Abstract
Iso-α-acids (IAAs) are hop-derived bitter acids of beer. Epidemiologic studies suggest that moderate alcohol consumption is beneficial for cognitive function, but they do not show the ingredients in alcoholic beverages. Previously, we reported that long-term consumption of IAAs prevents inflammation and Alzheimer pathologies in mice, but their effects on cognitive function have not been evaluated. In the present study, we demonstrated that the consumption of IAAs improves spatial and object recognition memory functions not only in normal Crl:CD1(ICR) male mice but also in mice with pharmacologically induced amnesia. IAA consumption increased the total and extracellular levels of dopamine in the hippocampus of mice and Sprague-Dawley male rats, respectively. Dopamine D1 receptor antagonist treatment and knockdown of dopamine D1 receptor expression in the hippocampus attenuated IAA-induced spatial memory improvement. Furthermore, vagotomy attenuated the effects of IAAs in improving spatial and object recognition memory functions and increasing the total level of dopamine in the hippocampus. These results suggest that the consumption of IAAs activates dopamine D1 receptor–signaling in the hippocampus in a vagus nerve–dependent manner and, consequently, improves spatial and object recognition memory functions. Vagal activation with food components including IAAs may be an easy and safe approach to improve cognitive functions.—Ano, Y., Hoshi, A., Ayabe, T., Ohya, R., Uchida, S., Yamada, K., Kondo, K., Kitaoka, S., Furuyashiki, T. Iso-α-acids, the bitter components of beer, improve hippocampus-dependent memory through vagus nerve activation.
Keywords: dopamine, amnesia, beer, hop
Dementia and cognitive impairment are burdens not only on patients and their families but also on national health care systems worldwide, and these burdens are increasing with the rapid growth in aged populations. Due to the lack of effective therapeutic agents for dementia, preventive approaches such as diet, exercise, and learning are receiving increased attention. Epidemiological studies reported that low-to-moderate consumption of alcohol, such as beer and wine, is associated with the risk of cognitive decline and the development of dementia. Individuals who consume low-to-moderate levels of alcoholic beverages on a daily basis have a significantly lower risk of developing neurodegenerative diseases compared with individuals who abstain from alcoholic beverages or drink heavily (1–3). Apart from the effects of alcohol itself, resveratrol, a polyphenolic compound in red wine, has neuroprotective activities (4, 5). Previously, we demonstrated that long-term consumption of iso-α-acids (IAAs), the bitter components in beer, prevents Alzheimer pathology in transgenic model mice (6). IAAs suppress microglial-based inflammation in the brain, resulting in protection from cognitive decline and Alzheimer disease. However, the effects of short-term consumption of IAA on cognitive function have not been examined.
IAAs are derived from hops, the female inflorescences of the hop plant (Humulus lupulus L.), and have been used in beer production since 822. Hops are used as both a preservative and a flavoring agent in the beer-brewing process. IAAs originating from α-acids in hops have potent agonist activity at the bitter taste receptors TAS2Rs (T2Rs) (7). T2Rs are abundant in gastrointestinal enteroendocrine cells (8–10). In these cells, increased Ca2+ from T2R activation leads to the release of a peptide hormone, cholecystokinin (CCK), which acts through CCK receptors on sensory fibers of the vagus nerve to transmit signals to the brain to control food intake (11–14). Recent studies have demonstrated that cognitive and mental functions are related closely to vagus nerve activation, including the gut-brain axis (15–17). It has been shown that vagus nerve activation enhances the release of norepinephrine and dopamine in the brain and improves memory function (18–22). In the present study, we examined the effects of short-term intake of IAAs on cognitive function, especially hippocampus-dependent memory.
MATERIALS AND METHODS
Animals
Six-week-old male Crl:CD1(ICR) mice, vagotomized male ICR mice, and Sprague-Dawley (SD) rats (Charles River Laboratories, Tokyo, Japan) were maintained at Kyowa Hakko Kirin Company or Kirin Company. Vagotomy was performed in 5-wk-old ICR mice in the laboratory of Charles River Laboratories Japan and were used for experiments after the mice were 6 wk old. All experiments were approved by the Animal Experiment Committee of Kyowa Hakko Kirin Co. or Kirin Co., and were conducted in strict accordance with their guidelines from 2012 to 2014. Mice were fed a standard rodent diet (CE-2; Central Institute for Experimental Animals, Tokyo, Japan) and were maintained at room temperature (23 ± 1°C) under constant 12-h light/dark cycles (light period from 8:00 am to 8:00 pm). All procedures were conducted in accordance with the guidelines of the Declaration of Helsinki.
Preparation of IAAs
α-Acids consist predominantly of 3 congeners: cohumulone, humulone, and adhumulone. During the brewing process, they are each isomerized into 2 epimeric isomers: cis- and trans-IAAs. A purchased isomerized hop extract stocked in potassium carbonate at 828 mM (Hopsteiner, Mainburg, Germany) with 30.5% (w/v) IAA comprising trans-isocohumulone (1.74% w/v), cis-isocohumulone (7.61% w/v), trans-isohumulone (3.05% w/v), cis-isohumulone (14.0% w/v), trans-isoadhumulone (0.737% w/v), and cis-isoadhumulone (3.37% w/v) was used to isolate individual IAAs by HPLC, as previously described (23).
Induction of memory impairment
To evaluate the effects of IAAs on scopolamine (SCP)-induced memory impairment, 6-wk-old ICR male mice were provided samples dissolved in distilled water at 1 h before evaluation. At 40 min after intragastric administration via a stomach tube, memory impairment was induced by intraperitoneal administration of 0.85 mg/kg (−) SCP hydrobromide trihydrate (MilliporeSigma, Burlington, MA, USA) dissolved in saline. At 1 hr after the intragastric administration, mice were subjected to the Y-maze trial (n = 10) or novel object recognition test (NORT; n = 10). In some experiments, mice were administered 0.85 mg/kg (−) SCP hydrobromide trihydrate and 0.05-mg/kg R(+)-SCH 23390 hydrochloride (dopamine D1 antagonist; MilliporeSigma) intraperitoneally at 40 min after the intragastric administration of 1 mg/kg IAA or its vehicle and then subjected to the Y-maze test at 1 h after the intragastric administration.
Next, to evaluate the effects of IAAs, trans-isohumulone, or donepezil hydrochloride monohydrate (MilliporeSigma) on oligomer amyloid-β (Aβ)-induced memory impairment, 6-wk-old ICR male mice were administered samples intragastrically at 1 h before the spontaneous alternation test and the NORT. To prepare Aβ oligomer, Aβ1-42 (MilliporeSigma) was dissolved in hexafluoro-2-propanol (Wako Pure Chemicals, Osaka, Japan) to 1 mM and incubated at room temperature for 30 min. Hexafluoro-2-propanol was removed by volatilization, and Aβ peptide film was obtained. Aβ was then dissolved in dimethyl sulfoxide to 5 mM and resuspended in PBS to 100 μM. This solution was incubated at 4°C for 24 h to induce aggregation of Aβ. The solution was centrifuged at 10,000 rpm for 15 min, and the supernatant was used as Aβ oligomer solution. Mice were anesthetized using pentobarbital (Somnopentyl; Kyoritsu, Tokyo, Japan), and 100 μM Aβ oligomer or PBS was injected intracerebroventricularly at 5 μl for both hemispheres. Mice were allowed to recover for 3 d and were then used for the spontaneous alternation test and the NORT after the intragastric administration of the test sample.
Spontaneous alternation test
The spontaneous alternation test was established in our experimental environment (24). The Y maze is a 3-arm maze with equal angles between all arms (25 cm long × 5 cm wide × 20 cm high). The maze walls were constructed of dark black polyvinyl plastic. Each mouse was placed initially in 1 arm, and the sequence and number of arm entries were counted for 8 min. The alternation score (%) for each mouse was defined as the ratio of the actual number of alternations to the possible number (defined as the total number of arm entries − 2) × 100 as follows: % alternation = [(no. of alternations)/(total arm entries − 2)] × 100.
NORT
The object recognition test was performed during the light period in a polyvinyl chloride box (25 × 40 × 20 cm) without a roof. For the acquisition trial, a pair of wooden triangle poles (4.5 × 4.5 × 4.5 cm3) or wooden pyramids (4.5 × 4.5 × 4.5 cm3) were used, and for the retention trial, a pair of poles or the pyramid and a golf ball (4.5-cm diameter) were used as familiar and novel objects, respectively, as previously described (25). In all trials, the objects were placed 7.5 cm apart from the corner of the box. In the acquisition trial, each mouse was allowed to explore the box with the 2 objects for 10 min at 1 h after intragastric administration of the test sample. A total of 24 h after the acquisition trial, the mouse was allowed to explore the box with the novel and familiar objects for 5 min at 1 h after an additional intragastric administration of the test sample. The discrimination index (DI) was calculated by dividing the difference in time for exploring the novel object and the familiar object by the total time spent exploring both objects: (novel object exploration time − familiar object exploration time)/(total exploration time). Thus, a DI of 0 indicates the equal exploration of both objects. The number of mice tested was the same as that in the spontaneous alternation test.
Monoamine analysis
To evaluate the levels of dopamine and its metabolites [3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)] in the brain, tissue was homogenized in 0.2-M perchloric acid (Wako Pure Chemicals) containing 100-μM EDTA-Na2 (MilliporeSigma). After centrifugation, the supernatant was analyzed by HPLC using an Eicompak SC-5ODS column and PrePak column (Eicom, Kyoto, Japan) with electrochemical detection (ECD). The mobile phase consisted of 83% 0.1-M acetic acid in citric acid buffer (pH 3.5), 17% methanol (Wako Pure Chemicals), 190 mg/ml of sodium 1-octanesulfonate sodium (Wako Pure Chemicals), and 5-mg/ml EDTA-Na2. For ECD, the applied voltage was 750 mV vs. an Ag/AgCl reference electrode.
Microdialysis
Eight-week-old male SD rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The anesthetized animals were placed in a stereotaxic apparatus. The skull was exposed, and a small hole was made using a dental drill. A guide cannula (AG-8; Eicom) was implanted into the hippocampus (from bregma: posterior, −5.8 mm; lateral, −4.8 mm; ventral, −4.0 mm) according to the atlas of Paxinos and Watson (26). The guide cannula was fixed to the skull with cranioplastic cement. A total of 3–5 d after the surgery, microdialysis probes (AI-8-3, 3-mm membrane length; Eicom) were inserted slowly into the hippocampus through guide cannulas under anesthesia with diethyl ether, and rats were settled in the experimental cages (30 cm wide × 30 cm deep × 30 cm high). The probes were perfused continuously at a flow rate of 2 μl/min with Ringer’s solution (147.1 mM NaCl, 4.0 mM KCl, and 2.3 mM CaCl2).
Animals were allowed a 3-h stabilization period before the baseline sample was collected. The outflow fractions were taken every 20 min. During the second sampling, test sample was administered intraperitoneally to the rats according to the following designated treatment groups (n = 9–12 rats/region): vehicle or IAA (0.5 mg/kg). Dialysis fractions were collected for 2 h after sample administration. These fractions were then analyzed using an HPLC-ECD system (700 series; Eicom). Dopamine and 5-hydroxytryptamine (5-HT) were separated by a column (Eicompak Cax, φ-2.0 × 200 mm; Eicom), with the mobile phase containing ammonium acetate (5.4 g/L), sodium sulfonate (7.1 g/L), EDTA (2Na; 50 mg/L), and 30% methanol. The mobile phase was delivered at a flow rate of 250 μl/min. DOPAC, 3-methoxytyramine (3-MT), and HVA were separated by a column (Eicompak SC-5ODS, φ-2.1 × 150 mm; Eicom), with the mobile phase as sodium acetate (4.00 g/L), citric acid monohydrate (10.25 g/L), sodium 1-octane sulfonate (140 mg/L), EDTA (2Na; 5 mg/L), and 17% methanol. The mobile phase was delivered at a flow rate of 230 μl/min. The identification of monoamine was performed according to the retention times of standards, and these amounts were quantified by calculating peak areas. The peaks were recorded using a Powerchrom integrator (Eicom).
Adeno-associated virus injection
The methods of adeno-associated virus (AAV) injection used in the present study to knock down dopamine D1 receptor were in accordance with our previous study (27). AAV-expressing artificial microRNA (miR) targeting dopamine D1 receptor with emerald green fluorescent protein (EmGFP) under the eukaryotic translation elongation factor 1–α (EF1-α) promoter only in the presence of Cre recombinase [AAV10–EF1-α–double-floxed inverted (DIO)-EmGFP-dopamine D1 receptor (D1miR)] as well as AAV-expressing control miRNA in the same condition (AAV10–EF1-α–DIO-EmGFP-control) were produced as previously described (27). These AAVs were mixed with another AAV-expressing Cre under the cytomegalovirus promoter (AAV10-cytomegalovirus-Cre). Eight-week-old ICR mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). These mice were placed in a stereotaxic apparatus. The skull was exposed, and a small hole was made using a dental drill. A glass capillary was inserted, and 0.5-μl of AAV solution (1.0 × 1012 genomics copies/ml/site) was injected into 2 sites in the hippocampal regions of both hemispheres (4 sites/mouse; from bregma: posterior, −3.5 mm; lateral, ± 3 mm; ventral, −3.8 mm and −1.8 mm), according to the atlas of Franklin and Paxinos (28), using a PV830 Pneumatic PicoPump (World Precision Instruments, Sarasota, FL, USA). Mice were allowed to recover for at least 3 wk and then used for the Y-maze test. After the experiments, mice were euthanized and coronal brain sections (10 μm thickness) were made by cryostat (CM3050 S; Danaher Corp., Nussloch, Germany). The expression of miRNA was confirmed by detecting the fluorescence of EmGFP (Supplemental Fig. S1).
Statistical data analysis
All data represent means ± se (error bars). The statistical significance of differences between the groups was assessed by 1-way ANOVA, followed by the Tukey-Kramer test. Other data presented (as indicated) were assessed by 2-way ANOVA, followed by the Bonferroni test. All statistical analyses were performed using the Ekuseru-Toukei 2012 software program (Social Survey Research Information, Tokyo, Japan).
RESULTS
Effects of IAA intake on memory performance
To evaluate the effects of IAAs on short-term spatial memory, mice were intragastrically administered IAA at 0.02–2 mg/kg once and subjected to the Y-maze test after treatment with SCP to induce amnesia. SCP treatment significantly reduced the spontaneous alternation (Fig. 1A), but a single administration of IAA at 0.2 and 2 mg/kg significantly attenuated SCP-induced reduction in spontaneous alternation (Fig. 1B), consistent with previous reports (24). This result indicates that IAAs prevent SCP-induced deficit in short-term spatial memory in mice. Next, to evaluate the effects of IAAs on long-term object recognition memory, mice were subjected to the NORT, with a 24-h interval between the acquisition and retention steps. Either IAA or its vehicle was administered at both the acquisition and retention steps. IAA administration at 0.2 and 2 mg/kg increased the time spent approaching a novel object and reduced the time spent approaching a familiar object, thus increasing the DI of NORT (Fig. 1C, D). These results indicate that IAAs enhance long-term object recognition memory in normal mice.
Figure 1.
Effects of IAAs on memory function. A, B) Effects of IAAs on spatial memory. Mice were administered 0, 0.02, 0.2, or 2 mg/kg IAA intragastrically, followed by intraperitoneal injection with 0.85 mg/kg of SCP at 40 min after IAA administration. At 1 h after IAA administration, each mouse was allowed to explore the Y maze for 8 min. Spontaneous alternations and arm entries were recorded. The effects of IAAs on spontaneous alternations (A) and arm entries (B), respectively, are shown. C, D) Effects of IAAs on episodic memory (NORT). The time spent exploring novel and familiar objects during 5 min of reexploration (C) was measured and DI [(time spent with object A− time spent with object B)/(total time exploring both objects)] (D) was calculated. E, F) Effects of IAAs on Aβ-induced memory impairment. Mice were injected intracerebroventricularly with oligomer Aβ, administered donepezil or trans-isohumulone intragastrically, and subjected to the Y-maze test or NORT. Spontaneous alternation (E) and DI (F) are shown. Data represent the means ± sem of 10 mice/group. *P < 0.05, **P < 0.01.
We then examined whether IAAs ameliorate memory deficits in an Aβ-derived diffusible ligands model, in which intracerebroventricular injection with Aβ oligomer significantly reduced the number of spontaneous alternations in the Y-maze test and the DI in NORT compared with vehicle treatment (Fig. 1E, F). Trans-isohumulone, a major isomer of IAAs in beer, at 0.3 and 1 mg/kg increased the number of the spontaneous alternations and the DI to the levels with 1-mg/kg donepezil (Fig. 1E, F). This result indicates that IAAs improve memory impairments induced by Aβ oligomer.
IAA-induced increase in the total and extracellular levels of dopamine in the hippocampus
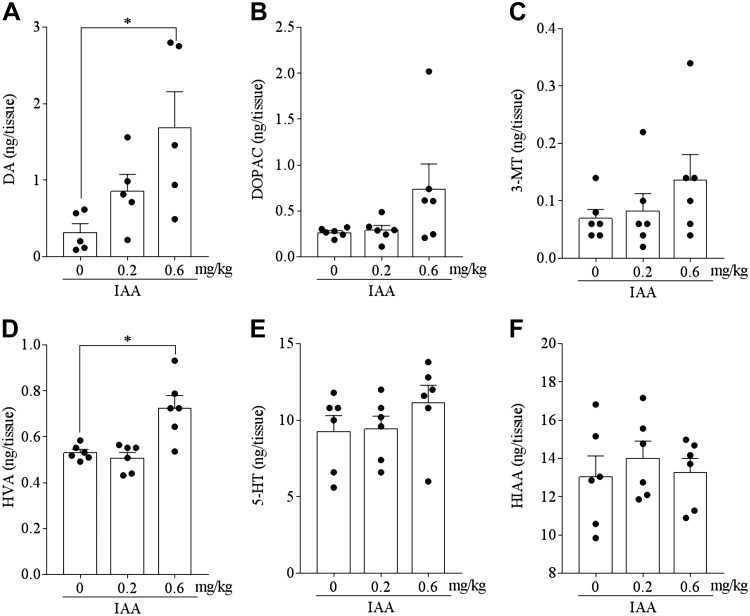
Dopamine neurotransmission in the hippocampus has been shown to play a crucial role in the Y-maze test and the NORT (29–31). To evaluate the effects of IAAs on monoamine contents in the hippocampus, the levels of monoamine and its metabolites in hippocampal tissue homogenates of mice administered once with IAA at 0.2 and 0.6 mg/kg were measured using HPLC-ECD. IAA administration at 0.6 mg/kg significantly increased the levels of dopamine and HVA in hippocampal tissue homogenates (Fig. 2A, D, respectively). The levels of DOPAC, 3-MT, 5-HT, and 5-hydroxyindole acetic acid (HIAA) were not significantly different between IAA-treated and control mice (Fig. 2B, C, E, F, respectively), whereas epinephrine and norepinephrine concentrations were not detectable. These results indicate that IAA treatment increases the total levels of dopamine and its metabolites in the hippocampus.
Figure 2.
Effects of IAAs on the levels of monoamines and metabolites in the hippocampus. The levels of dopamine (DA) (A), DOPAC (B), 3-MT (C), HVA (D), 5-HT (E), and 5-hydroxyindole acetic acid (HIAA) (F) in the hippocampus of mice at 1 h after intragastric administration of 0, 0.2, or 0.6 mg/kg IAA. The levels of monoamines and metabolites were measured using an HPLC-ECD system. Data represent the means ± sem of 5 mice/group. *P < 0.05.
To evaluate the effects of IAAs on the extracellular levels of monoamine in the hippocampus, brain interstitial fluid (ISF) monoamine was measured using microdialysis systems. A single intragastric administration of 0.5-mg/kg IAAs significantly increased the extracellular level of dopamine in the hippocampus (Fig. 3A). This increase peaked at 40 min and returned to the baseline at 100 min after administration. Administration of IAAs also significantly increased the extracellular level of DOPAC from 20 to 80 min and that of HVA at 20 min after administration (Fig. 3B, D, respectively). The extracellular levels of 3-MT and 5-HT were not affected (Fig. 3C, E, respectively). These results indicate that a single administration of IAA increases the extracellular levels of dopamine and its metabolites in the hippocampus.
Figure 3.
Effects of IAAs on the levels of monoamines and metabolites in ISF of the hippocampus. SD rats were administered 0 or 0.5 mg/kg IAA intragastrically (0 min indicated by arrow), and ISF from the hippocampus was collected using the probe between −20 and 120 min relative to the intragastric administration. The levels of dopamine (DA) (A), DOPAC (B), 3-MT (C), HVA (D), and 5-HT (E) were measured using the respective HPLC-ECD system. Data represent the means ± sem of 9–12 rats/group. **P < 0.01 (baseline vs. time point).
Memory improvement by IAA through the dopamine D1 receptor in the hippocampus
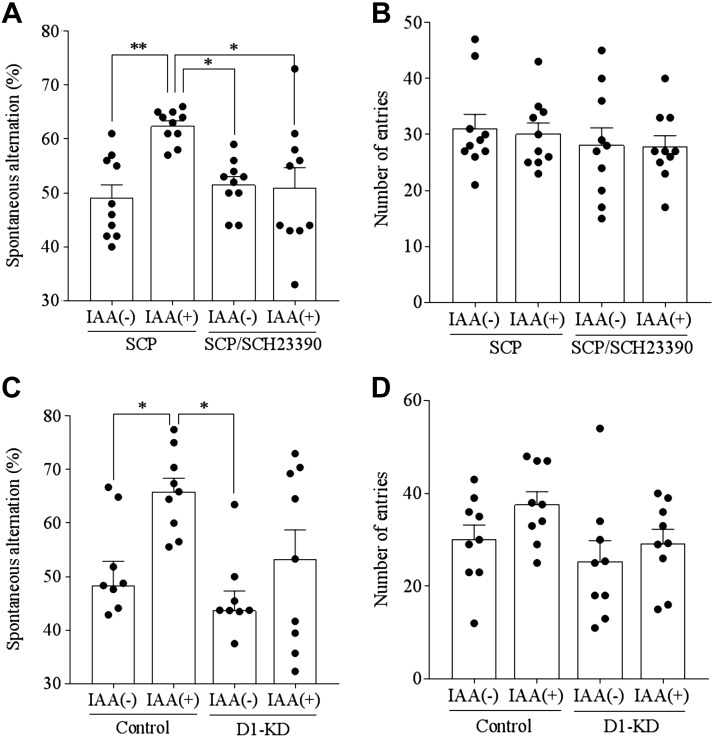
To elucidate the involvement of dopamine in the hippocampus in IAA-induced memory improvements after intragastric administration of IAA, SCP-induced memory deficits were examined with or without simultaneous treatment with a dopamine D1–like receptor antagonist, SCH23390. Prior treatment with IAA at 1 mg/kg increased the number of spontaneous alternations in the SCP-treated mice, but this increase was absent with the SCH23390 treatment (Fig. 4A). Arm entries were not affected (Fig. 4B), indicating that the locomotor activity was spared. These results suggest that dopamine D1–like receptors are involved in IAA-induced memory improvement.
Figure 4.
Involvement of dopamine D1 receptor with memory improvement induced by IAAs. A, B) Effects of IAAs on spatial memory. Mice were administered 0 or 1 mg/kg IAA intragastrically and then injected intraperitoneally with 0.85 mg/kg of SCP and 0.05 mg/kg SCH23390 at 40 min after IAA consumption. At 1 h after IAA consumption, each mouse was allowed to explore the Y maze for 8 min. Spontaneous alternations and arm entries were measured. Effects of IAAs on spontaneous alternations (A) and arm entries (B), respectively, are shown. Data represent the means ± sem of 10 mice/group. *P < 0.05, **P < 0.01. C, D) Effects of dopamine D1 receptor knockdown (D1-KD) in the hippocampus on memory improvement by IAAs. Mice were administered control miRNA or dopamine D1 receptor miRNA containing AAV to the hippocampus to suppress the expression of dopamine D1 receptor. One hour after the intragastric administration of 1 mg/kg IAAs, mice were tested in the Y maze for 8 min. Spontaneous alternations (C) and arm entries (D) are shown. Data analyzed using 2-way ANOVA and Bonferroni test. Data represent the means ± sem of 9 mice/group. *P < 0.05.
To further elucidate the involvement of dopamine in the memory improvement induced by IAAs, dopamine D1 receptors in hippocampal neurons were knocked down using the AAV system expressing artificial miRNA targeting this receptor (27). IAA treatment increased the number of spontaneous alternations in control mice expressing control miRNA, but the D1 receptor knockdown in hippocampal neurons inhibited this IAA-induced increase (Fig. 4C). The number of arm entries was not affected (Fig. 4D). These results indicate that dopamine D1 receptors mediate memory improvement caused by IAAs.
Role of the vagus nerve in memory improvement by IAAs
To elucidate whether IAA acts through the blood brain barrier or the vagus nerve, mice with vagotomy or sham surgery were intragastrically administered IAAs and subjected to the Y-maze test and the NORT. Consumption of IAAs significantly increased the number of spontaneous alternations and the DI in sham-treated mice, but not in vagotomized mice (Fig. 5A, B, respectively). IAA administration increased the total level of dopamine in the hippocampus of sham-treated mice, but this increase was not observed in the hippocampus of vagotomized mice (Fig. 5C). These results suggest that the vagus nerve is involved in the effects of IAA in improving spatial and object recognition memory functions and dopamine increase in the hippocampus.
Figure 5.
Involvement of the vagus nerve on memory improvement induced by IAAs. A, B) Vagotomized or sham-treated mice were administered IAAs intragastrically and subjected to the Y-maze test and NORT. At 1 h after intragastric administration, each mouse was subjected to the Y-maze test and NORT. Spontaneous alternations (A) and DI (B) are shown. C) The levels of dopamine (DA) in the hippocampus treated with IAAs as assessed using HPLC-ECD. Data represent the means ± sem of 7–12 mice/group. *P < 0.05.
DISCUSSION
In the present study, we demonstrated that an intragastric administration of IAAs, the bitter components in beer, increases the total and extracellular levels of dopamine in the hippocampus in a manner dependent on the vagus nerve and, consequently, improves hippocampus-dependent memory. Epidemiologic studies have reported that a moderate consumption of alcoholic beverages is beneficial for cognitive decline (1–3). However, the ingredients in beer have not been examined sufficiently for their benefits to cognitive function, despite the massive consumption. Our results are the first to demonstrate the effects of IAA on memory improvement.
IAAs improved spatial memory and object recognition memory, both of which are hippocampus-dependent memory functions. IAAs and trans-isohumulone, an IAA isomer, improved cognitive decline induced by oligomer Aβ as well as SCP. These improvements were equivalent to that observed with donepezil.
Analysis of monoamines showed that a single administration of IAA increases the levels of dopamine and its metabolites in the hippocampus, but not in frontal cortex (Supplemental Fig. S2). Microdialysis revealed that the extracellular level of dopamine increased between 20 and 80 min after a single administration of IAA, with a peak at 40 min. Dopamine and dopamine receptors in the hippocampus are crucial for long-term potentiation and spatial memory (32, 33). Previous reports using a dopamine receptor antagonist demonstrated that the dopamine D1 receptor is involved in hippocampus-dependent spatial memory (34–36). It has been reported that the dopamine D1 receptor activated the ERK1/2 signaling pathway, which is critically involved in molecular adaptations that are necessary for long-term behavioral and neuronal plasticity (37). The phosphorylation of ERK has been shown to be a correlate for plasticity (38) and long-term memory for taste (39), fear conditioning (40), and spatial tasks (41, 42). In addition, we demonstrated a role of the dopamine D1 receptor in IAAs-induced memory improvements using SCP-induced amnesia model mice and a dopamine D1–like receptor antagonist, SCH23390. In addition, we demonstrated that the dopamine D1 receptor in the hippocampus is crucial for the memory improvement induced by IAAs using the AAV-mediated knockdown of the D1 receptor. In the present study, we did not check the involvement of dopamine D2 receptor in the memory improvement by IAAs, which will be investigated in a further study. Taken together, these results show that oral intake of IAAs improves spatial and object recognition memory by enhancing the dopaminergic system in the hippocampus.
Previously, we evaluated the kinetics of IAAs in the brain and found that little IAA crosses the blood brain barrier (6). In the present study, a single intragastric administration of IAA improved memory functions. Recent studies demonstrated that the vagus nerve is involved in hippocampus-dependent spatial and object recognition memory, as assessed by the NORT and a spatial memory test (43–45). These results suggest that IAAs activate peripheral nerves, including the vagus nerve, resulting in neuronal activation in the brain rather than IAAs directly activating neurons in the brain. To evaluate the involvement of the vagus nerve on the effects of IAAs to improve memory functions, we used vagotomized mice. The consumption of IAAs improved spatial and object recognition memory in sham-treated mice, but this improvement was attenuated in vagotomized mice. The consumption of IAAs increased the total level of dopamine in the hippocampus, but this increase was also attenuated in vagotomized mice. These results suggest that IAAs increase dopamine release in the hippocampus through the vagus nerve and then improve memory functions. The vagus nerve regulates neural activation in the brain stem, including the locus coeruleus (46). It was reported that not only norepinephrine but also dopamine released from the locus coeruleus to the hippocampus promotes spatial learning and memory (20–22). In addition, IAAs have potent agonist activity at the T2R (7). T2Rs are abundant in enteroendocrine cells, and increased Ca2+ by T2R activation leads to the release of CCK, which acts through CCK receptors in afferent fibers of the vagus nerve to transmit signals to the brain to control food intake (11–14). Recent studies have demonstrated that cognitive and mental functions are closely related to the activation of the vagus nerve, including the gut-brain axis (15–17). It is reported that vagus nerve stimulation increased expression of tyrosine hydroxylase and improved locomotion and neuronal populations in a model of Parkinson disease (47); therefore, the gut-brain axis is increasing attention for the therapeutic approaches of Parkinson disease (48). Vagus nerve stimulation is connected to the neuronal activation in the locus coeruleus. It has also been reported that vagus nerve stimulation improves cognitive function via regulating hippocampal hyperactivity (18). Some groups also reported that dopamine in the hippocampus is derived from locus coeruleus neurons (20, 21). Taken together, we assumed that consumption of IAAs activates the T2R and increases the production of CCK, which activates the vagal nerve. Activated vagal nerves stimulate the locus coeruleus neurons, which are projected to hippocampus neurons. Whether IAA intake stimulates the vagus nerve and hippocampal dopamine release from the locus coeruleus remains to be studied.
In the present study, we demonstrated that IAAs, the bitter components in beer, improve memory functions through the vagus nerve and dopamine signaling in the hippocampus. These results are consistent with previous epidemiologic studies of cognitive function and alcoholic beverage consumption. Administrations of IAAs at 0.2–2 mg/kg improve memory function, which is equivalent to 0.03–3 mg/kg for humans according to the allometric scaling to human dose. For a 70-kg person, this would be 2 and 20 mg. IAAs are present in an average beer at 15 ppm, so this would require ∼0.13–1.3 L of consumption. Stimulation of the vagus nerve by food material is safe and easy and may be a new strategy for improving cognitive functions and reversing cognitive declines. Further studies are needed to elucidate the effects of IAAs or hop-derived food materials on cognitive function in human subjects, including amnestic patients.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by Kirin Co. and, in part by a Centers of Research Excellence in Science and Technology (CREST) Grant from Japan Agency for Medical Research and Development (AMED; JP18gm0910012 to T.F.); Grants-in-Aid for Scientific Research (16H05132 and 17K19457 to T.F., 17K08593 to S.K.) from the Japan Society for the Promotion of Science; and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (18H05429 to T.F.). The authors declare no conflicts of interest.
Glossary
- 3-MT
3-methoxytyramine
- 5-HT
5-hydroxytryptamine
- AAV
adeno-associated virus
- Aβ
amyloid-β
- CCK
cholecystokinin
- DI
discrimination index
- DOPAC
3,4-dihydroxyphenylacetic acid
- ECD
electrochemical detection
- EF1-α
eukaryotic translation elongation factor 1–α
- EmGFP
emerald green fluorescent protein
- HVA
homovanillic acid
- IAA
iso-α-acid
- ICR
Crl:CD1(ICR) mice
- ISF
interstitial fluid
- miRNA
microRNA
- NORT
novel object recognition test
- SCP
scopolamine
- SD
Sprague-Dawley
- T2R
bitter taste receptor TAS2R
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Ano, S. Kitaoka, and T. Furuyashiki designed research; Y. Ano, A. Hoshi, T. Ayabe, and R. Ohya performed research; Y. Ano and A. Hoshi analyzed data; Y. Ano, A. Hoshi, S. Kitaoka, and T. Furuyashiki wrote the manuscript; and S. Uchida and K. Yamada supervised experimental technique.
REFERENCES
- 1.Horvat P., Richards M., Kubinova R., Pajak A., Malyutina S., Shishkin S., Pikhart H., Peasey A., Marmot M. G., Singh-Manoux A., Bobak M. (2015) Alcohol consumption, drinking patterns, and cognitive function in older Eastern European adults. Neurology 84, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsui T., Yoshimura A., Toyama T., Matsushita S., Higuchi S. (2011) Preventive effect of moderation in drinking on dementia [in Japanese]. Nihon Rinsho 69 (Suppl 10 Pt 2), 217–222 [PubMed] [Google Scholar]
- 3.Neafsey E. J., Collins M. A. (2011) Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 7, 465–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arntzen K. A., Schirmer H., Wilsgaard T., Mathiesen E. B. (2010) Moderate wine consumption is associated with better cognitive test results: a 7 year follow up of 5033 subjects in the Tromsø Study. Acta Neurol. Scand. Suppl. 190, 23–29 [DOI] [PubMed] [Google Scholar]
- 5.Witte A. V., Kerti L., Margulies D. S., Flöel A. (2014) Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 34, 7862–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ano Y., Dohata A., Taniguchi Y., Hoshi A., Uchida K., Takashima A., Nakayama H. (2017) Iso-α-acids, bitter components of beer, prevent inflammation and cognitive decline induced in a mouse model of Alzheimer’s disease. J. Biol. Chem. 292, 3720–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes J. E., Wallace M. R., Knopik V. S., Herbstman D. M., Bartoshuk L. M., Duffy V. B. (2011) Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem. Senses 36, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan J. M., Margolskee R. F. (2008) Taste cells of the gut and gastrointestinal chemosensation. Mol. Interv. 8, 78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S. V., Rozengurt N., Yang M., Young S. H., Sinnett-Smith J., Rozengurt E. (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. USA 99, 2392–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmanov A. A., Beauchamp G. K. (2007) Taste receptor genes. Annu. Rev. Nutr. 27, 389–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon T. I., Seo Y. K., Osborne T. F. (2011) Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem. J. 438, 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon T. I., Zhu B., Larson J. L., Osborne T. F. (2008) SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Invest. 118, 3693–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran T. H., Baldessarini A. R., Salorio C. F., Lowery T., Schwartz G. J. (1997) Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 272, R1245–R1251 [DOI] [PubMed] [Google Scholar]
- 14.MacIntosh C. G., Morley J. E., Wishart J., Morris H., Jansen J. B., Horowitz M., Chapman I. M. (2001) Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. J. Clin. Endocrinol. Metab. 86, 5830–5837 [DOI] [PubMed] [Google Scholar]
- 15.Breit S., Kupferberg A., Rogler G., Hasler G. (2018) Vagus nerve as modulator of the brain-gut Axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez A. N., Hsu T. M., Liu C. M., Noble E. E., Cortella A. M., Nakamoto E. M., Hahn J. D., de Lartigue G., Kanoski S. E. (2018) Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 9, 2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu A. F., Zhao F. B., Wang J., Lu Y. F., Tian J., Zhao Y., Gao Y., Hu X. J., Liu X. Y., Tan J., Tian Y. L., Shi J. (2016) Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J. Transl. Med. 14, 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smucny J., Visani A., Tregellas J. R. (2015) Could vagus nerve stimulation target hippocampal hyperactivity to improve cognition in schizophrenia? Front. Psychiatry 6, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Follesa P., Biggio F., Gorini G., Caria S., Talani G., Dazzi L., Puligheddu M., Marrosu F., Biggio G. (2007) Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 1179, 28–34 [DOI] [PubMed] [Google Scholar]
- 20.Kempadoo K. A., Mosharov E. V., Choi S. J., Sulzer D., Kandel E. R. (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 113, 14835–14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi T., Duszkiewicz A. J., Sonneborn A., Spooner P. A., Yamasaki M., Watanabe M., Smith C. C., Fernández G., Deisseroth K., Greene R. W., Morris R. G. (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasaki M., Takeuchi T. (2017) Locus coeruleus and dopamine-dependent memory consolidation. Neural Plast. 2017, 8602690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi Y., Matsukura Y., Ozaki H., Nishimura K., Shindo K. (2013) Identification and quantification of the oxidation products derived from α-acids and β-acids during storage of hops (Humulus lupulus L.). J. Agric. Food Chem. 61, 3121–3130 [DOI] [PubMed] [Google Scholar]
- 24.Ano Y., Ayabe T., Kutsukake T., Ohya R., Takaichi Y., Uchida S., Yamada K., Uchida K., Takashima A., Nakayama H. (2018) Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 72, 23–31 [DOI] [PubMed] [Google Scholar]
- 25.Ayabe T., Ohya R., Kondo K., Ano Y. (2018) Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 8, 4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G., Watson C. (2006) The rat brain in stereotaxic coordinates, Academic Press, San Diego: [DOI] [PubMed] [Google Scholar]
- 27.Shinohara R., Taniguchi M., Ehrlich A. T., Yokogawa K., Deguchi Y., Cherasse Y., Lazarus M., Urade Y., Ogawa A., Kitaoka S., Sawa A., Narumiya S., Furuyashiki T. (2017) Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatry 23, 1717–1730 [DOI] [PubMed] [Google Scholar]
- 28.Franklin K., Paxinos G. (2008) The Mouse Brain in Stereotaxic Coordinates, Compact, Academic Press, San Diego [Google Scholar]
- 29.Lee K. N., Chirwa S. (2015) Blocking dopaminergic signaling soon after learning impairs memory consolidation in Guinea pigs. PLoS One 10, e0135578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang K., Broussard J. I., Levine A. T., Jenson D., Arenkiel B. R., Dani J. A. (2017) Dopamine receptor activity participates in hippocampal synaptic plasticity associated with novel object recognition. Eur. J. Neurosci. 45, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Da Silva W. C., Köhler C. C., Radiske A., Cammarota M. (2012) D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol. Learn. Mem. 97, 271–275 [DOI] [PubMed] [Google Scholar]
- 32.Chan J., Guan X., Ni Y., Luo L., Yang L., Zhang P., Zhang J., Chen Y. (2017) Dopamine D1-like receptor in lateral habenula nucleus affects contextual fear memory and long-term potentiation in hippocampal CA1 in rats. Behav. Brain Res. 321, 61–68 [DOI] [PubMed] [Google Scholar]
- 33.Li S., Cullen W. K., Anwyl R., Rowan M. J. (2003) Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat. Neurosci. 6, 526–531 [DOI] [PubMed] [Google Scholar]
- 34.Papaleonidopoulos V., Kouvaros S., Papatheodoropoulos C. (2018) Effects of endogenous and exogenous D1/D5 dopamine receptor activation on LTP in ventral and dorsal CA1 hippocampal synapses. Synapse 72, e22033 [DOI] [PubMed] [Google Scholar]
- 35.Hagena H., Manahan-Vaughan D. (2016) Dopamine D1/D5, but not D2/D3, receptor dependency of synaptic plasticity at hippocampal mossy fiber synapses that is enabled by patterned afferent stimulation, or spatial learning. Front. Synaptic Neurosci. 8, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furini C. R., Myskiw J. C., Schmidt B. E., Marcondes L. A., Izquierdo I. (2014) D1 and D5 dopamine receptors participate on the consolidation of two different memories. Behav. Brain Res. 271, 212–217 [DOI] [PubMed] [Google Scholar]
- 37.Nagai T., Takuma K., Kamei H., Ito Y., Nakamichi N., Ibi D., Nakanishi Y., Murai M., Mizoguchi H., Nabeshima T., Yamada K. (2007) Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn. Mem. 14, 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweatt J. D. (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 14, 311–317 [DOI] [PubMed] [Google Scholar]
- 39.Berman D. E., Hazvi S., Rosenblum K., Seger R., Dudai Y. (1998) Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 18, 10037–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafe G. E., Atkins C. M., Swank M. W., Bauer E. P., Sweatt J. D., LeDoux J. E. (2000) Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 20, 8177–8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blum S., Moore A. N., Adams F., Dash P. K. (1999) A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J. Neurosci. 19, 3535–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebert A. E., Dash P. K. (2002) Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn. Mem. 9, 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broadbent N. J., Gaskin S., Squire L. R., Clark R. E. (2009) Object recognition memory and the rodent hippocampus. Learn. Mem. 17, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen S. J., Stackman R. W., Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 285, 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pioli E. Y., Gaskill B. N., Gilmour G., Tricklebank M. D., Dix S. L., Bannerman D., Garner J. P. (2014) An automated maze task for assessing hippocampus-sensitive memory in mice. Behav. Brain Res. 261, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorr A. E., Debonnel G. (2006) Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 318, 890–898 [DOI] [PubMed] [Google Scholar]
- 47.Farrand A. Q., Helke K. L., Gregory R. A., Gooz M., Hinson V. K., Boger H. A. (2017) Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimul. 10, 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Pardo P., Kliest T., Dodiya H. B., Broersen L. M., Garssen J., Keshavarzian A., Kraneveld A. D. (2017) The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur. J. Pharmacol. 817, 86–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.