Abstract
Strontium (Sr) is a natural element, ubiquitous in the environment and known to occur in water, food, air, and soils. Strontium is present in media as a salt or an ionized divalent cation. The Sr ion (dissociated) is toxicokinetically important because it is easily absorbed into systemic circulation when inhaled with particulates or ingested with water or foods. Dietary exposure can be influenced by using tap water containing dissolved Sr in food preparation. Research was conducted to determine the amount of Sr transferred from water to individual foods during preparation. Strontium transferred to broccoli, lentils, and spaghetti at all levels tested (1.5, 10, and 50 mg/L) as evidenced by the residual Sr in the pour-off water following food preparation (33 – 64%). The data from the cooking study support the hypothesis that cooking of foods with water containing Sr adds to total dietary exposure. This information can inform the determination of the relative source contribution (RSC) that is typically used in developing drinking water advisory guidelines. These cooking study results indicate that food prepared in water containing Sr should be considered as part of the food in a dietary exposure assessment.
Keywords: strontium, transfer, water, foods, relative source contribution
Introduction
The natural element, Strontium (Sr), is ubiquitous in the environment and can be found in water, food, air, and soils. Strontium is present in all relevant media as a salt or an ionized divalent cation. Some Sr salts are more soluble than others. The Sr ion (dissociated) is toxicokinetically important because it is easily absorbed into systemic circulation when inhaled with air particulates or ingested with water or foods.
Strontium is an element of potential toxicological concern, because of its ability to substitute for calcium in bone, thereby possibly impacting bone density [1]. The potential for strontium to have an adverse effect on bone is strongly impacted by the calcium: strontium ratio [2]. The risk increases when the ratio decreases and is diminished when calcium intakes increase. The likelihood for strontium to have a detrimental impact on bone is greatest during periods of active bone growth in children and adolescents where it can contribute to a condition sometimes referred to as strontium rickets [3,4]. An increased fracture risk among children with low calcium intakes has been determined [5]. The risk can be increased through competition when high intakes of strontium is combined with lower dietary calcium uptake. In addition to strontium’s impact on bone, it also can substitute for calcium in teeth during their formation causing permanent visible staining of the tooth surface [6].
Strontium ions carry a 2+ charge and can bind to the biopolymers (polysaccharides, proteins) in foods with areas of negative or partially negative charge. Thus, cooking foods in the presence of water containing Sr may increase the strontium content of the food as served for meats (slow cooked with water), cooked grains (e.g. rice), vegetables (e.g. broccoli), legumes (e.g. lentils), and fruits (e.g., applesauce). Preparation of foods (handling, washing, and rinsing) can also influence residue levels that become part of the diet [7–10]. This is more likely to occur with dissolved minerals in water than volatile compounds, such as alcohols that volatilize during cooking or baking [11].
Development of drinking water guidelines such as a Maximum Contaminant Level Goal (MCLG) used to support a regulation or non-regulatory Drinking Water Health Advisory considers exposures from ingestion of drinking water as it compares to other sources, such as, diet, ambient air and incidental ingestion from soils and dusts when developing a guideline value [12, 13]. The latter are important considerations for crawling infants and toddlers [7, 8].
Identifying all routes of exposure to Sr can provide important information when determining the relative source contribution (RSC). The dietary data for strontium collected by the U.S. Food and Drug Administration as part of their Total Diet Study (TDS) program were provided to the U.S. EPA to use in the RSC analysis. However, TDS foods that require water for cooking or during preparation prior to analysis are prepared with deionized water, so chemicals in the preparation water will not be incorporated in the food prior to analysis [14–16].
It is also important to realize that the Sr in the local tap water impacts not only the foods prepared at home with water, but those commercially prepared and packaged with water, e.g., products canned with water or syrup when the local tap water without additional treatment at the processing facility is used for preparation. However, in most of those cases the chemicals added during commercial preparation will be reflected in the TDS results. The exception would be foods prepared at local food establishments that use the water directly from the public water system (e.g. restaurants, school lunch programs, hospitals).
The amount of Sr transferred from drinking water (in tap) to the food items during preparation could be complete, none, or somewhere in between. Research to determine the amount of Sr transferred from water to individual foods during preparation was conducted. The results can provide a more complete estimation of potential intakes of Sr that originate from a local public water supply.
Methods and Materials
Reagents and solutions
All reagents were ACS reagent grade. All sample preparation solutions were created with ultrapure water at 18.2 Ω obtained from a Millipore water purification system (Milli Q Plus, Millipore, Bedford, MA). The working solution was prepared by diluting 10,000 mg/L certified stock solution of Sr (GFS Chemicals, Columbus, OH) to the desired concentrations to use as the Sr containing water for the experiments.
Samples
Three food items were purchased at a local market to boil in ultrapure water containing three levels of Sr. The food items were a fresh vegetable (broccoli), dried pasta (spaghetti), and dried lentils which were chosen to represent a range of types of foods that are commonly boiled during preparation. Three Sr concentrations were tested in water; approximately 1.5 mg/L (reference level used in the Third Unregulated Contaminant Monitoring Rule (UCMR3) as a value established as protective for skeletal effects) [17], 10 mg/L, and 50 mg/L (representing high concentrations found in the UCMR3 [17]). Exact concentrations were determined by direct measurement (see Analysis section below). Blanks were generated by boiling Sr containing water with no foods and boiling food samples in ultrapure water containing no Sr. Samples were generated in triplicate for each Sr concentration level.
Preparation of samples
A measured amount of each food item was weighed (top-load balance, AND Electronic Balance, FX-4000, Denver, CO) to obtain the four separate, 30 to 50 g weighed samples (depending on the amount that would fit in the water) that were needed for each set of experiments. Six beakers were weighed dry with 15 glass beads (to aid in boiling), received approximately 250 mL of water (either with or without Sr, as needed), weighed again, then were set on hot plates (Corning, New York, USA) and allowed to boil, separately (see Figure 1). Two beakers contained no food. The other four beakers contained the weighed amount of food. Two beakers contained Sr free water; one with no food (designated as no food blank), the other with food (designated as a matrix blank). Four beakers contained Sr contaminated water at the designated level; one had no food and was used to obtain the actual concentration of Sr in the water, three beakers contained the weighed food item to allow for replicate analysis.
Figure 1:
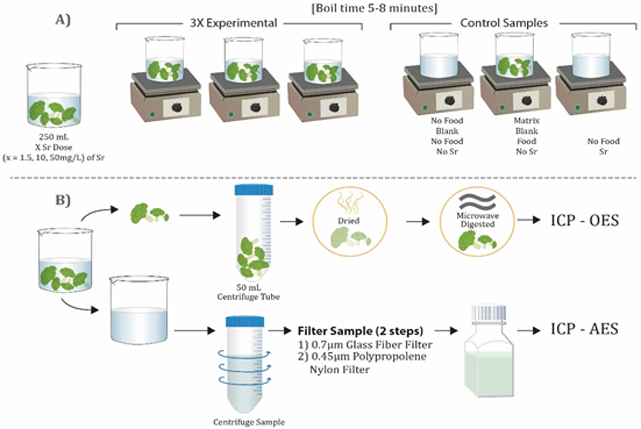
Sample preparation Scheme
After reaching a boiling state, the food item was placed into the beaker, allowed to return to boiling, then boiled for at least 5 minutes. The spaghetti required a longer boiling time of 8 minutes to allow for complete preparation. Following completion of the boiling, the beakers were removed from the hot plates and allowed to cool for 2 minutes. The water containing food was separated into a new, clean, and weighed beaker and allowed to cool completely. The final weight of water was returned to the original, pre-boiled weight using ultrapure water.
Because of boiling the food items, the water contained particles of food, starch, and lentil husks. To obtain a clear sample amenable to analysis, the water was poured into centrifuge tubes (VWR.com, Brooklyn, NY) and centrifuged (Sorvall Super T 21, Newtown, CT) at 10,000 rpm at 20°C for 10 minutes, filtered first through a 0.7 μm glass fiber filter (Whatman, Maidstone, England), and then filtered through a 0.45 μm polypropylene nylon disc (Millipore, Darmstadt, Germany). Water samples were stored at room temperature in labeled 60 mL plastic sampling containers (Thermo Scientific, Waltham, MA) and acidified with concentrated nitric acid (0.15% v/v). Food samples were placed into plastic tubes (FisherBrand, Waltham, MA) and frozen at −80°C until preparation for analysis.
Analysis of samples
a). Pour-off Water-
The pour-off waters were preserved with analytical grade ultrapure nitric acid (0.15% v/v) and were analyzed for Sr on an Inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Thermo Elemental model 7600 Duo, Waltham, MA) using U.S. EPA Method 200.7 [18]. A peristaltic pump delivers the sample into a nebulizer and the sample is injected directly into the plasma. The sample collides with electrons and charged ions in the plasma, breaking itself down into charged ions. Samples are modified with a 1:1 matrix modifier (10% HNO3/HCl) solution. Strontium is measured at wavelength 421.552 nm with no interferences. Results were expressed as the concentration of Sr recovered (mg/L).
b). Boiled Food Samples-
The boiled food samples were dried at 60°C in a drying oven. One half of a gram of dried food item was microwave digested using a digester (CEM MARS Xpress, Mathews, NC), following U.S. EPA Method 3051A [19]. The samples were digested in nitric acid and hydrochloric acid (9 mL:3 mL) under pressure of 12 atm. The contents were heated to 175 ℃ within 5 minutes and 30 seconds, held for 4 minutes 30 seconds for a tray of samples (24 samples in a tray). The moisture content of the food samples was measured using Method CLC-MOI.03 from the U.S. Department of Agriculture Food Safety and Inspection Service, Office of Public Health Science [20]. Food sample digests were analyzed by inductively coupled plasma optical emission spectrometry (ICP/OES) Optima 2100 (Perkin Elmer, Waltham, MA) for Sr and reported as mg Sr/kg food using U.S. EPA Methods 200.7 and 6010C [18, 19], as stated above. Food results were converted to wet weight.
c). Mass Balance Calculation
The concentrations determined from the analysis of the pour-off water and the boiled foods were converted into milligram weights to evaluate the mass balance. The concentration (mg/L or mg/kg) was multiplied by the amount of water or food used (g) and divided by the conversion factor (1 L/1000 g or 1 kg/1000 g) to reach the amount of Sr in each sample for water or food item. The sum of the mass from the pour-off water and food item should be equivalent to the initial amount of Sr in the water. Differences between the total available Sr and the amount in the pour-off water and food items could be attributed to volatilization of the Sr that may occur during the boiling process.
Quality Assurance
Quality control (QC) was strictly adhered to and all sample results were within the appropriate specified criteria. For the pour-off water samples, laboratory controls and blanks were analyzed simultaneously with each analysis for each batch of samples. Laboratory reagent blanks were analyzed every 10 samples, with QC limits of less than the method detection limit and continuous calibration checks were within 90-110% recovery. Fortified Sr water samples were all within an acceptable percent recovery of 85-115% of the true value. This included both laboratory fortified blanks and laboratory fortified matrices. Analytical water duplicates were also conducted with results within 10% of the original samples.
For food samples, laboratory controls and blanks were analyzed simultaneously with each analysis batch of 10 samples. All QC samples fell within the specified criteria of ±25% for fortified matrix, ±15% for fortified blanks, ±10% for standard checks, ≤20% difference for duplicates, and ≤0.025 ppm for reagent blanks. Several samples failed the QC criteria when analyzing the broccoli samples, therefore, the broccoli data are not used or reported.
Results and Discussion
The results from the analysis of the pour-off water varied by food item and initial Sr concentration (mg/L) in the water (Table 1). No food type tested absorbed the entire amount of Sr from the water. The concentration of Sr remaining in the pour-off water rose as the concentration increased. Broccoli and spaghetti had similar Sr absorption. Unexpectedly, the replicate pour-off water from the cooking of lentils consistently contained around 32%, regardless of the initial concentration.
Table 1:
Residual Strontium in Pour-off Water following Food Preparation, mg/L ± standard deviation (percent recovery)
| Theoretical water mg/L | 1.5 | 2 | 10 | 50 |
|---|---|---|---|---|
| Actual water mg/L | 1.2 – 2.1 | 2.4 | 9.3 – 12 | 44 - 49 |
| Blank | 0.020 | * | 0.022 | 0.033 |
| Broccoli | 0.87 ± 0.39 (41) | * | 5.8 ± 0.36 (47) | 28 ± 1.2 (64) |
| Blank | 0.021 | 0.020 | 0.026 | 0.020 |
| Lentils | 0.44 ± 0.04 (36) | 0.77 ± 0.02 (32) | 3.7 ± 0.02 (38) | 14 ± 0.44 (32) |
| Blank | 0.021 | * | 0.13 | 0.007 |
| Spaghetti | 0.62 ± 0.08 (33) | * | 4.0 ± 0.11 (44) | 27 ± 1.1 (56) |
indicates no sample
The amount of Sr in the pour-off water from each food type was statistically different from that of the other food types (p<0.0001) using the Student t test [21], except for the broccoli and spaghetti samples cooked in water with 50 mg Sr/L. At the 50 mg/L concentration, the p values for Sr left in the pour off water were the same (p=0.492) for the broccoli and spaghetti. The concentration of Sr in each food type differed for each concentration level (p<0.0028). Based on the results of the pour off water, it appears that broccoli and spaghetti reached a saturation point and could no longer absorb the Sr from the water as the concentration increased. Lentils, on the other hand, continued to absorb more Sr from the water as the concentration increased, as evidenced by the sequentially lower recoveries in the pour-off water. For broccoli and spaghetti, Sr absorption was dependent on the initial concentration available, broccoli – r2 = 0.9993; spaghetti – r2 = 0.8853 (Figure 2), whereas, for lentils, Sr absorption was not dependent on the initial concentration, r2 = 0.2019. Strontium was always absorbed at 57±1%, resulting in a flat line across all concentrations (Figure 2).
Figure 2:
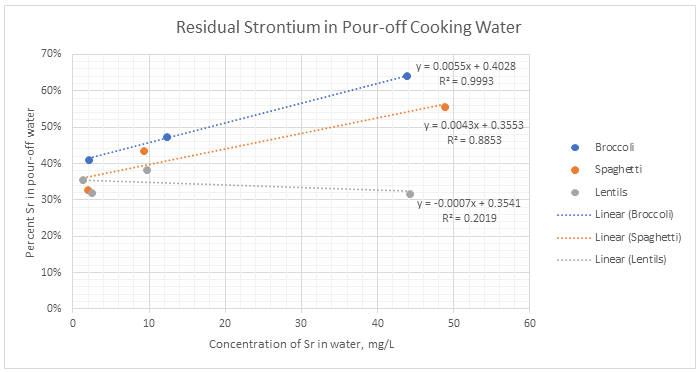
Residual Strontium in Pour-off Cooking Water
From the results of the analysis of the pour-off cooking water, Sr appeared to be absorbed by the foods. To ensure that the Sr transferred to the food items during cooking, the post preparation foods were analyzed. Table 2 summarizes the analytical results for Sr in lentils and spaghetti. As explained earlier issues arose with the broccoli samples and the results did not meet the QC requirements. Broccoli data are not reported.
Table 2:
Concentration of Strontium in Boiled Food, mg/kg (wet weight) ± standard deviation
| Theoretical water mg/L | 1.5 | 2 | 10 | 50 |
|---|---|---|---|---|
| Actual water mg/L | 1.2 – 2.1 | 2.4 | 9.3 – 12 | 44 - 49 |
| Blank | 1.1 | 1.1 | 0.83 | 1.0 |
| Lentils | 3.6 ± 0.36 | 6.4 ± 0.29 | 18 ± 1.4 | 100 ± 8.8 |
| Blank | 0.81 | * | 0.79 | 0.83 |
| Spaghetti | 5.2 ± 0.25 | * | 16 ± 0.74 | 55 ± 2.0 |
indicates no sample
Strontium did not volatilize as demonstrated by a mass balance calculation. Taking into consideration the actual weight of the amount of water used, amount of food used, and the analytical concentration, the mass of Sr (mg) in the pour-off water and food items accounted for most of the available Sr. The difference between the total amount of Sr measured and the amount of Sr in the pour-off water and food item combined falls well within the analytical error so would not be considered volatilized. These results are summarized in Table 3.
Table 3:
Mass Balance Calculations
| Theoretical Concentration, mg/L | Total Sr Available, mg | Sr in Pour-off Water, mg | Sr in Lentils, mg | Difference |
|---|---|---|---|---|
| 1.5 | 0.31 | 0.11 | 0.26 | −0.06 |
| 2 | 0.60 | 0.19 | 0.44 | −0.03 |
| 10 | 2.4 | 0.92 | 1.6 | −0.07 |
| 50 | 11 | 3.5 | 7.4 | 0.14 |
| Total Sr Available, mg | Sr in Pour-off Water, mg | Sr in Spaghetti, mg | ||
| 1.5 | 0.47 | 0.16 | 0.32 | 0.00 |
| 10 | 2.3 | 1.0 | 1.5 | −0.17 |
| 50 | 12 | 6.8 | 8.5 | −3.04 |
The strontium in the food items retained increasing amounts as the concentration in the cooking water increased. The blanks did contain some Sr, but the foods contained additional amounts indicating that the Sr from the water was absorbed by the food items. It is clear that Sr in the cooking water could become incorporated in the food as served adding to the total intake from the diet because of the use of the local tap water during cooking. This is a far greater concern when the water contains > 50 mg/L than it is for the 1.5 mg/L concentration of Sr, the current health reference level [17].
Conclusions
The data from the cooking study support the hypothesis that cooking of foods with water containing Sr that is poured off before serving adds to the total dietary Sr. Food groups where this needs to be considered include vegetables, grains, legumes and probably some mixed foods (e.g., soups) that are cooked or prepared using tap water. As shown in this study, intake through foods may be higher than what would be predicted from analysis of FDA’s TDS data.
The RSC calculation uses the mean national concentration from public water systems (if known), not the high-end concentrations. In the case of Sr, the mean concentration observed during the monitoring under UCMR3 was less than 1 mg/L, while the concentrations at some systems exceeded 50 mg/L [17]. Accordingly, uptake of Sr from foods is a greater concern for some locations than others, such as, the parts of the country with elevated strontium concentrations including the states that surround the Great Lakes, Texas, New Mexico, Arizona and Florida [22]. Cooking studies, such as this one, assist toxicologists to quantify the contribution of the local tap water to total exposure, especially in cases where parts of the country receive concentrations in their tap water that are considerably higher than the average. Under the 1996 amendments to the Safe Drinking Water Act, consideration of sensitive populations during decision making is required [23]. Populations can be classified as sensitive based on their exposure potential, age (e.g. infants and children), life stage (e.g. women of childbearing age) and health status (e.g. those with autoimmune diseases). Some drinking water regulations require public notification for sensitive populations because of exposure concerns, most notably copper (Wilson’s disease), fluoride (dental fluorosis) [24], sodium (salt-sensitive hypertension) [25, 26]. Thus, when some populations that are highly exposed through drinking water from their public system, public notification can be considered as a regulatory option.
Abbreviations:
- ACS
American Chemical Standard
- EPA
U.S. Environmental Protection Agency
- FDA
U.S. Food and Drug Administration
- ICP-AES
inductively coupled plasma atomic emission spectroscopy
- ICP-OES
inductively coupled plasma optical emission spectrometry
- MCLG
maximum contaminant level goal
- QC
quality control
- RSC
relative source contribution
- Sr
strontium
- TDS
total diet study
- UCMR3
unregulated contaminant monitoring
Footnotes
Disclaimer
The research described in this article was funded by the U.S. Environmental Protection Agency through its Office of Research and Development. It has been subjected to Agency’s administrative review and approved for publication. The views expressed in this paper are those of the author[s] and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute an endorsement or recommendation for use.
References
- [1].Marie PJ, Garba MT, Holt M, and Miravel L. 1985. Effects of low doses of stable strontium on bone metabolism in rates. Mineral and Electrolyte Metabolism 11(1) : 5–13. [PubMed] [Google Scholar]
- [2].Grynpas MD, Hamilton E, Cheung R, Tsouderos Y, Deloffre P, Hott M, and Marie PJ. 1996. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone. 18(3):253–259. [DOI] [PubMed] [Google Scholar]
- [3].Özgür S, Sümner H, and Kocoglu G. 1996. Rickets and soil strontium. Archives of Disease in Childhood. 75:524–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Storey E 1961. Strontium “rickets”: Bone, calcium and strontium changes. Australasian Annals of Medicine. 10:213–222. [DOI] [PubMed] [Google Scholar]
- [5].Rockell JE, Williams SM, Taylor RW, Grant AM, Jones IE, and Goulding A. 2005. Two-year changes in bone and body composition in young children with a history of prolonged milk avoidance. Osteoporosis International. 16: 1016–1023. [DOI] [PubMed] [Google Scholar]
- [6].Curzon MEJ and Spector PC. 1977. Enamel mottling in a high strontium area of the USA. Community Dentistry and Oral Epidemiology. 5:243–247. [DOI] [PubMed] [Google Scholar]
- [7].Melnyk LJ, Byron M, Brown G, Clayton A, and Michael L. 2011a. Pesticides on household surfaces may influence dietary intake of children. Environmental Science & Technology, 45: 4594–4601. [DOI] [PubMed] [Google Scholar]
- [8].Melnyk LJ, Hieber TE, Turbeville T, Vonderheide AP, and Morgan JN. 2011b. Influences on transfer of selected synthetic pyrethroids from treated Formica® to foods. Journal of Exposure Science and Environmental Epidemiology, 21 (2): 186–196. [DOI] [PubMed] [Google Scholar]
- [9].Asami M, Yoshida N, Kosaka K, Ohno K, and Matsui Y. 2013. Contribution of tap water to chlorate and perchlorate intake: A market basket study. Science of the Total Environment, 463-464: 199–208. [DOI] [PubMed] [Google Scholar]
- [10].Rahman MA, Hasegawa H, Rahman MA, Rahman MM, and Majid Miah MA. 2006. Influence of cooking method on arsenic retention in cooked rice related to dietary exposure. Science of the Total Environment, 370: 51–60. [DOI] [PubMed] [Google Scholar]
- [11].Augustin J, Augustin E, Cutrufelli RL, Hagen SR, and Teitzel C. 1992. Alcohol retention in food preparation. Research and Professional Briefs, 92(4): 486–488. [PubMed] [Google Scholar]
- [12].U.S. EPA. 1989. National primary and secondary drinking water regulations. Proposed Rule. Federal Register. Vol. 54, No. 97 p. 22062, January 30, 1991. [Google Scholar]
- [13].U.S. EPA. 2000. Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (2000). EPA-822-B-00-004.
- [14].U.S. Food and Drug Administration. 2017. TDS results. www.fda.gov, last accessed 1/26/2018.
- [15].Egan SK, Bolger PM, and Carrington CD. 2007. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Environ Epidemiol 17: 573–582. [DOI] [PubMed] [Google Scholar]
- [16].Pennington J and Jones J 1987. Molybdenum, nickel, cobalt, vanadium and strontium in total diets J Am Dietet Assoc, 87: 1644–1650. [PubMed] [Google Scholar]
- [17].U.S. EPA, 2017. UCMR3 Monitoring Results 2017. https://www.epa.gov/sites/production/files/2017-02/documents/ucmr3-data-summary-january-2017.pdf, last accessed 10/3/2017.
- [18].U.S. EPA. 1994. Method 200.7: Determination of metals and trace elements in water and wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry, Revision 4.4. www.epa.gov/homeland-security-research/method-2007-determination-metals-and-trace-elements-water-and-wastes, last accessed 1/31/18.
- [19].U.S. EPA. SW-846 Methods. https://www.epa.gov/hw-sw846/sw-846-compendium, last accessed 1/31/18.
- [20].U.S.DA. Chemical Laboratory Guidebook. https://www.fsis.usda.gov/wps/portal/fsis/topics/science/laboratories-and-procedures/guidebooks-and-methods/chemistry-laboratory-guidebook, last accessed 7/2/18.
- [21].U.S. EPA. ProUCL. https://www.epa.gov, last accessed 10/13/2017.
- [22].U.S. EPA, 2017. Occurrence Data for the Unregulated Contaminant Monitoring Rule. https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule#3 last accessed 12/18/18.
- [23].U.S. EPA. 1996a. Save Drinking Water Act Amendments of 1996: General guide to Provisions. Office of Water (EPA 810-S-96-001) p.7. [Google Scholar]
- [24].U.S. EPA. 2011. Questions and Answers on Fluoride. https://www.epa.gov/sites/production/files/2015-0/documents/2011_fluoride_questionsanswers.pdf
- [25].EPA US. 1996b. Code of Federal Regulations: Protection of the Environment. Parts 126-149. Section 141.41 Office of the Federal Register, National Archives and Records Administration; Washington DC: pp. 352–353. [Google Scholar]
- [26].U.S. EPA. 2003. Drinking Water Advisory: Consumer acceptability advice and health effects on sodium. https://www.epa.gov/sites/production/files/2014-09/documents/support_cc1_sodium_dwreport.pdf.


