the Norfolk soil with PL at 500 C (Figure 5B). Shoot dry weight increased for tomato planted in Coxville soil with the 82 mixture, PL and SS at 350°C, with the 55 mixture at 500°C, and with PL and SG at 700°C (Figure 5F). However, there were no significant shoot dry weight responses for tomato planted in the Norfolk soil (Figure 5G). Overall, the most consistent and greatest responses were the increases in shoot dry weight for the manure-based biochars, PL and SS. This was in contrast to the results reported by Gascó et al. (2016) who found no effect of wood chip, paper sludge, and wheat husks, or sewage sludge biochars on shoot biomass in lettuce; and a soil-depend response to these biochars in lentil.
Figure 4.
Effects of biochar on shoot dry weight per plant for cabbage with the Coxville (A) and (B) Norfolk soil, carrot across soils (C), cucumber with Coxville (D) and Norfolk (E) soil, and lettuce with Coxville (F) and Norfolk (G) soil. Bars are means plus upper bound standard error for four replicate trays. An “*” indicates a significant difference vs. the controls at p < 0.05 using Dunnett’s test. There were no significant differences between biochar treatments and controls for cabbage. Biochar treatment abbreviations are: C = control (no biochar), PC = pine chips, PL = poultry litter, 55 = 50% PC and 50% PL, 82 = 80% PC and 20% PL, SG = switchgrass, and SS = swine solids. The boxes in panel “A” indicate no biochar controls, and biochars pyrolyzed at 350°, 500° and 700°C.
Figure 5.
Effects of biochar on shoot dry weight per plant for oat with the Coxville (A) and Norfolk (B) soil, onion with Coxville (C) and Norfolk (D) soil, and ryegrass across soils (E), and tomato with (F) Coxville and (G) Norfolk soil. Bars are means plus upper bound standard error for four replicate trays, except for three for oat, Norfolk SG 700. An “*” indicates a significant difference vs. the controls at p < 0.05 using Dunnett’s test. There were no significant differences between biochar treatments and controls for onion and ryegrass. Biochar treatment abbreviations are: C = control (no biochar), PC = pine chips, PL = poultry litter, 55 = 50% PC and 50% PL, 82 = 80% PC and 20% PL, SG = switchgrass, and SS = swine solids. The boxes in panel “A” indicate no biochar controls, and biochars pyrolyzed at 350°, 500° and 700°C.
Biochar pH, EC, EP and TP
Biochar pH and EC generally were highest with PL followed by the PL and PC mixtures; medium for SS and lowest with PC and SG alone (Table 2). The pH and EC generally increased with increasing pyrolysis temperature, except for the SS where the EC decreased with increasing temperature. The highest biochar pH was > 10 for PL, and 55 and 82 mixtures of PC and PL pyrolyzed at 700°C; and lowest pH < 6 for PC and SG pyrolyzed at 350°C. The EC was much higher with PL (> 16 mS/cm), especially pyrolyzed at 700°C, than the other biochars; and was very low (< 0.4 mS/cm) for the PC and SG pyrolyzed at 350°C. The high EC and pH with PL was related to its much higher ash content (> 30%) compared with <8% ash for SG and PC. The higher ash content with the manure based feedstocks PL and SS also was related to their higher mineral content (e.g. N, S, and TP) compared with the cellulosic feedstocks.
Biochar EP was highest with the SS and mixtures of PC and PL (Table 2). The PL had an intermediate level of EP, and SG and PC biochars had the lowest EP. The high EP with the mixtures was surprising since both PL and especially PC alone had lower EP concentration. Increasing the pyrolysis temperature generally decreased the EP, especially for PC at 700°C, where EP reached a very low level of 0.04 mg L−1. For SS, increasing temperature had less effect on EP than for the other biochar; where the 500°C EP was actually higher than the 350°C, and the 700°C only showed a 25% decrease in EP compared with the 350°C biochar. For SG, increasing temperature from 350°C to 500°C resulted in increased EP. Previously Novak et al. (2009) had indicated a much higher exchangeable-P (similar to EP) for the PL than for the SG biochar. They also reported a higher exchangeable-P level with biochar pyrolyzed at 700°C than at 350°C.
Biochar TP was highest with the biochars derived from manures; SS (≥ 5%) and PL (2.5–3.5%) with slightly higher concentrations with the higher temperatures (Table 2). The PL and PC mixtures had intermediate TP concentrations while the TP was low (0.02–0.15%) for the biochars derived from cellulosic feedstocks, SG, and PC.
Soil pH, EC and EP
The soil health parameters were affected by species and nearly all factors based on the across-species ANOVA using the pseudoreplicates (Table 3). Because the F values were so much larger for feedstock and soil than for species and were generally consistent across species, the Dunnett’s tests were across species in the text of this paper, with results by species in the supplementary figures.
Soil pH, either was raised by biochar or unchanged compared to no biochar controls depending on biochar type and soil. For the Coxville soil, all biochars significantly increased pH except for PC at 350°C (Figure 6A). For the Norfolk soil, the response was similar, but there were fewer significant effects for the 82 mixture, PC and SG (Figure 6B). In general, PL increased pH to the greatest extent of any biochar, and the biochar effects increased with pyrolysis temperature for all biochars.
Figure 6.
Effects of biochar on soil health across species for pH with the Coxville (A) and Norfolk (B) soil, electrical conductivity (EC) with the Coxville (C) and Norfolk (D), and extractable phosphorus (EP) with the Coxville (E) and Norfolk (F) soil. Biochar treatment abbreviations: C = control (no biochar), PC = pine chips, PL = poultry litter, 55 = 50% PC and 50% PL (to left), 82 = 80% PC and 20% SS, SG = switchgrass, and SS = swine solids. Bars are means plus upper bound standard error for a target of 32 replicate trays per treatment, except as follows: for pH, EC, and EP there were 31 trays for Coxville 82 at 350°, for PL and SS at 700°; and for Norfolk PL and SG at 700°. There were also 31 trays for Coxville pH and EC 55 at 350°, P for PC at 350° and 500°; and for Norfolk P controls, PL at 350° and 500°; 55 at 500°, SS at 700°; and Norfolk pH for PC at 350°. There were 30 trays for Norfolk P SS at 500° and 29 trays for Coxville P 55 at 350°. An “*” indicates a significant difference from the controls at p < 0.05 using Dunnett’s test. The boxes in panel “A” indicate no biochar controls, and biochars pyrolyzed at 350°, 500° and 700°C.
There were possible differences in response for individual species from the general pattern across species (Supplementary Figures 1 and 2). In terms of some noteworthy examples, the increased pH with nearly all biochars for the Coxville soil seen across species was only evident for carrot (with some minor differences shown in Supplementary Figure 1C). Cucumber pH in the Norfolk soil was only affected by SS and PL at 350°, SS at 500°, and PL SS and 55 at 700°C (Supplementary Figure 1F). Onion pH increased in the Norfolk soil only with PL at all temperatures and 55 at 500° and 700°C (Supplementary Figure 2C). Ryegrass pH in the Coxville soil was only affected by PL at the higher temperatures (Supplementary Figure 2D), and ryegrass in the Norfolk was only affected by PL, and 55 at the highest temperature (Supplementary Figure 2E). These differences among specific species likely occurred due to slightly different treatment means and variances, which were not important across species.
Soil EC increased dramatically with PL, SS and 55 for both soils, (Figures 6C and 6D). The EC also increased with 82 at 350°C in the Coxville soil (Figure 6C) and with 82 for all temperatures in the Norfolk soil (Figure 6D). The EC tended to decrease with decreasing pyrolysis temperatures, except for PL; and was significantly reduced vs. the controls for PC at 500°C and 700°C for the Coxville soil (Figure 6C) and SG at 700°C for the Norfolk soil (Figure 6D). The greatest differences in individual species vs. across species effects were for cabbage and tomato in the Coxville soil where EC increased only with PL (Supplementary Figures 3A and 4G, respectively), for cucumber in the Coxville soil where there was only a decrease in EC with 82 at 500°C (Supplementary Figure 3E), and for oat in the Coxville soil where there were no treatment effects (Supplementary Figure 4A). There were no effects on EC with 82 for cabbage in the Coxville soil (Supplementary Figure 3A), cucumber in the Norfolk soil (Supplementary Figure 3F), lettuce in either soil (Supplementary Figure 3G,H), oat in either soil (Supplementary Figure 4A,B), or onion, ryegrass or tomato in the Coxville soil (Supplementary Figure 4C,E,G, respectively).
Across species and for both soils, soil EP increased with PL, SS and the 55 and 82 mixtures of PL and PC (Figure 6E,F). The largest increase was with SS, especially for the Norfolk soil, which had substantially lower EP in the control soil (~ 3 mg/L); compared with the control Coxville soil (~13 mg/L). The smallest increases were with the 82 mixtures. Soil EP was not affected by PC for either soil or SG for the Coxville soil. Soil P also increased slightly with 500° and 700° for SG and the Norfolk soil (Figure 6F). However, there were few other differences in temperature response for a feedstock.
Based on the per species analysis, the pattern of increase in EP was similar across species (Supplementary Figure 5,6). The most notable differences were the lack of a consistent increase in soil EP with the 82 mixture in the Coxville soil for cucumber at the higher temperatures (Supplementary Figure 5E) and ryegrass at all temperatures (Supplementary Figure 6E). There were a few small increases in EP with PC for Cabbage in the Coxville soil (Supplementary Figure 5A), carrot in the Norfolk soil (Supplementary Figure 5D), and tomato in both soils (Supplementary Figure 6G,H); and with SG for cabbage (Supplementary Figure 5B) and ryegrass in the Norfolk soil (Supplementary Figure 6F), and tomato in both soils (Supplementary Figure 6G,H).
Success of the test
This study provided a rapid screening test to determine biochar suitability for initial plant growth. An advantage of our method is that it provided uniform seedlings and adequate replication to detect biochar effects on plants, using a minimum amount of soil. Furthermore, the test had the added benefit of providing material to determine key soil health characteristics in response to biochar treatments (e.g., pH, EC, and EP), with the presence of germinating seeds and young seedlings.
Even though there are commercially available test kits, they are more useful for short-term evaluation of a limited number of seeds and treatments. For example, the Microbiotests Inc. Phytotoxkit™ uses only a few seeds per replicated unit, is meant to evaluate germination after a few days, and comes with only one of three plant species (http://www.microbiotests.be/SOPs/Phytotoxkit%20SOP%20-%20A5.pdf). In contrast, our rapid-test provides for a large number of replicate seeds and containers, can go for up to 2 weeks or longer depending on the amount of soil used, the size of the seedlings, and length of time to germination, and can be used with a variety of species.
While this rapid-test indicated the early effects of biochar on seed germination, which is critical for seedling establishment and plant growth; it was difficult to tell whether those effects correspond to longer term seedling establishment and growth effects. Ellis (1992) reviewed the relationship between seed germination and crop growth and yield and found both indirect (percentage emergence and time from sowing to emergence) effects, as well as direct effects. However, comparative studies where seed germination and plant growth are compared are rare. In a separate study, we determined the effects of biochar on crop seedling growth using plants in pots (Olszyk et al. Unpublished data). Seedling growth data from the pot study indicated increased shoot dry weights with many biochar treatments for lettuce in both soil types, especially with the 55 mixture of PC and PL, and the SS. Carrots also showed increases in shoot dry weights primarily with the 55 mixture and SS, but only for the Norfolk soil. In contrast, the germination test underestimated the biochar effects found in pots. In the germination test, lettuce had significant increases in early seedling growth for the Norfolk soil, primarily with the 55 and PL and SS treatments, but had few effects of biochar on early seedling growth for the Coxville soil. Carrot only had a significant increase in shoot dry weight with the 500°C PL treatment across soils. In a similar study, Van Zwieten et al. (2010) found that biochar increased wheat seed germination in the acidic ferrosol, but had no effect on wheat seed germination in the higher pH calcarosol or for radish or soybean in either soil. Visioli et al. (2016) used a Petri dish test to determine the phytotoxicity of biochar mixed with soil and reported that seed germination was a less sensitive indicator of adverse effects from biochar than root elongation.
Other research comparing rapid germination test results with longer term plant studies with environmental stressors also indicated differences in responses. In a study of a rapid-test using seeds in Petri dishes, Ghanizadeh et al. (2015) compared the rapid-test results to those from a greenhouse pot study to determine dicamba herbicide response of different weed populations. They found that the rapid-test indicated the same differences in weed population responses as the pot study, but that the rapid-test overestimated weed herbicide resistance. In a study Brassica napa, Meng et al. (2009) also noted no effect on seed germination but an effect on older seedling fresh weight with 50 μM Cd. When studying the effects of salt stress on Salsola ikonnikovii, Xing et al. (2013) reported inhibition of germination with 100 mmol L−1 NaCl, but growth stimulation with the same salt concentration in a greenhouse pot study. The germination study used a filter paper and Petri dish method with salt solutions without soil; thus, the soil in the pot study likely mediated the salt effects. In another study with salt stress, Almansouri, Kinet, and Lutts (2001) reported no differences among 3 wheat cultivars in terms of seed germination responses using a Petri dish test, even though there were large differences in salt sensitivity among these cultivars based on a greenhouse pot study.
Test as indicator of changes in soil health
While seed germination and early plant growth can be indicated by this test, the test provided more substantial evidence of the effects of biochar and possible interactions with crop species and the soil characteristics, pH, EC, and EP. The pH is important to measure after biochar additions because biochars themselves can vary greatly in pH; which impacts soil pH, and, hence, nutrient availability and turnover processes. The literature indicates that biochar produced from manures had higher pH values than plant-based biochars (Rajkovich et al. 2012). For example, Chan et al. (2008) reported an increase in soil pH with PL biochar in a pot study evaluation of the growth of radish. As a result of the inherent differences in biochar properties, soil and biochar incubation experiments indicate that soil pHs were raised more by animal manure-based biochars because they have higher salt contents and are more alkaline than lignocellulosic biochars (Novak et al. 2014). Biochar may also increase pH more for acidic vs. more neutral or calcareous soils (McDonald et al. 2014; Van Zwieten et al. 2010) and for sandy soils vs. heavier soils (Butnan et al. 2015). However, in our study pH was raised to a similar extent (as a % of the control pH) for the sandier Norfolk soil as in the Coxville soil. The study by McDonald et al. (2014) is especially relevant as they also used acidic soils, measured similar soil parameters and had a similar rate of 1.33% (w/w) of PL or wheat straw (WS) biochar. We found some effects of the woody biochar PC or SG on pH (Figure 6A,B); similar to the increase in pH with woody biochar reported by Reverchon et al. (2015), or with grass (rice) straw reported by Yang et al. (2016). In the future, we may consider biochar measuring calcium carbonate equivalents (Van Zwieten et al. 2010), as a better indicator of acid neutralizing capacity than pH.
The higher EC with the PL and to a lesser extent the 55 mixture and SS in our study likely were due to the soluble salts content reported in these manure-based biochars (Novak et al. 2014). Novak et al. (2014) reported a biochar K concentration of approximately 5.9% on a dry weight basis for PL produced at 350°C, and 4.1% for a 50:50 mixture of PC and PL produced at 350°C. In their study, addition of PL produced at 350°C resulted in an increase in soil K to 209 mg kg−1 compared to 19 mg kg−1 for the Norfolk soil without biochar. In contrast, the SG biochar produced at 500°C had a K concentration of only 1.16%, which raised the soil K to 85 mg kg−1 (Novak et al. 2014), which would account for the lack of an effect of EC from this and similar cellulosic biochar treatments.
Researchers in other studies reported similar results for the different biochars. McDonald et al. (2014) found an increase in soil EC with either PL or WS biochar, primarily for acidic soils. Chan et al. (2008) also reported an increase in soil EC after incubation with PL biochar. In contrast with a cellulosic biochar, Brewer et al. (2011) reported that no change in soil EC for SG biochars produced by fluidized bed fast pyrolysis between 450 and 550°C, and a possible decrease (though not statistically significant) in soil EC with wood product biochars compared with controls. Similarly, Reverchon et al. (2015) reported no change in soil EC with 2.5% wood biochar. Yang et al. (2016) also reported no change in EC for 1% course rice straw biochar, while fine rice straw biochar increased EC. However, while we used 1% biochar in our study, with higher application rates of these materials (e.g., 3%) both grass straw and woody biochar may increase both soil EC and pH (Kloss et al. 2014).
The large increases in soil EP with SS, PL and mixtures of PL and PC indicated that this rapid-test is sensitive to this important soil parameter. Our large increase in EP with PL was similar to the increases seen for the Norfolk soil after a 127-day pot incubation study (Novak et al. 2014). Also, similar to our results, Novak et al. (2014) reported only a slight increase in Norfolk soil P with SG as the biochar feedstock. McDonald et al. (2014) found an increase in soil Olsen P with either poultry litter or wheat straw biochar. A short-term test such as ours is well suited to detect increases in soil EP with biochar due to the rapid release rate of EP from soils (e.g. 55% from rice hull biochar in 3–4 days, Qian et al. 2013). Increases in extractable soil P also were observed in other studies. For example, Laird et al. (2010) reported an increase in Mehlich III extractable P with mixed hardwood biochar addition to a Hapludoll soil in a 500-day incubation study, and Kloss et al. (2014) found an increase in calcium-acetate-lactate extractable P with grass straw and woody biochars in a green- house plant/pot study.
An increase in soil EP is important as it would likely increase the availability of this important nutrient which can benefit plant growth, especially for relatively P infertile soils such as those from the southern coastal plain of the US. However, application of high EP biochars such manures could exacerbate P leaching problems (Troy et al. 2014). There was little change in soil EP with increasing temperature for a given biochar, which reflected the uniform biochar TP with different pyrolysis, but was in contrast to general decrease in biochar EP with increasing temperatures. The apparent decrease in biochar EP may reflect a change to more recalcitrant forms of P as temperature increases, rather than any actual loss of P due to volatilization as P4O10 as reported when woody material is combusted in the presence of oxygen above its sublimation point of 300°C (Raison, Khanna, and Woods 1985, https://pubchem.ncbi.nlm.nih.gov/compound/phosphorus_pentoxide, downloaded 20 March 2018). However, despite the decreases in biochar EP with increasing temperature, there still was enough biochar EP with all temperatures to increase the soil EP.
Other uses for the test
While this rapid-test for seed germination was developed assessing the effects of biochar on productivity of agricultural soils, it could be used to study other soil environmental stressors. For example, this test could be used to enhance restoration of abandoned mine sites (Beesley et al. 2011), by testing the effects of biochar on seed germination and growth prior to fieldwork. González-Valdez et al. (2016) used petri dishes with main tailings to determine their effect on seed germination. Our rapid-test system could use different types of biochar and mine tailings or mine soils to determine if biochar can alleviate adverse effects on seed germination. Our method could be used as an early screen of effects of other amend- ments in addition to biochar on soil characteristics. For example, the rapid-test could improve on laboratory studies on the effects of lime and other amendments in addition to biochar on soils such as conducted by (Qayyum et al. 2015) and (Holes et al. 2014); and before going to field trials (Slavich et al. 2013) could indicate the effects of adding lime and/or fertilizer to biochar. In addition, to effects on shoots, the rapid-test can be adapted to include effects on root systems, which are an important endpoint which may be more sensitive to biochar contaminants than shoots (Visioli et al. 2016). Finally, because of its ability for detecting germination in a controlled, replicated way, it also could provide an option for an improved pesticide and chemical toxicity seed germination text (OECD 2006; US EPA 2012).
Conclusions
This study indicated a rapid test which can provide an early measure of possible beneficial or detrimental effects of biochar on seed germination and early seedling growth, and early effects on salient soil health characteristics such as pH, EC, and EP. Biochar had few effects on seed germination, but increased shoot dry weight for carrot, cucumber, lettuce, oat, and tomato; primarily with biochars produced from PL. The test detected primarily increased soil pH and EC, especially with PL and SS, and the response varied with plant species. The test provided an early indicator that biochar increased EP, especially with the PL, SS, 55 and 82 biochars, with very little difference among species. The test could suggest possible responses of older plants, even though it may underestimate responses which occur over time. Benefits of the test include multiple replicated units with automated seed placement, a large number of observations (target of 25 seeds in each of 4 replicates per treatment), can be completed in a small space under controlled conditions to reduce environmental variability, and indicate biochar impacts on soil characteristics after a relatively brief period of time (2 weeks). Because of its flexibility, it could be used for other soil-based stressors using a limited amount of soil and contaminant.
Supplementary Material
Acknowledgements
The authors would like to thank Mr. Dale Brown of the Oregon State University Seed Laboratory for his assistance in guiding us in the development of the seed germination procedure. Would also thank Dr. E. Henry Lee of the US EPA, National Health and Environmental Effects Research Laboratory, Western Ecology Division; and Dr. Maliha Nash of the US EPA, National Exposure Research Laboratory, System Exposure Division for their assistance in developing the experimental design for this study and statistical analysis procedures, and Mr. Leon Reece, of the National Asian Pacific Center Senior Environmental Employment Program, for assistance in monitoring and maintaining growth chamber conditions and data collection. We also thank the EPA Greater Research Opportunity Intern Ms. Amber White for her valuable assistance with the plant experiments and soil pH analysis.
Footnotes
Disclaimer
The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency or the U.S. Department of Agriculture. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the U.S. Environmental Protection Agency or the US Department of Agriculture. The EPA or the USDA does not endorse any commercial products, services, or enterprises. The USDA is an equal opportunity employer.
References
- Almansouri M, Kinet JK, and Lutts S 2001. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant and Soil 231:243–54. [Google Scholar]
- Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, and Sizmur T 2011. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environmental Pollution 159:3269–82. [DOI] [PubMed] [Google Scholar]
- Brewer CE, Unger R, Schmidt-Rohr K, and Brown RC 2011. Criteria to select biochars for field studies based on biochar chemical properties. Bioenergy Research 4:312–23. [Google Scholar]
- Buss W, and Mašek O 2014. Mobile organic compounds in biochar - A potential source of contamination - Phytotoxic effects on cress seed (Lepidium sativum) germination. Journal of Environmental Management 137:111–19. [DOI] [PubMed] [Google Scholar]
- Butnan S, Deenik JL, Toomsan B, Antal MJ, and Vityakona P 2015. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 237–238:105–16. [Google Scholar]
- Chan KY, Van Zwieten L, Meszaros I, Downie A, and Joseph S 2008. Using poultry litter biochars as soil amendments. Australian Journal of Soil Research 46:437–44. [Google Scholar]
- Chan KY, and Xu Z 2009. Biochar: Nutrient properties and their enrichment In Biochar for environmental management: Science and technology, ed. Lehmann J, and Joseph S, 67–84. London: Earthscan. [Google Scholar]
- DeLuca TH, MacKenzie MD, and Gundale MJ 2009. Biochar effects on soil nutrient transformations In Biochar for environmental management: Science and technology, ed. Lehmann J, and Joseph S, 251–70. London: Earthscan. [Google Scholar]
- Deska J, Jankowski K, Bombik A, and Jankowska J 2011. Effect of growing medium pH on germination and initial development of some grassland plants. Acta Scientiarum Polonorum Agricultura 10:45–56. [Google Scholar]
- Ellis RH 1992. Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regulation 11:249–55. [Google Scholar]
- Free HF, McGill CR, Rowarth JS, and Hedley MJ 2010. The effect of biochars on maize (Zea mays) germination. New Zealand Journal of Agricultural Research 53:1–4. [Google Scholar]
- Gascó G, Cely P, Paz-Ferreiro J, Plaza C, and Méndez A 2016. Relation between biochar properties and effects on seed germination and plant development. Biological Agriculture & Horticulture 32:237–47. [Google Scholar]
- Ghanizadeh H, Harrington KC, James TK, and Woolley DJ 2015. A quick test using seeds for detecting dicamba resistance in fathen (Chenopodium album). Australian Journal of Crop Science 9:337–43. [Google Scholar]
- Gong P, Wilke B-M, Strozzi E, and Fleischmann S 2001. Evaluation and refinement of a continuous seed germination and early seedling growth test for the use in the ecotoxicological assessment of soils. Chemosphere 44:491–500. [DOI] [PubMed] [Google Scholar]
- González-Valdez E, Alarcón A, Ferrera-Cerrato R, Vega-Carrillo HR, Maldonado-Vega M, and Salas- Luévano MA 2016. Seed germination and seedling growth of five plant species for assessing potential strategies to stabilizing or recovering metals from mine tailings. Water Air and Soil Pollution 227:24. [Google Scholar]
- Holes A, Szegi T, Fuchs M, Gulyás M, and Aleksza L 2014. Effects of different biochars, compost and lime treatments on the chemical properties of sandy soils. Columella - Journal of Agricultural and Environmental Sciences 1:49–55. [Google Scholar]
- ISTA. 2016. International rules for seed testing. Bassersdorf, Switzerland: International Seed Testing Association (ISTA). [Google Scholar]
- Kloss S, Zehetner F, Wimmer B, Buecker J, Rempt F, and Soja G 2014. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. Journal of Plant Nutrition and Soil Science 177:3–15. [Google Scholar]
- Laird DA, Fleming P, Davis DD, Horton R, Wang B, and Karlem DL 2010. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–49. [Google Scholar]
- Lehmann J, and Joseph S 2009. Biochar for environmental management: Science and technology. 1st ed. Sterling, VA: Earthscan. [Google Scholar]
- McDonald LM, Farrell M, Van Zwieten L, and Krull ES 2014. Plant growth responses to biochar addition: An Australian soils perspective. Biology and Fertility of Soils 50:1035–45. [Google Scholar]
- Meng H, Hua S, Shamsi IH, Jilani G, Li Y, and Jiang L 2009. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regulation 58:47–59. [Google Scholar]
- Niu G, Rodriguez DS, Cabrera R, Jifon J, Leskovar D, and Crosby K 2010. Salinity and soil type effects on emergence and growth of pepper seedlings. HortScience 45:1265–69. [Google Scholar]
- Novak JM, Busscher WJ, Watts DW, Amonette J, Ippolito JA, Lima IM, Gaskin J, Das KC, Steiner C, Ahmedna M, Rehrah D, and Schomberg H 2012. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Science 177:310–20. [Google Scholar]
- Novak JM, Cantrell KB, and Watts DW 2013. Compositional and thermal evaluations of lignocellulosic and poultry litter chars via high and low temperature pyrolysis. BioEnergy Research 6:114–30. [Google Scholar]
- Novak JM, Cantrell KB, Watts DW, Busscher WJ, and Johnson MG 2014. Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. Journal of Soils and Sediments 14:330–43. [Google Scholar]
- Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das KC, Ahmedna M, Rehrah D, Watts DW, Busscher WJ, and Schomberg H 2009. Characterization of designer biochar produced at different temperatures and their effects on loamy sand. Annals of Environmental Science 3:195–206. [Google Scholar]
- Oleszczuk P, Jośko I, and Kuśmiez M 2013. Biochar properties regarding to contaminants content and ecotox- icological assessment. Journal of Hazardous Materials 260:375–82. [DOI] [PubMed] [Google Scholar]
- Olsen SR, and Sommers LE 1982. Phosphorus. Methods of soil analysis. Part 2 In Chemical and microbiological properties, ed. Page AL, 2nd ed., 403–30. Madison, WI, USA: America Society of Agronomy, Inc., Soil Science Society of America, Inc. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). 2006. Test No. 208: Terrestrial plant test: 1 Seedling emergence and seedling growth. Paris, France: OECD Guidelines for the Testing of Chemicals. [Google Scholar]
- Qayyum MF, Ashraf I, Abid M, and Steffens D 2015. Effect of biochar, lime, and compost application on phosphorus adsorption in a Ferralsol. Journal of Plant Nutrition and Soil Science 178:576–81. [Google Scholar]
- Qian T, Xuesong Z, Jianyang H, and Jaing H 2013. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 93:2069–75. [DOI] [PubMed] [Google Scholar]
- Raison RJ, Khanna PK, and Woods PV 1985. Mechanisms of element transfer to the atmosphere during vegetation fires. Canadian Journal of Forest Research 15:132–40. [Google Scholar]
- Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, and Lehmann J 2012. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biology and Fertility of Soils 48:271–84. [Google Scholar]
- Reverchon F, Yang H, Ho TY, Yan G, Wang J, Xu Z, Chen C, and Zhang D 2015. A preliminary assessment of the potential of using an acacia—Biochar system for spent mine site rehabilitation. Environmental Science and Pollution Research 22:2138–44. [DOI] [PubMed] [Google Scholar]
- Rogovska N, Laird D, Cruse RM, Trabue S, and Heaton E 2011. Germination tests for assessing biochar quality. Journal of Environmental Quality 41:1014–22. [DOI] [PubMed] [Google Scholar]
- Rosende M, Beesley L, Moreno-Jimenez E, and Miró M 2016. Automatic flow-through dynamic extraction: A fast tool to evaluate char-based remediation of multi-element contaminated mine soils. Talanta 148:686–93. [DOI] [PubMed] [Google Scholar]
- Sigua GC, Novak JM, Watts DW, Cantrell KB, Shumaker PD, Szögi AA, and Johnson MG 2014. Carbon mineralization in two ultisols amended with different sources and particle sizes of pyrolyzed biochar. Chemosphere 103:313–21. [DOI] [PubMed] [Google Scholar]
- Slavich PG, Sinclair K, Morris SG, Kimber WL, Downie A, and Van Zwieten L 2013. Contrasting effects of manure and green waste biochars on the properties of an acidic ferralsol and productivity of a subtropical pasture. Plant and Soil 366:213–27. [Google Scholar]
- Solaiman ZM, Murphy DV, and Abbott LK 2012. Biochars influence seed germination and early growth of seedlings. Plant and Soil 353:273–87. [Google Scholar]
- Soudek P, Rodriquez Valesca IM, Petrová Š, Song J, and Vanĕk T 2017. Characteristics of different types of biochar and effects on the toxicity of heavy metals to germinating sorghum seeds. Journal of Geochemical Exploration 182 Part B:157–65. [Google Scholar]
- Thies JE, and Rillig MC 2009. Characteristics of biochar: Biological properties In Biochar for environmental management: Science and technology, ed. Lehmann J, and Joseph S, 85–105. London: Earthscan. [Google Scholar]
- Troy SM, Lawlor PG, O’ Flynn CJ, and Healy MG 2014. The impact of biochar addition on nutrient leaching and soil properties from tillage soil amended with pig manure. Water Air and Soil Pollution 225:1900. [Google Scholar]
- U.S. Environmental Protection Agency. 2012. Ecological effects test guidelines. OCSPP 850.4100, Seedling Emergence and Growth. Office of Chemical Safety and Pollution Prevention (7101) EPA 712-C-012. [Google Scholar]
- Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, and Cowie A 2010. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant and Soil 327:235–46. [Google Scholar]
- Visioli G, Conti FD, Menta C, Bandiera M, Malchevschi A, Jones DL, and Vamerali T 2016. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environmental Monitoring and Assessment 188:166. doi: 10.1007/s10661-016-5173-y. [DOI] [PubMed] [Google Scholar]
- Xing J, Cai M, Chen S, Chen L, and Lan H 2013. Seed germination, plant growth and physiological responses of Salsola ikonnikovii to short-term NaCl stress. Plant Biosystems 147:285–97. [Google Scholar]
- Yang X, Liu J, McGrouther K, Huang H, Lu K, Guo X, He L, Lin X, Che L, Ye Z, and Wang H 2016. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environmental Science and Pollution Research 23:974–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.