Abstract
Increased levels of the calcium-binding protein neuronal calcium sensor 1 (NCS1) predict an unfavorable patient outcome in several aggressive cancers, including breast and liver tumors. Previous studies suggest that NCS1 overexpression facilitates metastatic spread of these cancers. To investigate this hypothesis, we explored the effects of NCS1 overexpression on cell proliferation, survival, and migration patterns in vitro in 2- and 3-dimensional (2/3-D). Furthermore, we translated our results into an in vivo mouse xenograft model. Cell-based proliferation assays were used to demonstrate the effects of overexpression of NCS1 on growth rates. In vitro colony formation and wound healing experiments were performed and 3-D migration dynamics were studied using collagen gels. Nude mice were injected with breast cancer cells to monitor NCS1-dependent metastasis formation over time. We observed that increased NCS1 levels do not change cellular growth rates, but do significantly increase 2- and 3-D migration dynamics in vitro. Likewise, NCS1-overexpressing cells have an increased capacity to form distant metastases and demonstrate better survival and less necrosis in vivo. We found that NCS1 preferentially localizes to the leading edge of cells and overexpression increases the motility of cancer cells. Furthermore, this phenotype is correlated with an increased number of metastases in a xenograft model. These results lay the foundation for exploring the relevance of an NCS1-mediated pathway as a metastatic biomarker and as a target for pharmacologic interventions.—Apasu, J. E., Schuette, D., LaRanger, R., Steinle, J. A., Nguyen, L. D., Grosshans, H. K., Zhang, M., Cai, W. L., Yan, Q., Robert, M. E., Mak, M., Ehrlich, B. E. Neuronal calcium sensor 1 (NCS1) promotes motility and metastatic spread of breast cancer cells in vitro and in vivo.
Keywords: cell migration, xenograft model, calcium binding protein, metastasis, Ca2+ signaling
A hallmark of aggressive tumors is their ability to invade tissues and metastasize to distant organs (1). It is well known that the majority of tumor-related deaths are attributable to dissemination of cancer cells throughout the body (2, 3). Nevertheless, many of the mechanisms that favor the spread of tumor cells to distant sites in the body remain to be elucidated (1, 4).
Calcium (Ca2+) is a crucial second messenger molecule. It enters the cytoplasm via voltage- or ligand-gated channels (5, 6) from 2 major sources, the extracellular space and intracellular Ca2+ storage compartments such as the endoplasmic reticulum (7) and the mitochondria (8). Release of Ca2+ from intracellular compartments often follows oscillatory patterns, which can lead to reprogramming of the transcriptional machinery of mammalian cells (9–11). Alterations in cytoplasmic Ca2+ regulate critical cellular processes such as proliferation, cell growth, cell cycle progression (12), neurogenesis (6, 13, 14), and apoptotic cell death (12, 15).
The coordinated movement of cells largely depends on tightly regulated spatiotemporal Ca2+ signals (16–20). Given these properties of the physiologic function of Ca2+, dysregulated Ca2+ pathways were recently recognized to be possible drivers of aggressive, highly metastatic cancers (21–24). A variety of proteins that are involved in regulating and amplifying Ca2+ signals in mammalian cells have been implicated in cancer progression, including S100 Ca2+-binding proteins (25) and visinin-like protein 1 (VILIP1) (26). The fact that cell motility is regulated by Ca2+ as a second messenger suggests that molecules which bind Ca2+ and mediate its downstream effects could be potential cancer biomarkers as well as therapeutic targets.
One example of a Ca2+ regulated kinase involved in cell movement is LIM domain kinase 1 (LIMK1) (16). LIMK1 regulates the organization of the actin cytoskeleton via phosphorylation of its downstream effector cofilin (27). Cancer cells rely on increased levels of LIMK1 to be able to invade the tissue that surrounds the tumor (28) and inhibition of LIMK1 reduces their invasiveness (29, 30).
Neuronal calcium sensor 1 (NCS1) is a ubiquitously expressed Ca2+ binding protein (31, 32) with the highest levels of expression being found in the CNS (33). It is closely related to other members of the NCS family of proteins (34) such as hippocalcin or recoverin. On the structural level, NCS proteins are composed of 4 EF-hand domains that are canonical Ca2+ binding sites and a myristoylation site at the N terminus (31). NCS1 interacts with a wide range of proteins, including the inositol 1,4,5-trisphosphate receptor (InsP3R), dopamine receptor type 2 (D2R), and phosphatidylinositol 4-OH kinase (PI4K) (35, 36). Through its protein–protein interactions, NCS1 regulates vital cellular processes such as neurotransmitter release (32), neurite outgrowth and neuronal survival (37, 38), spatial memory formation (31), and the InsP3R signaling pathway (39, 40).
We have previously described NCS1 as a prognostic biomarker in cohorts of breast (41) and liver (42) cancer patients and demonstrated that the overexpression of NCS1 leads to a marked increase in invasion and motility in vitro (41) using 2-dimensional (2-D) assays. Furthermore, NCS1 expression levels are highly correlated with other components of Ca2+ signaling as well as LIMK1 expression (42). In this study, we investigated the hypothesis that increased expression of NCS1 facilitates the formation of distant metastases by enhancing cellular motility. In vitro cell culture models of NCS1 overexpression were used to demonstrate that NCS1 levels do not modulate proliferation rates but do modulate cell motility in 2- and 3-D environments. We validated these results in a mouse model, showing that NCS1 facilitates early metastatic spread of tumor cells and increases the survival of cancer cells in more mature tumors.
MATERIALS AND METHODS
Cell culturing
MDA-MB-231 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). ATCC validates all cell lines by Short Tandem Repeat Analysis. The MDA-MB-231 cells were transduced with a NCS1 overexpression vector and a control vector as previously described (41). The MDA-MB-231 cell lines were maintained at 37°C, 5% CO2 in DMEM medium supplemented with 10% fetal bovine serum, 1% l-glutamine and 1% penicillin/streptomycin.
Cell proliferation assays
For the CellTiter-Glo assay, 1000 cells/well were plated into sterile 96-well plates and grown over a period of 5 d. The relative number of viable cells was determined every day for 10 wells of such a plate using CellTiter-Glo reagent (Promega, Madison, WI, USA) and a microplate reader (Tecan Infinite M1000 Pro; Tecan Trading, Männedorf, Switzerland) according to the manufacturers’ instructions. Every well was used just once and the marginal wells were never used. Three independent experiments were performed using NCS1-overexpressing (OE) MDA-MB-231 cells and control cells, and all measurements were normalized to the average luminescence on d 1.
For the AlamarBlue assay, 8 replicates of 1250, 2500, 5000, and 10,000 cells/well were plated into sterile 96-well plates. After a 24-h incubation period, medium was removed and 100 μl fresh medium with an additional 10 μl AlamarBlue reagent (Thermo Fisher Scientific, Waltham, MA, USA) was added to each well. After another 2 h of incubation, a fluorescence signal was measured using the aforementioned microplate reader.
Scratch assay and colony formation assay
Scratch assays were performed as previously described (41). Cells were serum starved 12 h prior to the experiment to inhibit cell proliferation. For quantification, ImageJ (National Institutes of Health, Bethesda, MD, USA) was used and the distance traveled was calculated after 24 h. The mean distance traveled was plotted for n = 3 independent experiments.
For colony formation assays, cells were cultured per standard protocol in T75 flasks. Once cell confluence approached 80–90%, the cells were detached by the addition of 2 ml TrypLE (Thermo Fisher Scientific, Rockford, IL, USA), followed by dilution in 5 ml of fresh medium. Cell concentration was determined using a hematocytometer. Subsequently, a total number of 100, 200 or 500 cells was added to each well of a 12-well plate. Cells were then left undisturbed in the incubator for 14 d. After 14 d, colonies were fixed and stained with 2.5% crystal violet solution and were subsequently washed to remove excess dye and scanned with a conventional scanner. The total area covered was determined with ImageJ (43). Data were obtained from 3 independent experiments with 4 replicates in each experiment. Data were represented as total area covered in each individual well.
Quantitative RT-PCR
RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Using a High-Capacity cDNA Reverse Transcription Kit (4368814; Thermo Fisher Scientific) according to the manufacturer’s protocol, 0.5–1 µg of RNA was then transcribed to cDNA. Quantitative real-time PCR was performed using Power SYBR Green Master Mix reagent and a 7300 Real-Time PCR System (Thermo Fisher Scientific). The ΔΔCt method (44) was used to calculate expression fold changes with ACTB (β-actin) and ribosomal protein S18 as control genes. The following primers were used at a concentration of 5 µM: NCS1 (forward, 5′-GATGCTGGACATTGTGGATG-3′; reverse, 5′-CTTGGAACCCTCCTGGAACT-3′), ACTB (forward, 5′-GTCTTCCCCTCCATCGTGG-3′; reverse, 5′-GATGCCTCTCTTGCTCTGGG-3′), and S18 (forward, 5′-TTCGAACGTCTGCCCTATCAA-3′; reverse, 5′-ATGGTAGGCACGGCGACTA-3′).
Assessment of NCS1 protein levels
MDA-MB-231 cells were lysed in ice-cold M-PER Mammalian Protein Extraction Reagent buffer (Thermo Fisher Scientific) supplemented with a protease inhibitor. Protein concentrations were determined using the Bio-Rad protein assay reagent (San Diego, CA, USA). SDS-PAGE was performed with 30 μg of protein. Briefly, the protein was transferred to a nitrocellulose membrane (GE Healthcare, Chicago, IL, USA), the resulting blots were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBST), and were then incubated with a primary NCS1-specific antibody (sc-13037, diluted 1:5000; Santa Cruz Biotechnology, Dallas, TX, USA) or β-actin–specific antibody (sc-47778, diluted 1:1000; Santa Cruz Biotechnology) over night at 4°C. All dilutions are vol/vol. Blots were then incubated with horseradish peroxidase and labeled goat anti-rabbit IgG (diluted 1:10,000; Santa Cruz Biotechnology) at room temperature for 1 h. Ultimately, protein bands were visualized using electrochemiluminescence detection reagents (Thermo Fisher Scientific).
Immunofluorescence microscopy
Control and NCS1-OE cells were seeded on sterile 22 × 22-mm glass coverslips at a density of 50,000 cells/coverslip. Medium was removed 24 h after seeding, and each coverslip was briefly washed twice with 2 ml of 1× PBS (pH 7.4; AmericanBio, Natick, MA, USA) each time. Fixation was performed for 15 min at room temperature with a 4% paraformaldehyde solution (pH 7.4). Following 3 washes with 2 ml PBS, cells were permeabilized and blocked in PBS solution containing 1% bovine serum albumin (0.1% Triton-X 100; AmericanBio) for 1 h at room temperature. Following blocking, cells were incubated with a rabbit anti-NCS1 pAb diluted in blocking solution (sc-13037, diluted 1:100; Santa Cruz Biotechnology) overnight at 4°C. After extensive washing with PBS, cells were incubated with an AlexaFluor-488 goat anti-rabbit secondary antibody (diluted 1:1000; Thermo Fisher Scientific) and a rhodamine-conjugated phalloidin (diluted 1:1000 dilution; Thermo Fisher Scientific) for 2 h at room temperature in the dark. Cells were then washed extensively with PBS before being mounted on glass slides with antifade medium ProLong Gold with DAPI (Thermo Fisher Scientific). Slides were cured overnight before images were captured with a confocal microscope using the ×100 lens (LSM 710 Duo; Carl Zeiss, Oberkochen, Germany). A laser power of 0.5% was used to detect NCS1 in overexpressing cells and 10% to detect NCS1 in control cells. All other settings were kept the same among all coverslips.
Transduction of cells with a reporter for bioluminescent imaging
Previously (41) generated NCS1-OE and control MDA-MB-231 cells were retro-virally infected with a triple-fusion protein reporter. The reporter encodes for herpes simplex virus thymidine kinase 1, green fluorescent protein (GFP) and firefly luciferase (45). Human embryonic kidney 293 (HEK-293) cells were used to produce viruses. They were transduced using the Clontech Calcium Phosphate Transfection Kit (Clontech Laboratories, Mountain View, CA, USA) and a polybrene-facilitated infection of MDA-MB-231 cells. Briefly, HEK-293 cells were plated on a 10 cm dish 1 d before the transfection for producing retrovirus and grown to 60% confluence. The next day, 20 μg of retroviral vector DNA and packaging plasmids [envelope, vesicular stomatitis virus (VSVG) 6 µg; 10μg pMDLg/pRRE 3rd generation lentiviral packaging plasmid containing Gag and Pol, 5μg pRSV-Rev 3rd generation lentiviral packaging plasmid containing Rev (or HIV1gp6)] were mixed with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–buffered saline to obtain a 1-time solution and were then incubated for 15 min. Then, the plasmid-containing solution was added drop-wise to the HEK-293 cells and fresh medium was applied after 6 h. The HEK-293 cells were examined for GFP positivity 2 d after transfection by using a microscope to measure green fluorescence. The retrovirus-containing supernatant was collected on d 2 and 3 after transfection and was centrifuged and filtered using a 0.45-µm filter.
MDA-MB-231 cells were incubated with the virus-containing solution and 4 μg/ml polybrene for 6 h. The medium was changed after the 6 h incubation and later on d 4. On d 6, the MDA-MB-231 cells were checked for GFP expression. The MDA-MB-231 cells were harvested 48 h posttransduction in ice-cold PBS and were passed through a 70-µM filter. After 2 washing cycles, cells were prepared in PBS at a concentration of 5–10 million/ml. Cells were sorted for GFP positivity using a FACSAria-B high-speed cell sorter (BD Biosciences, San Jose, CA, USA). GFP positive cells were collected and transferred to a sterile plastic flask for further culturing.
Assessment of bioluminescence
The Promega Luciferase Assay System was used to quantify bioluminescence. Cells were prepared in a 96-well plate according to the manufacturer’s instructions. Luciferin was added to the wells 20 s before measuring luminescence using a Tecan Infinite M1000 Pro microplate reader.
Cell migration assay
Embedding MDA-MB-231 cancer cells in a 3-D collagen I matrix
Engineered MDA-MB-231 cells were suspended at a concentration of 10 million cells/ml in tissue culture media. A collagen gel was made by adding a calculated amount of 0.5 N NaOH to neutralize a mixture of double-distilled H2O and acetic acid–solubilized type I rat tail collagen (Corning, Corning, NY, USA) on ice for a final collagen concentration of 4 mg/ml. Suspended cells were then added to the gel at a 1:10 dilution for a final cell concentration of 1 × 106 cells/ml. The gels were then transferred to a 24-well glass-bottomed cell culture plate (MatTek, Ashland, MA, USA) kept on an ice pack. Once all gels were transferred to the 24-well plate, the plate was transferred to an incubator at 37°C with 5% CO2. The sample was flipped several times during gelation to prevent cell sediment from forming on the bottom of the plate. After 1 h, tissue culture media was added to the gels, which were subsequently maintained at 37°C with 5% CO2.
Analysis of cell shape, velocity, and mean squared displacement
A Leica SP8 confocal microscope (Wetzlar, Germany) using a ×20 objective was used to image the cells. A temperature of 37°C and a 5% CO2 atmosphere were maintained using a humidified OKO labs live cell imaging incubator. For each well, 250 µm z stacks were created using 10 slices ≥50 µm from the bottom of the well. Images were taken every 5 min for 8 h to create the final hyperstacks. The perimeter, circularity, and aspect ratio of the cells were measured by tracing the edges of z projections of 20 cells using ImageJ. Cell migration was tracked by first taking a z projection of the hyperstack to create a 2-D representation of 3-D migration of the cells. Cell migration was then manually tracked using the point selection tool in ImageJ across 8 h of hyperstack data. The resulting output was analyzed using custom scripts in MatLab (MathWorks, Natick, MA, USA). The average speed of each condition was determined by measuring the average speed of each cell at each time step, and then averaging this average cell speed for 40 cells across all conditions. The mean squared displacement (MSD) was calculated as
where Δt is time interval, x(t) and y(t) are spatial coordinates at time t, and < … > indicates the average over all available starting times. An average of the MSDs at the longest interval (8 h) was calculated for 40 cells per condition. Static trajectories and graphs of MSDs were also created using custom scripts in MatLab.
Animal studies: tail vein injection
All mouse work was done in accordance with the Yale University Institutional Animal Care and Use Committee. Female athymic nude mice (7–9 wk old) were obtained from Envigo (Somerset, NJ, USA) for the xenografting study. For each mouse, 4 × 105 MDA-MB-231 cells were harvested, washed in PBS, resuspended in 0.1 ml sterile saline, and injected into the lateral tail vein. The mice were imaged directly after the tail vein injection and unsuccessfully xenografted mice were excluded from the study.
Mouse imaging studies, data analysis, and lung harvest
After anesthetizing mice by intraperitoneal injection of 0.2 ml 10% ketamine/1% xylazine in sterile saline, they were retro-orbitally injected with 0.1 ml luciferin. The mice were imaged within 2–5 min of the retro-orbital injection using a PerkinElmer Ivis system coupled with Live Image acquisition and analysis software. The photon flux from the xenografted cells in the lungs of each mouse was evaluated by selecting a rectangular region of interest over the lung. All obtained values were normalized to the photon flux obtained immediately after xenografting, resulting in an initial bioluminescence signal of 1 for every mouse.
Lungs for histopathologic evaluation were harvested on d 3 and 7 and at the end of the study. The lungs were harvested after the aforementioned in vivo imaging of xenografted mice and after perfusion with 10 ml ice-cold Dulbecco’s PBS. The harvested lungs were washed in ice-cold Dulbecco’s PBS and imaged with Ivis to obtain an ex-vivo lung bioluminescence signal.
Histopathologic assessment of lung specimens
Harvested lung tissue was placed in 10% formalin for fixation. After fixation, the lungs were paraffin embedded and hematoxylin and eosin (H&E)–stained slides were generated by Yale Mouse Pathology. Anti-NCS1 immunohistochemical staining using a previously described antibody (41) was performed by Yale Research Histology to confirm the presence of human NCS1-positive MDA-MB-231 cells in the xenografted lungs. All slides underwent blind evaluation by an experienced pathologist.
Statistical analysis
Unless noted otherwise, all analyses were done using the Python programming language (v.3.6; https://www.python.org/). An independent 2-sample Student’s t test was used to compare the mean values of 2 independent datasets; values of P < 0.05 were considered significant. Whenever possible, error bars were plotted indicating either 95% confidence intervals or the means ± sem.
RESULTS
Overexpression of NCS1 changes the cellular phenotype without affecting proliferation rates
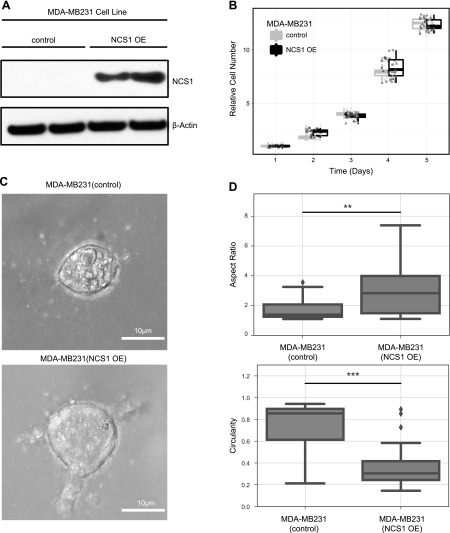
To explore the in vitro function of NCS1 in malignant tumors, we stably overexpressed NCS1 in MDA-MB-231 breast cancer cells (referred to as NCS1-OE) using a previously described protocol (41). Immunoblotting was performed to confirm successful overexpression of the target gene (Fig. 1A). Real-time quantitative PCR measurements confirmed that NCS1 mRNA expression levels were, on average, 4-fold higher in NCS1-OE cells than in the controls. This result was consistent when normalized to 2 different housekeeping genes (P < 0.01, Supplemental Fig. S1).
Figure 1.
NCS1 overexpression changes cellular morphology of MDA-MB231 cells without affecting proliferation rates. A) Immunoblot showing control and NCS1-OE MDA-MB231 cells. Actin is used as a loading control. Longer exposure shows NCS1 expression in control cells, as previously demonstrated (41). B) Box plots demonstrating proliferation rates of MDA-MB231 control and NCS1-OE cells continuously over 5 d. Circles, triangles, and squares represent different biologic replicates. An ATP dye was used to measure the absolute cell number and all values were normalized to the mean on d 0 before plotting. C) Brightfield microscopy images of single MDA-MB231 control and NCS1-OE cells showing the different morphologies of these genotypes. Cells were grown in 3-D collagen gels identical to the gels used for subsequent experiments (e.g., Fig. 3C–E). D) Box plots showing the aspect ratio and circularity of MDA-MB231 control and NCS1-OE cells (n = 20). **P < 0.01, ***P < 0.001.
To determine whether NCS1 overexpression enhances the aggressiveness of tumor cells by increasing their proliferation rates, cell growth of NCS1-OE and control cells was measured over a period of 5 d (Fig. 1B) using an ATP-based growth assay. As expected from previously reported results (41), no differences in proliferation rates were observed. To validate this result, we performed an AlamarBlue assay (Supplemental Fig. S2). Again, proliferation rates of NCS1-OE and control cells were similar.During the course of these experiments it became clear that the overexpression of NCS1 led to a marked change in cellular morphology in a 3-D environment (Fig. 1C). Specifically, NCS1-OE cells were significantly less rounded with a higher aspect ratio than the control (**P < 0.01, ***P < 0.001, Fig. 1D). Furthermore, NCS1-OE cells had a significantly higher cell perimeter (Supplemental Fig. S3). Large cellular protrusions were seen exclusively in the NCS1-OE context (Supplemental Fig. S4), suggesting that this newly acquired phenotype predicts the functional consequences of cellular motility, metastatic behavior, and survival (46, 47).
Immunofluorescence microscopy shows that NCS1 preferentially localizes to cell protrusions in control and NCS1-OE cells
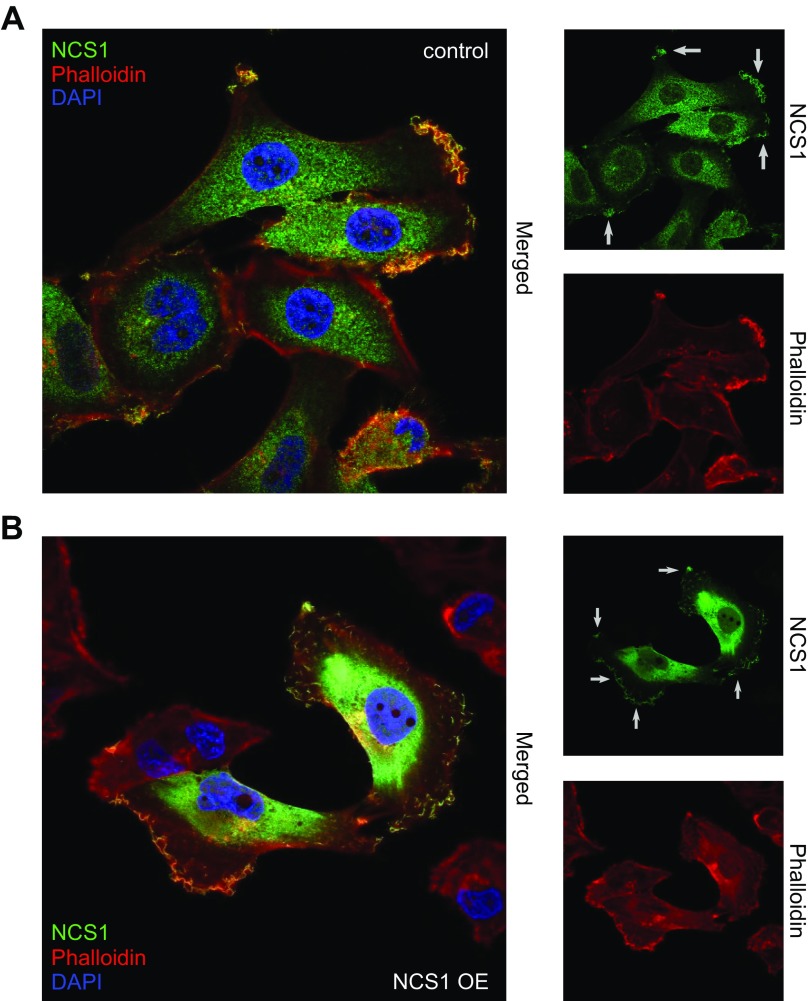
To further investigate the localization of NCS1 in control and NCS1-OE MDA-MB231 cells, immunofluorescence imaging was performed (Fig. 2A, B). As a result, NCS1 was found to be localized at cellular protrusions, including the lamellopodia. NCS1 also colocalizes extensively with actin at the leading edge, but not with cytoplasmic actin puncta or stress fibers.
Figure 2.
NCS1 localizes to the leading edge of MDA-MB231 control and NCS1-OE cells. A) Immunofluorescence microscopy image of control MDA-MB231 cells. The large panel shows a merged image of DAPI (blue), phalloidin (to stain for actin; red), and anti-NCS1 (green) stainings. The smaller panels show the same anti-NCS1 and Phalloidin stainings but separately. Small gray arrows point at localized NCS1. B) Immunofluorescence microscopy image of NCS1-OE MDA-MB231 cells. The large panel shows a merged image of DAPI (blue), Phalloidin (red), and anti-NCS1 (green) stainings. The smaller panels show the same anti-NCS1 and Phalloidin stainings but separately. Small gray arrows point at localized NCS1. Note that the laser power used to image the control cells in A was the original magnification and is ×20 larger than the NCS1-OE cells in B.
NCS1 overexpression increases colony formation and cell motility in 2- and 3-D in vitro assays
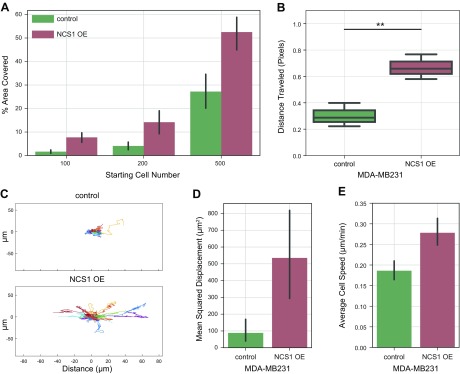
To further investigate the hypothesis that NCS1 favors tumor growth and metastatic spread through increasing survival and motility instead of proliferation, a colony formation assay was performed. Different numbers of cells were plated in a cell culture dish and grown for 14 d. After fixing and staining the resulting colonies, the total area covered by cells was calculated. Compared with the control, the NCS1-OE cells showed a significantly increased ability to form colonies (P < 0.01; Fig. 3A). These differences were consistently found with different starting cell numbers (Fig. 3A). Thus, high NCS1 expression increases the capacity of cancer cells to form colonies in vitro, which mimics a metastatic setting.
Figure 3.
NCS1 overexpression increases cellular motility in 2- and 3-D cell culture experiments. A) Bar plot of a colony formation assay with MDA-MB231 control and NCS1-OE cells. The assay was performed with 100, 200, and 500 initial cells, and the plot shows the percentage area covered at the end of the experiment as mean values ± 95% confidence intervals (P < 0.01 for all comparisons). B) Scratch assay demonstrating the wound healing capacity of NCS1-OE and control MDA-MB231 cells. The distance traveled (in pixels) was assessed after 24 h. Box plots represent n = 3 independent experiments per genotype. **P < 0.01. C) Line plots showing the movement of MDA-MB231 control and NCS1-OE cells in collagen gels over a period of 8 h (in micrometers) as measured using time-lapse microscopy. Each colored trace represents an individual cell. D, E) Bar plots showing the MSD (μM2) (D) and average velocity (μM/min) (E) of MDA-MB231 control and NCS1-OE cells in collagen gels over a period of 8 h. Values are means of n = 40 cells ± 95% confidence intervals (P < 0.005 and P < 0.0001, respectively).
To monitor in vitro 2-D motility, assay cells were placed in a cell culture dish and a standardized wound was applied to the cell monolayer. Then, wound closure was quantified using the relative distance that control and NCS1-OE cells had traveled after 24 h. NCS1-OE cells closed the scratched area in the monolayer significantly (P < 0.01) more than the control cells, indicating an enhanced 2-D migration of NCS1-OE cells (Fig. 3B).
Next, a 3-D migration assay was performed to validate these findings. NCS1-OE and control MDA-MB231 cells were placed in a collagen matrix and time-lapse microscopy was performed to capture the movement of many cells simultaneously over time. The overall trajectory of 20 cells per condition is shown in Fig. 3C, where each colored trace is a single cell that was tracked over time, demonstrating that there was considerably more movement in the NCS1-OE condition. This movement did not have a directional bias. NCS1-OE cells showed increased MSD after 8 h, indicating that the NCS1-OE cells experienced significantly more net displacement (P < 0.005; Fig. 3D). Also, the average cell velocity in the NCS1-OE cells was significantly higher than in the control cells (P < 0.001; Fig. 3E). These results show that the NCS1-OE cells are better able to migrate through a 3-D collagen gel, which indicates that these cells would be more prone to metastatic migration in vivo.
NCS1-OE cells exhibit an increased capacity to metastasize in vivo
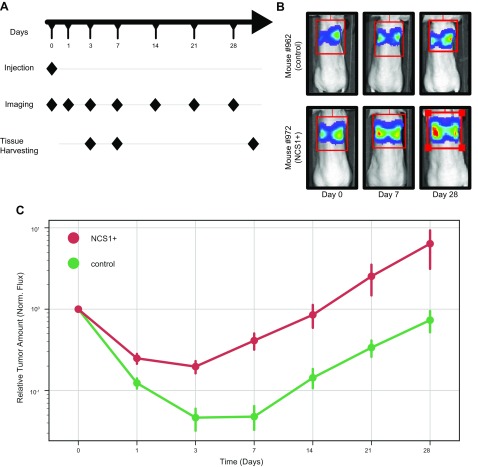
Given these changes in cellular phenotype, colony formation, and migratory capacity that are induced by overexpression of NCS1, we next studied NCS1 in an in vivo setting. Female nude mice were injected with MDA-MB231 cells that were engineered to express a firefly luciferase reporter and either NCS1 (NCS1+) or an empty vector (control). Photon flux was utilized as a metric of relative tumor amount in the mouse lungs. The total flux was calculated from lung imaging studies on d 0, 1, 3, 7, 14, 21, and 28 (Fig. 4A) after tail vein injection with the respective tumor cells. The majority of cells were observed in the lungs. No luminescence signal was found in other organs (Supplemental Fig. S5). All measurements were normalized to the total flux on d 0 to permit comparisons among individual mice. Figure 4B shows representative results for 2 mice and Fig. 3C shows the relative flux for all mice (see Supplemental Fig. S6 for a validation experiment with a longer follow-up).
Figure 4.
Mouse xenograft experiments show larger lung tumors from NCS1+-containing cells when compared with the control. A) Workflow schematic; n = 16 mice per group were injected via the tail vein with MDA-MB231 breast cancer cells overexpressing either NCS1 or a control vector. Lung tissue of 4 mice per group was harvested after 3 and 7 d and all mice were euthanized at the end of the study after d 28. Imaging studies measuring the photon flux of a luciferase reporter was performed on d 1, 3, 7, 14, 21, and 28. B) The panels show representative mouse images of a control mouse (962) and an NCS1+ mouse (972). The color-coded luminescence signal indicates the relative amount of tumor in the mouse lungs with blue being a weak signal and red being a strong signal. C) Line plot showing the relative luminescence signal after normalization at each imaging time point. Until d 7, the fluorescence measurements decreased less in NCS1+ tumors (red line plot) than in control tumors (green line plot). All tumors grow at similar rates from d 7 onwards.
Whereas the growth rates of NCS1+ and control tumors are comparable from d 7 onwards, the biggest difference between the groups can be observed between d 0 and 7. Consistent with our in vitro results, these in vivo findings suggest that NCS1-OE tumor cells have a survival advantage in the early phase of tumor development (Fig. 4C).
Histopathologic assessment of lung specimens confirms the presence of multiple tumor cell clusters in the NCS1+ group and small numbers of single tumor cells in the control
During the aforementioned mouse study (Fig. 5A), mouse lungs were harvested after d 3 and 7, and again at the end of the study. Because the biggest differences between NCS1+ and control tumors were found in the early phase (defined as the first 7 d) of tumor development, histopathologic assessment was conducted with a focus on these early tumors.
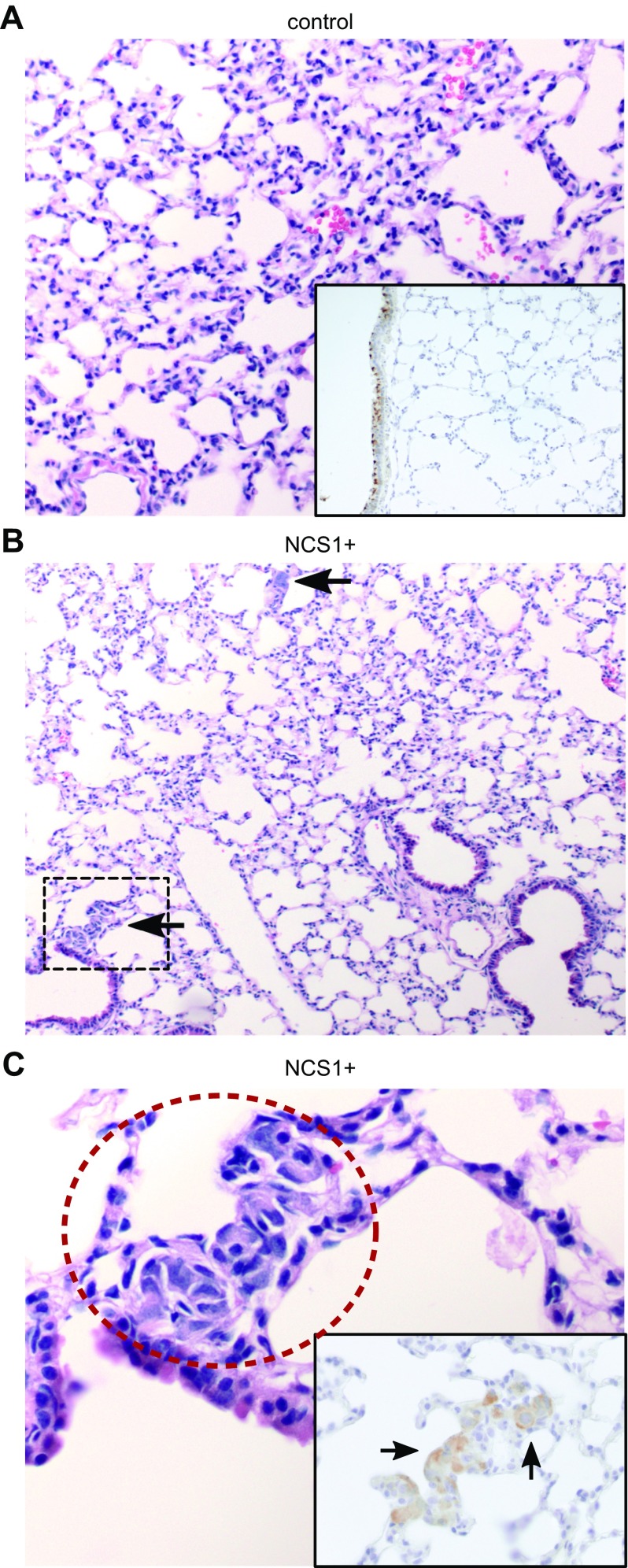
Figure 5.
Lung specimens of NCS1+ mice contain large tumor cell clusters after 7 d. A) Tumor cells were not found in an H&E-stained lung specimen from a control mouse (966) that was euthanized after 7 d (medium magnification). Anti-NCS1 IHC staining confirms the absence of tumor cells (insert, high magnification). NCS1 staining within normal bronchial epithelium served as a positive control in all samples. B) Two prominent foci of tumor cells are visible (black arrows) in an H&E-stained lung specimen from an NCS1+ mouse (987) that was euthanized after 7 d (low magnification). C) High-magnification image of the region in B that is marked with a black box. Tumor cells are circled in red. The insert shows an anti-NCS1 IHC-stained specimen of mouse 987 (high magnification) demonstrating that the tumor cells were stained positively for NCS1 (black arrows).
Lung tissue collected 7 d after tumor cell injection showed only a rare appearance of cancer cells in the lung specimens isolated from control mice and stained with H&E (Fig. 5A and Supplemental Table S2). Only 3 of the 8 control mouse lungs were found to contain a few single tumor cells (Supplemental Fig. S7 and Supplemental Table S2), whereas all lungs from NCS1+ mice harbored tumor cells and 6 of these 8 lungs contained multiple clusters of tumor cells (Fig. 5B, C and Supplemental Table S2). Anti-NCS1 immunohistochemistry (IHC) staining was used to validate the expression of NCS1 in the cells that were identified as cancer cells in the H&E-stained slides (inset in Fig. 5C and Supplemental Table S3). These results indicate that overexpression of NCS1 causes a higher early incidence of metastasis in mouse lungs after tail-vein injection with a tumor cell suspension. This can be explained by an increase of the number of cells which survived to form colonies in the lung tissue or an increase of cell invasiveness upon NCS1 overexpression.
NCS1 overexpression confers a long-term survival advantage to tumor cells
Mouse lung specimens that were obtained at the end of the study were stained with H&E and inspected to investigate the question whether NCS1 plays a role in mature tumors as well. Because all mice were euthanized after the respective lung tumor reached a large, predetermined size (109 absolute flux as measured using the method described above), a homogeneous histologic appearance of NCS1+ and control tumors was anticipated.
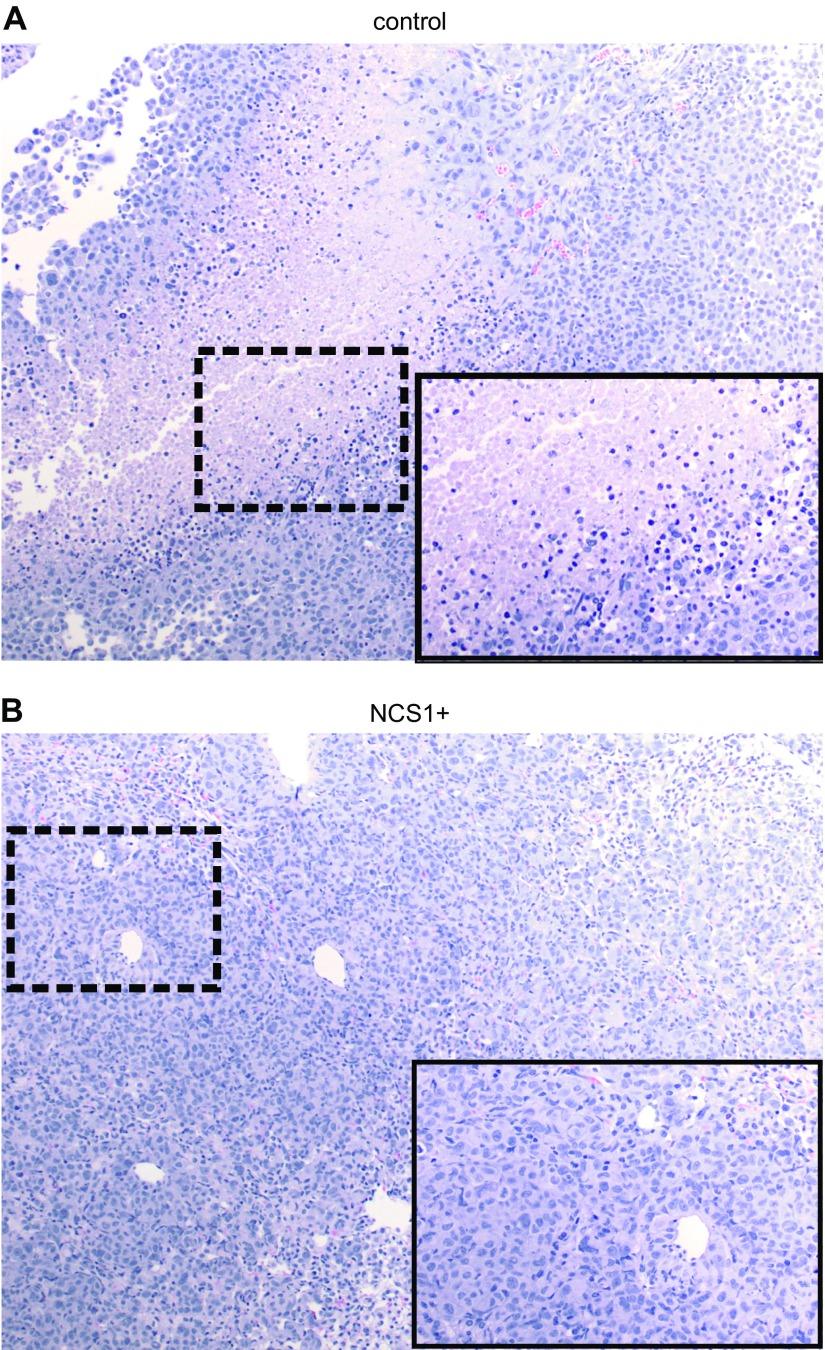
Contrary to our expectations, we found large areas of necrosis in the control tumors (Fig. 6A), whereas no necrotic cells could be identified in 3 of the 4 specimens from NCS1+ tumors (Fig. 6B and Supplemental Table S1). A fourth specimen exhibited limited amounts of necrotic material as opposed to the large areas of necrosis in the control tumors. Histologically, no difference with regard to the overall tumor volume was found between NCS1+ and control lungs, most likely because all tumors had reached a size where the lungs were completely filled with tumor cells (Supplemental Fig. S8). The absence of tumor cell death in the context of NCS1 overexpression suggests that even in a larger tumor, high NCS1 levels confer a survival advantage. This may impact the reactivity of the tumor to treatment by preserving more living cells in the tumor’s core.
Figure 6.
Lung specimens of NCS1+ mice show no necrotic areas after more than 28 d of tumor growth. A) H&E-stained lung specimen (medium magnification) of a control mouse (952) at the end of the study. The insert shows an area of necrosis (black box) at high magnification. B) Picture of an H&E-stained lung specimen (medium magnification) of an NCS1+ mouse (973) at the end of the study. Note the absence of necrotic cells. The insert shows a high magnification view of the area in the black box.
DISCUSSION
In this study, we examined the effects of increased levels of NCS1 on tumor cell migration and survival in vitro and in vivo. We have previously observed that high NCS1 expression was significantly associated with an unfavorable prognosis in 2 independent breast cancer cohorts (41) as well as 2 publicly available liver cancer cohorts (42). Interestingly, NCS1 levels were highly correlated with expression levels of LIMK1, an enzyme associated with regulation of cell motility (27), when examining RNA sequencing data of liver samples from the Cancer Genome Atlas (48) and the International Cancer Genome Consortium (49). LIMK1 is a key regulator of the actin cytoskeleton and its high expression was previously identified as a potential driver of invasion in a variety of tumors (28–30). Other components of physiologic Ca2+ signaling are also known to regulate physiologic cell movement as well as tumor cell motility (21, 22, 24). Thus, we hypothesized that high levels of NCS1 may confer enhanced metastatic capability on tumor cells.
To investigate the molecular effects of increased NCS1, this Ca2+-binding protein was stably overexpressed in the MDA-MB231 breast cancer cell line. First, we validated (41) that high levels of NCS1 do not alter cellular proliferation rates using 2 cell based growth assays. Another hallmark of aggressive, metastatic tumor cells shown to be regulated by NCS1 is their motility, which would enhance a cell’s ability to spread to distant organs within the body. Accordingly, we found that upon overexpression of NCS1, cells exhibited a profoundly different morphology. In particular, they were less rounded and displayed an increased number of large cellular protrusions. This phenotype is consistent with enhanced motility and the capacity to form colonies.
To further study the effects of NCS1 overexpression on tumor cell morphology, immunofluorescence imaging was performed. We found that NCS1 preferentially localizes to the leading edge of migrating cells. Although this effect was independent of absolute expression levels, we found more NCS1 at cellular protrusions in cells that highly overexpressed NCS1. This finding supports the hypothesis that NCS1 might facilitate the movement of cancer cells via regulation of local Ca2+ at cell extensions. The colocalization between NCS1 and actin, specifically at the leading edge, suggests that NCS1 assists in regulating the continuous turnover of the actin cytoskeleton necessary for cell migration to occur.
We performed 2-D colony formation and wound healing assays to confirm that this morphologic change is also accompanied by a functional change. Indeed, NCS1-OE cells were more motile compared with the controls. In an attempt to more closely mimic the physiologic 3-D microenvironment in which cancers grow and to analyze the dynamics of MDA-MB231 cell movement in vitro (50, 51), we placed NCS1-OE and control cells in type I collagen gels and performed time-lapse microscopy to monitor their movement. Again, increased motility, as measured using the MSD and average speed, was observed.
Thus, the in vitro experiments performed in this study suggest that tumor cells that acquire high levels of NCS1 during tumorigenesis gain the advantage of being more motile. To explore the question of whether this in vitro phenotype also leads to an increased number of metastases in vivo, we used a mouse xenograft model. Engineered MDA-MB231 cells were injected into the tail vein of nude mice and a luciferase reporter was used to monitor tumor growth in the mouse lungs over time. Mice injected with high NCS1 levels presented with increased numbers of nascent tumors between d 0 and 7, but after this initial period, the growth rates of NCS1+ and control tumors were similar. This finding in an intact mouse is in close alignment with our observations from in vitro experiments. If NCS1 impacted cell proliferation, we would have expected to see a difference in the overall tumor growth rates.
That NCS1 facilitates early tumor cell engraftment in the mouse lung suggests that NCS1 promotes cell survival as well as metastasis. In addition to examining mouse specimens at early time points during the experiment, specimens from larger tumors after more than 28 d were analyzed to gain more insight into how NCS1 overexpression impacts tumor behavior. Although all of the tumors were large, we observed differences between the groups. Control tumors presented with large necrotic zones in all specimens, whereas NCS1+ tumors did not. This difference indicates a survival advantage of cells with high NCS1 levels that extends beyond the initial metastatic expansion.
It is well known that major oncogenic pathways are regulated by cytoplasmic Ca2+. Enhanced signaling via the PI3K/Akt pathway facilitates cell movement and increases cellular survival (52). NCS1 physically binds to PI4K (35, 36) and via this protein–protein interaction, it regulates the production of the second messenger molecule inositol 1,4,5 trisphosphate (53). InsP3 in turn activates the PI3K pathway. Furthermore, NCS1 interacts with InsP3Rs at the endoplasmic reticulum (ER) and upon binding, there is increased Ca2+ efflux from the ER (54). Previous research has shown that InsP3Rs are important contributors to an aggressive, prometastatic phenotype in cancer cells (21). The combination of these studies indicates that NCS1 enhances cell migration and survival via several routes. Upon overexpression, NCS1 binds to InsP3Rs and increases cytoplasmic Ca2+ concentrations. Ca2+ itself can act as a second messenger molecule and can facilitate cell movement. In addition, NCS1 may activate the PI3K pathway upon binding to PI4K.
Our previous study used 2 different breast cancer cell lines (MDA-MB231 and MCF-7), and similar responses to NCS1 overexpression were observed in both cell lines (41). In this study we focused on the triple-negative MDA-MB231 cells derived from plural effusion, as these cells are better able to metastasize to various organs (55). Various in vitro and in vivo experiments were performed to demonstrate the effects of NCS1 overexpression on several aspects of the cellular phenotype. In-depth mechanistic studies of the molecular effects of NCS1 are currently in preparation. Our future work will also reveal which parts of the Ca2+ signaling complex associated with NCS1 (42) are best suited for pharmacologic intervention.
CONCLUSIONS
This study demonstrates that overexpression of the Ca2+ binding protein NCS1 increases cellular motility and the invasive capacity of tumor cells in vitro and in vivo without altering growth rates. It lays the groundwork for studying the molecular mechanisms of NCS1- and Ca2+-driven metastasis in cancers such as breast and liver tumors. Furthermore, it describes a set of experiments that can be used to test pharmacologic interventions to inhibit metastatic spread of tumor cells with high levels of NCS1.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Sabine Lang (Yale University) for technical and administrative support. The authors acknowledge helpful discussions with Allison Brill, Dr. David Calderwood, and Dr. Tamar Taddei (Yale University). J.E.A., D.S., J.A.S., and H.K.G. received a scholarship from the German Academic Scholarship Foundation. M.Z. received a Brown Coxe Postdoctoral Fellowship from Yale University. W.L.C. received NSF Graduate Research Fellowship DGE-1122492. This work was supported, in part, by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant 5P01DK057751 (to B.E.E. and M.E.R.), NIH National Institute of Biomedical Imaging and Bioengineering (Grant 1R21EB026630 to M.M.), and U.S. Department of Defense Grant W81XWH-15-1-0117 (to Q.Y.). B.E.E. is a founder of Osmol Therapeutics, a company that is targeting NCS1 for therapeutic purposes. Primary data are maintained in the Ehrlich Laboratory at Yale University. Reagents not commercially available are available from the Ehrlich Laboratory. The authors declare no conflicts of interest.
Glossary
- 2/3-D
2-dimensional
- GFP
green fluorescent protein
- H&E
hematoxylin and eosin
- HEK-293
human embryonic kidney 293
- IHC
immunohistochemistry
- InsP3R
inositol 1,4,5-trisphosphate receptor
- MSD
mean squared displacement
- NCS1
neuronal calcium sensor 1
- NCS1-OE
NCS1-overexpressing
- PI4K
phosphatidylinositol 4-OH kinase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. E. Apasu, D. Schuette, and B. E. Ehrlich designed the study; J. E. Apasu, D. Schuette, R. LaRanger, J. A. Steinle, L. D. Nguyen, H. K. Grosshans, M. Zhang, and W. L. Cai contributed to experiments; M. E. Robert provided all pathology assessments; D. Schuette and B. E. Ehrlich wrote the first draft; and all authors edited the manuscript and have consented to publication.
REFERENCES
- 1.Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 2.Mehlen P., Puisieux A. (2006) Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458 [DOI] [PubMed] [Google Scholar]
- 3.Taketo M. M. (2011) Reflections on the spread of metastasis to cancer prevention. Cancer Prev. Res. (Phila.) 4, 324–328 [DOI] [PubMed] [Google Scholar]
- 4.Lambert A. W., Pattabiraman D. R., Weinberg R. A. (2017) Emerging biological principles of metastasis. Cell 168, 670–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge M. J. (1993) Inositol trisphosphate and calcium signalling. Nature 361, 315–325 [DOI] [PubMed] [Google Scholar]
- 6.Berridge M. J. (1998) Neuronal calcium signaling. Neuron 21, 13–26 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T., Chin W. C., Verdugo P. (1998) Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+. Nature 395, 908–912 [DOI] [PubMed] [Google Scholar]
- 8.Gunter T. E., Pfeiffer D. R. (1990) Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. J. (1995) Calcium signalling and cell proliferation. BioEssays 17, 491–500 [DOI] [PubMed] [Google Scholar]
- 10.Smedler E., Uhlén P. (2014) Frequency decoding of calcium oscillations. Biochim. Biophys. Acta 1840, 964–969 [DOI] [PubMed] [Google Scholar]
- 11.Dolmetsch R. E., Xu K., Lewis R. S. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 [DOI] [PubMed] [Google Scholar]
- 12.Clapham D. E. (2007) Calcium signaling. Cell 131, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 13.Augustine G. J., Santamaria F., Tanaka K. (2003) Local calcium signaling in neurons. Neuron 40, 331–346 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A., Greenberg M. E. (1995) Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268, 239–247 [DOI] [PubMed] [Google Scholar]
- 15.Hajnóczky G., Davies E., Madesh M. (2003) Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 304, 445–454 [DOI] [PubMed] [Google Scholar]
- 16.Takemura M., Mishima T., Wang Y., Kasahara J., Fukunaga K., Ohashi K., Mizuno K. (2009) Ca2+/calmodulin-dependent protein kinase IV-mediated LIM kinase activation is critical for calcium signal-induced neurite outgrowth. J. Biol. Chem. 284, 28554–28562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna S., El-Sibai M. (2013) Signaling networks of Rho GTPases in cell motility. Cell. Signal. 25, 1955–1961 [DOI] [PubMed] [Google Scholar]
- 18.Zheng J. Q., Poo M. M. (2007) Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 23, 375–404 [DOI] [PubMed] [Google Scholar]
- 19.Swaney K. F., Huang C.-H., Devreotes P. N. (2010) Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi M., Weaver D., Hajnóczky G. (2004) Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando H., Kawaai K., Bonneau B., Mikoshiba K. (2018) Remodeling of Ca2+ signaling in cancer: regulation of inositol 1,4,5-trisphosphate receptors through oncogenes and tumor suppressors. Adv. Biol. Regul. 68, 64–76 [DOI] [PubMed] [Google Scholar]
- 22.Florea A. M., Büsselberg D. (2009) Anti-cancer drugs interfere with intracellular calcium signaling. Neurotoxicology 30, 803–810 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y. F., Chen Y. T., Chiu W. T., Shen M. R. (2013) Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 20, 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart T. A., Yapa K. T. D. S., Monteith G. R. (2015) Altered calcium signaling in cancer cells. Biochim. Biophys. Acta 1848, 2502–2511 [DOI] [PubMed] [Google Scholar]
- 25.Xiao M., Li T., Ji Y., Jiang F., Ni W., Zhu J., Bao B., Lu C., Ni R. (2018) S100A11 promotes human pancreatic cancer PANC-1 cell proliferation and is involved in the PI3K/AKT signaling pathway. Oncol. Lett. 15, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez Guerrico A. M., Jaffer Z. M., Page R. E., Braunewell K. H., Chernoff J., Klein-Szanto A. J. (2005) Visinin-like protein-1 is a potent inhibitor of cell adhesion and migration in squamous carcinoma cells. Oncogene 24, 2307–2316 [DOI] [PubMed] [Google Scholar]
- 27.Prunier C., Prudent R., Kapur R., Sadoul K., Lafanechère L. (2017) LIM kinases: cofilin and beyond. Oncotarget 8, 41749–41763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott R. W., Hooper S., Crighton D., Li A., König I., Munro J., Trivier E., Wickman G., Morin P., Croft D. R., Dawson J., Machesky L., Anderson K. I., Sahai E. A., Olson M. F. (2010) LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J. Cell Biol. 191, 169–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott R. W., Olson M. F. (2007) LIM kinases: function, regulation and association with human disease. J. Mol. Med. (Berl.) 85, 555–568 [DOI] [PubMed] [Google Scholar]
- 30.Li R., Doherty J., Antonipillai J., Chen S., Devlin M., Visser K., Baell J., Street I., Anderson R. L., Bernard O. (2013) LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clin. Exp. Metastasis 30, 483–495 [DOI] [PubMed] [Google Scholar]
- 31.Boeckel G. R., Ehrlich B. E. (2018) NCS-1 is a regulator of calcium signaling in health and disease. Biochim. Biophys. Acta. Mol. Cell Res. 1865, 1660–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss J. L., Hui H., Burgoyne R. D. (2010) Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell. Mol. Neurobiol. 30, 1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Onofrio S., Kezunovic N., Hyde J. R., Luster B., Messias E., Urbano F. J., Garcia-Rill E. (2015) Modulation of gamma oscillations in the pedunculopontine nucleus by neuronal calcium sensor protein-1: relevance to schizophrenia and bipolar disorder. J. Neurophysiol. 113, 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgoyne R. D., Weiss J. L. (2001) The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 353, 1–12 [PMC free article] [PubMed] [Google Scholar]
- 35.Rajebhosale M., Greenwood S., Vidugiriene J., Jeromin A., Hilfiker S. (2003) Phosphatidylinositol 4-OH kinase is a downstream target of neuronal calcium sensor-1 in enhancing exocytosis in neuroendocrine cells. J. Biol. Chem. 278, 6075–6084 [DOI] [PubMed] [Google Scholar]
- 36.Haynes L. P., Fitzgerald D. J., Wareing B., O’Callaghan D. W., Morgan A., Burgoyne R. D. (2006) Analysis of the interacting partners of the neuronal calcium-binding proteins L-CaBP1, hippocalcin, NCS-1 and neurocalcin δ. Proteomics 6, 1822–1832 [DOI] [PubMed] [Google Scholar]
- 37.Blasiole B., Kabbani N., Boehmler W., Thisse B., Thisse C., Canfield V., Levenson R. (2005) Neuronal calcium sensor-1 gene ncs-1a is essential for semicircular canal formation in zebrafish inner ear. J. Neurobiol. 64, 285–297 [DOI] [PubMed] [Google Scholar]
- 38.Koizumi S., Rosa P., Willars G. B., Challiss R. A., Taverna E., Francolini M., Bootman M. D., Lipp P., Inoue K., Roder J., Jeromin A. (2002) Mechanisms underlying the neuronal calcium sensor-1–evoked enhancement of exocytosis in PC12 cells. J. Biol. Chem. 277, 30315–30324 [DOI] [PubMed] [Google Scholar]
- 39.Choe C. U., Ehrlich B. E. (2006) The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci. STKE 2006, re15 [DOI] [PubMed] [Google Scholar]
- 40.Boehmerle W., Splittgerber U., Lazarus M. B., McKenzie K. M., Johnston D. G., Austin D. J., Ehrlich B. E. (2006) Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc. Natl. Acad. Sci. USA 103, 18356–18361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore L. M., England A., Ehrlich B. E., Rimm D. L. (2017) Calcium sensor, NCS-1, promotes tumor aggressiveness and predicts patient survival. Mol. Cancer Res. 15, 942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuette D., Moore L. M., Robert M. E., Taddei T. H., Ehrlich B. E. (2018) Hepatocellular carcinoma outcome is predicted by expression of neuronal calcium sensor 1. Cancer Epidemiol. Biomarkers Prev. 27, 1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzmán C., Bagga M., Kaur A., Westermarck J., Abankwa D. (2014) ColonyArea: an ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS One 9, e92444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 45.Ponomarev V., Doubrovin M., Serganova I., Vider J., Shavrin A., Beresten T., Ivanova A., Ageyeva L., Tourkova V., Balatoni J., Bornmann W., Blasberg R., Gelovani Tjuvajev J. (2004) A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur. J. Nucl. Med. Mol. Imaging. 31, 740–751 [DOI] [PubMed] [Google Scholar]
- 46.Stuelten C. H., Parent C. A., Montell D. J. (2018) Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer 18, 296–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedl P., Gilmour D. (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 [DOI] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research Network (2017) Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto A., Furuta M., Totoki Y., Tsunoda T., Kato M., Shiraishi Y., Tanaka H., Taniguchi H., Kawakami Y., Ueno M., Gotoh K., Ariizumi S., Wardell C. P., Hayami S., Nakamura T., Aikata H., Arihiro K., Boroevich K. A., Abe T., Nakano K., Maejima K., Sasaki-Oku A., Ohsawa A., Shibuya T., Nakamura H., Hama N., Hosoda F., Arai Y., Ohashi S., Urushidate T., Nagae G., Yamamoto S., Ueda H., Tatsuno K., Ojima H., Hiraoka N., Okusaka T., Kubo M., Marubashi S., Yamada T., Hirano S., Yamamoto M., Ohdan H., Shimada K., Ishikawa O., Yamaue H., Chayama K., Miyano S., Aburatani H., Shibata T., Nakagawa H. (2016) Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 48, 500–509 [DOI] [PubMed] [Google Scholar]
- 50.Riching K. M., Cox B. L., Salick M. R., Pehlke C., Riching A. S., Ponik S. M., Bass B. R., Crone W. C., Jiang Y., Weaver A. M., Eliceiri K. W., Keely P. J. (2014) 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 107, 2546–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu P.-H., Giri A., Sun S. X., Wirtz D. (2014) Three-dimensional cell migration does not follow a random walk. Proc. Natl. Acad. Sci. USA 111, 3949–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vivanco I., Sawyers C. L. (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- 53.Balla A., Balla T. (2006) Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 16, 351–361 [DOI] [PubMed] [Google Scholar]
- 54.Schlecker C., Boehmerle W., Jeromin A., DeGray B., Varshney A., Sharma Y., Szigeti-Buck K., Ehrlich B. E. (2006) Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J. Clin. Invest. 116, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fantozzi A., Christofori G. (2006) Mouse models of breast cancer metastasis. Breast Cancer Res. 8, 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.