Abstract
Overexpression of mouse neurogenin (Neurog)2 alone or in combination with mouse Neurog2/1 in human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) can rapidly produce high-yield excitatory neurons. Here, we report a detailed characterization of human neuronal networks induced by the expression of human NEUROG2 together with human NEUROG2/1 in hESCs using molecular, cellular, and electrophysiological measurements over 60 d after induction. Both excitatory synaptic transmission and network firing activity increased over time. Strikingly, inhibitory synaptic transmission and GABAergic cells were identified from NEUROG2/1 induced neurons (iNs). To illustrate the application of such iNs, we demonstrated that the heterozygous knock out of SCN2A, whose loss-of-function mutation is strongly implicated in autism risk, led to a dramatic reduction in network activity in the NEUROG2/1 iNs. Our findings not only extend our understanding of the NEUROG2/1-induced human neuronal network but also substantiate NEUROG2/1 iNs as an in vitro system for modeling neuronal and functional deficits on a human genetic background.—Lu, C., Shi, X., Allen, A., Baez-Nieto, D., Nikish, A., Sanjana, N. E., Pan, J. Q. Overexpression of NEUROG2 and NEUROG1 in human embryonic stem cells produces a network of excitatory and inhibitory neurons.
Keywords: neurogenin, induced neuronal network, GABAergic, SCN2A
One of the major challenges of studying human neurologic disorders is the lack of access to the affected neural tissue, which limits molecular and cellular examinations of pathogenesis. Although animal models are widely used and may capture certain neurologic phenotypes consistent with human diseases, they do not fully recapitulate complex human behaviors because of evolutionary divergence in brain circuits and structures. As a result, successes in ameliorating phenotypes in preclinical animal models often do not translate to clinical efficacy (1, 2). In addition, it is not practical to use animal models for large-scale screening assays. Some of these limitations may be addressed by using cultured human neurons in an appropriate genetic context relevant to human neurologic diseases.
Both human embryonic stem cells (hESCs) (3–5) and human induced pluripotent stem cells (hiPSCs) (6, 7) can be induced to differentiate into human neurons and serve as cellular resources for modeling neurologic disorders in the human genomic context. Several protocols have been developed and refined in recent years for induced neuron (iN) differentiation from hESCs/hiPSCs (8, 9). In 2013, Zhang et al. (8) reported that a single mouse transcription factor [mouse neurogenin (Neurog)2 or Neurog1] could efficiently convert hESCs and hiPSCs into functional excitatory neurons. In 2014, an independent study showed that overexpression of Neurog2/1 in hiPSCs caused rapid neuronal differentiation (10). Recently, the electrical properties of iNs induced by Neurog2/1 were further characterized over 2 mo of culture, supporting the glutamatergic nature of Neurog2/1 iNs (11). Here, we established iNs induced by human NEUROG2/1 from hESCs and performed a detailed characterization of their molecular, cellular, and electrophysiological properties over 60 d after induction. We found that NEUROG2/1 iNs can be easily maintained in culture and that their synaptic and firing activities robustly increased with development. Strikingly, we identified GABA-positive cells and inhibitory transmission later in development, indicating the development of a complex iN network with both excitatory and inhibitory neurotransmission, which has not been reported before for other neurogenin-induced neuronal differentiation strategies.
To explore the application of NEUROG2/1 iNs, we evaluated the functional impact of disrupting SCN2A on such a network. SCN2A mutation has been shown to be associated with autism (12, 13), developmental delays (14) and infantile seizure disorders (15). We utilized clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) genome editing to produce hESCs with heterozygous loss of function (LOF) of SCN2A (16). Using the NEUROG2/1 differentiation protocol, we found a dramatic reduction in network activity throughout the culture period in iNs with a heterozygous knock out of SCN2A. Our findings not only extend our understanding of the dynamic changes in NEUROG2/1-induced human neuronal networks but also demonstrate the utility of this system for modeling specific aspects of neurologic disorders in human neurons.
MATERIALS AND METHODS
Cell culture
The hESC line 66 (HUES66) was obtained from the Harvard Stem Cell Institute (Cambridge, MA, USA). As previously described (10), hESCs were plated in standard tissue culture dishes coated with Geltrex (A1413301; Thermo Fisher Scientific, Waltham, MA, USA) diluted 1:100 in DMEM (10566024; Thermo Fisher Scientific). The cells were dissociated by Accutase (07920; StemCell Technologies, Vancouver, BC, Canada) and maintained in mTeSR medium (05850; StemCell Technologies) in the presence of Normocin (Ant-nr-1; InvivoGen, San Diego, CA, USA) to reduce the risk of bacterial and mycoplasma contamination. Rho kinase inhibitor (Y-27632) (688001; Millipore Sigma, Burlington, MA, USA) was added to the mTeSR medium at a final concentration of 10 μM during subculture to increase the survival rate of single cells. Mouse glial cells were obtained from the cortex of wild-type B6/C57 postnatal day (P)0 mice. Cortices from P0 mice were cut into pieces and digested with papain for 30 min; the cells were dissociated by harsh trituration using a glass pipette and plated onto 10-cm cell culture dishes in DMEM supplemented with 10% fetal bovine serum. Upon reaching confluence, the glial cells were trypsinized and subcultured at a 1:5 dilution at least 3 times before being used for coculture with iNs.
Generation of iNs from hESCs
Lentiviral delivery was used to introduce constitutive expression of reverse tetracycline-controlled transactivator 3 driven by the human elongation factor-1α promoter and inducible expression of NEUROG2/1 driven by a tetracycline response element promoter into hESCs. To select for cells expressing NEUROG2/1, a puromycin resistance gene encoding puromycin-N-acetyltransferase (also known as pac) was linked to NEUROG2/1 by a porcine teschovirus-1 2A (P2A) linker (Fig. 1A). On d 0, the transduced hESCs were plated in the presence of doxycycline (1 μg/ml) (D3447; MilliporeSigma) in mTeSR medium. The cells were plated in Geltrex-coated 12-well Axion microelectrode array (MEA) plates (Axion Biosystems, Atlanta, GA, USA) at a density of 1 × 104/well for MEA recording or in 6-well cell culture plates at a density of 1 × 105/well for quantitative PCR (qPCR), immunocytochemistry (ICC), and patch-clamp recording. Glass coverslips (354087; Corning, Corning, NY, USA) were included in the 6-well cell culture plates for ICC and patch-clamp recording. The cells were selected in puromycin (1 μg/ml) from d 1 to 4 in mTeSR medium with gradually increasing neural basal (NB) medium (mTeSR to NB ratio: 3:1 on d 1, 1:1 on d 2, and 1:3 on d 4). On d 4, the puromycin was removed, and the culture medium was changed to 100% NB. Mouse glial cells (4 × 104 cells/well in a 12-well MEA plate or 4 × 105/well in a 6-well cell culture plate for ICC and patch-clamp recording) were added on d 5 to enhance neuron maturation. On d 7, Ara-C (C1768, 1 μM; MilliporeSigma) was added to control the growth of the glial cells. After d 7, 1/4 of the medium was replaced every 2–3 d. Doxycycline (1 μg/ml) was present throughout the entire culture period.
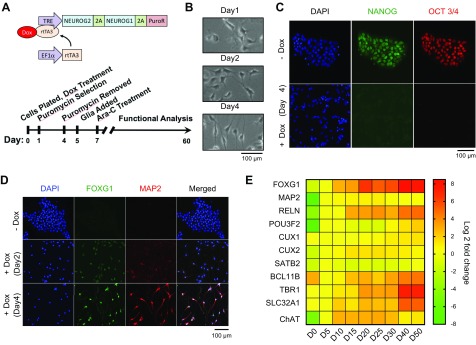
Figure 1.
Rapid differentiation of human iNs induced by overexpression of NEUROG2/1 in hESCs. A) Schematic outline of the lentiviral constructs and flow diagram of the differentiation protocol. B) Representative phase-contrast images illustrating the changes in cell morphology on d 1, 2, and 4 after doxycyclin (Dox) induction. Scale bar, 100 μm. C) Representative immunofluorescence images of hESCs and iN cells immunostained with the stem cell markers Nanog and Oct3/4. One hundred percent of iNs lost staining for both Nanog and Oct3/4 on d 4. Scale bar, 100 μm. D) Representative images of hESCs and iN cells immunostained for the neuronal markers FoxG1 and Map2. One hundred percent of iNs were stained for FoxG1 and Map2 on d 4. Scale bar, 100 μm. E) Heatmap of gene expression levels of cortical layer markers and neuronal markers in iNs at different days after induction. All expression levels are normalized to those on d 5 (D5). EF1α, elongation factor-1 alpha; PuroR, puromycin resistance gene; rtTA3, reverse tetracycline-controlled transactivator 3; TRE, tetracycline response element.
MEA recordings and data analysis
The electrical activity of iNs was recorded using the Axion MEA system (Axion Biosystems) with an integrated amplifier. Each MEA dish (M768-GLx; Axion Biosystems) contained 12 wells with 64 microelectrodes (Au, 30 µm diameter) arranged over an 8 × 8 square grid with a 200-µm center-to-center spacing in each well. The recordings started 5 min after the MEA plates were placed on the Maestro recording chamber, which was set to 35°C and supplied with a continuous perfusion of 5% carbon dioxide-balanced air (Airgas, Cambridge, MA, USA) throughout recording. For all time points, each MEA recording lasted 15 min. All MEA recordings were performed in the culture medium.
The electrical signals were collected at 10 kHz and analyzed offline using Axion’s Integrated Studio software. Raw MEA data were first high-pass filtered at 200 Hz to remove low-frequency local field potentials. Spikes were detected using a threshold-based detector set to an upward or downward excursion beyond 6× sd above the peak-to-peak noise level. Spike sorting was not performed; multiunit activity may therefore contribute to the spikes that were detected from 1 electrode. The timestamps of the spikes were used for further analysis. The spike rate was calculated by dividing the total number of spikes by the recording time (15 min) per electrode. Electrodes without any spike activity were excluded from spike rate analysis. Raster plots were generated from timestamp data using Neuroexplorer (Madison, AL, USA). Cross correlations were calculated by comparing timestamp data collected from electrodes in the same MEA well with MatLab (MathWorks, Natick, MA, USA).
Patch-clamp recording
Coverslips with iNs were placed in the recording chamber on the stage of an inverted microscope (Axiovert.A1; Zeiss, Oberkochen, Germany) equipped with phase-contrast optics. All recordings were performed at room temperature (24°C–26°C). The continuously perfused extracellular solution contained (mM) 145 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 5 glucose (all from MilliporeSigma), and the pH was adjusted to 7.4 with NaOH. Electrodes were pulled using a horizontal P-1000 pipette puller (Sutter Instrument, Novato, CA, USA). Membrane potentials were recorded in the current-clamp whole-cell configuration using pipettes filled with K-gluconate recording solution containing (mM) 131 K-gluconate, 17.5 KCl, 1 MgCl2, 10 HEPES, 1 EGTA, 2 Mg-ATP, and 0.2 Na3GTP, with the pH adjusted to 7.4 with KOH. The same internal recording solution was also used for whole-cell recording of the Na-K current voltage clamp in the voltage-clamp configuration. For Ca2+ current recordings, the extracellular solution contained (mM) 130 NaCl, 10 BaCl2, and 10 HEPES, and the pH was adjusted to 7.3 with NaOH. To block Na+ currents, all Ca2+ current recordings were performed in the presence of 0.5 μM tetrodotoxin (TTX). The internal solution contained (mM) 126 CsCl, 10 EGTA, 1 EDTA, 10 HEPES, and 4 Mg-ATP, and the pH was adjusted to 7.3 with CsOH. The synaptic transmissions were recorded in the whole-cell patch-clamp mode using pipettes filled with CsCl recording solution containing (mM) 120 CsCl, 5 NaCl, 1 MgCl2, 10 HEPES, 10 EGTA, 3 Mg-ATP, 0.3 Na4GTP, and 10 QX-314, with the pH adjusted to 7.2 with NaOH. The excitatory synaptic transmissions were recorded in the whole-cell patch-clamp mode using pipettes filled with K-gluconate recording solution, as previously described. Whole-cell voltage-clamp recordings were made at −70 mV with a MultiClamp 700B Amplifier (Molecular Devices, Sunnyvale, CA, USA) in gap-free mode for at least 5 min for each cell, and access resistance was monitored throughout the recordings. The data were digitized at 10 kHz with a 2-kHz low-pass filter. The liquid junction potential was not corrected and was calculated as 14.8 mV at a recording temperature of 25°C. Data were analyzed offline using Clampfit 10.02 (Molecular Devices) and the MiniAnalysis Program (Synaptosoft, Fort Lee, NJ, USA).
Pharmacological experiments
TTX (1078; Tocris Bioscience, Bristol, United Kingdom) (final concentration of 0.5 μM) was used to isolate miniature synaptic events. NBQX (ab120045; Abcam, Cambridge, MA, USA) (final concentration of 10 μM) was used to remove α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated excitatory synaptic responses. Picrotoxin (1128, PTX; Tocris Bioscience) (final concentration of 20 μM) was used to dissect GABA receptor-mediated inhibitory responses.
Immunocytochemistry
The cells were fixed by incubation with 4% paraformaldehyde and 4% sucrose in PBS at room temperature for 5 min and then washed 3 times with PBS. The fixed cells were permeabilized with 0.1% Triton X-100/PBS for 5 min, washed 3 times with PBS, and incubated in 3% bovine serum albumin plus 10% normal goat serum in PBS for 1 h to block nonspecific binding. The cells were then incubated overnight with the following primary antibodies at 4°C: mouse anti-NeuN (MAB377, 1:1000; MilliporeSigma), rabbit anti-Nanog (ab80892, 1:500; Abcam), mouse anti-Oct3/4 (sc-5279, 1:500; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-FoxG1 (ab18259, 1:1000; Abcam), mouse anti-Map2 (M1406, 1:500; MilliporeSigma), guinea pig anti-Map2 (188004, 1:1000; SYnaptic SYstems, Goettingen, Germany) and rabbit anti-GABA (A2052, 1:1000; MilliporeSigma). After the cells were washed 3 times with PBS, they were incubated with a secondary goat anti-mouse, Alexa Fluor 555 (A28180, 1:1000; Thermo Fisher Scientific), goat anti-rabbit, Alexa Fluor 555 (A21428, 1:1000; Thermo Fisher Scientific), goat anti-mouse, Alexa Fluor 488 (A32723, 1:1000; Thermo Fisher Scientific), goat anti-rabbit, Alexa Fluor 488 (A11034, 1:1000; Thermo Fisher Scientific), or donkey anti-guinea pig, Alexa 647 (706-605-148, 1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) antibody. The images were collected using an Axio Cam MRm (Zeiss).
RT-qPCR
No mouse glial cells were added in this set of experiments to avoid amplifying mouse glial genes. At the designated time points for collection, hESCs or iNs were harvested in RLT buffer (Qiagen, Germantown, MD, USA) and stored at −80°C until all time points were collected. Total RNA was extracted using the RNeasy Plus Mini Kit (74134; Qiagen). A total of 90 ng of total RNA was used to generate random-hexamer-primed cDNA from each sample using the Transcriptor First Strand cDNA Synthesis Kit (04896866001; Roche, Basel, Switzerland). qPCR was carried out in technical quadruplicate and biologic triplicate for each time point. Briefly, the samples were processed in 384-well format using a 10-μl total volume with 300 nM forward and reverse primers and FastStart SYBR Green 2X Master Mix (04673484001; Roche) using the following PCR conditions: 10 min at 95°C, followed by 45 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C on a LightCycler 480 System (Roche). Raw Ct values were averaged and normalized to a sample-specific ACTB control, and fold changes in gene expression were calculated using the ΔΔCt method. The primer sequences are listed in Supplemental Table S1. All primer sets were previously validated using RNA from the third trimester human fetal brain (Clontech Laboratories, Mountain View, CA, USA) and HUES66 cells (data not shown), and results from qPCR analysis that did not yield consistent Ct values above 37 cycles were deemed undetectable in our system. Heatmaps were generated by Prism using the log2 fold changes of the (RT-qPCR results normalized to the level measured on d 5 after NEUROG2/1 induction.
Generating hESC lines with heterogeneous knockout of SCN2A
hESCs (HUES66) were transiently transfected with EF1a-hSpCas9-T2A-EGFP (48138, pX458; Addgene, Watertown, MA, USA) and U6-sgRNA (52963; Addgene), which targets human SCN2A. Similar to the previously described method (17), 24 h later, the transfected cells were enriched for green fluorescent protein–positive cells by fluorescence-activated cell sorting and replated at a low density to encourage the growth of single-cell-derived colonies. Seven to ten days later, each colony was picked, and genomic DNA was extracted and sequenced to obtain all allele genotypes. The karyotypes of hESC lines carrying LOF in 1 allele were validated by TaqMan CNV probes (4400291 and 4403326; Applied Biosystems, Foster City, CA, USA) and used in this study. One allele from line 1 contains an 8 bp exonic deletion together with a 1 bp intron deletion, whereas the other allele is wild type (WT)-like with a silent mutation. One allele of line 2 contains a 4 bp exonic deletion, and the other allele is WT. The off-target sequences for both sgRNAs were identified using MIT CRISPR tool (http://crispr.mit.edu). SCN2A mRNA expression levels were analyzed using RT-qPCR, as previously described, with forward 5′-GGTTTTATTGTGAGCCTTAG-3′ and reverse 5′-CTTGAAAACTCGGAGCAGCCG-3′ primers.
Statistical analysis
All data were collected from 3 independent batches of cultures. Box and whisker plots were used to show the median and the 5th to 95th percentiles. Data in other plots were shown as means ± sem. Statistical analyses were performed as indicated in each result.
RESULTS
Generation of iNs by overexpression of NEUROG2/1 in hESCs
hESCs with constitutive expression of reverse tetracycline-controlled transactivator 3 and inducible expression of NEUROG2/1 were generated using a lentiviral delivery system (Fig. 1; see Materials and Methods for details). On d 0, NEUROG2/1-transduced hESCs were plated on Geltrex-coated plates in the presence of doxycycline. The cells were then selected with puromycin from d 1 to 4 for neurogenin expression. Except for the q experiments, mouse glial cells were added on d 5 to support the growth of iNs. Ara-C was added on d 7 to control the growth of the glial cells (Fig. 1A).
Upon overexpression of NEUROG2/1, the hESCs rapidly changed their colonial morphology to a more scattered pattern (Fig. 1B) and completely lost expression of the pluripotency markers Nanog and Oct3/4 on d 4 (Fig. 1C). This change was concurrent with the complete conversion of hESCs to FoxG1- and Map2-positive cells (Fig. 1D). To gain insight into the types of neurons produced, we analyzed the expression of a series of neuronal markers at different time points during the 2-mo culture (Fig. 1E). Our results showed that the expression of FOXG1 and MAP2 increased dramatically within 5 d after NEUROG2/1 induction and remained at high levels throughout the culture period. Similar to Neurog2- and Neurog2/1-differentiated neurons (8, 10), NEUROG2/1 iNs also expressed several superficial cortical layer markers (RELN, POU3F2, CUX2, and SATB), as well as the cholinergic marker choline acetyltransferase. The expression of deep layer marker TBR1 (18, 19) was found to be insignificant (2.4-fold up-regulated expression on d 10 and 11.9-fold up-regulated expression on d 20 compared to that on d 5). Strikingly, we detected the expression of the GABA vesicular transporter SLC32A1 (also known as VGAT) (20) (4-fold up-regulated expression on d 10, 6-fold up-regulated expression on d 20 and 38-fold up-regulated expression on d 40 compared to that on d 5), which was not reported in either Neurog2 or Neurog2/1 iNs (8, 10). To avoid amplifying genes from glial cells, pure iNs in the absence of glial cell coculture were used for gene expression measurements. As glial cells are crucial for synaptic development (21), the gene expression measured in pure iNs may not fully reflect the function of iNs cocultured with glial cells, which were used for functional analysis later in this report. However, the gene expression in pure NEUROG2/1 iNs supported the notion that overexpression of NEUROG2/1 produced a network of both excitatory and inhibitory neurons, even in the absence of glial cells.
Firing properties of NEUROG2/1 iNs
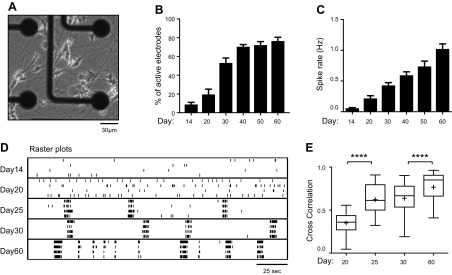
To characterize the spontaneous firing activity of NEUROG2/1 iNs, we performed MEA recording (Fig. 2A) and observed reliable firings from approximately d 14 after induction, with a steady increase in activity until the end of our study on d 60 (Fig. 2B, C). In comparison, primary mouse neurons in culture have been reported to display spontaneous firing activity within the first 7 d after plating (22). Using patch-clamp recording, we further characterized the spontaneous action potentials recorded from NEUROG2/1 iNs and found them to have an amplitude of 54 ± 1 mV and a rise time of 1.13 ± 0.05 ms on d 60 (Supplemental Fig. S1). As shown in the raster plot in Fig. 2D, in addition to an increased spike rate, NEUROG2/1 iN networks developed synchronized firing starting on d 25. We calculated the correlation coefficient for the spikes detected from different electrodes on the same MEA at different developmental stages and found that the correlation coefficient increased significantly with development (Fig. 2E), indicating an increase in network synchrony over time and suggesting that stronger network connections occur later in development.
Figure 2.
Characterization of the firing activity of iN networks during development. A) Phase-contrast image of NEUROG2/1 iNs on an MEA on d 14. Scale bar, 30 μm. B) Quantification of the percentage of active electrodes from iN networks at different time points during development. Data were collected from 9 MEAs. P < 0.0001, F(5, 48) = 50.2, 1-way ANOVA. C) Quantification of the mean spike rate from iN networks at different time points during development. Data were collected from 9 MEAs. P < 0.0001, F(5, 766) = 17.82, 1-way ANOVA. D) Representative raster plots show the spontaneous firing activities recorded from iN networks during development. E) Cross correlation analysis of the synchrony of spontaneous firing activities from iN networks on d 20, 25, 30, and 60 after induction. Data were collected from 9 MEAs. Box and whisker plots are used to show the median and the 5th to 95th percentiles. Plus sign indicates the means; 1-way ANOVA, F(3, 3966) = 385.7, P < 0.0001. ****P < 0.0001 Tukey’s multiple comparisons test.
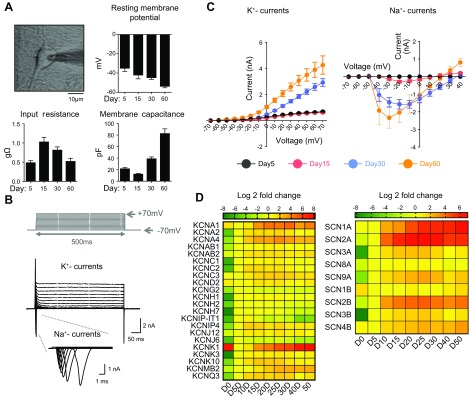
To better understand the excitability of NEUROG2/1 iNs, we characterized their membrane properties using whole-cell patch-clamp recordings. As shown in Fig. 3A, the resting membrane potential of NEUROG2/1 iNs steadily hyperpolarized from −35 ± 3 mV on d 5 to −54 ± 1 mV on d 60, suggesting that neuronal maturation occurred during this period. Input resistance analysis showed an increase from d 5 to 15, followed by a decrease from d 15 to 60, whereas the membrane capacitance analysis showed a small reduction from d 5 to 15, followed by an increase from d 15 to 60. Voltage-gated K+ currents appeared on d 5, remained steady between d 5 and 15, and then dramatically increased over time (Fig. 3C, left). Unlike voltage-gated K+ currents, voltage-gated Na+ currents were hardly detectable on d 5. On d 15, Na+ currents (0.3 ± 0.1 nA) were present, coinciding with the earliest time point of spontaneous firing activity recorded by MEA (Fig. 2C). A dramatic increase in the voltage-gated Na+ current (1.6 ± 0.3 nA) was observed on d 30 and was maintained at a similar level until d 60 (Fig. 3C, right). As voltage-gated Na+ and K+ currents play key roles in neuronal excitability, these observations suggest that NEUROG2/1 iNs undergo dynamic molecular regulation of membrane excitability during development.
Figure 3.
Membrane properties of NEUROG2/1 iNs. A) Phase-contrast image of a NEUROG2/1 iN during patch-clamp recording (upper left); resting membrane potential (RMP, upper right), input resistance (IR, lower left), and membrane capacitance (MC, lower left) measured by whole-cell patch-clamp recording for iNs on d 5, 15, 30, and 60 after induction. Scale bar, 10 μm. Data were collected from iNs on d 5 (n = 12), 15 (n = 10), 30 (n = 27), and 60 (n = 33). F(3,74) = 17.61, P < 0.0001, 1-way ANOVA for RMP; F(3,36) = 9.37, P < 0.0001, 1-way ANOVA for IR; F(3,61) = 67.95, P < 0.0001, 1-way ANOVA for MC. B) Representative traces of whole-cell voltage-gated K+ and Na+ currents recorded from iNs. Current traces were elicited by depolarizing voltage steps from −70 to +70 mV for 500 ms from a holding potential of −70 mV. C) Quantification of current/voltage (I/V) relationships of K+ (left) and Na+ (right) currents from iNs at different time points during development. The K+ currents were recorded on d 5 (n = 12), 15 (n = 20), 30 (n = 20), and 60 (n = 13). The Na+ currents were recorded on d 5 (n = 10), 15 (n = 16), 30 (n = 16), and 60 (n = 6). D) Heatmap of the expression levels of genes encoding voltage-gated potassium (left) and sodium (right) channels in iNs during development. All expression levels are normalized to those on d 5 (D5).
To further understand the molecular identity of the ion channels that contribute to the excitability of NEUROG2/1 iNs, we performed RT-qPCR at different developmental stages using primers targeting different voltage-gated K+ and Na+ channels expressed in the brain (Fig. 3D). With the exception of KCND2 and KCNK1, we found that the expression of most neuronal voltage-gated K+ channel genes peaked between 5 and 15 d after NEUROG2/1 induction and remained at elevated levels (Fig. 3D, left). Similarly, the expression of most voltage-gated Na+ channel genes was induced on d 5 (SCN2A, SCN3A, SCN8A, SCN9A, SCN2B, and SCN3B) or d 10 (SCN1A and SCN4B) and remained elevated throughout the culture period (Fig. 3D, right). The time course for gene expression of K+ channels was largely consistent with their functional conductance, as measured by patch-clamp recordings; however, there was an apparent delay in detecting functional Na+ currents (on d 15) after the expression of Na+ channel genes (on d 5), which might be due to regulatory factors specific to Na+ channels, including the time required for translation, posttranslational modification, and translocation of functional Na+ channels to the plasma membrane.
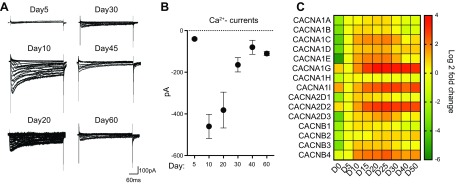
Because voltage-gated Ca2+ channels are important in multiple cellular functions and have been implicated in the risk of several psychiatric disorders, we next characterized their properties in NEUROG2/1 iNs (Fig. 4). Unlike voltage-gated K+ or Na+ currents, which continued to increase after d 15, the observed Ca2+ current plateaued between d 10 and 20 and then decreased (Fig. 4A, B). Overall, the timeline for the expression of Ca2+ channels is consistent with their functional conductance (Fig. 4C).
Figure 4.
Characterization of voltage-gated calcium channels in NEUROG2/1 iNs. A) Representative traces of whole-cell Ca2+ currents recorded in NEUROG2/ iNs at different time points during development. Current traces were elicited by depolarizing voltage steps from −80 to +20 mV for 200 ms at a holding potential of −100 mV. B) Quantification of Ca2+ currents. The Ca2+ currents were recorded on d 5 (n = 6), 10 (n = 5), 20 (n = 6), 30 (n = 7), 45 (n = 4), and 60 (n = 3). C) Heatmap of expression levels of genes encoding voltage-gated calcium channels in iNs during development. All expression levels are normalized to those on d 5 (D5).
AMPA-excitatory postsynaptic currents dominate synaptic transmissions in NEUROG2/1 iN networks
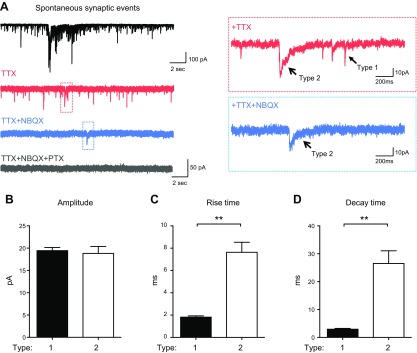
To investigate synaptic transmission in NEUROG2/1 iN networks, we performed whole-cell patch-clamp recordings. Because MEA studies indicated that the spontaneous firing activity of NEUROG2/1 iNs continued to increase during the 2-mo culture (Fig. 2C), we started to evaluate the synaptic events of iNs on d 60 (Fig. 5A, black trace). We applied 0.5 μM TTX to isolate miniature synaptic transmission. In 2 of 12 iNs recorded, 2 types of synaptic events, with either fast rise and decay kinetics (type 1) or slow rise and decay kinetics (type 2), were isolated upon TTX treatment (Fig. 5A, red traces). Type 1 events were blocked by the AMPA receptor antagonist NBQX1 (10 μM) (Fig. 5A, blue traces), whereas type 2 events were blocked by the GABA receptor antagonist PTX (20 μM) (Fig. 5A, gray trace). In the other 10 iNs recorded in the presence of TTX, only type 1 synaptic events were present, which were blocked by NBQX. Quantification of type 1 and type 2 events showed similar peak amplitudes, with type 1 events displaying significantly faster rise and decay kinetics than type 2 events (Fig. 5B–D). Taken together, our data suggest that type 1 events represent AMPA receptor-mediated excitatory postsynaptic currents (EPSCs) and that type 2 events represent GABA receptor-mediated inhibitory postsynaptic currents (IPSCs).
Figure 5.
Characterization of the synaptic activities of NEUROG2/1 iN networks. A) Representative traces of spontaneous (black trace) and pharmacologically isolated synaptic events (red, blue, and gray traces) recorded from iN networks by whole-cell patch-clamp recording on d 60. Two types of miniature synaptic events were observed after TTX (0.5 μM) treatment (red traces). Type 1 miniature synaptic events were depleted upon NBQX (10 μM) treatment (blue traces). Type 2 miniature synaptic events were depleted by additional PTX treatment (20 μM) (gray trace). B–D) Quantification of the amplitude (B), rise time (C), and decay time (D) for the type 1 and type 2 synaptic events. The analysis of type 1 and type 2 events included recordings of 12 and 2 iNs, respectively. **P < 0.01 (unpaired Student’s t test).
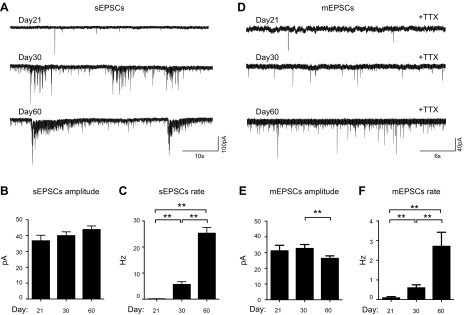
Given our finding that EPSCs dominated the synaptic transmissions in the iN networks on d 60, we next focused on characterizing the EPSCs during development. As shown in Fig. 6A–C, EPSCs were detectable on d 21. Although no obvious changes were observed in the average peak amplitude for the spontaneous EPSCs (sEPSCs) over time, the frequency for sEPSCs significantly increased from 0.15 ± 0.02 Hz on d 21 to 25.5 ± 2.01 Hz on d 60. In the presence of TTX treatment, we measured miniature excitatory postsynaptic currents (mEPSCs) from d 21 to 60. As shown in Fig. 6D–F, mEPSCs had small yet significant reductions in peak amplitude from d 30 to 60, whereas their frequency dramatically increased over time (from 0.12 ± 0.03 Hz on d 21 to 2.7 ± 0.52 Hz on d 60), suggesting that increased synapse formation is the dominant functional change between d 21 and 60.
Figure 6.
Developmental changes in the excitatory synaptic transmission of iN networks. A) Representative traces of sEPSCs recorded from iNs on d 21, 30, and 60. B, C) Quantification of the amplitude (B) and frequency (C) of the sEPSCs during development. D) Representative traces of mEPSCs recorded from iN cells on d 21, 30, and 60 after induction. E, F) Quantification of the amplitude (E) and frequency (F) of the mEPSCs during development. The analyses of sEPSCs included recordings of 16, 27, and 27 iNs on d 21, 30, and 60, respectively; analyses of mEPSCs included recordings of 5, 12, and 22 iNs on d 21, 30, and 60, respectively. **P < 0.01 (unpaired Student’s t test).
GABAergic iNs play a significant role in regulating spontaneous network firing activity
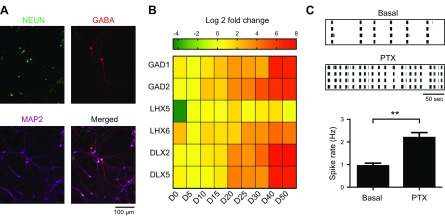
As we identified the expression of GABAergic markers in RT-qPCR analysis (Fig. 1E) and IPSCs in patch-clamp recordings (Fig. 5A), we hypothesized that functional GABAergic neurons were present in the NEUROG2/1 iN network. We performed ICC with an anti-GABA antibody and found GABA-positive cells in the iN networks (Fig. 7A). The percentage of GABA-positive iNs on d 30 was analyzed and found to be 2.3% ± 0.3%. To exclude contamination with GABAergic neurons from cocultured mouse glial cells, we cultured iNs alone and confirmed that immunostaining of GABA was also present in pure iN cultures (Supplemental Fig. S2). To understand the developmental profile of the GABAergic iNs, we performed RT-qPCR to evaluate the expression of GABAergic markers in pure iN cultures at different time points (Fig. 7B). Similar to the expression of the GABA vesicular transporters SLC32A1, GAD1, and GAD2, the proteins that catalyze the conversion of glutamate to GABA also showed a modest but steady increase in expression with development (2.1- and 1.8-fold increased expression, respectively, on d 15 and 6.8- and 6.2-fold increased expression, respectively, on d 40 compared to that on d 5). The expression of LHX5, which has been found in dorsal spinal cord interneurons and in Cajal-Retzius cells that play important roles in the lamination of the mammalian cortex (23, 24), increased 19-fold on d 5 and then remained at the same level throughout the culture. The expression of LHX6, which has been reported to be involved in the migration and specification of cortical interneurons (25), was found to be insignificant (10-fold on d 25 compared to that on d 5). The expression of the transcription factors DLX2 and DLX5 in GABAergic neurons (26) showed 13- and 4-fold increases on d 20, respectively, and 184- and 7.2-fold increases on d 40, respectively, compared to that on d 5. These data collectively showed that multiple molecular inhibitory neuronal markers are present in NEUROG1/2 iNs. To evaluate the functional role of GABAergic iNs in the network, we treated NEUROG2/1 iN networks with the GABA receptor antagonist PTX (20 μM) on d 60 and found that the network firing rate significantly increased upon PTX treatment (Fig. 7C), suggesting that inhibitory synaptic transmission played a significant role in regulating the spontaneous firing of NEUROG2/1 iN networks during the later developmental stage.
Figure 7.
GABAergic neurons developed in the NEUROG2/1 iN networks. A) Representative iNs immunostained for NeuN (green), GABA (red), and Map2 (purple) on d 30. Scale bar, 100 μm. B) Heatmap of the expression levels of genes encoding inhibitory molecular markers at different days after induction. All expression levels are normalized to those on d 5 (D5). C) Representative raster plots (top) and analysis of the spike rate (bottom) of iN networks recorded by MEA on d 60 before and after PTX (20 μM) treatment. Data were collected from 6 MEAs. **P < 0.01 (unpaired Student’s t test).
Heterozygous knockout of SCN2A led to hypoactive NEUROG2/1 iN networks
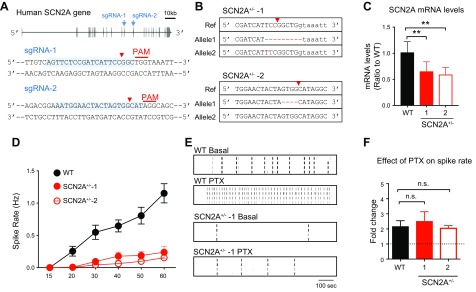
To explore the application of NEUROG2/1 iNs, we studied the functional impact of disrupting SCN2A on the NEUROG2/1 iN network. SCN2A encodes the voltage-gated sodium channel α subunit Nav1.2, and its heterozygous LOF has been associated with autism spectrum disorders (ASD) and other neurologic disorders (12–15). As SCN2A is expressed in NEUROG2/1 iNs (Fig. 3D), we set out to model SCN2A LOF using NEUROG2/1 iNs. As shown in Fig. 8A, 2 hESC lines with heterozygous knockout of SCN2A (SCN2A+/−) were produced with CRISPR/Cas9-mediated genome engineering (16). Sanger sequencing indicated that both lines contained 1 WT-like allele and 1 edited allele that was predicted to produce premature stop codons (Fig. 8B) (premature stop codon at aa 872 for line 1 and aa 1077 for line 2; RefSeq NM_021007.2). The raw Sanger sequencing reads for both lines around the engineered locations are shown in Supplemental Fig. S3. To evaluate the potential off-target effects, we identified the top 5 predicted off-target sequences for both sgRNAs and Sanger sequencing verified that those sites were free of mutations (Supplemental Fig. S4). Using the same method described in Fig. 1A, we transduced SCN2A+/− hESCs with NEUROG2/1 and differentiated them into neurons. RT-qPCR studies revealed an ∼50% reduction in the SCN2A mRNA level for both SCN2A+/− iNs, validating the LOF of SCN2A in both lines (Fig. 8C). Subsequently, we monitored the spontaneous firing activities in SCN2A+/− iN networks during development and found that the spike rates of SCN2A+/− networks were reduced compared to those of WT networks throughout development (Fig. 8D). These data, for the first time, demonstrated significant functional deficits in neurons with LOF of SCN2A on a human genomic background. After PTX treatment on d 60, the spike rate of SCN2A+/− iN networks increased ∼2-fold, similar to the observation in WT iN networks (Fig. 8F). Taken together, our data suggested that heterozygous knock out of SCN2A led to a significant reduction in spontaneous network firing activity but did not impair the functional role of inhibitory synaptic transmission in the network.
Figure 8.
Impaired network firing in SCN2A+/− iN networks. A) Schematic illustration of the strategy for establishing SCN2A+/− hESCs using CRISPR/Cas9 genome editing. The locations of sgRNAs targeting the human SCN2A gene are indicated by blue arrows (top panel) and highlighted in light blue, with the corresponding protospacer-adjacent motif (PAM) in red (bottom panels). Red arrowheads indicate predicted double-stranded break (DSB) sites. B) Sequencing results from 2 monoclonal hESC lines (SCN2A+/−-1 and SCN2A+/−-2) produced with sgRNA-1 and sgRNA-2, respectively. Red arrowheads indicate predicted DSB sites. Mutant sequences are highlighted in red. Uppercase sequence indicates exons, and lowercase sequence indicates introns. C) The mRNA expression levels of SCN2A in the 2 SCN2A+/− lines, normalized to the expression of SCN2A in WT iNs. D) Quantification of the mean spike rate of WT, SCN2A+/−-1, and SCN2A+/−-2 iN networks at different time points during development. Data were collected from 9 MEAs for each line. E) Representative raster plots of network activities of WT and SCN2A+/−-1 iN networks recorded by MEA at d 60 in the absence and presence of PTX (20 μM) treatment. F) Analysis of the fold change in the spike rate for WT and SCN2A+/− iN networks in response to PTX (20 μM) treatment. Data were collected from 9 MEAs for each line. N,s., not significant. **P < 0.01 (unpaired Student’s t test).
DISCUSSION
Previous studies have shown that overexpression of Neurog2 or Neurog2/1 in hESCs and hiPSCs can rapidly and efficiently produce excitatory neurons (8, 10, 11, 27, 28). Here, we established NEUROG2/1 iNs and monitored their molecular, cellular, and electrophysiological properties over 60 d after induction. We found that overexpression of NEUROG1 and NEUROG2 in hESCs induced a network with both glutamatergic excitatory and GABAergic inhibitory neurons.
Although only a small portion of NEUROG2/1 iNs were found to be GABAergic (2.3 ± 0.3% on d 30), MEA recordings revealed a 2-fold increase in the network spiking rate in response to PTX treatment to block inhibitory transmission (Fig. 7C), demonstrating the significant functional role of inhibitory neurons in the NEUROG2/1 iN network. GABAergic cells were not previously reported to develop from Neurog2 (8) or Neurog2/1 iNs (10, 11). Last year, a report showed that forced expression of Neurog2 in the presence of Dlx2 could produce a network with glutamatergic and GABAergic neurons, whereas Neurog2 in combination with any other transcription factors, such as Lhx1 and Pax2, induced only glutamatergic excitatory neurons (25). Our data indicated that NEUROG2 in combination with NEUROG1 produced a neuronal network with both excitatory and inhibitory neurons (Figs. 5A and 7). Because many conditions were changed in our experiments compared to previous experiments, such as the cells used for differentiation, the source of glial cells, and the culture conditions, it is not clear which particular factor/factors triggered the induction of GABAergic cells in our protocol. Among all these variations, having human, not mouse, neurogenins might play an important role because the protein sequences encoded by NEUROG2/1 and Neurog2/1 are not identical (Supplemental Fig. S5). Neurog2, as a pioneer transcription factor, can open chromatin structures to allow the binding of secondary transcription factors that facilitate the expression of more mature or subtype-specific proteins (29). The difference in protein sequence might allow NEUROG2 alone or in combination with NEUROG1 to activate additional transcription factors compared to Neurog2/1, which could promote the induction of GABAergic neurons. It will be important to perform more experiments in the future to test this hypothesis, which might result in new strategies to generate exclusively GABAergic neurons.
In this study, we followed NEUROG2/1 iNs up to 60 d after induction and found that they could be easily maintained in culture. As shown in the MEA and patch-clamp characterizations, spontaneous firing and synaptic transmission became more active with development, showing no plateau (or decrease) throughout the 60-d period (Figs. 2 and 6). Compared to Neurog2/1 iNs (11), the NEUROG2/1 iNs showed some nonsignificant differences in their intrinsic membrane properties (∼1.3-fold depolarization of the resting membrane potential, 1.5-fold decrease in input resistance, and 1.6-fold increase in membrane capacitance on approximately d 60). Interestingly, the excitatory synaptic transmissions detected from NEUROG2/1 iNs were dramatically more active than those reported from Neurog2/1 iNs (an ∼32-fold increase in the sEPSC rate on approximately d 60). The increase in the sEPSC rate suggested that the NEUROG2/1 iNs developed into a network with much more spontaneous activity. As discussed earlier, different experimental conditions, such as the type of cells used for differentiation (hESCs vs. iPSCs), the source of glial cells (mouse vs. rat) for coculture, and the culture protocol (plating glial cells after iNs vs. plating glial cells before iNs), might cause the observed differences in spontaneous activity.
It is known that the construction of neural circuits in the CNS is a dynamic process involving the formation and elimination of synapses to sharpen the neural circuitry (30, 31). Although the spontaneous firing and synaptic activity of NEUROG2/1 iNs continued to increase over time, the observed activities never reached a plateau (or decrease) during the 60-d culture period. It is likely that the NEUROG2/1 iN network still reflects the early developmental stages of neuronal assembly before the synaptic connections are saturated. Nevertheless, given that reliable robust synaptic and firing activities developed in such networks with both excitatory and inhibitory neurons (Figs. 2 and 6), NEUROG2/1 iNs could be useful for modeling neurodevelopmental disorders.
To demonstrate the utility of NEUROG2/1 iNs, we engineered hESCs with LOF in 1 allele of SCN2A and differentiated them into neurons by overexpressing NEUROG2/1. SCN2A encodes the voltage-gated sodium channel Nav1.2, which is critical for the generation and propagation of action potentials. Gain-of-function variants in the SCN2A gene have been implicated in infantile seizures (12, 32, 33), whereas LOF variants have been implicated in ASDs (13, 34, 35). Homozygous LOF of SCN2A is lethal in mice (36), and haploinsufficiency of SCN2A produces seizure-like activities (37). A recent study using HEK293 cells expressing WT or ASD-associated mutant SCN2A showed that ASD-associated mutations impaired Nav1.2 channel function, and a predicted ASD-associated SCN2A mutation led to a reduction in neuronal excitability using computational models (12). Our data showed that heterogeneous knockout of SCN2A produced a hypoactive human neuronal network with a drastic reduction in neuronal firing frequency, as revealed by MEA. Such a cellular phenotype could be used to study the mechanism through which SCN2A influences network excitability and may serve as a cellular phenotype applicable to screening compounds for rescuing defects. Our study not only identified a cellular phenotype caused by the LOF of SCN2A but also substantiated NEUROG2/1 iNs as a neuronal model system that may be useful for studying the function of human genes implicated in neurodevelopmental disorders.
Over the past decade, genetic studies have identified a growing list of de novo mutations in patients with neurodevelopmental and neuropsychiatric diseases (38–40). A key challenge is the lack of relevant, efficient, and accessible human neuronal models for studying the causative effects of those mutations. Genome engineering combined with stem cell reprogramming enables fast and accurate engineering of hESCs with desired mutations for modeling psychiatric disorders in a human neuronal context. Through in-depth characterization of iN networks with overexpression of NEUROG2/1, we found that this model system contained both excitatory and inhibitory neurons and exhibited robust spontaneous network activity within 1 mo. In combination with high-throughput assays, such as MEA, NEUROG2/1 human iNs could be valuable for many applications, such as neurophenotyping the de novo mutations identified from genetic studies of disease and modeling neurodevelopmental diseases with the appropriate human genetic background.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Wenting Wang (Institute of Neuroscience, Fourth Military Medical University, Xi’an, China) for his advice on electrophysiological experiments, Dr. Qian Chen (McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, USA), and Rhiannon Macrae (Broad Institute, Cambridge, MA, USA) for help with the manuscript. This work was supported by funding from the Stanley Center for Psychiatric Research, and U.S. National Institutes of Health, National Institute of Mental Health Grants R21 MH099448-02 (to J.Q.) and R01 MH115045-01 (to J.Q.). The authors declare no conflicts of interest.
Glossary
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9
- EPSC
excitatory postsynaptic current
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- hESC
human embryonic stem cell
- hiPSC
human induced pluripotent stem cell
- ICC
immunocytochemistry
- iN
induced neuron
- IPSCs
inhibitory postsynaptic currents
- LOF
loss of function
- MEA
microelectrode array
- mEPSC
miniature excitatory postsynaptic current
- NB
neural basal
- Neurog
neurogenin
- PTX
picrotoxin
- qPCR
quantitative PCR
- sEPSC
spontaneous excitatory postsynaptic current
- TTX
tetrodotoxin
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Lu, X. Shi, and J. Q. Pan designed the study; C. Lu conducted electrophysiology experiments other than Ca2+ current recordings; D. Baez-Nieto conducted Ca2+ current recordings; A. Allen conducted RT-qPCR experiments; C. Lu and A. Nikish conducted ICC; N. E. Sanjana generated the NEUROG2/1 lentivirus; X. Shi and N. E. Sanjana initiated the project on iNs and generated SCN2A+/− hESCs; C. Lu, X. Shi, and J. Q. Pan drafted the manuscript; J. Q. Pan supervised the study; and all authors contributed to the editing of the manuscript.
REFERENCES
- 1.Hafezparast M., Ahmad-Annuar A., Wood N. W., Tabrizi S. J., Fisher E. M. C. (2002) Mouse models for neurological disease. Lancet Neurol. 1, 215–224 [DOI] [PubMed] [Google Scholar]
- 2.Chesselet M.-F., Carmichael S. T. (2012) Animal models of neurological disorders. Neurotherapeutics 9, 241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avior Y., Sagi I., Benvenisty N. (2016) Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 17, 170–182 [DOI] [PubMed] [Google Scholar]
- 4.Hibaoui Y., Feki A. (2012) Human pluripotent stem cells: applications and challenges in neurological diseases. Front. Physiol. 3, 267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J., Xu W., Yang N., Danko T., Chen L., Wernig M., Südhof T. C. (2013) Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascón S., Masserdotti G., Russo G. L., Götz M. (2017) Direct neuronal reprogramming: achievements, hurdles, and new roads to success. Cell Stem Cell 21, 18–34 [DOI] [PubMed] [Google Scholar]
- 10.Busskamp V., Lewis N. E., Guye P., Ng A. H. M., Shipman S. L., Byrne S. M., Sanjana N. E., Murn J., Li Y., Li S., Stadler M., Weiss R., Church G. M. (2014) Rapid neurogenesis through transcriptional activation in human stem cells. Mol. Syst. Biol. 10, 760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam R. S., Töpfer F. M., Wood P. G., Busskamp V., Bamberg E. (2017) Functional maturation of human stem cell-derived neurons in long-term cultures. PLoS One 12, e0169506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Shalom R., Keeshen C. M., Berrios K. N., An J. Y., Sanders S. J., Bender K. J. (2017) Opposing effects on NaV1.2 function underlie differences between SCN2A variants observed in individuals with autism spectrum disorder or infantile seizures. Biol. Psychiatry 82, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders S. J., He X., Willsey A. J., Ercan-Sencicek A. G., Samocha K. E., Cicek A. E., Murtha M. T., Bal V. H., Bishop S. L., Dong S., Goldberg A. P., Jinlu C., Keaney J. F., III, Klei L., Mandell J. D., Moreno-De-Luca D., Poultney C. S., Robinson E. B., Smith L., Solli-Nowlan T., Su M. Y., Teran N. A., Walker M. F., Werling D. M., Beaudet A. L., Cantor R. M., Fombonne E., Geschwind D. H., Grice D. E., Lord C., Lowe J. K., Mane S. M., Martin D. M., Morrow E. M., Talkowski M. E., Sutcliffe J. S., Walsh C. A., Yu T. W.; Autism Sequencing Consortium (2015) Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deciphering developmental disorders study (2015) Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell K. B., McMahon J. M., Carvill G. L., Tambunan D., Mackay M. T., Rodriguez-Casero V., Webster R., Clark D., Freeman J. L., Calvert S., Olson H. E., Mandelstam S., Poduri A., Mefford H. C., Harvey A. S., Scheffer I. E. (2015) SCN2A encephalopathy: a major cause of epilepsy of infancy with migrating focal seizures. Neurology 85, 958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A., Zhang F. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters D. T., Cowan C. A., Musunuru K. (2008) Genome editing in human pluripotent stem cells. In StemBook, Harvard Stem Cell Institute, Cambridge, MA, USA: [PubMed] [Google Scholar]
- 18.Molyneaux B. J., Arlotta P., Menezes J. R. L., Macklis J. D. (2007) Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J., Jiao J. (2015) Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. BioMed Res. Int. 2015, 727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M. (1997) Identification and characterization of the vesicular GABA transporter. Nature 389, 870–876 [DOI] [PubMed] [Google Scholar]
- 21.Neniskyte U., Gross C. T. (2017) Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat. Rev. Neurosci. 18, 658–670 [DOI] [PubMed] [Google Scholar]
- 22.Lu C., Chen Q., Zhou T., Bozic D., Fu Z., Pan J. Q., Feng G. (2016) Micro-electrode array recordings reveal reductions in both excitation and inhibition in cultured cortical neuron networks lacking Shank3. Mol. Psychiatry 21, 159–168 [DOI] [PubMed] [Google Scholar]
- 23.Pillai A., Mansouri A., Behringer R., Westphal H., Goulding M. (2007) Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 134, 357–366 [DOI] [PubMed] [Google Scholar]
- 24.Miquelajáuregui A., Varela-Echavarría A., Ceci M. L., García-Moreno F., Ricaño I., Hoang K., Frade-Pérez D., Portera-Cailliau C., Tamariz E., De Carlos J. A., Westphal H., Zhao Y. (2010) LIM-homeobox gene Lhx5 is required for normal development of Cajal-Retzius cells. J. Neurosci. 30, 10551–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liodis P., Denaxa M., Grigoriou M., Akufo-Addo C., Yanagawa Y., Pachnis V. (2007) Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 27, 3078–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang N., Chanda S., Marro S., Ng Y.-H., Janas J. A., Haag D., Ang C. E., Tang Y., Flores Q., Mall M., Wapinski O., Li M., Ahlenius H., Rubenstein J. L., Chang H. Y., Buylla A. A., Südhof T. C., Wernig M. (2017) Generation of pure GABAergic neurons by transcription factor programming. Nat. Methods 14, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frega M., van Gestel S. H. C., Linda K., van der Raadt J., Keller J., Van Rhijn J.-R., Schubert D., Albers C. A., Nadif Kasri N. (2017) Rapid neuronal differentiation of induced pluripotent stem cells for measuring network activity on micro-electrode arrays. J. Vis. Exp. 119, e54900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho S.-M., Hartley B. J., Tcw J., Beaumont M., Stafford K., Slesinger P. A., Brennand K. J. (2016) Rapid Ngn2-induction of excitatory neurons from hiPSC-derived neural progenitor cells. Methods 101, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens J., Marchetto M. C., Bardy C., Gage F. H. (2016) Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtman J. W., Colman H. (2000) Synapse elimination and indelible memory. Neuron 25, 269–278 [DOI] [PubMed] [Google Scholar]
- 31.Hua J. Y., Smith S. J. (2004) Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 7, 327–332 [DOI] [PubMed] [Google Scholar]
- 32.Xu R., Thomas E. A., Jenkins M., Gazina E. V., Chiu C., Heron S. E., Mulley J. C., Scheffer I. E., Berkovic S. F., Petrou S. (2007) A childhood epilepsy mutation reveals a role for developmentally regulated splicing of a sodium channel. Mol. Cell. Neurosci. 35, 292–301 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz N., Hahn A., Bast T., Müller S., Löffler H., Maljevic S., Gaily E., Prehl I., Biskup S., Joensuu T., Lehesjoki A.-E., Neubauer B. A., Lerche H., Hedrich U. B. S. (2016) Mutations in the sodium channel gene SCN2A cause neonatal epilepsy with late-onset episodic ataxia. J. Neurol. 263, 334–343 [DOI] [PubMed] [Google Scholar]
- 34.Iossifov I., O’Roak B. J., Sanders S. J., Ronemus M., Krumm N., Levy D., Stessman H. A., Witherspoon K. T., Vives L., Patterson K. E., Smith J. D., Paeper B., Nickerson D. A., Dea J., Dong S., Gonzalez L. E., Mandell J. D., Mane S. M., Murtha M. T., Sullivan C. A., Walker M. F., Waqar Z., Wei L., Willsey A. J., Yamrom B., Lee Y. H., Grabowska E., Dalkic E., Wang Z., Marks S., Andrews P., Leotta A., Kendall J., Hakker I., Rosenbaum J., Ma B., Rodgers L., Troge J., Narzisi G., Yoon S., Schatz M. C., Ye K., McCombie W. R., Shendure J., Eichler E. E., State M. W., Wigler M. (2014) The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders S. J., Murtha M. T., Gupta A. R., Murdoch J. D., Raubeson M. J., Willsey A. J., Ercan-Sencicek A. G., DiLullo N. M., Parikshak N. N., Stein J. L., Walker M. F., Ober G. T., Teran N. A., Song Y., El-Fishawy P., Murtha R. C., Choi M., Overton J. D., Bjornson R. D., Carriero N. J., Meyer K. A., Bilguvar K., Mane S. M., Sestan N., Lifton R. P., Günel M., Roeder K., Geschwind D. H., Devlin B., State M. W. (2012) De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planells-Cases R., Caprini M., Zhang J., Rockenstein E. M., Rivera R. R., Murre C., Masliah E., Montal M. (2000) Neuronal death and perinatal lethality in voltage-gated sodium channel alpha(II)-deficient mice. Biophys. J. 78, 2878–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogiwara I., Miyamoto H., Tatsukawa T., Yamagata T., Nakayama T., Atapour N., Miura E., Mazaki E., Ernst S. J., Cao D., Ohtani H., Itohara S., Yanagawa Y., Montal M., Yuzaki M., Inoue Y., Hensch T. K., Noebels J. L., Yamakawa K. (2018) Nav1.2 haplodeficiency in excitatory neurons causes absence-like seizures in mice. Commun Biol. 1, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu T. W., Chahrour M. H., Coulter M. E., Jiralerspong S., Okamura-Ikeda K., Ataman B., Schmitz-Abe K., Harmin D. A., Adli M., Malik A. N., D’Gama A. M., Lim E. T., Sanders S. J., Mochida G. H., Partlow J. N., Sunu C. M., Felie J. M., Rodriguez J., Nasir R. H., Ware J., Joseph R. M., Hill R. S., Kwan B. Y., Al-Saffar M., Mukaddes N. M., Hashmi A., Balkhy S., Gascon G. G., Hisama F. M., LeClair E., Poduri A., Oner O., Al-Saad S., Al-Awadi S. A., Bastaki L., Ben-Omran T., Teebi A. S., Al-Gazali L., Eapen V., Stevens C. R., Rappaport L., Gabriel S. B., Markianos K., State M. W., Greenberg M. E., Taniguchi H., Braverman N. E., Morrow E. M., Walsh C. A. (2013) Using whole-exome sequencing to identify inherited causes of autism. Neuron 77, 259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krumm N., O’Roak B. J., Shendure J., Eichler E. E. (2014) A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 37, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gratten J., Visscher P. M., Mowry B. J., Wray N. R. (2013) Interpreting the role of de novo protein-coding mutations in neuropsychiatric disease. Nat. Genet. 45, 234–238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.