Abstract
Aurora kinase A (AURKA) is necessary for proper primary cilium disassembly before mitosis. We found that depletion of caveolin-1 expression promotes primary cilia formation through the proteasomal-dependent degradation of aurora kinase A and induces premature senescence in human fibroblasts. Down-regulation of intraflagellar transport-88, a protein essential for ciliogenesis, inhibits premature senescence induced by the depletion of caveolin-1. In support of these findings, we showed that alisertib, a pharmacological inhibitor of AURKA, causes primary cilia formation and cellular senescence by irreversibly arresting cell growth. Suppression of primary cilia formation limits cellular senescence induced by alisertib. The primary cilium must be disassembled to free its centriole to form the centrosome, a necessary structure for mitotic spindle assembly and cell division. We showed that the use of the centriole to form primary cilia blocks centrosome formation and mitotic spindle assembly and prevents the completion of mitosis in cells in which cellular senescence is caused by the inhibition of AURKA. We also found that AURKA is down-regulated and primary cilia formation is enhanced when cellular senescence is promoted by other senescence-inducing stimuli, such as oxidative stress and UV light. Thus, we propose that impaired AURKA function induces premature senescence by preventing reabsorption of the primary cilium, which inhibits centrosome and mitotic spindle formation and consequently prevents the completion of mitosis. Our study causally links the inability of the cell to disassemble the primary cilium, a microtubule-based cellular organelle, to the development of premature senescence, a functionally and pathologically relevant cellular state.—Jeffries, E. P., Di Filippo, M., Galbiati, F. Failure to reabsorb the primary cilium induces cellular senescence.
Keywords: caveolae, caveolin-1, aurora kinase A, alisertib, mitotic spindle
Primary cilia are sensory antennae found on the surface of nearly every type of eukaryotic cell. The cilium, which is composed of 9 doublet microtubules termed the axoneme, elongates directly from the mother centriole. Ciliogenesis, the process of primary cilia formation, is coordinated with progression of the cell cycle. The process has been characterized in full detail by examination of ultrastructural data. A cell forms a primary cilium in the G1/G0 phase of the cell cycle and disassembles it as the cell re-enters the cell cycle at the G2/M phase boundary. Failure to disassemble cilia may restrict cell cycle progression, as the cilia must be disassembled in mitosis to liberate the captive centriole and promote mitotic spindle formation (1–3). Intraflagellar transport (IFT)-88 is one of the best-studied positive regulators of primary cilia formation (4), whereas AURKA, whose expression peaks at the G2/M boundary, is necessary for ciliary disassembly and mitotic spindle formation (5). Defects in ciliary formation lead to diverse disease states, and inability to form primary cilia is often associated with carcinogenesis (6).
Replicative senescence is an irreversible form of cell cycle arrest that is achieved by somatic cells and is dependent on telomere shortening (7, 8). A variety of cellular stimuli can induce a premature form of cellular senescence (i.e., premature cellular senescence) that is independent of telomere shortening. Examples of senescence-inducing stimuli include oxidative stress, UV light, cytotoxic drugs, DNA damaging agents, and oncogenes (9–13). The growth of senescent cells is permanently arrested, and senescent cells are characterized by enlarged and flat cell morphology. Additional hallmarks of cellular senescence include increased β-gal activity at pH 6, p53 activity, p21Waf1/Cip1, and p16 protein expression, as well as γ-H2A histone family member X (H2A.X, indicative of activation of a DNA damage response), and expression of the senescence-associated secretory phenotype (7, 8, 10, 14).
Caveolin-1 is one of the major protein components of caveolae, flask-shaped invaginations of the plasma membrane (15, 16). Caveolin-1 acts as a scaffolding protein that concentrates and functionally regulates a variety of signaling molecules within caveolar membranes (17–21). Our laboratory was the first to show that caveolin-1 promotes oxidative stress-induced premature senescence in fibroblasts through the modulation of functions of mouse double minute 2, ataxia telangiectasia mutated, protein phosphatase 2A–C, nuclear factor (erythroid 2)–related factor 2, and sirtuin-1 (22–29). Oxidative stress–induced premature senescence is inhibited in mouse embryonic fibroblasts derived from caveolin-1–null mice (22) and in the lungs of caveolin-1–null mice (26). Since our original observations, multiple laboratories have independently confirmed that caveolin-1–mediated signaling is necessary for the development of stress-induced cellular senescence. Thus, caveolin-1 is a key regulator of signal transduction mechanisms linking oxidative stress to the development of a senescent phenotype. Data from the Lisanti group show that cancer-associated fibroblasts isolated from patients with breast cancer display reduced caveolin-1 expression and increased expression of senescence markers (30, 31); however, the researchers did not address the underlying mechanism or whether there is a causal relationship between reduced caveolin-1 expression and the development of cellular senescence in fibroblasts when the cells are not hit by a senescence-inducing stimulus.
In the present study, we found that the inability to disassemble the primary cilium irreversibly arrests cell growth and induces cellular senescence by preventing the formation of the mitotic spindle. Mechanistically, we show that senescence-inducing stimuli promote primary cilia formation and cellular senescence through the down-regulation and inhibition of AURKA expression. Our study causally links the formation of the primary cilium, a microtubule-based cellular organelle, to the development of cellular senescence, a functionally and pathologically relevant cellular state.
MATERIALS AND METHODS
Materials
Antibodies were obtained from the following sources: anti-caveolin-1 IgG (pAb N-20), anti-β-actin (mAb C4), anti-p21 from Santa Cruz Biotechnology (Dallas, TX, USA); anti-IFT88 (pAb) from Proteintech (Rosemont, IL, USA); anti-aurora kinase A (mAb D3E4Q) and anti-phospho histone H2A.X (Ser139) (mAb 20E3) from Cell Signaling Technology (Danvers, MA, USA); anti-p16 ARC (mAb EP1551Y) from Abcam (Cambridge, MA, USA); and anti-α-tubulin (mAb DM1A) and anti-acetylated-α-tubulin (mAb 6-11B-1) from MilliporeSigma (Burlington, MA, USA). Alisertib (MLN8237) was obtained from Selleckchem (Houston, TX, USA). Scrambled IFT88 (s15625) and caveolin-1 (299945) small interfering RNA (siRNA) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Scrambled and human caveolin-1 short hairpin (sh)RNAs (cloned into the pLKO lentiviral vector) were a generous gift from Dr. Paul Pilch (Boston University School of Medicine, Boston, MA, USA). Human caveolin-1 cDNA was cloned into the lentiviral vector pLVX by using standard cloning techniques. Lentivirus was generated by cotransfecting lentiviral vectors with pMD2G and psPAX2 into 293T cells with the calcium phosphate method. RO-3306, 2′-deoxycytidine hydrate, MG-132, and chloroquine were from MilliporeSigma. All other biochemical reagents were of the highest available purity and were commercially obtained.
Cell culture and oxidative stress
WI-38, IMR-90 human diploid fibroblasts and normal human lung fibroblasts (NHLFs; kindly provided by Dr. Mauricio Rojas, Simmons Center for Interstitial Lung Diseases, University of Pittsburgh, Pittsburgh, PA, USA) were cultured in Eagle’s minimum essential medium supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum. HeLa cells (a generous gift from Dr. Peter Friedman; University of Pittsburgh), MDA-MB-231 and MCF-7 breast cancer cells were cultured in DMEM, supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum. HUVECs (kindly provided by Shiva Swamynathan; University of Pittsburgh School of Medicine) and human lung microvascular endothelial cells (HMVECs) and human pulmonary artery endothelial cells (HPAECs; a generous gift from Dr. Adam Straub, Vascular Medicine Institute, University of Pittsburgh) were grown in endothelial cell growth medium 2 (EGM-Plus Endothelial Cell Growth Medium BulletKit; Lonza, Basel, Switzerland). Proteasomal (0.6 μΜ MG-132) or lysosomal (50 μΜ chloroquine and 10 mM NH4Cl) inhibitors were added to the cells 24 h before they were collected. Oxidative stress was induced by subcytotoxic levels of H2O2 (450 µM) for 2 h. After induction of stress by H2O2, cells were recovered in complete medium for the duration specified by the manufacturer.
Immunoblot analysis
Cells were collected in boiling sample buffer. Cellular proteins were resolved by SDS-PAGE (12.5% acrylamide) and transferred to Amersham Protran 0.2 μm nitrocellulose blotting membrane (GE Healthcare Life Sciences, Marlborough, MA, USA). Blots were incubated for 1 h 15 min in Tris-buffered saline-Tween 20 [TBST; 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.2% Tween 20] containing 2% powdered skim milk and 1% bovine serum albumin (BSA). After 3 washes with TBST, membranes were incubated overnight with the primary antibody and washed 3 more times with TBST. Blots were then incubated for 1 h 15 min with horseradish peroxidase–conjugated goat anti-rabbit/mouse IgG. Bound proteins were detected with an ECL detection kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Representative images of at least 3 independent experiments are shown.
Measurement of cellular senescence
Cellular senescence was measured with the Senescence β-Galactosidase Staining Kit according to the manufacturer’s protocol (Cell Signaling Technology). The mean percentage of senescence was calculated from quantification of total cells and senescent cells in 10 fields of view per condition, using an upright epifluorescence microscope (Leica Microsystems, Buffalo Grove, IL, USA). At least 3 independent experiments were performed in triplicate. Representative images of microscope fields are shown.
Immunofluorescence microscopy
WI-38, IMR-90, and HeLa cells were washed 3 times with PBS and fixed for 30 min at room temperature with 2% paraformaldehyde in PBS. Fixed cells were rinsed with PBS and permeabilized with 0.1% Triton X-100 and 0.2% BSA for 10 min. Cells were then treated with 10 μg/ml RNAse A with 0.1% Triton X-100 and 0.2% BSA for 10 min at room temperature, washed with PBS, and incubated for 20 min with 1 µg/ml DAPI. Cells were treated with 25 mM NH4Cl in PBS for 10 min at room temperature to quench free aldehyde groups. After three 10-min washes with 0.1% Triton X-100 and 0.2% BSA in PBS, the cells were incubated with the primary antibody diluted in PBS with 0.1% Triton X-100 and 0.2% BSA. After 3 washes with 0.1% Triton X-100 and 0.2% BSA in PBS (10 min each), cells were incubated with lissamine rhodamine B sulfonyl chloride–conjugated goat anti-rabbit antibody (5 μg/ml) and FITC-conjugated goat anti-mouse antibody (5 μg/ml) for 1 h at room temperature. Cells were washed 3 times with 0.1% Triton X-100 and 0.2% BSA in PBS (10 min each). Slides were mounted with slow-fade anti-fade reagent and examined using an epifluorescent microscopy at ×40 magnification. Quantification was performed by analyzing randomly chosen fields from at least 3 independent slides.
Transfections of siRNA
siRNA was introduced into fibroblasts by using Lipofectamine RNAiMax Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol, using 45 pmol per well of 6-well culture plate.
RNA isolation and RT-PCR
Cells were collected, and total RNA was isolated by using the RNeasy Mini Kit from Qiagen (Valencia, CA, USA). Equal amounts of RNA were treated with RNase-free DNase and subjected to reverse transcription with the Advantage RT-for-PCR kit from Takara Bio (Mountain View, CA, USA), according to the manufacturer’s protocol. PCR was then performed for each gene studied in its linear zone of amplification.
Bromodeoxyuridine-incorporation assay
Cell proliferation was measured and quantified with the Cell Proliferation ELISA, Bromodeoxyuridine (BrdU) Colorimetric Kit (Roche, Indianapolis, IN, USA). The kit was used according to the manufacturer’s protocol. Cells were incubated with BrdU labeling solution overnight at 37°C. The absorbances were measured at 370 nm on an ELISA plate reader, after the specified duration of substrate incubation. Experiments were performed in triplicate.
Cell synchronization by double thymidine block
HeLa cells were plated at 30% confluence. Thymidine was added in complete medium to a final concentration of 5 mM, and cells were placed in incubator for 20 h. Medium containing thymidine was removed, and the cells were washed 3 times with PBS and cultured in complete medium for 8 h. Thymidine was then added in complete medium at a final concentration of 5 mM, and cells were placed in incubator for 16 h. Medium containing thymidine was removed, and cells were washed 3 times with PBS. Complete medium containing 25 μM 2′-deoxycytidine was added, and cells were placed in an incubator for 4 h. RO-3306 was added at a final concentration of 10 μM, and cells were placed in an incubator for 6 h. Medium containing 2′-deoxycytidine and RO-3306 was removed, and cells were washed with PBS 3 times. Complete medium was added to the cells, and they were placed in incubator for 1 h, then fixed with paraformaldehyde, the nuclei were stained with DAPI, and the mitotic spindles were detected with α-tubulin antibody. α-Tubulin was visualized with immunofluorescence.
Statistical analysis
Senescence and ciliation quantification studies were performed at least in triplicate and percentages shown are the averaged percentages of 10 independent microscope fields. The means ± sem is shown. Significance was calculated with Student’s t test, and was set at P < 0.05.
RESULTS
Caveolin-1 deficiency inhibits primary cilium absorption through the proteasomal-mediated degradation of aurora kinase A
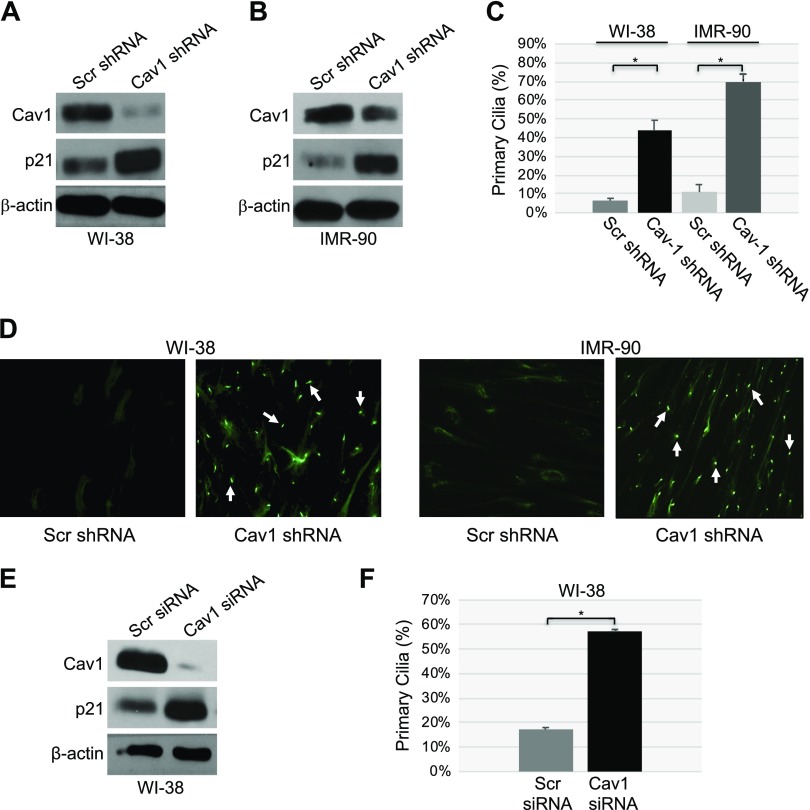
To investigate the functional consequence of a loss of caveolin-1 expression in human cells under resting conditions, we achieved more than 95% down-regulation of caveolin-1 expression by shRNA in WI-38 (Fig. 1A) and IMR-90 (Fig. 1B) human diploid fibroblasts. Primary cilia formation was quantified by staining the cells with acetylated α-tubulin, a marker of primary cilia. A loss of caveolin-1 expression induced a dramatic increase in the percentage of cells carrying a primary cilium (Fig. 1C, D). In support of these findings, transfection of WI-38 cells with caveolin-1 siRNA decreased endogenous caveolin-1 expression (Fig. 1E) and increased the percentage of cells with a primary cilium (Fig. 1F).
Figure 1.
Down-regulation of caveolin (Cav)-1 promotes ciliogenesis. A, B) WI-38 (A) and IMR-90 (B) human diploid fibroblasts were infected with a lentivirus carrying caveolin-1 shRNA. Infection with a lentivirus carrying scrambled (Scr) shRNA was used as the control. Cells were cultured for 10 d, and cell lysates were collected for immunoblot analysis with antibody probes specific for caveolin-1 and p21. Immunoblot with anti-β-actin IgGs was performed to show equal loading. C, D) Caveolin-1 expression was down-regulated by shRNA in WI-38 and IMR-90 fibroblasts, as described in A and B. After 10 d, primary cilia formation was quantified by immunofluorescence staining with an antibody probe specific for acetylated α-tubulin. Quantification of ciliogenesis (C) and representative images (D) are shown. E, F) Caveolin-1 expression was down-regulated in WI-38 human diploid fibroblasts by caveolin-1 siRNA. Transfection of WI-38 cells with scrambled siRNA was performed as the control. After 10 d, caveolin-1, p21 and β-actin protein expression was determined by immunoblot analysis (E), whereas primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin IgGs (F). Values are means ± sem. *P < 0.001 (Student’s t test).
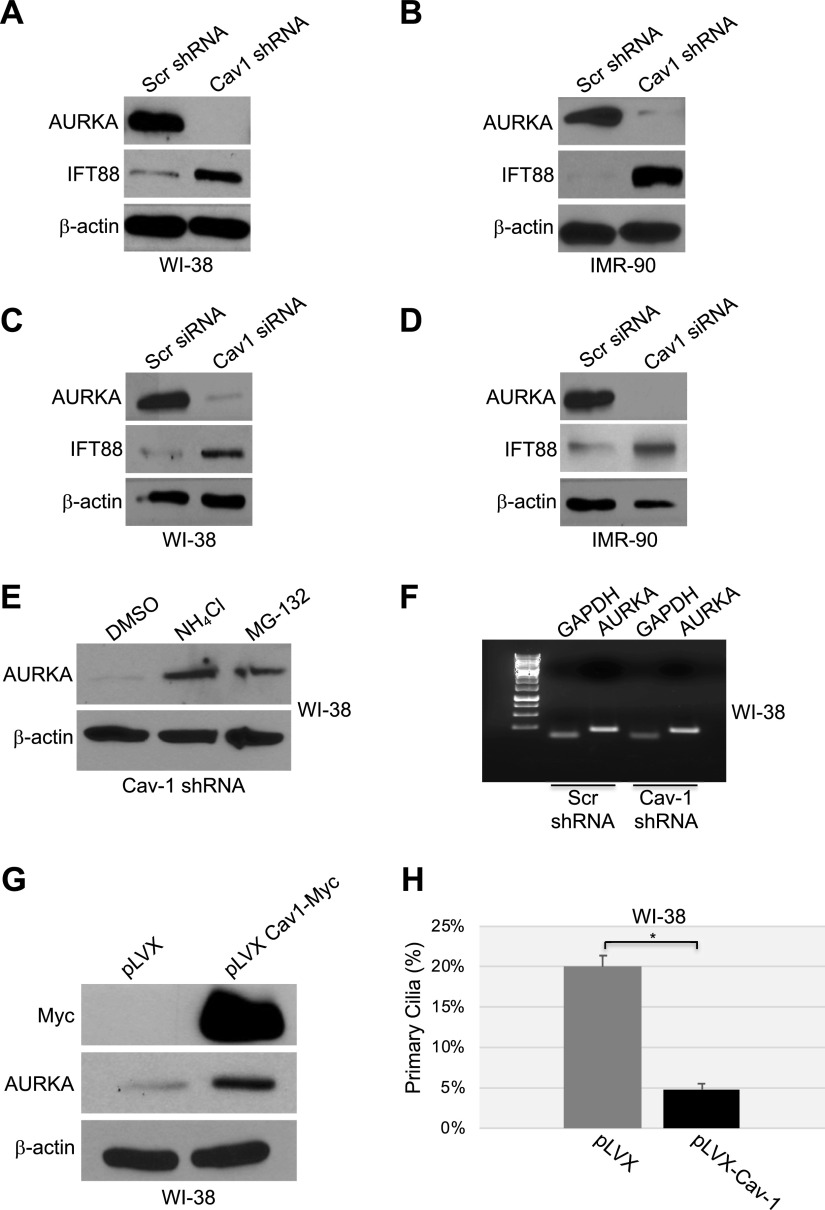
How does caveolin-1 deficiency promote primary cilia formation? Because AURKA is a well-established negative regulator of primary cilia formation, we asked whether a loss of caveolin-1 would down-regulate AURKA levels. To this end, caveolin-1 deficiency was achieved by shRNA in both WI-38 and IMR-90 cells (as described in Fig. 1A, B), and the protein levels of AURKA were quantified by immunoblotting analysis. AURKA was virtually lost in caveolin-1–lacking cells (Fig. 2A, B). Identical results were obtained when caveolin-1 deficiency was achieved by siRNA (Fig. 2C, D). The dramatic down-regulation of AURKA protein expression, after a loss of caveolin-1 expression, occurred through both the lysosome and proteasome pathways. In fact, AURKA protein expression made a partial recovery when the cells were treated with either a proteasomal (MG-132) or lysosomal (NH4Cl) inhibitor (Fig. 2E). Down-regulation of AURKA expression in caveolin-1–lacking cells did not occur at the transcriptional level, as demonstrated by RT-PCR analysis (Fig. 2F). Thus, we conclude that caveolin-1 is a positive regulator of AURKA, whose dramatically compromised expression after a loss of caveolin-1 inhibits primary cilium disassembly. In support of this conclusion, we find that overexpression of exogenous caveolin-1 in WI-38 cells increased the level of AURKA (Fig. 2G) and inhibited ciliogenesis (Fig. 2H).
Figure 2.
A–D) Down-regulation of caveolin-1 expression promotes degradation of AURKA. WI-38 (A, C) and IMR-90 (B, D) fibroblasts were infected with a lentivirus carrying caveolin (Cav)-1 shRNA (A, B) or transfected with caveolin-1 siRNA (C, D). Infection with a lentivirus carrying scrambled (Scr) shRNA (A, B) and transfection with scrambled siRNA (C, D) were used as controls. Cells were collected after 10 d, and cell lysates were subjected to immunoblot analysis using antibody probes specific for AURKA and IFT88. Immunoblotting of anti-β-actin IgGs was performed to show equal loading. E, F) WI-38 human diploid fibroblasts were infected with caveolin-1 shRNA and cultured for 10 d in the presence or absence of ammonium chloride (NH4Cl) or the proteasome inhibitor MG-132. Infection with scrambled shRNA was performed as the control. E) Cells were collected, and the AURKA expression level was determined by immunoblot analysis with anti-AURKA IgGs. Immunoblot of anti-β-actin IgGs was performed as the control. F) Cells were collected, and AURKA mRNA level was determined by RT-PCR with specific primers. RT-PCR with primers specific for GAPDH was performed as an internal control. G, H) WI-38 fibroblasts were infected with a lentiviral vector expressing myc-tagged caveolin-1. Infection with the empty vector pLVX was performed as the control. G) After 6 d, cells were collected, and the AURKA protein level was determined by immunoblot analysis with an antibody probe specific for AURKA. Immunoblot with anti-c-myc IgGs was performed to determine exogenous caveolin-1 expression. Immunoblot of anti-β-actin IgGs was performed to show equal loading. H) Primary cilia formation was quantified by immunofluorescence analysis with anti-acetylated α-tubulin IgGs 6 d after infection. Values represent means ± sem. *P < 0.001 (Student’s t test).
Failure to reabsorb the primary cilium after the down-regulation of caveolin-1 promotes premature senescence
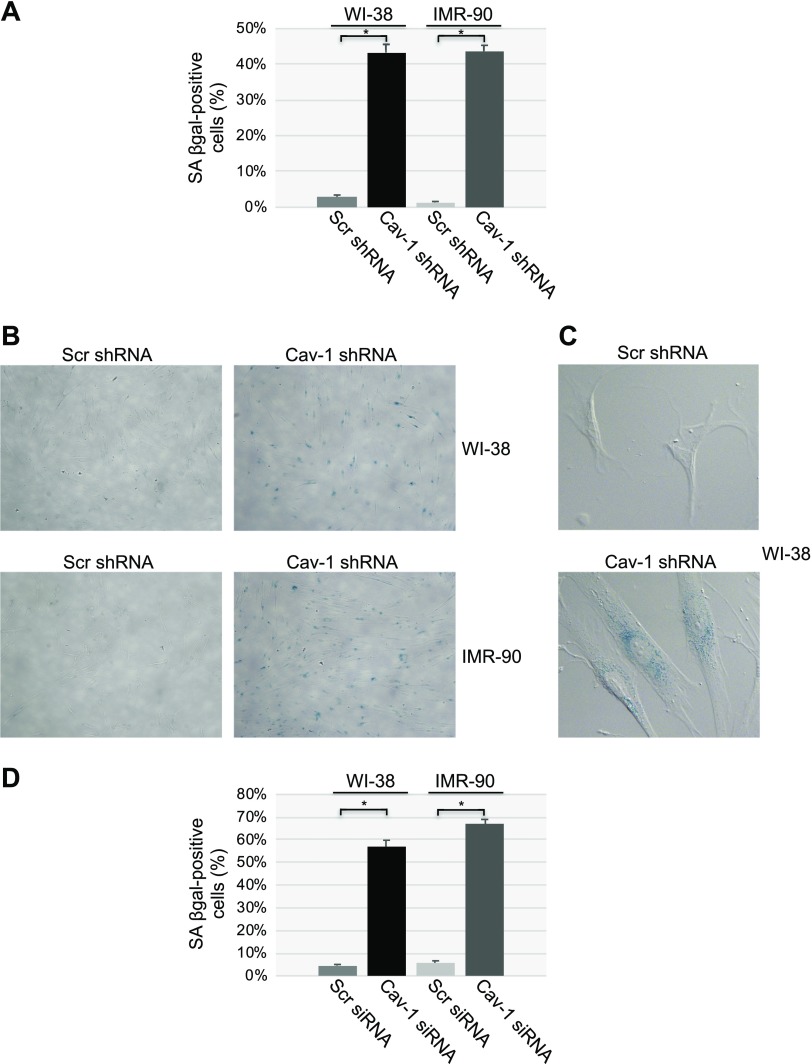
What is the functional consequence of the increased ciliogenesis after down-regulation of caveolin-1 expression? Because primary cilia formation occurs when the cells exit the cell cycle and cellular senescence is characterized by an irreversible cell cycle arrest, we first asked whether cellular senescence was induced by caveolin-1 deficiency. We found that down-regulation of caveolin-1 by shRNA was sufficient to induce cellular senescence in both WI-38 and IMR-90 cells, as quantified by senescence-associated β-galactosidase activity (SA-β-gal) staining (Fig. 3A–C) and immunoblot analysis for p21 (Fig. 1A, B), a marker of cellular senescence. These results were independently confirmed when we down-regulated caveolin-1 expression by siRNA (Figs. 1E and 3D).
Figure 3.
Cellular senescence is promoted by the down-regulation of caveolin-1 expression. A–C) Caveolin (Cav)-1 expression was down-regulated in WI-38 and IMR-90 fibroblasts by shRNA. Cells expressing scrambled (Scr) shRNA were used as the control. Ten days after the infection, cellular senescence was quantified by SA-β-gal staining. Quantification (A), representative low-magnification images (B) and representative high-magnification images (C) are shown. D) WI-38 and IMR-90 cells were transfected with caveolin-1 siRNA. Transfection with scrambled (Scr) siRNA was used as the control. After 10 d, cellular senescence was quantified by SA-β-gal staining. Values represent means ± sem. *P < 0.001 (Student’s t test).
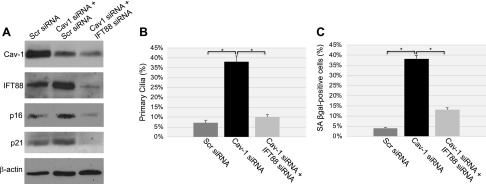
We then asked whether there was a causal relationship between primary cilia formation and induction of cellular senescence in caveolin-1–deficient cells. To answer this question, caveolin-1 deficiency was achieved in WI-38 cells in which primary cilia formation was prevented. More specifically, because down-regulation of IFT88 is known to inhibit ciliogenesis, IFT88 protein expression was down-regulated by siRNA in caveolin-1–lacking WI-38 cells (Fig. 4A). We found that, when primary cilia formation was prevented by IFT88 down-regulation in caveolin-1–lacking cells (Fig. 4B), cellular senescence was dramatically inhibited (Fig. 4C). This result is consistent with increased expression of endogenous IFT88 in cells in which caveolin-1 expression is down-regulated by both shRNA and siRNA (Fig. 2A–D). We conclude that the inability of the cells to reabsorb the primary cilium significantly contributes to the development of premature senescence following a loss of caveolin-1.
Figure 4.
Cellular senescence induced by the down-regulation of caveolin-1 is dependent on primary cilia formation. WI-38 human diploid fibroblasts were transfected with caveolin (Cav)-1 siRNA, together with either IFT88 siRNA or scrambled (Scr) siRNA. Transfection of scrambled siRNA alone was used as the control. Cells were collected for analysis 10 d after transfection. A) Cell lysates were subjected to immunoblot analysis with antibody probes specific for caveolin-1, IFT88, p16, and p21. Immunoblot with anti-β-actin IgGs was performed as the control. B) Primary cilia formation was determined by immunofluorescence analysis with anti-acetylated α-tubulin. Quantification of the staining is shown. C) Cells were subjected to SA-β-gal staining; quantification of the staining is shown. Values represent means ± sem. *P < 0.001 (Student’s t test).
Pharmacologic inhibition of AURKA prevents the absorption of the primary cilium and induces cellular senescence in human diploid fibroblasts
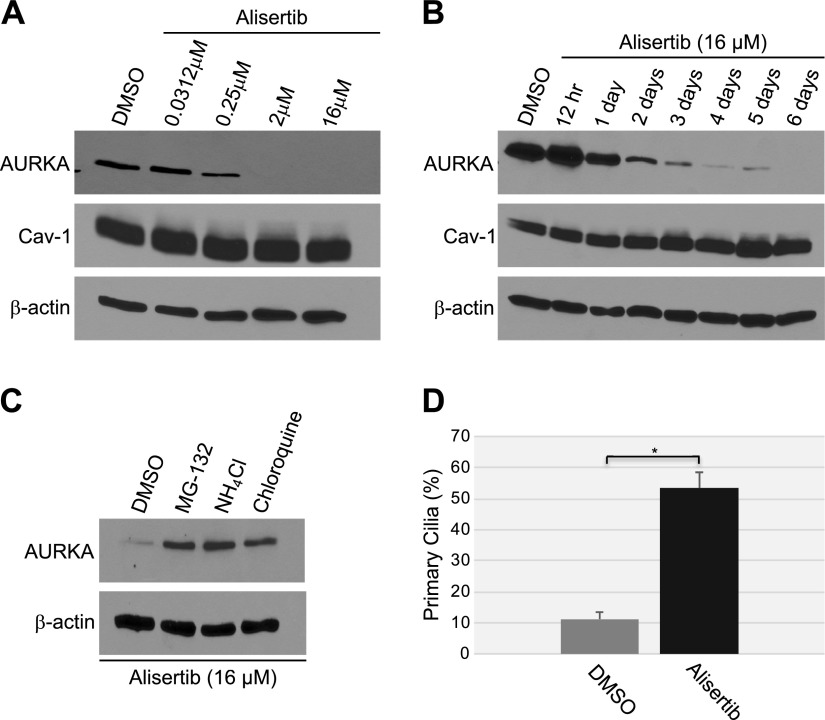
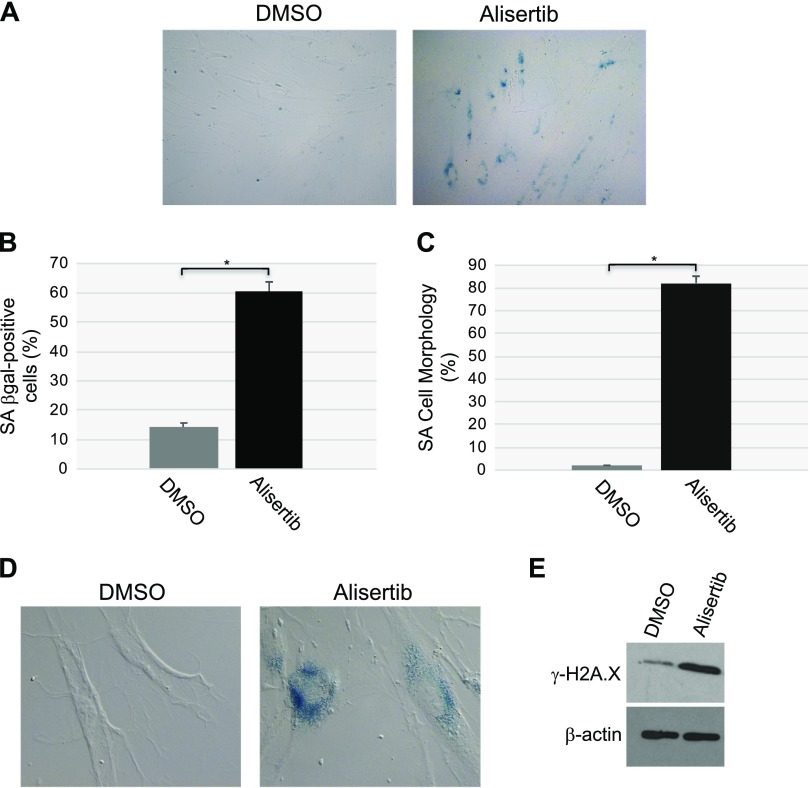
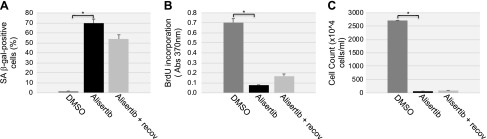
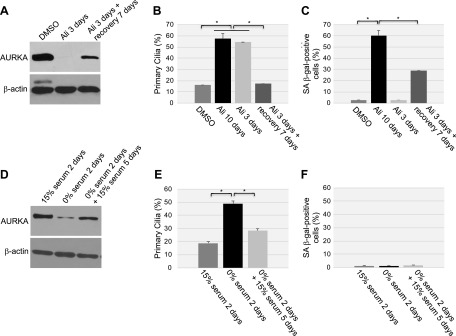
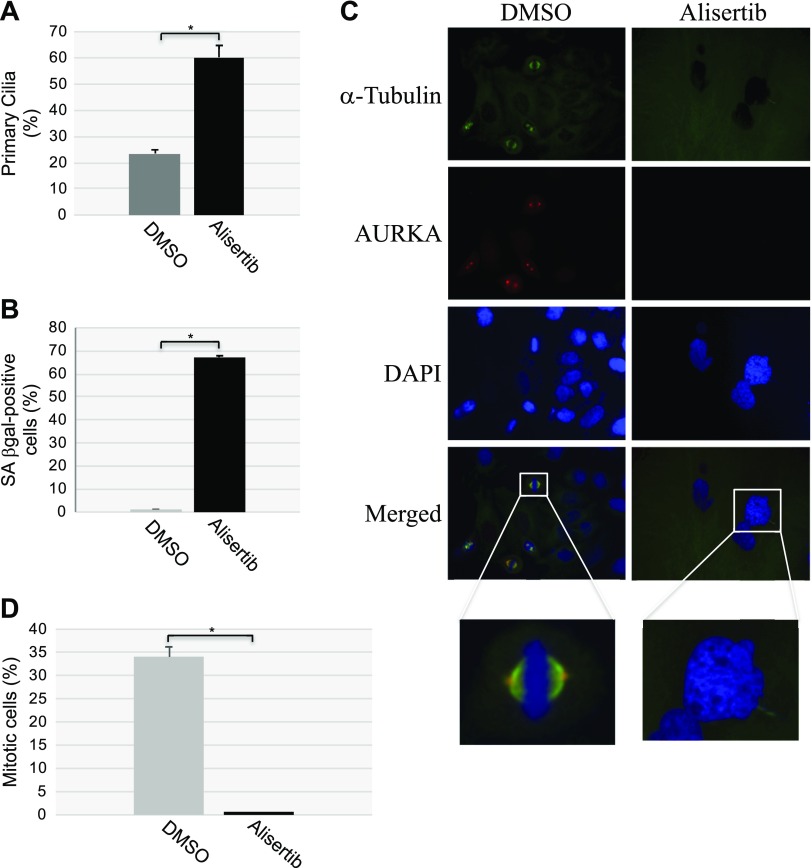
To independently confirm our data showing that down-regulation of caveolin-1 expression promotes primary cilia–dependent senescence through down-regulation of the negative regulator of ciliogenesis AURKA, we treated human diploid fibroblasts with alisertib, a selective AURKA inhibitor. We noted that inhibition of AURKA with alisertib induced a concentration- and time-dependent degradation of AURKA, which was independent of changes in caveolin-1 expression level (Fig. 5A, B). The degradation occurred through both lysosomal and proteasomal pathways, as shown by the partial rescue of AURKA protein expression when WI-38 cells were treated with alisertib in the presence of either MG-132 or NH4Cl/chloroquine (Fig. 5C). Consistent with the inhibitory role of AURKA in primary cilia formation, treatment with alisertib promoted ciliogenesis in WI-38 cells (Fig. 5D). Human diploid fibroblasts displayed a senescent phenotype 10 d after alisertib treatment, as demonstrated by alisertib-induced 1) increase of SA-β-gal–positive cells (Fig. 6A, B), 2) elevation of senescence-associated cell morphology (Fig. 6C, D), and 3) increase in activated histone H2A.X (Fig. 6E). In support of these data, we found that alisertib induced irreversible growth arrest. In fact, alisertib-induced enhancement of SA-β-gal-positivity (Fig. 7A), lack of BrdU incorporation (Fig. 7B), and inhibition of cellular proliferation (Fig. 7C) occurred after 10 d of treatment and persisted even after alisertib was removed from the cell culture medium and the cells were cultured for an additional 5 d (Fig. 7). Thus, alisertib treatment inhibits the absorption of the primary cilium and promotes senescence in human diploid fibroblasts.
Figure 5.
Alisertib induces degradation of AURKA and ciliogenesis. A) WI-38 human diploid fibroblasts were treated with different concentrations of alisertib (0.0312, 0.25, 2 and 16 μM) for 6 d. B) WI-38 fibroblasts were treated with 16 μM alisertib for different times (12 h and 1, 2, 3, 4, 5 and 6 d). Treatment with DMSO was used as the control. A, B) Cells were collected, and the expression levels of AURKA and caveolin (Cav)-1 were determined by immunoblot analysis with anti-AURKA and anti-caveolin-1 IgGs. Immunoblot with anti-β-actin IgGs was performed to show equal loading. C) WI-38 fibroblasts were treated with 16 μM alisertib for 6 d in the presence of MG-132 (0.6 μΜ), ammonium chloride (NH4Cl; 10 mM), or chloroquine (50 μΜ). Treatment with DMSO served as the control. Cells were collected, and cell lysates were subjected to immunoblot analysis with antibody probes specific for AURKA and β-actin (to show equal loading). D) WI-38 cells were treated with 16 μM alisertib for 6 d. Cells were then subjected to immunofluorescence analysis using anti-acetylated α-tubulin IgGs. Quantification of the staining is shown. Values represent means ± sem. *P < 0.001 (Student’s t test).
Figure 6.
Treatment with alisertib promotes accumulation of cells displaying SA-β-gal activity, senescent cell morphology, and elevation of phosphorylated H2A.X. A, B) WI-38 human diploid fibroblasts were treated with 16 μM alisertib for 10 d. DMSO-treated cells were used as the control. Cells were then subjected to SA-β-gal activity staining. Representative low-magnification images (A) and quantification of the staining (B) are shown;. C, D) WI-38 human diploid fibroblasts were treated with 16 μM alisertib for 10 d. Treatment with DMSO was used as control. Cells showing senescence-associated cell morphology were identified. Quantification of the percentage of cells showing senescence-associated cell morphology (C) and representative high-magnification images (D) are shown. D) SA-β-gal activity staining is also shown. E) WI-38 human diploid fibroblasts were treated with 16 μM alisertib for 10 d. DMSO-treated cells were used as the control. Cells were collected, and cell lysates were subjected to immunoblot analysis with an antibody probe specific for phosphorylated histone H2A.X (γ-H2A.X). Immunoblot analysis with anti-β-actin IgGs was performed to show equal loading. Values represent means ± sem. *P < 0.001 (Student’s t test).
Figure 7.
Alisertib causes irreversible growth arrest in human fibroblasts. WI-38 fibroblasts were treated with either DMSO or alisertib (16 μM) for 10 d. Cells were then washed and recovered in alisertib-free medium for an additional 5 d. A) Cells were stained for SA-β-gal activity. B) A BrdU-incorporation assay was performed. C) The number of cells in the cell culture dishes was counted. Values represent means ± sem. *P < 0.001, student’s t test.
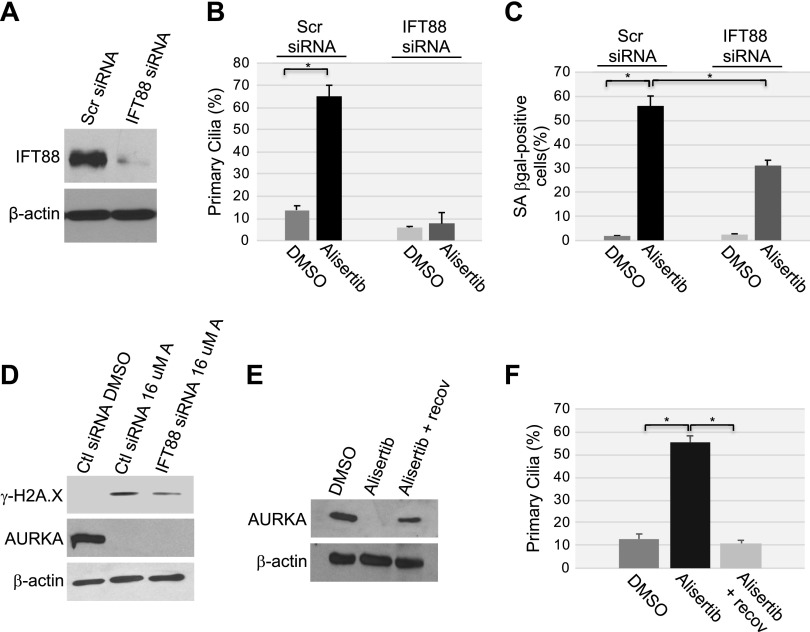
The incapacity to disassemble the primary cilium mediates alisertib-induced cellular senescence
To directly determine whether the forced maintenance of the primary cilium is causally linked to the development of cellular senescence induced by alisertib, we down-regulated IFT88, whose expression is essential for ciliogenesis, in WI-38 cells by siRNA (Fig. 8A). Cells were then cultured with or without alisertib. We found that down-regulation of IFT88 prevented ciliogenesis induced by alisertib treatment for 10 d (Fig. 8B). Alisertib-induced cellular senescence was inhibited only in cells in which ciliogenesis was prevented, as shown by the significantly reduced percentage of cells expressing SA-β-gal activity (Fig. 8C) and H2A.X (Fig. 8D) in alisertib-treated WI-38 cells transfected with IFT88 siRNA, as compared with cells transfected with scrambled siRNA. These findings directly support our data in Fig. 4 showing that inhibition of ciliogenesis reduced cellular senescence induced by the down-regulation of caveolin-1 expression. Further, when cells were cultured for 5 d in alisertib-free medium after being exposed to the drug for 10 d to induce senescence, the cells remained senescent (Fig. 7), and AURKA protein expression (Fig. 8E) and the percentage of cells carrying a primary cilium (Fig. 8F) returned to levels seen in untreated cells. We conclude from these experiments that the failure to reabsorb the primary cilium induced by AURKA inhibition contributes to the development of cellular senescence, but it is not necessary for the maintenance of the senescent phenotype.
Figure 8.
The inability to reabsorb the primary cilium contributes to cellular senescence induced by alisertib. A) WI-38 human diploid fibroblasts were transfected with either scrambled (Scr) or IFT88 siRNA. Cells were collected, and cell lysates were subjected to immunoblot analysis using anti-IFT88 IgGs. Immunoblot with anti-β-actin IgGs was performed to show equal loading. B–D) WI-38 fibroblasts were transfected with IFT88 siRNA. Transfection with scrambled (Scr) siRNA was used as the control. After 48 h, cells were treated with 16 μM alisertib and cultured for 10 d. Treatment with DMSO was used as the control. B) Primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin. C) Cells were subjected to SA-β-gal activity staining. D) The expression levels of phosphorylated histone H2A.X (γ-H2A.X), AURKA, and β-actin were determined by immunoblot analysis using antibody-specific probes. E, F) WI-38 fibroblasts were treated with DMSO or alisertib (16 μM) for 10 d. Cells were then washed and recovered in alisertib-free medium for an additional 5 d. E) Cell lysates were subjected to immunoblot analysis with anti-AURKA IgGs. Immunoblot with anti-β-actin IgGs was performed to show equal loading. F) Primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin. Values in B, C and F represent means ± sem. *P < 0.001 (Student’s t test).
Formation of the primary cilium per se does not induce premature senescence
To independently confirm that the chronic failure to disassemble the primary cilia, caused by the chronic down-regulation of AURKA, promotes premature senescence, we tested the hypothesis that a transient down-regulation of AURKA temporarily promotes ciliogenesis but is not sufficient to promote cellular senescence. To this end, the transient down-regulation of AURKA was achieved by treating WI-38 cells with alisertib for 3 d and then by culturing the cells for 7 additional days in the absence of alisertib. We found that treatment of WI-38 cells with alisertib for only 3 d was sufficient to down-regulate AURKA protein expression (Fig. 9A) and enhance ciliogenesis (Fig. 9B). When alisertib was removed from the culture medium and the cells were cultured for 7 additional days, AURKA down-regulation and enhanced ciliogenesis were reversed (Fig. 9A, B). The transient formation of the primary cilium was not sufficient to induce a robust premature senescent phenotype, as demonstrated by the significantly reduced SA-β-gal staining when WI-38 cells were treated with alisertib for only 3 d or for 3 d and then for an additional 7 d in the absence of alisertib, as compared with continuous treatment with alisertib for 10 d (Fig. 9C).
Figure 9.
Transient down-regulation of AURKA temporarily prevents the disassembly of the primary cilium but does not promote cellular senescence. A–C) WI-38 fibroblasts were continuously treated with DMSO or alisertib (16 μM) for 3 or 10 d. Cells were also treated with alisertib (16 μM) for 3 d, washed, and cultured for an additional 7 d in the absence of alisertib. A) Cell lysates were subjected to immunoblot analysis with an antibody probe specific for AURKA. Immunoblot analysis using anti-β-actin IgGs was performed to show equal loading. B) Primary cilia formation was quantified by immunofluorescence staining with an antibody probe specific for acetylated α-tubulin. C) Cellular senescence was quantified after cells were subjected to SA-β-gal activity staining. D–F) WI-38 cells were cultured in either serum-containing or serum-free medium for 2 d. Serum-starved cells were then cultured for an additional 5 d in the presence of serum. D) AURKA protein expression was determined by immunoblot analysis using anti-AURKA IgGs. Immunoblot analysis with anti-β-actin IgGs was performed as control. E) Primary cilia formation was quantified by immunofluorescence staining with an antibody probe specific for acetylated α-tubulin. F) Cells were subjected to senescence-associated (SA)-β-galactosidase activity staining. Values represent means ± sem. *P < 0.001 (Student’s t test).
Serum withdrawal induces a mitotically inactive state known as quiescence. To independently corroborate our results on the transient down-regulation of AURKA, we transiently induced quiescence in WI-38 cells by first culturing the cells in 0% serum for 2 d and then by adding serum back to the culture medium and culturing WI-38 cells for an additional 5 d. AURKA protein expression was down-regulated (Fig. 9D), and primary cilium formation was enhanced (Fig. 9E) in quiescent WI-38 cells (that is, after culturing the cells in 0% serum for 2 d). AURKA protein expression (Fig. 9D) and the number of WI-38 cells carrying a primary cilium (Fig. 9E) returned to normal levels 5 d after serum was added back to the culture medium. We did not observe development of premature senescence after serum starvation for 2 d or after serum-starved WI-38 cells were cultured in the presence of serum for 5 d (Fig. 9F). These results strengthen the conclusion that the chronic inability to reabsorb the primary cilium, not the formation of the primary cilium per se, induces premature senescence.
Centrosome and mitotic spindle formation are inhibited in senescent cells following inhibition of AURKA
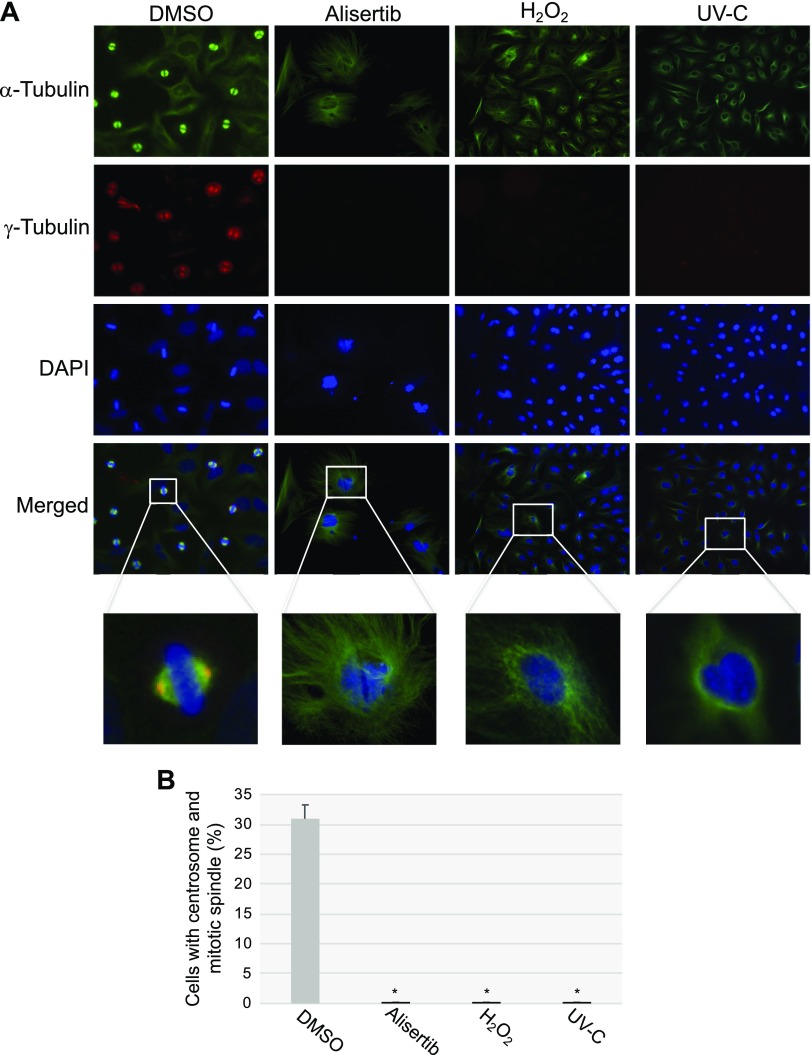
Why does the forced maintenance of the primary cilium contribute to the development of cellular senescence? During normal cell division, the primary cilium must be disassembled to free its centrioles to form centrosomes, and centrosomes are necessary structures for mitotic spindle assembly. Because our data show that alisertib treatment prevented primary cilium disassembly and promoted cellular senescence, and senescent cells were permanently growth arrested, we asked whether forced ciliogenesis would prevent the formation of the centrosome and mitotic spindle. To answer this question, we quantified mitotic spindle and centrosome formation in HeLa cells, cultured for 10 d in the presence or absence of alisertib, by immunofluorescence staining using antibody probes specific for α-tubulin, a marker of the mitotic spindle, and γ-tubulin, a centrosome marker. First, we demonstrated that alisertib treatment induced ciliogenesis (Fig. 10A) and cellular senescence (Fig. 10B) in HeLa cells. We showed that, although formation of the mitotic spindle was evident in control WI-38 cells, mitotic spindle formation was virtually undetectable in WI-38 cells that were induced to senesce by treatment with a selective AURKA inhibitor (Fig. 10C, D). Similarly, we observed centrosome formation in DMSO-treated HeLa cells but not in HeLa cells treated with alisertib (Fig. 11A, B). We also found inhibition of centrosome and mitotic spindle formation (Fig. 11A, B) when ciliogenesis and senescence (data not shown) were induced in HeLa cells by either oxidative stress or UV-C light. Thus, inhibition of AURKA promotes senescence by inhibiting primary cilia reabsorption, which makes the centrioles unavailable to form the centrosomes. The consequent lack of proper mitotic spindle assembly prevents the completion of mitosis.
Figure 10.
Alisertib inhibits mitotic spindle formation. HeLa cells were treated for 10 d with alisertib (16 μM). Cells were also treated with DMSO as the control. A, B) Primary cilia formation was quantified by immunofluorescence staining with anti-acetylated α-tubulin (A). Cells were subjected to SA-β-gal activity staining (B). C, D) Cells were synchronized in mitosis by double thymidine block. Immunofluorescence staining was then performed with antibody probes specific for α-tubulin (green), AURKA (red), and DAPI (blue). Representative images (C) and quantification of cells displaying a mitotic spindle (D) are shown. Values represent means ± sem. *P < 0.001 (Student’s t test).
Figure 11.
Senescence-inducing stimuli inhibit centrosome and mitotic spindle formation. HeLa cells were treated with alisertib (16 μM) for 10 d and H2O2 (450 μM) for 2 h and recovered in complete medium for 7 d, or subjected to UV-C radiation (10 J/m2) and cultured in complete medium for 7 d. Cells were also treated with DMSO as a control. Cells were synchronized in mitosis by double thymidine block. Immunofluorescence staining was then performed using antibody probes specific for α-tubulin (green), γ-tubulin (red), and DAPI (blue). Representative images (A) and quantification of cells displaying centrosome formation and a mitotic spindle (B) are shown. Values represent means ± sem. *P < 0.001 (Student’s t test).
The partial down-regulation of caveolin-1 expression sensitizes cells to alisertib-induced senescence
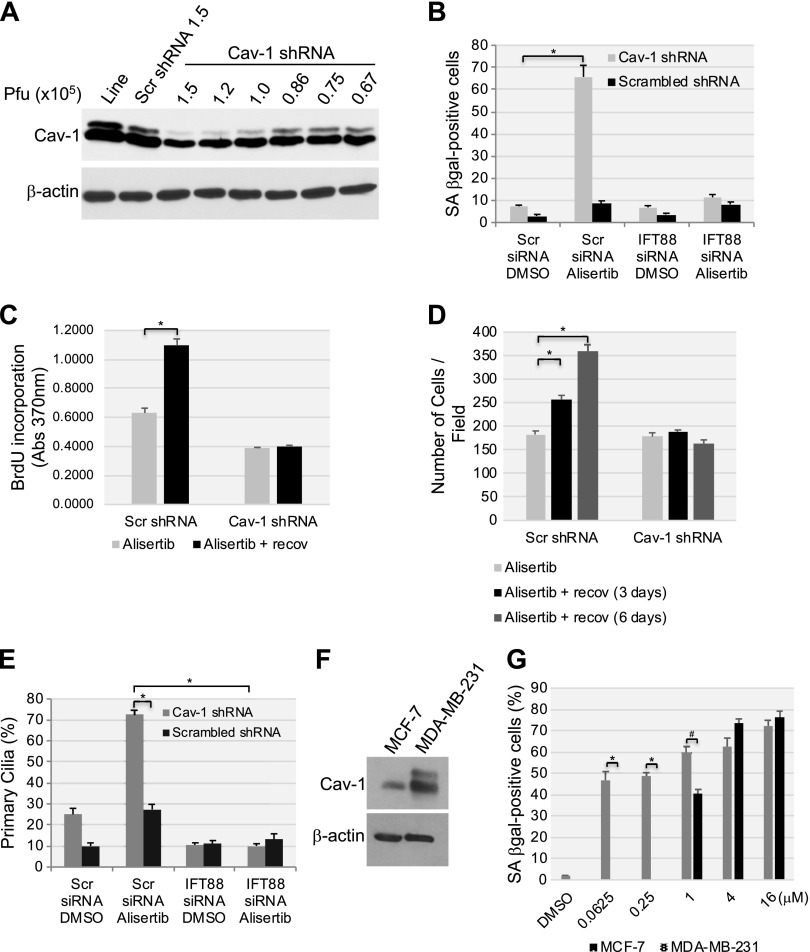
Our data show that both a lack of caveolin-1 expression and alisertib treatment for 10 d promoted senescence by preventing the disassembling of the primary cilia in human diploid fibroblasts. Thus, we asked whether a partial down-regulation of caveolin-1 expression sensitizes cells to cellular senescence induced by alisertib. To answer this question, we quantified cellular senescence after only 6 d of alisertib treatment in cells in which caveolin-1 expression was down-regulated by ∼50% (Fig. 12A). Neither the partial down-regulation of caveolin-1 expression, nor the treatment with alisertib for 6 d, was sufficient, per se, to induce senescence in WI-38 (Fig. 12B) and IMR-90 cells (data not shown). In contrast, alisertib treatment for 6 d promoted senescence in both WI-38 (Fig. 12B) and IMR-90 (data not shown) cells when caveolin-1 expression was partially down-regulated by siRNA, as quantified by SA-β-gal positivity. In support of these findings, only the combination of alisertib treatment in cells in which caveolin-1 expression was partially down-regulated by shRNA displayed irreversible growth arrest after 6 d, as shown by BrdU incorporation and cell proliferation assays (Fig. 12C, D). Consistent with these data, ciliogenesis was promoted only by the combination of alisertib treatment for 6 d and the partial down-regulation of caveolin-1 expression in both WI-38 (Fig. 12E) and IMR-90 (data not shown) cells. Sensitization to alisertib-induced senescence by the partial down-regulation of caveolin-1 expression was dependent on enhanced ciliogenesis, as senescence was abrogated (Fig. 12B) when primary cilia formation was prevented by IFT88 siRNA (Fig. 12E).
Figure 12.
Low endogenous caveolin-1 expression sensitizes human fibroblasts to alisertib-induced primary cilia formation and senescence. A) WI-38 human diploid fibroblasts were infected with a lentivirus-carrying caveolin (Cav)-1 shRNA in the indicated plaque-forming units. Infection with scrambled (Scr) shRNA and uninfected cells were used as controls. Caveolin-1 expression was determined by immunoblot analysis using anti-caveolin-1 IgGs. Immunoblot with anti-β-actin IgGs was performed as the internal control. B) WI-38 human fibroblasts were transfected with either scrambled or IFT88 siRNA. After 48 h, cells were infected with caveolin-1 shRNA at 0.86 × 105 pfu. Infection with scrambled shRNA (0.86 × 105 pfu) was performed as the control. Cells were treated with DMSO or 16 μM alisertib and cultured for 6 d. Cells were then subjected to SA-β-gal staining. C, D) WI-38 cells were transfected/infected and treated for 6 d as described in B. Cell proliferation was then quantified by BrdU incorporation assay (C) and by cell counting (D) at the end of the 6-d treatment or after the cells were washed and recovered in alisertib-free medium for an additional 6 d. E) WI-38 human diploid fibroblasts were transfected/infected and treated for 6 d, as described in B. Primary cilia formation was then quantified by immunofluorescence staining with an antibody probe specific for acetylated α-tubulin. F) Caveolin-1 expression in MCF-7 and MDA-MB-231 breast cancer cells was assessed by immunoblot analysis with a caveolin-1-specific antibody probe. Immunoblot analysis with anti-β-actin IgGs was performed to show equal loading. G) MCF-7 and MDA-MB-231 breast cancer cells were treated with different concentrations of alisertib (0.0625, 0.25, 1, 4 and 16 μM) for 6 d. Treatment with DMSO was the control. Cells were then subjected to SA-β-gal staining. Values represent means ± sem. *P < 0.001, #P < 0.005 (Student’s t test).
To independently confirm that low endogenous caveolin-1 expression sensitizes cells to alisertib-induced senescence, we investigated the effect of alisertib in 2 breast cancer cell lines that dramatically differ in caveolin-1 protein expression. More specifically, MDA-MB-231 cells, which express high levels of caveolin-1, and MCF-7 cells, which express low levels of caveolin-1 (Fig. 12F), were treated with alisertib, and cellular senescence was quantified by SA-β-gal positivity. We found that MCF-7 cells were dramatically more sensitive to alisertib-induced senescence than were MDA-MB-231 cells (Fig. 12G). Together, these data suggest that the level of endogenous caveolin-1 protein expression represents a key determinant of the cellular sensitivity to senescence induced by alisertib.
Down-regulation of AURKA and failure to disassemble the primary cilium contribute to stress-induced senescence
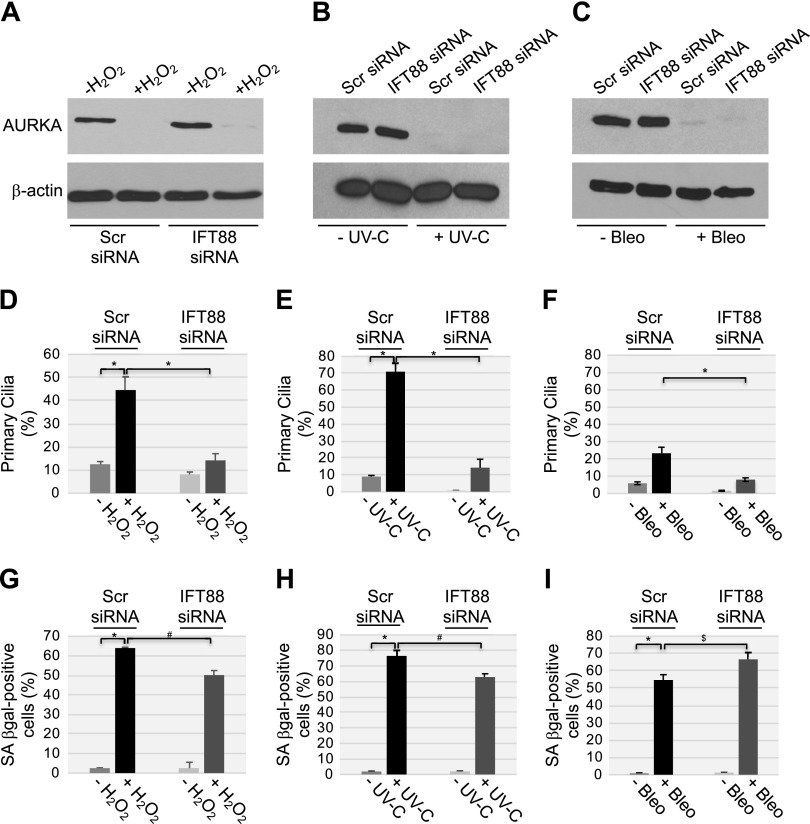
Are the down-regulation of AURKA and forced maintenance of the primary cilium limited to senescence induced by the down-regulation of caveolin-1 and pharmacological inhibition with alisertib? Do they mediate other forms of senescence? To answer these questions, we induced premature senescence in WI-38 human diploid fibroblasts by using sublethal doses of H2O2 and UV-C light as senescence inducers. Both H2O2 and UV-C light reduced AURKA expression to almost undetectable levels (Fig. 13A, B) in WI-38 cells, while bleomycin treatment down-regulated AURKA protein expression (Fig. 13C). H2O2 and UV-C light also enhanced ciliogenesis (Fig. 13D, E), although it was not enhanced with bleomycin treatment (Fig. 13F). Senescence was promoted in WI-38 cells by both H2O2 and UV-C light (Fig. 13G, H), as well as bleomycin treatment (Fig. 13I). Inhibition of primary cilia formation (Fig. 13D, E) by transfecting WI-38 cells with IFT-88 siRNA (Fig. 13A, B) significantly inhibited (∼20%) both H2O2- and UV-C light-induced premature senescence (Fig. 13G, H).
Figure 13.
Oxidative stress and UV-C radiation induce senescence that is partially dependent on the failure to disassemble the primary cilium. A–C) WI-38 human diploid fibroblasts were transfected with either scrambled (Scr) or IFT88 siRNA. A) Cells were treated with sublethal doses (450 μM) of H2O2 for 2 h, washed, and recovered in complete medium for 10 d. B) WI-38 fibroblasts were treated with UV-C radiation (10 J/m2) and cultured in complete medium for 10 d. C) WI-38 cells were treated with bleomycin (10 μg/ml) for 24 h. Cells were washed and cultured in complete medium for 10 d. Cell lysates were then subjected to immunoblot analysis with antibody probes specific for AURKA and β-actin to show equal loading. D–F) WI-38 fibroblasts were transfected and treated as described in A–C. Cells were then subjected to immunofluorescence staining with anti-acetylated α-tubulin IgGs to detect primary cilia. Quantification of cells carrying a primary cilium is shown. G–I) WI-38 fibroblasts were transfected and treated as described in A–C. Cells were then subjected to SA-β-galactosidase activity staining. Values represent means ± sem. *P < 0.001, #P < 0.005, $P < 0.05 (Student’s t test).
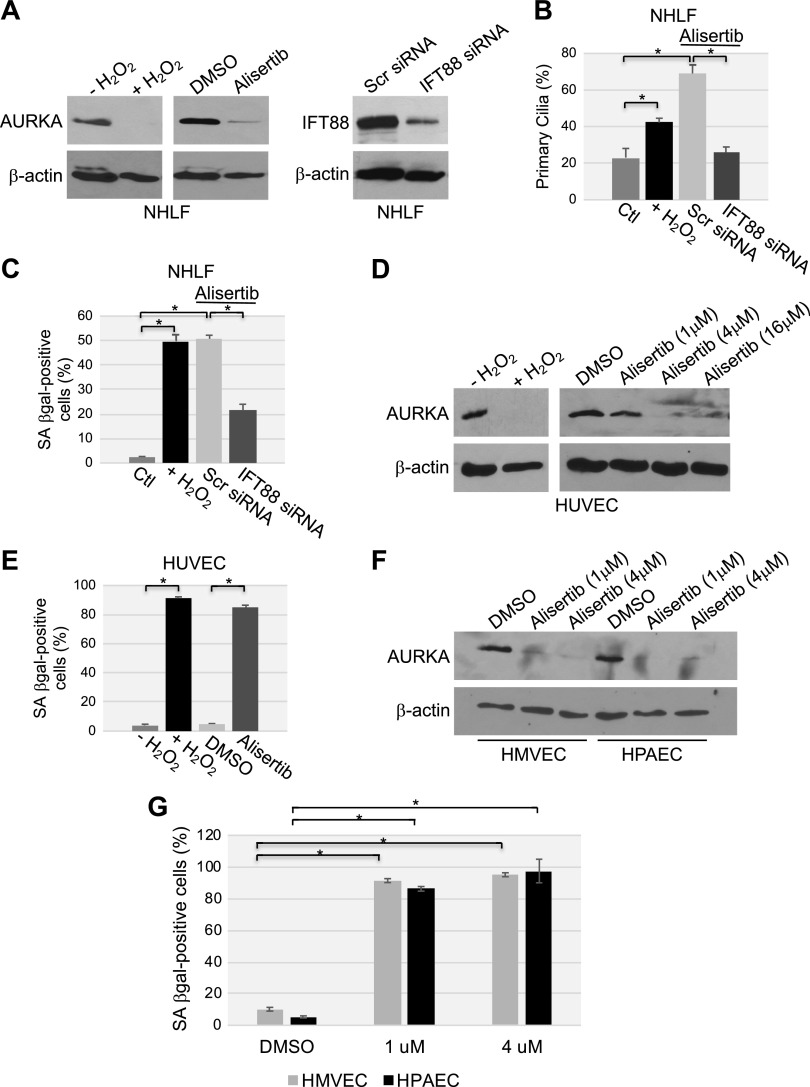
We confirmed these data in primary human fibroblasts derived from adult tissue. Oxidative stress and alisertib down-regulated AURKA protein expression to almost undetectable levels (Fig. 14A), inhibited primary cilium disassembly (Fig. 14B), and promoted premature senescence (Fig. 14C) in NHLFs. Inhibition of ciliogenesis with IFT88 siRNA (Fig. 14A, B) substantially prevented premature senescence induced by alisertib in NHLFs (Fig. 14C). Oxidative stress and alisertib down-regulated AURKA protein expression (Fig. 14D) and promoted premature senescence (Fig. 14E) but did not enhance ciliogenesis (data not shown) in HUVECs; the same was found to be true for HPAECs and HMVECs (Fig. 14F, G). Thus, we propose that loss of AURKA protein expression and the forced maintenance of the primary cilium as novel markers of oxidative stress- and UV-C light–induced senescence in fibroblasts, which are, at least in part, causally linked to the development of a stress-induced senescent phenotype.
Figure 14.
Oxidative stress and alisertib promote down-regulation of AURKA, ciliogenesis, and premature senescence in primary human fibroblasts. A–C) NHLFs were transfected with either scrambled (Scr) or IFT88 siRNA. Cells were then treated with either alisertib (16 μM) for 10 d or with sublethal doses (450 μM) of H2O2 for 2 h, washed, and recovered in complete medium for 10 d. A) cell lysates were subjected to immunoblot analysis with antibody probes specific for either AURKA or IFT88; immunoblot with anti-β-actin IgGs was performed to show equal loading. B) Cells were subjected to immunofluorescence staining with anti-acetylated α-tubulin IgGs to detect primary cilia. Quantification of cells carrying a primary cilium is shown. C) Cells were subjected to SA-β-gal staining. D, E) HUVECs were treated with either sublethal doses of H2O2 (450 μM) for 2 h and recovered in complete medium for 7 d or alisertib (1, 4 and 16 μM) for 10 d. D) AURKA protein expression was determined by immunoblot analysis with an AURKA-specific antibody probe; immunoblot with anti-β-actin IgGs served as the control. E) Cells were subjected to SA-β-gal activity staining. F, G) HPAECs and human lung microvascular endothelial cells were treated with alisertib (1 and 4 μM) for 10 d. F) Cell lysates were subjected to immunoblot analysis, with antibody probes specific for AURKA; immunoblot with anti-β-actin IgGs was performed as the control. G) Cells were subjected to SA-β-gal activity staining. Values represent means ± sem. *P < 0.001 (Student’s t test).
DISCUSSION
The primary cilium must be disassembled to free its centriole to form the centrosome, a necessary structure for mitotic spindle assembly. The mitotic spindle ensures the accurate segregation of chromosomes into the 2 daughter cells during cell division. Our data show that when the cell is forced to use the centriole to form the primary cilium, the cell cannot complete mitosis, because both the centrosome and the mitotic spindle cannot be properly assembled. A lack of a functional mitotic spindle initiates a DNA damage response that makes growth arrest irreversible and drives the development of a senescent phenotype. We also found that, although the inability to disassemble the primary cilium was causally linked to the development of cellular senescence, preservation of the primary cilium was not necessary for the maintenance of a senescent phenotype. Mechanistically, we describe how the forced maintenance of the primary cilium and development of cellular senescence are promoted by the down-regulation/inhibition of AURKA caused by either the down-regulation of caveolin-1 expression or the use of an AURKA-specific inhibitor. Together, our data link the inability to reabsorb the primary cilium, a microtubule-based cellular structure, to the development of premature senescence, a cellular state that is both functionally and pathologically relevant in humans (Fig. 15). These results are consistent with previous findings showing a correlation between increased number and length of primary cilia and reduced cellular proliferation in replicative senescent fibroblasts (32) and data associating ciliation with reduced proliferation (33). However, they are in contrast to the correlation found between reduced primary cilia formation and expression of the senescence marker p16 in the context of replicative senescence of human mammary epithelial cells (34). This discrepancy can be explained by either the different nature of senescence investigated [replicative senescence in (34) vs. premature senescence in our current study] or the distinct senescent programs that exist in fibroblasts and epithelial cells. An additional potential functional relationship between ciliogenesis and senescence comes from data showing that depolarization of the plasma membrane contributes to the inability of senescent cells to elongate the primary cilia upon starvation, which has been linked to constitutive PI3K/mammalian target of rapamycin complex-1 activity (35). Restoration of plasma membrane potential rescues primary cilia elongation in senescent cells and induces starvation-dependent inhibition of Akt and mammalian target of rapamycin complex-1 (35).
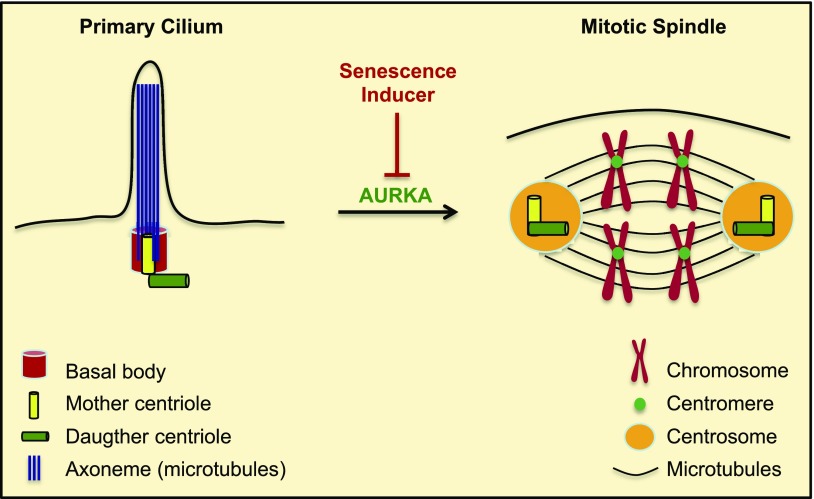
Figure 15.
Summary of the role of primary cilia in the development of cellular senescence. When the cell enters the cell cycle, the primary cilium is disassembled, a process that is regulated by AURKA. The mother and daughter centrioles duplicate and form the centrosomes, which organize the spindle fibers to form the mitotic spindle. The mitotic spindle allows proper chromosomal segregation to form 2 daughter cells during mitosis. Inhibition/degradation of AURKA by senescence inducers prevents primary cilium disassembly, forcing the cell to use the centrioles to maintain the primary cilium. As a result, the centrioles are not available to form the centrosomes and generation of a properly organized mitotic spindle is inhibited. This leads to an irreversible growth arrest/senescent status, because restoration of AURKA expression and absorption of the primary cilium to free the centrioles are not sufficient for cell cycle reentry.
We have previously shown that caveolin-1 expression is necessary for the development of premature senescence when the cell is hit by oxidative stress (22–29). In the current study, we provide evidence causally linking a lack of caveolin-1 expression to premature senescence through primary cilia formation in unstimulated cells. Molecularly, we show that down-regulation of caveolin-1 expression by either shRNA or siRNA leads to degradation of AURKA, a ciliogenesis inhibitor and the up-regulation of IFT88, a promoter of primary cilia formation. Our results are consistent with data showing accumulation of senescence markers in human breast cancer–associated fibroblasts in which caveolin-1 expression is significantly down-regulated (30, 31). Because senescent cells can promote cancer cell growth, we speculate that the selective inhibition of the signaling molecules that are responsible for the down-regulation of caveolin-1 expression in cancer-associated fibroblasts may represent an alternative therapeutic strategy to inhibit their protumorigenic features. We conclude that caveolin-1 is a novel pleiotropic regulator of cellular senescence: caveolin-1-mediated signaling is essential for oxidative stress-induced premature senescence, whereas caveolin-1 deficiency promotes senescence in resting cells by inhibiting the disassembling of the primary cilium. This pleiotropic function is not unique to caveolin-1, as other signaling molecules, such as members of the DNA damage signaling pathway (36, 37) and E2F transcription factors (38, 39), have been shown to either promote or inhibit senescence depending on their expression levels.
Our findings show that cellular senescence induced by the inability of the cells to disassemble the primary cilium, after inhibition of AURKA function, is a more generalized phenomenon and is not limited to cells in which caveolin-1 expression is down-regulated or AURKA is pharmacologically inhibited. In fact, we found that well-established senescence inducers, such as oxidative stress and UV-C light, promoted degradation of AURKA protein expression and premature senescence that is causally linked to the forced maintenance of the primary cilium. Inhibition of primary cilia formation using IFT88 siRNA, however, only partially abolished both oxidative stress– and UV-C light–induced senescence. This finding is not surprising, considering that these stimuli cause a wide range of cellular damage in addition to degradation of AURKA. Nevertheless, failure to reabsorb the primary cilium is responsible for the development of premature senescence in a significant fraction of human fibroblasts that are exposed to either oxidative stress or UV-C light.
Similarly, senescence induced by either a lack of caveolin-1 expression or the pharmacological inhibition of AURKA was significantly, but partially, inhibited when primary cilia formation was prevented with IFT88 siRNA. These data suggest that a loss of AURKA function promotes cellular senescence through 2 distinct mechanisms. On the one hand, a loss of AURKA function induces senescence through the inability to reabsorb the primary cilium because, by doing so, it does not allow the centrioles to be available for centrosome formation and mitotic spindle assembly. On the other hand, because AURKA is also localized at centrosomes and regulates the assembly of the mitotic spindle during mitosis, a lack of AURKA-mediated signaling promotes irreversible cell cycle arrest and senescence by directly inhibiting proper mitotic spindle assembly, even when ciliogenesis is inhibited with IFT88 siRNA and therefore the centrosomes are available to the cell. The existence of a senescence-inducing signaling pathway that is mediated by the loss of AURKA in a ciliogenesis-independent manner is supported by our data showing that treatment of WI-38 fibroblasts with bleomycin down-regulated AURKA protein expression and induced premature senescence but did not promote substantial primary cilia formation. Consistent with these results, inhibition of primary cilia formation with IFT88 siRNA did not inhibit but marginally increased bleomycin-induced premature senescence. IFT88 has been shown to positively regulate G1/S transition in nonciliated cells (40), and we therefore speculate that down-regulation of IFT88 enhanced bleomycin-induced senescence by interfering with G1/S transition in a primary-cilium–independent pathway.
Our data show that activation of this ciliogenesis-dependent senescence pathway occurs in primary fibroblasts derived from adult tissue as well. In fact, we found that oxidative stress and alisertib down-regulated AURKA protein expression, forced the maintenance of the primary cilium, and induced premature senescence in NHLFs. Inhibition of primary cilia formation by IFT88 siRNA dramatically prevented alisertib-induced premature senescence in these primary cells. Consistent with the existence of a ciliogenesis-independent pathway that is driven by a loss of AURKA function, we found that oxidative stress and alisertib down-regulated AURKA protein expression and induced premature senescence but did not inhibit primary cilium absorption in human primary endothelial cells (HUVECs, HMVECs, and HPAECs). These data suggest that failure to disassemble the primary cilium is a predominant mechanism through which fibroblasts, but not endothelial cells, promote the development of a senescent phenotype.
Our data strongly indicate that the forced use of the centriole to maintain the primary cilium, after inhibition of AURKA function, promotes premature senescence by preventing the formation of the mitotic spindle. However, we do not rule out that additional primary cilium-mediated signaling mechanisms may be causally linked to the development of a senescent phenotype. We speculate that modulation of hedgehog, Wnt, platelet-derived growth factor, or Notch signaling, which are known to occur in and to be functionally associated with the primary cilium, contributes to the development of cellular senescence that is initiated by ablation of caveolin-1 and alisertib treatment. In support of this scenario, both caveolin-1 and AURKA have been shown to modulate, through different mechanisms, signaling mediated by hedgehog, Wnt, PDGF, and Notch. Further investigations are necessary to directly test this hypothesis.
Alisertib is a second-generation and highly selective small molecule inhibitor of AURKA. Because aberrant expression of AURKA has been described in a variety of cancers, including breast cancer (41–45), the potential antineoplastic activity of alisertib is currently under investigation in a several clinical trials in patients with cancer. A loss of primary cilia has been reported in multiple malignant tumors (46–49). Given the well-established tumor-suppressive activity of cellular senescence, our data suggest that induction of senescence through failure to disassemble the primary cilium represents a novel molecular mechanism underlying the antitumorigenic properties of alisertib in human cancer.
ACKNOWLEDGMENTS
This work was supported by Grant R01-CA205165 from the U.S. National Institutes of Health (NIH) National Cancer Institute (to F.G.), and Grant R01-HL124747 from the NIH National Heart, Lung, and Blood Institute (to F.G.). The authors declare no conflicts of interest.
Glossary
- AURKA
aurora kinase A
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- H2A.X
H2A histone family member X
- HMVEC
human lung microvascular endothelial cell
- HPAEC
human pulmonary artery endothelial cell
- IFT
intraflagellar transport
- NHLF
normal human lung fibroblast
- SA-β-gal
senescence-associated-β-galactosidase
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
- TBST
Tris-buffered saline-Tween 20
AUTHOR CONTRIBUTIONS
F. Galbiati conceived the project; E. P. Jeffries, M. D. Filippo, and F. Galbiati designed the experiments; E. P. Jeffries and M. D. Filippo performed the experiments and analyzed the data; and E. P. Jeffries and F. Galbiati wrote the manuscript.
REFERENCES
- 1.Sorokin S. (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeley E. S., Nachury M. V. (2010) The perennial organelle: assembly and disassembly of the primary cilium. J. Cell Sci. 123, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa H., Marshall W. F. (2011) Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 [DOI] [PubMed] [Google Scholar]
- 4.Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korobeynikov V., Deneka A. Y., Golemis E. A. (2017) Mechanisms for nonmitotic activation of Aurora-A at cilia. Biochem. Soc. Trans. 45, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badano J. L., Mitsuma N., Beales P. L., Katsanis N. (2006) The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 [DOI] [PubMed] [Google Scholar]
- 7.Lundberg A. S., Hahn W. C., Gupta P., Weinberg R. A. (2000) Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12, 705–709 [DOI] [PubMed] [Google Scholar]
- 8.Sherr C. J., DePinho R. A. (2000) Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410 [DOI] [PubMed] [Google Scholar]
- 9.Jeyapalan J. C., Sedivy J. M. (2008) Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiling J. A., Tamamori-Adachi M., Sexton A. N., Jeyapalan J. C., Munoz-Najar U., Peterson A. L., Manivannan J., Rogers E. S., Pchelintsev N. A., Adams P. D., Sedivy J. M. (2011) Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell 10, 292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frippiat C., Chen Q. M., Zdanov S., Magalhaes J. P., Remacle J., Toussaint O. (2001) Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J. Biol. Chem. 276, 2531–2537 [DOI] [PubMed] [Google Scholar]
- 12.Robles S. J., Adami G. R. (1998) Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 16, 1113–1123 [DOI] [PubMed] [Google Scholar]
- 13.Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 [DOI] [PubMed] [Google Scholar]
- 14.Campisi J. (2013) Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. (1994) Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 4, 231–235 [DOI] [PubMed] [Google Scholar]
- 16.Okamoto T., Schlegel A., Scherer P. E., Lisanti M. P. (1998) Caveolins, a family of scaffolding proteins for organizing “pre-assembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273, 5419–5422 [DOI] [PubMed] [Google Scholar]
- 17.García-Cardeña G., Oh P., Liu J., Schnitzer J. E., Sessa W. C. (1996) Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc. Natl. Acad. Sci. USA 93, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P., Ying Y., Anderson R. G. (1997) Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc. Natl. Acad. Sci. USA 94, 13666–13670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smart E. J., Ying Y.-S., Anderson R. G. W. (1995) Hormonal regulation of caveolae internalization. J. Cell Biol. 131, 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnitzer J. E., Liu J., Oh P. (1995) Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J. Biol. Chem. 270, 14399–14404 [DOI] [PubMed] [Google Scholar]
- 21.Segal S. S., Brett S. E., Sessa W. C. (1999) Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am. J. Physiol. 277, H1167–H1177 [DOI] [PubMed] [Google Scholar]
- 22.Bartholomew J. N., Volonte D., Galbiati F. (2009) Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 69, 2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasari A., Bartholomew J. N., Volonte D., Galbiati F. (2006) Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 66, 10805–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volonte D., Galbiati F. (2009) Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep. 10, 1334–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volonte D., Galbiati F. (2011) Polymerase I and transcript release factor (PTRF)/cavin-1 is a novel regulator of stress-induced premature senescence. J. Biol. Chem. 286, 28657–28661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volonte D., Kahkonen B., Shapiro S., Di Y., Galbiati F. (2009) Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. J. Biol. Chem. 284, 5462–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volonte D., Zhang K., Lisanti M. P., Galbiati F. (2002) Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol. Biol. Cell 13, 2502–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volonte D., Liu Z., Musille P. M., Stoppani E., Wakabayashi N., Di Y. P., Lisanti M. P., Kensler T. W., Galbiati F. (2013) Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol. Biol. Cell 24, 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volonte D., Zou H., Bartholomew J. N., Liu Z., Morel P. A., Galbiati F. (2015) Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J. Biol. Chem. 290, 4202–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capparelli C., Guido C., Whitaker-Menezes D., Bonuccelli G., Balliet R., Pestell T. G., Goldberg A. F., Pestell R. G., Howell A., Sneddon S., Birbe R., Tsirigos A., Martinez-Outschoorn U., Sotgia F., Lisanti M. P. (2012) Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle 11, 2285–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercier I., Casimiro M. C., Wang C., Rosenberg A. L., Quong J., Minkeu A., Allen K. G., Danilo C., Sotgia F., Bonuccelli G., Jasmin J. F., Xu H., Bosco E., Aronow B., Witkiewicz A., Pestell R. G., Knudsen E. S., Lisanti M. P. (2008) Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 down-regulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol. Ther. 7, 1212–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breslin L., Prosser S. L., Cuffe S., Morrison C. G. (2014) Ciliary abnormalities in senescent human fibroblasts impair proliferative capacity. Cell Cycle 13, 2773–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto H., Inoko A., Inagaki M. (2013) Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell. Mol. Life Sci. 70, 3893–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop C. L., Bergin A. M., Fessart D., Borgdorff V., Hatzimasoura E., Garbe J. C., Stampfer M. R., Koh J., Beach D. H. (2010) Primary cilium-dependent and -independent Hedgehog signaling inhibits p16(INK4A). Mol. Cell 40, 533–547 [DOI] [PubMed] [Google Scholar]
- 35.Carroll B., Nelson G., Rabanal-Ruiz Y., Kucheryavenko O., Dunhill-Turner N. A., Chesterman C. C., Zahari Q., Zhang T., Conduit S. E., Mitchell C. A., Maddocks O. D. K., Lovat P., von Zglinicki T., Korolchuk V. I. (2017) Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 216, 1949–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L. V., Kolettas E., Niforou K., Zoumpourlis V. C., Takaoka M., Nakagawa H., Tort F., Fugger K., Johansson F., Sehested M., Andersen C. L., Dyrskjot L., Ørntoft T., Lukas J., Kittas C., Helleday T., Halazonetis T. D., Bartek J., Gorgoulis V. G. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 [DOI] [PubMed] [Google Scholar]
- 37.Mallette F. A., Gaumont-Leclerc M. F., Ferbeyre G. (2007) The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 21, 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maehara K., Yamakoshi K., Ohtani N., Kubo Y., Takahashi A., Arase S., Jones N., Hara E. (2005) Reduction of total E2F/DP activity induces senescence-like cell cycle arrest in cancer cells lacking functional pRB and p53. J. Cell Biol. 168, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park C., Lee I., Kang W. K. (2006) E2F-1 is a critical modulator of cellular senescence in human cancer. Int. J. Mol. Med. 17, 715–720 [PubMed] [Google Scholar]
- 40.Robert A., Margall-Ducos G., Guidotti J. E., Brégerie O., Celati C., Bréchot C., Desdouets C. (2007) The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 120, 628–637 [DOI] [PubMed] [Google Scholar]
- 41.Cirak Y., Furuncuoglu Y., Yapicier O., Aksu A., Cubukcu E. (2015) Aurora A overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J. BUON 20, 1414–1419 [PubMed] [Google Scholar]
- 42.Do T. V., Xiao F., Bickel L. E., Klein-Szanto A. J., Pathak H. B., Hua X., Howe C., O’Brien S. W., Maglaty M., Ecsedy J. A., Litwin S., Golemis E. A., Schilder R. J., Godwin A. K., Connolly D. C. (2014) Aurora kinase A mediates epithelial ovarian cancer cell migration and adhesion. Oncogene 33, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferchichi I., Sassi Hannachi S., Baccar A., Marrakchi Triki R., Cremet J. Y., Ben Romdhane K., Prigent C., Ben Ammar El Gaaied A. (2013) Assessment of Aurora A kinase expression in breast cancer: a tool for early diagnosis? Dis. Markers 34, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang A., Gao K., Chu L., Zhang R., Yang J., Zheng J. (2017) Aurora kinases: novel therapy targets in cancers. Oncotarget 8, 23937–23954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang G., Chang B., Yang F., Guo X., Cai K. Q., Xiao X. S., Wang H., Sen S., Hung M. C., Mills G. B., Chang S., Multani A. S., Mercado-Uribe I., Liu J. (2010) Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin. Cancer Res. 16, 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Dabiri S., Seeley E. S. (2011) Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One 6, e27410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeger-Nukpezah T., Little J. L., Serzhanova V., Golemis E. A. (2013) Cilia and cilia-associated proteins in cancer. Drug Discov. Today Dis. Mech. 10, e135–e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeley E. S., Carrière C., Goetze T., Longnecker D. S., Korc M. (2009) Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 69, 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan K., Frolova N., Xie Y., Wang D., Cook L., Kwon Y. J., Steg A. D., Serra R., Frost A. R. (2010) Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J. Histochem. Cytochem. 58, 857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]