Abstract
Tribbles 3 (TRB3) is a pseudokinase that has been found in multiple tissues in response to various stress stimuli, such as nutrient deprivation and endoplasmic reticulum (ER) stress. We recently found that TRB3 has the potential to regulate skeletal muscle mass at the basal state. However, it has not yet been explored whether TRB3 regulates skeletal muscle mass under atrophic conditions. Here, we report that food deprivation for 48 h in mice significantly reduces muscle mass by ∼15% and increases TRB3 expression, which is associated with increased ER stress. Interestingly, inhibition of ER stress in C2C12 myotubes reduces food deprivation–induced expression of TRB3 and muscle-specific E3-ubiquitin ligases. In further in vivo experiments, muscle-specific TRB3 transgenic mice increase food deprivation–induced muscle atrophy compared with wild-type (WT) littermates presumably by the increased proteolysis. On the other hand, TRB3 knockout mice ameliorate food deprivation–induced atrophy compared with WT littermates by preserving a higher protein synthesis rate. These results indicate that TRB3 plays a pivotal role in skeletal muscle mass regulation under food deprivation–induced muscle atrophy and TRB3 could be a pharmaceutical target to prevent skeletal muscle atrophy.—Choi, R. H., McConahay, A., Silvestre, J. G., Moriscot, A. S., Carson, J. A., Koh, H.-J. TRB3 regulates skeletal muscle mass in food deprivation–induced atrophy.
Keywords: protein synthesis, protein degradation, Akt, mTOR, FOXO
Skeletal muscle is a highly malleable tissue that can transform its characteristics depending on anabolic or catabolic stimuli. Anabolic signals, including insulin or IGF-1, activate protein synthesis, leading to muscle hypertrophy, whereas catabolic stimuli such as energy imbalance and inflammation activate protein degradation, resulting in muscle atrophy. These phenomena are important for the regulation of skeletal muscle mass in order to maintain proper muscle function and strength for a population undergoing aging or diseases including obesity, type 2 diabetes, and cancer.
Muscle atrophy occurs in various pathophysiological conditions, such as starvation, inactivity, denervation, and sepsis, leading to poor quality of life among patients (1). Multiple mechanisms can induce muscle wasting, including an imbalance between the rate of protein synthesis and breakdown (2). The IGF-1/PI3K/protein kinase B (Akt) pathway has been considered as a main mechanism to manage muscle protein homeostasis (3, 4). Phosphorylation and activation of Akt provokes protein synthesis through the activation of mechanistic target of rapamycin (mTOR) and inhibits protein breakdown via the phosphorylation of forkhead box O families (FOXOs) (5). Phosphorylation of mTOR mediates a subsequent phosphorylation of ribosomal S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) to induce protein synthesis (6–8). FOXOs, another downstream target of Akt, are transcription factors that induce transcription of the genes involved in protein degradation, including muscle-specific E3 ubiquitin ligases, muscle atrophy F-box (atrogin-1) and muscle RING finger-1 (MuRF-1) (9, 10). In addition to the activation of the ubiquitin-proteasome proteolysis system by FOXOs, dephosphorylation of FOXOs has been associated with the up-regulation of autophagy-lysosomal protein degradation (11–13). These studies have established the idea that the regulation of Akt signaling is a significant target to control protein synthesis and degradation in skeletal muscle. However, the mechanism that manages the activation of Akt to regulate skeletal muscle mass has not been fully described.
Tribbles 3 (TRB3), a mammalian Drosophila tribbles homolog 3, is a pseudokinase that contains a kinase domain without catalytic function (14). Its expression has been associated with several stress conditions, such as endoplasmic reticulum (ER) stress, insulin resistance, glucose toxicity, and tumorigenesis (15–19). Functionally, TRB3 has been known as a negative regulator of Akt in metabolic tissues—including liver, fat, and skeletal muscle—through disrupting phosphorylation of Akt (16, 20). In skeletal muscle, increased TRB3 has been associated with insulin resistance. A study that utilized TRB3 knockout (KO) mice fed a high-fat diet found that the KO mice had improved glucose tolerance and insulin-stimulated Akt activation, resulting in the prevention of high-fat diet–induced obesity (16). Previously, we found that TRB3 in mouse skeletal muscle plays a critical role in the regulation of protein turnover at the basal state using both muscle-specific TRB3 transgenic (TG) and KO mice. TG mice had an inhibited protein synthesis rate confirmed by decreased phosphorylation of mTOR and S6K1 and puromycin incorporation, whereas the KO mice had an enhanced protein synthesis rate in soleus (SOL) muscle (21). TG mice also showed an increase in the protein degradation pathway, evidenced by up-regulation of atrogin-1 and MuRF-1, whereas KO mice had a decreased expression of atrogin-1 and MuRF-1 (21). Although skeletal muscle TRB3 has the potential to regulate skeletal muscle mass at basal levels, the effect of TRB3 on skeletal muscle mass regulation under atrophic conditions has not yet been defined.
Atrophic conditions, such as starvation and denervation, can prompt ER stress and the unfolded protein response (UPR) (22, 23). These processes have been associated with the regulation of skeletal muscle mass and function (24), but the mechanism is not completely understood. TRB3 interacts with activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP), which are ER stress markers, under ER stress conditions and nutrient deprivation conditions (15, 25, 26). Moreover, TRB3 has been known to respond to ER stress in skeletal muscle (16). The activation of ER stress via ER stress inducers, such as tunicamycin or thapsigargin, increases TRB3 expression (15, 16, 27). The ER stress–induced TRB3 has been related to the activation of ATF4 and CHOP, which is an important arm among 3 UPR pathways to regulate global translation (15, 25, 27, 28). Although a direct mechanism of stress-induced TRB3 expression in skeletal muscle has not been revealed, based on previous findings, it is promising that ER stress could up-regulate TRB3 expression in response to food deprivation. Therefore, in this study, we withdrew food to induce muscle atrophy. In addition to the protein turnover, we also investigated whether food deprivation–induced ER stress mediates TRB3 expression.
Here, we show that 48 h of food deprivation increased TRB3 expression in mouse skeletal muscle and blunted the phosphorylation of Akt and its downstream signaling molecules, resulting in an up-regulation of muscle-specific E3 ubiquitin ligases. Furthermore, food deprivation induced ER stress markers in vivo and in vitro. Blocking of ER stress using 4-phenylbutric acid (4-PBA) attenuated ER stress and TRB3 expression in mouse myoblast C2C12 cells after 6 h of PBS-induced starvation. TG mice had significantly higher levels of food deprivation–induced muscle atrophy along with greater activation of FOXOs and atrogenes. In contrast, the knockout of TRB3 prevented mice from food deprivation–induced atrophy, resulting from maintenance of protein synthesis rate after 48 h of food deprivation with higher activation of the protein synthesis pathway. Therefore, our study reveals that TRB3 plays an important role in food deprivation–induced skeletal muscle atrophy and may be a potential target strategy for the maintenance of skeletal muscle mass during atrophic conditions.
MATERIALS AND METHODS
Animal care and food deprivation procedure
Ten- to 13-wk-old inbred mouse strain C57BL/6 wild-type (WT), muscle-specific TG and KO mice were used for the current study. All animals were maintained in the animal facility at the University of South Carolina and were housed in an environmentally controlled room on a 12-h light/dark cycle. During the 48 h of food-deprivation experiment, standard chow diet (Envigo, Somerset, NJ, USA) was removed from the food-deprivation group with ad libitum access to water. For the fed group, mice had free access to food and water. All protocols were approved by the Institutional Animal Care and Use Committee of the University of South Carolina.
Cell culture
C2C12 myoblasts were grown in DMEM containing 10% fetal bovine serum. When the cells reached ∼90% confluence, differentiation was induced by replacing the growth medium with medium containing 2% horse serum for 4 d as previously described by Koh et al. (16). For cell-starvation experiments, myotubes were incubated in sterile PBS for 6 h. To block ER stress during cell starvation, additional myotubes were preincubated with 5 mM 4-PBA (P21005; MilliporeSigma, Burlington, MA, USA) 30 min prior to PBS starvation. Cells were then starved in PBS in the presence of 4-PBA for 6 h.
Protein extraction and Western blot analysis
Frozen tibialis anterior (TA) muscles were grinded by a homogenizer (Next Advance, Troy, NY, USA) and lysed in a buffer containing 20 mM Tris-HCl pH 7.4, 5 mM EDTA, 10 mM Na3PO4, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, and Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (Thermo Fisher Scientific, Waltham, MA, USA). To collect proteins from C2C12 myotubes, the medium from each well was removed and washed twice with ice-cold PBS. Then, the lysis buffer was added and the myotubes were scrapped by using a sterile cell scrapper. Tissue and cell lysates (30–60 mg) were separated by 15–8% SDS-PAGE and transferred to PVDF membrane. The membranes were incubated in 5% bovine serum albumin blocking buffer for 1 h, and then primary antibodies were applied for overnight incubation at 4°C. Blots were washed 3 times for 10 min each with Tris-buffered saline with Tween, and then incubated with a specific secondary antibody (1:2000–5000). Blots were visualized with chemiluminescent substrates (PerkinElmer, Waltham, MA, USA) and quantified by Image Studio Lite (Li-Cor Biosciences, Lincoln, NE, USA). All primary and secondary antibodies used in this study were commercially available, including protein kinase B (pAktT308) (9275), Akt (9271), mechanistic target of rapamycin (pmTORS2448) (2971), mTOR (2972), forkhead box O family (pFOXO1S256) (9461), FOXO1 (2880), pFOXO3aS253 (13129), FOXO3a (12829), glyceraldehyde 3-phosphate dehydrogenase (2118) from Cell Signaling Technology (Danvers, MA, USA), atrogin-1 (AP2041), MuRF-1 (MP3401) from ECM Biosciences (Versailles, KY, USA), TRB3 (ST1032; MilliporeSigma), anti-puromycin (MABE343; MilliporeSigma), horseradish peroxidase–lined anti-rabbit secondary antibody (NA934; GE Healthcare, Chicago, IL, USA), and IgG2a-horseradish peroxidase conjugate anti-mouse secondary antibody (61–0220; Thermo Fisher Scientific).
RNA extraction and real-time PCR
Total RNA was extracted from TA muscles and C2C12 myotubes using a Trizol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. cDNA was synthesized from 4 µg of total RNA with the High Capacity cDNA Kit (Thermo Fisher Scientific). cDNA and primers for real-time PCR were carried by SYBR green PCR Master Mix (Thermo Fisher Scientific). Primer sequences are as shown in Table 1. All genes were normalized to the level of TATA box-binding protein (TBP) for in vivo tissues and the level of 18S for in vitro samples.
TABLE 1.
Primer sequences for RT-PCR
| Primer sequence, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| TBP | ACCCTTCACCAATGACTCCTATG | TGACTGCAGCAAATCGCTTGG |
| TRB3 | TCTCCTCCGCAAGGAACCT | TCTCAACCAGGGATGCAAGAG |
| ATF4 | TGGCGAGTGTAAGGAGCTAGAAA | TGGCGAGTGTAAGGAGCTAGAAA |
| CHOP | CCACCACACCTGAAAGCAGAA | GGTGCCCCCAATTTCATCT |
| ATF6 | TGGGCAGGACTATGAAGTAATG | CAACGACTCAGGGATGGTGCTG |
| XBP1s | AAGAACACGCTTGGGAATGG | CTGCACCTGCTGCGGAC |
| XBP1t | GACAGAGAGTCAAACTAACGTGG | GTCCAGCAGGCAAGAAGGT |
| GRP78 | CTGGACTGAATGTCATGAGGATCA | CTCTTATCCAGGCCATATGCAATAG |
| eIF2α | ATGGAAGCCAAAGCTGAAG | CTGACATGAAGGAGGGCA |
| Atrogin-1 | GCAGAGAGTCGGCAAGTC | CAGGTCGGTGATCGTGAG |
| MuRF-1 | GGAACCTGCTGGTGGAAAACATC | CGTCTTCGTGTTCCTTGCACATC |
eIF2α, eukaryotic translation initiation factor 2α; GRP78, glucose-regulated protein 78; TBP, TATA box-binding protein.
In vivo protein synthesis measurement
The in vivo protein synthesis rate was measured with a nonradioactive technique using puromycin incorporation into a nascent peptide as previously described by Goodman et al. (29). Briefly, mice were given an intraperitoneal injection of 0.04 µmol/g puromycin (MilliporeSigma) dissolved in 100 µl of PBS 30 min before euthanasia. Thirty minutes after the injection, tissues were collected and frozen immediately in liquid nitrogen and then stored at –80°C until the analysis. Details of puromycin immunoblotting can be found in the Protein Extraction and Western Blot Analysis sections.
Statistical analysis
All results are presented as means ± sem. Statistical analysis was performed using Prism 7 (GraphPad, La Jolla, CA, USA). Two-tailed, unpaired Student’s t tests were used for comparisons between 2 groups. One- or 2-way ANOVA was conducted to determine significance between more than 2 group followed by Tukey’s or Sidak post hoc analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
Food deprivation for 48 h increases TRB3 expression in mouse skeletal muscle
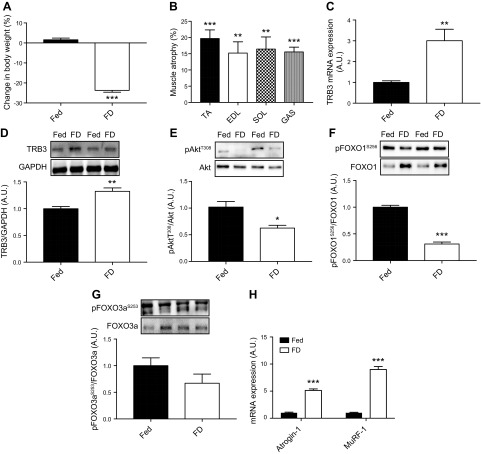
To determine the role of TRB3 in food deprivation–induced muscle atrophy, we first determined TRB3 expression in response to 48 h of food deprivation. C57BL/6 WT mice were divided into 2 groups. One group freely consumed standard chow diet (the fed group), whereas the other group was deprived of food for 48 h. Water was accessible ad libitum for both groups. After 48 h, body weight was measured before euthanasia. Mice that had undergone 48 h of food deprivation had significantly decreased body weight more than 20% compared with fed controls (Fig. 1A). Consistently, multiple skeletal muscles, including TA, extensor digitorum longus (EDL), SOL, and gastrocnemius (GAS), from the food-deprivation group showed a 15% decrease in muscle weight compared with those from the fed group (Fig. 1B). There was no significant difference in the percentage of muscle atrophy among 4 muscles after 48 h of food deprivation. Our method, food deprivation for 48 h, was sufficient to induce skeletal muscle atrophy in mice. Interestingly, 48 h of food deprivation significantly increased TRB3 mRNA and protein expression in mouse skeletal muscle by 300 and 30%, respectively (Fig. 1C, D). These data demonstrate that food deprivation for 48 h induces skeletal muscle atrophy and increases TRB3 expression in mouse skeletal muscle.
Figure 1.
Food deprivation increases TRB3 expression and decreases Akt and FOXO phosphorylation. Ten- to 13-wk-old female C57BL/6 WT mice underwent either free access to food (Fed) or food deprivation (FD) for 48 h. Body weight was measured before and after the procedure. A) Percentage difference in body weight before and after 48 h of each treatment was calculated. B) Percentage of skeletal muscle atrophy after 48 h of food deprivation was determined in TA, EDL, SOL, and GAS. C, D) TRB3 mRNA (C) and protein expression (D) were determined in TA muscles. E–G) Phosphorylation and total expression of Akt (E), FOXO1 (F), and FOXO3a (G) were determined by Western blot analysis. H) mRNA expression of muscle-specific E3 ubiquitin ligases was determined by RT-PCR. A.U., arbitrary unit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; pAktT308, protein kinase B; pFOXO1S256, forkhead box O family 1; pFOXO3aS253, forkhead box O family 3a. Data are the means ± sem (n = 4/group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Fed.
Food deprivation for 48 h inhibits Akt phosphorylation and activates protein degradation
Muscle wasting is regulated by the balance between protein synthesis and protein degradation (2). The IGF-1/PI3K/Akt pathway is critical for the control of protein turnover through mTOR and FOXOs signaling (3, 4). Because our method was sufficient to decrease muscle mass (Fig. 1B), we next examined whether Akt and FOXOs are involved in food deprivation–induced skeletal muscle atrophy. After 48 h of food deprivation, phosphorylation of Akt at T308 in the food-deprivation group was significantly decreased compared with the fed group (Fig. 1E). Phosphorylation of Akt at S473 did not respond to 48 h of food deprivation (Supplemental Fig. S1A). Further, phosphorylation of FOXO1 and FOXO3a was dramatically decreased in the food-deprivation group, suggesting the activation of FOXOs (Fig. 1F, G). Dephosphorylated FOXOs are required for up-regulation of atrogin-1 and MuRF-1, skeletal muscle-specific E3 ubiquitin ligases (9, 10, 30). In line with this, we also found that 48 h of food deprivation significantly increased atrogin-1 and MuRF-1 mRNA expression in mouse skeletal muscle (Fig. 1H). Together, these results demonstrate that 48 h of food deprivation inhibits Akt signaling but activates FOXOs and muscle-specific E3 ubiquitin ligases, which may be responsible for 48 h of food deprivation–induced skeletal muscle atrophy.
Food deprivation induces ER stress in vivo and in vitro and ER stress meditates food deprivation–induced TRB3 expression
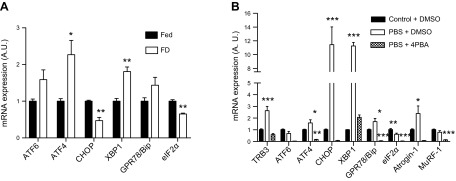
It has been previously determined that TRB3 interacts with ATF4 and CHOP, which are ER stress markers and one of the UPR pathways, and coordinates ATF4-related apoptosis during ER stress (15, 25, 26). Therefore, we next hypothesized that food deprivation–induced TRB3 expression is mediated by increased ER stress. We first analyzed ER stress markers in skeletal muscles from food-deprived WT mice. The expression of ATF4 and X-box binding protein 1 (XBP1) was significantly increased in skeletal muscles from the food-deprivation group (Fig. 2A). The mRNA expression of ATF6 and glucose-regulated protein 78 tended to be higher in response to food deprivation (Fig. 2A). To further determine whether ER stress triggers the up-regulation of TRB3 in response to food deprivation, we incubated C2C12 myotubes with PBS, which can mimic food deprivation–induced muscle atrophy (9, 22). After 6 h of PBS incubation, the expression of ER stress markers, including ATF4, CHOP, XBP1, glucose-regulated protein 78 and binding Ig protein, and atrogin-1 was robustly elevated (Fig. 2B). Interestingly, TRB3 mRNA expression was also increased by more than 2.6-fold (Fig. 2B). To test whether ER stress is required to increase TRB3 expression in response to food deprivation, C2C12 myotubes were treated with 4-PBA, an ER stress blocker, and TRB3 and ER stress markers were determined. The 4-PBA treatment significantly blunted ER stress responses in starved cells (Fig. 2B). Interestingly, PBS incubated C2C12 myotubes with 4-PBA treatment had significantly reduced expression of TRB3 and muscle-specific E3 ubiquitin ligases (Fig. 2B). These data suggest that ER stress is necessary to induce TRB3 expression in response to food deprivation, and TRB3 may play a regulatory role in the expression of atrogenes.
Figure 2.
ER stress is responsible for food deprivation–induced TRB3 expression. A) mRNA expression of multiple ER stress markers was determined in TA muscles by RT-PCR after 48 h starvation. B) mRNA expression of several ER stress markers was analyzed in C2C12 cells after 6 h PBS or 4-PBA treatment. FD, food deprivation group; Fed, group with free access to food; A.U., arbitrary unit; GRP78/Bip, glucose-regulated protein 78 and binding Ig protein; eIF2α, eukaryotic translation initiation factor 2α. Data are the means ± sem (n = 4/group for in vivo, n = 9/group for in vitro). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Fed or control + DMSO.
Overexpression of TRB3 aggravates food deprivation–induced skeletal muscle atrophy
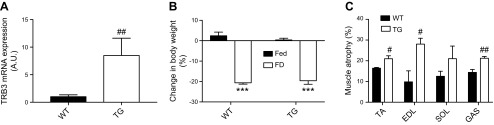
To further determine the effect of TRB3 in skeletal muscle atrophy, we used a TG mouse model (Fig. 3A). In our previous studies, we found that TG mice displayed smaller muscle weight and cross-sectional area with suppressed protein synthesis and accelerated protein degradation compared with WT littermates at the basal state (21). Therefore, we hypothesized that TG mice are more prone to food deprivation–induced atrophy compared with WT. WT and TG mice were deprived of food for 48 h and body weight was measured. Forty-eight hours of food deprivation significantly decreased body weight in both WT and TG mice and there was no difference between genotypes (Fig. 3B). Interestingly, TG mice had markedly increased muscle atrophy by ∼22% in various muscles compared with WT (Fig. 3C). These findings suggest that overexpression of TRB3 in skeletal muscle exacerbates skeletal muscle atrophy in response to 48 h of food deprivation.
Figure 3.
Muscle-specific TRB3 overexpression increases food deprivation–induced skeletal muscle atrophy. Ten- to 13-wk-old female TG mice and their WT littermate control mice were used to determine the role of TRB3 in skeletal muscle mass regulation under food deprivation–induced atrophy conditions. A) TRB3 overexpression was confirmed by RT-PCR with increased TRB3 mRNA expression in TA muscles. TG and WT mice were divided into groups with free access to food (Fed) and groups deprived of food for 48 h (FD). B) Percentage change in body weight between Fed and FD groups in both WT and TG mice was determined. C) Percentage of food deprivation–induced atrophy was determined in TA, EDL, SOL, and GAS muscles. A.U., arbitrary unit. Data are the means ± sem (n = 6/group). ***P < 0.001 vs. Fed, #P < 0.05, ##P < 0.01 vs. WT.
TRB3 overexpression activates FOXOs and ubiquitin-proteasome proteolysis
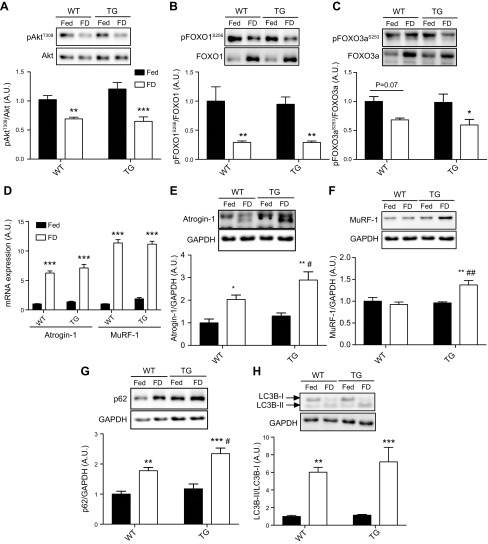
Based on our data indicating that overexpression of TRB3 aggravated food deprivation–induced muscle atrophy (Fig. 3C), we next investigated whether muscle-specific TRB3 overexpression may influence the Akt-FOXOs-ubiquitin-proteasome protein degradation pathway. The phosphorylation of Akt (T308) and subsequently FOXO1 (S256) phosphorylation was significantly diminished after 48 h of food deprivation (Fig. 4A, B), whereas Akt phosphorylation at S473 was not altered (Supplemental Fig. S1B). Additionally, there was a trend for activation and dephosphorylation of FOXO3a in WT after 48 h of food deprivation, whereas TG mice had significantly activated FOXO3a (Fig. 4C). In agreement with increased FOXOs activation, atrogin-1 and MuRF-1 mRNA expression was highly elevated independently of genotype (Fig. 4D). In protein expression, however, TG mice displayed a greater increase in atrogin-1 and MuRF-1 after 48 h of food deprivation compared with WT (Fig. 4E, F). These data suggest that TRB3 may accelerate the ubiquitin-proteasome proteolysis system.
Figure 4.
TRB3 overexpression in skeletal muscle facilitates the protein degradation pathways, including ubiquitin-proteasomal and autophagy-lysosomal systems after 48 h of food deprivation. TA muscles from groups with free access to food (Fed) and food-deprived groups (FD) in both genotypes were used for analysis. A–C) The phosphorylation of Akt (A), FOXO1 (B), and FOXO3a (C) were determined by Western blot analysis. D) mRNA expression of atrogenes was measured by RT-PCR in both genotypes. E, F) Protein expression of atrogin-1 (E) and MuRF-1 (F) was determined by Western blot analysis. G, H) The markers of autophagy-lysosomal protein degradation, including p62 (G) and LC3B (H), were analyzed after 48 h of food deprivation. A.U., arbitrary unit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; pFOXO1S256, forkhead box O family 1; pFOXO3aS253, forkhead box O family 3a. Data are the means ± sem (n = / group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Fed. #P < 0.05, ##P < 0.01 vs. WT.
It has been well established that autophagy plays an important role in skeletal muscle mass regulation under atrophic conditions (11, 31). To determine the effect of TRB3 on food deprivation–induced autophagy, we measured autophagic markers, including polyubiquitin-binding protein (p62) and microtubule-associated protein 1 light chain-3B (LC3B), which have been previously reported to increase in skeletal muscle in response to food deprivation (11, 22). Recently, Hua et al. (32) demonstrated that TRB3 interacts with p62 to induce an accumulation in the human branchial epithelial cell line. In our current study, 48 h of food deprivation greatly increased p62 in both genotypes, but TG mice displayed a further increase in p62 protein expression (Fig. 4G). In addition to p62, conversion of LC3B-I to LC3B-II is a critical marker of autophagosome formation (33). We measured LC3B by Western blot and found that 48 h of food withdrawal dramatically increased LC3B conversion in both genotypes with a trend to be higher in TG mice (Fig. 4H). These results suggest that TRB3 plays a role in the up-regulation of food deprivation–induced autophagy.
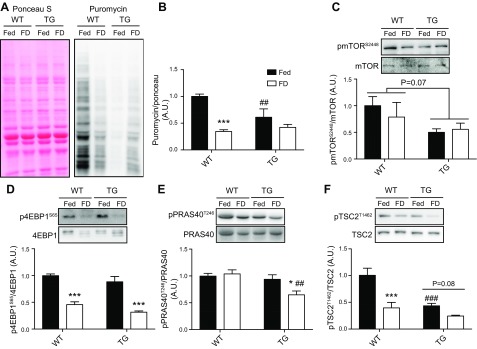
Muscle-specific TRB3 overexpression does not augment the effect of food deprivation on protein synthesis rate and signaling pathway
Loss of muscle mass is stimulated by not only increased protein degradation but also decreased protein synthesis (2). Our previous study has reported that TRB3 overexpression in mouse skeletal muscle results in a decrease in protein synthesis rate at the basal condition (21). In our current study, we observed that TRB3 overexpression in mouse skeletal muscle promoted atrogin-1 and MuRF-1 expression after 48 h of food deprivation compared with WT (Fig. 4E, F). Therefore, we further determined whether TRB3 overexpression would have an effect on Akt-downstream molecules related to protein synthesis under atrophic conditions. Forty-eight hours of food deprivation decreased the protein synthesis rate by 60–65% in WT (Fig. 5A, B). Consistent with our previous study (21), overexpression of TRB3 decreased protein synthesis rate at fed state (Fig. 5A, B). Although TG mice showed significantly suppressed protein synthesis rate in fed state, TG mice had no further effect on protein synthesis rate in response to 48 h of food deprivation (Fig. 5B). There was no significant difference in protein synthesis rate between genotypes after 48 h of food deprivation. Next, we determined the effect of TRB3 on signaling molecules related to protein synthesis. Forty-eight hours of food withdrawal did not affect mTOR phosphorylation in either genotype (Fig. 5C). Although a statistical significance was not found, phosphorylation of mTOR in TG mice at the basal state was 50% less than WT (Fig. 5C). To understand what factors may influence the decreased protein synthesis rate in TG mice, we examined the proteins upstream and downstream of mTOR, including the proline-rich Akt substrate of 40 kDa (PRAS40), tuberous sclerosis complex 2 (TSC2), and 4EBP1. Forty-eight hours of food deprivation resulted in a significant increase in dephosphorylation of 4EBP1 (S65) in both genotypes, suggesting diminished initiation of protein synthesis (Fig. 5D). It is known that Akt regulates mTOR activity by phosphorylating PRAS40 and TSC2 (34, 35). Therefore, we tested both signaling molecules to evaluate whether TRB3 affects them to regulate mTOR activity. Phosphorylated PRAS40 (T246) was significantly decreased only in TG mice after 48 h of food deprivation (Fig. 5E). Phosphorylation of TSC2 (T1462) was significantly decreased in TG mice at fed state like mTOR (Fig. 5F). Forty-eight hours of food deprivation decreased TSC2 phosphorylation in both WT and TG mice, and there was no difference in TSC2 phosphorylation between genotypes (Fig. 5F). These data support the result that TG mice showed the decreased protein synthesis rate under fed condition but did not augment the rate in response to 48 h of food deprivation. Taken together, these findings demonstrate that the worsening food deprivation–induced skeletal muscle atrophy in TG mice is not due to the change in protein synthesis rate.
Figure 5.
Muscle-specific TRB3 overexpression does not augment the effect of food deprivation on protein synthesis rate and signaling pathway. TA muscles from groups with access to food (Fed) and food-deprived (FD) TG and WT littermate control mice were processed for analysis. A) Western blot with antipuromycin antibody was used to analyze puromycin incorporation into nascent peptides in WT and TG mice. Ponceau S staining was used to demonstrate an equal loading of protein. Methodology details can be found in Materials and Methods. B) The amount of puromycin incorporation was normalized by ponceau S staining. C–F) The phosphorylation and total expression of mTOR (C), 4EBP1 (D), PRAS40 (E), and TSC2 (F) were measured by Western blot analysis. A.U., arbitrary unit; p4EBP1S65, eukaryotic translation initiation factor 4E-binding protein 1; pmTORS2448, mechanistic target of rapamycin; PRAS40T246, proline-rich Akt substrate of 40 kDa; pTSC2T1462, tuberous sclerosis complex 2. Data are the means ± sem (n = 6–9/group). *P < 0.05, ***P < 0.001 vs. Fed. ##P < 0.01 vs. WT, ###P < 0.001 vs. WT.
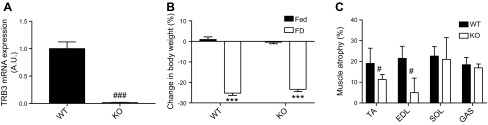
TRB3 knockout prevents 48 h of food deprivation–induced muscle atrophy
We found that TRB3 overexpression in mouse skeletal muscle notably decreased muscle mass in response to 48 h of food deprivation (Fig. 3C). This augmented muscle atrophy in TG mice could result more likely from an increased expression of muscle-specific E3 ubiquitin ligases (Fig. 4E, F) but less likely from protein synthesis rate and activity of signaling molecules (Fig. 5). To determine whether TRB3 has the potential to be a pharmaceutical target to ameliorate skeletal muscle wasting, we next examined KO mice. With the 48 h of food deprivation, we first confirmed that KO mice expressed significantly lower amounts of TRB3 in TA muscle compared with WT littermates (Fig. 6A). Next, WT and KO mice underwent 48 h of food deprivation and displayed reduced body weight more than 20% after food withdrawal (Fig. 6B). Intriguingly, we observed a smaller degree of muscle wasting in some muscles from KO mice compared with WT (Fig. 6C). These findings indicate that the knockout of TRB3 in skeletal muscle prevents food deprivation–induced muscle atrophy.
Figure 6.
KO mice were protected from food deprivation–induced atrophy. Ten- to 13-wk-old female KO and WT littermate mice were used to demonstrate whether TRB3 is a key molecule to induce food deprivation–induced atrophy. A) TRB3 mRNA expression in TA muscle was determined to confirm the knockout of TRB3. KO and WT mice were divided into Fed and FD groups. B) Percentage change in body weight between groups with access to food (Fed) and food-deprived groups (FD) in both WT and KO mice was determined. C) Percentage of food deprivation–induced atrophy was determined in TA, EDL, SOL, and GAS muscles. A.U., arbitrary unit. Data are the means ± sem (n = 4–6/group). ***P < 0.001 vs. Fed, #P < 0.05, ###P < 0.001 vs. WT.
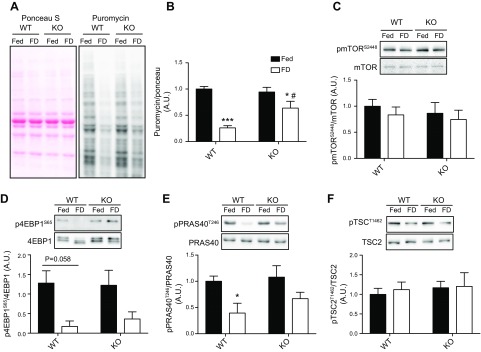
TRB3 knockout blunts a reduction of protein synthesis after 48 h of food deprivation
The preventive effect of TRB3 knockout on food deprivation–induced muscle loss may be associated with increased protein synthesis based on our previous report (21). To examine the mechanisms by which knockout of TRB3 prevented food deprivation–induced muscle atrophy, we measured the protein synthesis rate and signaling pathway in TA muscle. As previously shown, protein synthesis rate was dramatically reduced after 48 h of food withdrawal in both WT and KO mice (Fig. 7A, B). However, the knockout of TRB3 ameliorated the food deprivation–induced reduction in the protein synthesis rate (Fig. 7B). This phenomenon could promote maintenance of muscle mass in KO mice and protect the KO mice from food deprivation–induced atrophy compared with WT. Next, we examined signaling molecules involved in protein synthesis, including mTOR, 4EBP1, PRAS40, and TSC2. However, there were no significant differences between genotypes (Fig. 7C–F). The same results were observed in EDL muscle (Supplemental Figs. S2 and S3). This may be because of a flaw in our methodology. We may not be able to capture the correct time point for detection of alterations in signaling pathways because we euthanize mice 30 min after puromycin injection. On the basis of the increased protein synthesis rate in KO mice, there is the possibility that the protein synthesis pathway may be affected by genotype. These data suggest that increased muscle mass in KO mice could be due to preserving protein synthesis rate.
Figure 7.
TRB3 knockout maintains protein synthesis rate after 48 h of food deprivation. Frozen TA muscles were processed for analysis. A) Protein synthesis rate was measured by Western blot, and ponceau S staining revealed that an equal amount of proteins was loaded for each sample. B) All puromycin blots were normalized by ponceau S staining. C–F) Protein synthesis pathway factors, including mTOR, 4EBP1, PRAS40, and TSC2, were analyzed by Western blot. FD, food deprivation group; Fed, group with free access to food; A.U., arbitrary unit; p4EBP1S65, eukaryotic translation initiation factor 4E-binding protein 1; pmTORS2448, mechanistic target of rapamycin pPRAS40T246, proline-rich Akt substrate of 40 kDa; pTSCT1462, tuberous sclerosis complex 2. Data are the means ± sem (n = 6/group). *P < 0.05, ***P < 0.001 vs. Fed, #P < 0.05 vs. WT.
DISCUSSION
TRB3 has been known as a negative regulator of the insulin signaling pathway focused on Akt phosphorylation (20, 36). Its expression is involved in pathophysiology of multiple diseases, including diabetes and obesity, resulting in insulin resistance (16, 18). Recently, we demonstrated that the overexpression of TRB3 in mouse skeletal muscle decreases protein synthesis and increases protein degradation through the disruption of Akt and its downstream molecules at the basal state (21). However, it has not yet been elucidated whether TRB3 affects skeletal muscle mass regulation under atrophic conditions. Therefore, in this study we examined the role of TRB3 in the regulation of skeletal muscle mass under 48 h of food deprivation–induced skeletal muscle atrophy.
This study demonstrated that TRB3 mRNA and protein expressions were significantly elevated in mouse skeletal muscle after 48 h of food deprivation (Fig. 1C, D). Previously, it has been established that food deprivation for 24 h can induce TRB3 expression in liver and fat (20, 37). Our report is the first showing that 48 h of food deprivation is sufficient to induce TRB3 expression in mouse skeletal muscle (Fig. 1C, D). In addition, our study and others demonstrated that an incremental increase of TRB3 expression in multiple tissues impairs the insulin-stimulated Akt (16, 38). Here, we showed that 48 h of food deprivation significantly reduced skeletal muscle mass along with a decrease in phosphorylation of Akt and FOXOs, leading to up-regulation of atrogin-1 and MuRF-1 (Fig. 1B, E–H). Taken together, food deprivation–induced TRB3 expression in mouse skeletal muscle may decrease Akt phosphorylation and activate FOXOs and muscle-specific E3 ubiquitin ligases.
A significant reduction in the phosphorylation of Akt in response to food withdrawal (Fig. 2A) has been observed in other studies (9, 22). Blunted Akt phosphorylation subsequently leads to the dephosphorylation of FOXO1 and 3a, which translocate to the nucleus to promote the transcriptions of protein degradation genes, such as atrogin-1 and MuRF-1 (4, 9). In line with this, we observed that 48 h of food deprivation significantly reduced the phosphorylation of FOXO1, but not of FOXO3a (Fig. 1F, G), leading to increased muscle-specific E3 ubiquitin ligases (Fig. 1H). This phenomenon has previously been observed by Ogata et al. (31), and there has been evidence that different isoforms of FOXOs can coordinately assist their activation (9).
Our finding that ER stress mediates TRB3 and atrogenes expression in response to starvation suggests that ER stress is an important regulator of TRB3 expression in skeletal muscle (Fig. 2B). Although earlier studies have shown a tight relationship between ER stress and TRB3 in regulatory mechanisms, it has not been determined in skeletal muscle and food deprivation conditions. For example, tunicamycin-treated HepG2 cells have an increase in TRB3 expression following an elevation of ATF4 and CHOP (15), whereas ER stress–induced TRB3 expression is completely blocked in ATF4 knockdown mouse embryonic fibroblasts (25). Whereas these studies suggest that ER stress may play a critical role in TRB3 expression, our results indicate that ER stress may be required to induce TRB3 expression in skeletal muscle under food-deprivation conditions. Furthermore, our data suggest that food deprivation–induced atrophy may be mediated by ER stress–induced TRB3 expression. In the current experiment, however, we could not determine whether TRB3 is required for food deprivation–induced or ER stress–mediated atrophy. To examine a precise mechanism, it will be necessary to utilize TRB3 knockdown in C2C12 cells or isolated primary cells from skeletal muscle of KO mice to demonstrate whether food deprivation–induced atrophy is prevented in the absence of TRB3. These experiments will be performed in our future studies.
In this study, we demonstrated that muscle-specific TRB3 overexpression resulted in increased 48 h of food deprivation–induced muscle atrophy compared with WT (Fig. 3C). Previously, we have reported that TG mice present smaller muscle weights and a reduced rate of protein synthesis compared with WT at the basal condition (21). Our previous and current studies consistently demonstrate that muscle-specific TRB3 overexpression significantly increased atrogin-1 and MuRF-1 at basal and under food deprivation–induced atrophic conditions (Fig. 4E, F) (21). In contrast, An et al. (39) reported that the same mice display larger muscle mass in SOL and GAS compared with WT littermates. This may be due to differences in the number of TG mice backcrossing and diet as previously reported (21). Although the reason for the discrepancy is not clear, our current results were consistent with our previous study. Therefore, based on our findings, TRB3 may have the potential to be a critical regulator of skeletal muscle mass at basal and also under food deprivation–induced atrophy conditions.
Given that TRB3 impairs Akt signaling in skeletal muscle and other tissues (16, 20, 40), we anticipated the decreased Akt phosphorylation in TG mice after 48 h of food deprivation. However, we did not observe any differences in Akt phosphorylation between WT and TG mice. The discrepancy is likely due to the different experimental settings. Our previous study and others have examined Akt phosphorylation after insulin stimulation (16, 20, 40). In our current study, we measured the basal level of Akt phosphorylation in order to precisely determine the role of TRB3 in food deprivation–induced skeletal muscle atrophy because insulin can initiate anabolic signaling pathways to alter protein turnover (2). Although we did not detect differences in Akt phosphorylation between genotypes, our data demonstrate that TRB3 overexpression in skeletal muscle could affect Akt-downstream proteins associated with protein turnover. For future studies, it will be important to determine Akt phosphorylation in insulin-stimulated conditions. In addition, more sensitive methodology to detect definite changes in Akt activity will be necessary, such as Akt kinase assays.
In addition to up-regulating the ubiquitin-proteasome system in response to food withdrawal, autophagy-lysosomal protein degradation is activated under atrophic conditions (11, 22). In this study, TG mice displayed significantly increased p62 accumulation compared with WT (Fig. 4G). Recently, it has been reported that the overexpression of TRB3 in human bronchial epithelial cells and diabetic mice results in the accumulation of p62 (32). Although the current and previous findings have been confirmed in different tissues and experimental conditions, TRB3 expression seems to play an essential role in the autophagic process. The activation of FOXOs has been considered as an important regulator of not only ubiquitin-proteasome proteolysis (9, 10) but also autophagy-lysosomal protein degradation (11–13). However, TG mice did not show any differences in FOXO1 and FOXO3a activation in response to food deprivation compared with WT (Fig. 4B, C). These results represent only a small piece of the process. Although we could not find any differences in the activation of FOXOs between WT and TG mice in response to food deprivation, we cannot fully exclude the possibility that the overexpression of TRB3 may affect the activation of FOXOs to induce ubiquitin-proteasome and autophagy-lysosomal protein degradations.
Along with protein degradation, skeletal muscle mass is also regulated by protein synthesis. Food deprivation for 48 h diminished protein synthesis rate by more than 60% in WT mice (Fig. 5A, B). In accordance with our previous study (21), TG mice showed significantly suppressed protein synthesis rate at fed state (Fig. 5B). However, we did not detect further reduction in protein synthesis rate in TG mice after 48 h of food deprivation. This may be because the level of protein synthesis was already decreased in TG mice in fed state (60% of fed WT mice), and no further decrease could be made by 48 h of food deprivation. To determine how TRB3 overexpression hinders protein synthesis, we further investigated the expression of signaling molecules related to mTOR regulation, such as 4EBP1, PRAS40, and TSC2. TRB3 overexpression in mouse skeletal muscle significantly reduced the phosphorylation of PRAS40 and TSC2 during food deprivation and at fed conditions, respectively (Fig. 5E, F). Both PRAS40 and TSC2 are known to be negative regulators of mTOR and are inhibited by Akt phosphorylation (34, 35). In line with our results, TRB3 overexpression in primary mouse hepatocytes has been shown to down-regulate the Akt-TSC2-mTOR pathway and activate 4EBP1, resulting in an inhibition of nutrient- and insulin-induced S6K1 activity (41). These findings suggest that TRB3 plays a critical role in the regulation of not only the Akt but also Akt-associated proteins, including PRAS40 and TSC2. Taken together, our data indicate that the worsening skeletal muscle atrophy in response to 48 h of food deprivation may be because of the increased proteolysis pathways rather than changing protein synthesis rate.
We utilized our KO mouse model to determine the role of TRB3 in skeletal muscle mass regulation under atrophic conditions. We collected multiple hindlimb muscles and measured their weights to study whether there were variations of food deprivation–induced muscle atrophy in a muscle fiber type–specific manner. In contrast to TG mice, the knockout of TRB3 protected mice from food deprivation–induced muscle atrophy, especially in TA and EDL (Fig. 6C). This variation may be caused by various compositions of muscle fiber type in specific muscles. For instance, TA and EDL contain more fast-glycolytic type IIb fiber than SOL, which mainly contains slow-oxidative type I and fast-oxidative type IIa (42). In addition, it has been revealed that food deprivation could preferentially affect protein turnover in fast-glycolytic fibers (43, 44). Based on our previous study, KO mice have greater muscle mass and higher protein synthesis rate compared with WT mice (21). Therefore, we measured the protein synthesis rate in the skeletal muscle from food-deprived mice to examine whether KO mice can maintain a higher protein synthesis rate. Puromycin incorporation was highly preserved in KO mice compared with WT after 48 h of food deprivation (Fig. 7B). This may support the result that food deprivation–induced muscle atrophy was inhibited in KO mice. However, in this study, we did not find any differences in the expression of signaling molecules related to protein synthesis pathways (Fig. 7C–F). Furthermore, there were no differences in protein degradation pathways, including FOXO1 and 3a, and atrogenes expression (data not shown). It is possible that changes in signaling activities at the molecular level may have already occurred prior to euthanization, which occurred 30 min after puromycin injection. In addition, unlike TG mice, the KO mice were a global knockout, meaning that TRB3 expression is down-regulated in a whole-body manner. Therefore, it is difficult to observe muscle-specific roles of TRB3 in the regulation of skeletal muscle mass under food-deprivation conditions. Nonetheless, our data suggest a possibility that TRB3 has the potential to be a therapeutic target for skeletal muscle atrophy.
In summary, 48 h of food deprivation induced the expression of TRB3 in skeletal muscle and resulted in muscle atrophy. ER stress was required to induce TRB3 expression in response to food deprivation. Overexpression of TRB3 in mouse skeletal muscle increased food deprivation–induced atrophy via activating ubiquitin-proteasomal and autophagy-lysosomal protein degradation. KO mice were protected from food deprivation–induced atrophy via the maintenance of proteins synthesis rate. We conclude that TRB3 plays a pivotal role in food deprivation–induced skeletal muscle atrophy. Our data also suggest that TRB3 may be a potential target for maintaining skeletal muscle mass under food deprivation–induced atrophic condition.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank M. Montminy (Salk Institute, La Jolla, CA, USA) for providing the muscle-specific TRB3 TG mice and Regeneron (Tarrytown, NY, USA) for KO mice. This work was supported by U.S. National Institues of Health grants to H.-J.K. [R03AR066825 (National Institute of Arthritis and Musculoskeletal and Skin Diseases) and P20GM10909 (National Institute of General Medical Sciences)], and a departmental start-up grant to H.-J.K. The authors declare no conflicts of interest.
Glossary
- 4EBP1
eukaryotic translation initiation factor 4E-binding protein 1
- 4-PBA
4-phenylbutric acid
- Akt
protein kinase B
- ATF4
activating transcription factor 4
- atrogin-1
muscle atrophy F-box
- CHOP
C/EBP homologous protein
- EDL
extensor digitorum longus
- ER
endoplasmic reticulum
- FOXO
forkhead box O family
- GAS
gastrocnemius
- KO
whole-body TRB3 knockout
- LC3B
microtubule-associated protein 1 light chain-3B
- mTOR
mechanistic target of rapamycin
- MuRF-1
muscle RING finger-1
- p62
polyubiquitin-binding protein
- PRAS40
proline-rich Akt substrate of 40 kDa
- S6K1
ribosomal S6 kinase 1
- SOL
soleus
- TA
tibialis anterior
- TG
muscle-specific TRB3 transgenic
- TRB3
Tribbles 3
- TSC2
tuberous sclerosis complex 2
- UPR
unfolded protein response
- WT
wild type
- XBP1
X-box binding protein 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. H. Choi designed the research, performed the experiments, analyzed the data, and wrote and revised the manuscript; A. McConahay performed the experiments and edited the manuscript; J. G. Silvestre performed the experiments and edited the manuscript; A. S. Moriscot provided feedback on the experiments and edited the manuscript; J. A. Carson designed the experiments and edited the manuscript; and H.-J. Koh designed the research, analyzed the data, and wrote and edited the manuscript.
REFERENCES
- 1.Cohen S., Nathan J. A., Goldberg A. L. (2015) Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 [DOI] [PubMed] [Google Scholar]
- 2.Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314 [DOI] [PubMed] [Google Scholar]
- 3.Latres E., Amini A. R., Amini A. A., Griffiths J., Martin F. J., Wei Y., Lin H. C., Yancopoulos G. D., Glass D. J. (2005) Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 280, 2737–2744 [DOI] [PubMed] [Google Scholar]
- 4.Stitt T. N., Drujan D., Clarke B. A., Panaro F., Timofeyva Y., Kline W. O., Gonzalez M., Yancopoulos G. D., Glass D. J. (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 [DOI] [PubMed] [Google Scholar]
- 5.Glass D. J. (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 37, 1974–1984 [DOI] [PubMed] [Google Scholar]
- 6.Rommel C., Bodine S. C., Clarke B. A., Rossman R., Nunez L., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 7.Bodine S. C., Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J. C., Glass D. J., Yancopoulos G. D. (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 8.Gingras A. C., Kennedy S. G., O’Leary M. A., Sonenberg N., Hay N. (1998) 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S. H., Goldberg A. L. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waddell D. S., Baehr L. M., van den Brandt J., Johnsen S. A., Reichardt H. M., Furlow J. D., Bodine S. C. (2008) The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am. J. Physiol. Endocrinol. Metab. 295, E785–E797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S. J., Di Lisi R., Sandri C., Zhao J., Goldberg A. L., Schiaffino S., Sandri M. (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6, 458–471 [DOI] [PubMed] [Google Scholar]
- 12.Zhao J., Brault J. J., Schild A., Cao P., Sandri M., Schiaffino S., Lecker S. H., Goldberg A. L. (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483 [DOI] [PubMed] [Google Scholar]
- 13.Milan G., Romanello V., Pescatore F., Armani A., Paik J. H., Frasson L., Seydel A., Zhao J., Abraham R., Goldberg A. L., Blaauw B., DePinho R. A., Sandri M. (2015) Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 6, 6670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosshans J., Wieschaus E. (2000) A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101, 523–531 [DOI] [PubMed] [Google Scholar]
- 15.Ohoka N., Yoshii S., Hattori T., Onozaki K., Hayashi H. (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 24, 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh H. J., Toyoda T., Didesch M. M., Lee M. Y., Sleeman M. W., Kulkarni R. N., Musi N., Hirshman M. F., Goodyear L. J. (2013) Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nat. Commun. 4, 1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Wu X., Franklin J. L., Messina J. L., Hill H. S., Moellering D. R., Walton R. G., Martin M., Garvey W. T. (2010) Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 298, E565–E576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Liu J., Tian L., Liu Q., Fu Y., Garvey W. T. (2013) TRIB3 mediates glucose-induced insulin resistance via a mechanism that requires the hexosamine biosynthetic pathway. Diabetes 62, 4192–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Lv S., Qin Y., Shu F., Xu Y., Chen J., Xu B. E., Sun X., Wu J. (2007) TRB3 interacts with CtIP and is overexpressed in certain cancers. Biochim. Biophys. Acta 1770, 273–278 [DOI] [PubMed] [Google Scholar]
- 20.Du K., Herzig S., Kulkarni R. N., Montminy M. (2003) TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 21.Choi R. H., McConahay A., Jeong H. W., McClellan J. L., Hardee J. P., Carson J. A., Hirshman M. F., Goodyear L. J., Koh H. J. (2017) Tribbles 3 regulates protein turnover in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 493, 1236–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul P. K., Bhatnagar S., Mishra V., Srivastava S., Darnay B. G., Choi Y., Kumar A. (2012) The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z., Wang A. M., Adachi H., Katsuno M., Sobue G., Yue Z., Robins D. M., Lieberman A. P. (2011) Macroautophagy is regulated by the UPR-mediator CHOP and accentuates the phenotype of SBMA mice. PLoS Genet. 7, e1002321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnert K. R., McMillan J. D., Kumar A. (2018) Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell. Physiol. 233, 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jousse C., Deval C., Maurin A. C., Parry L., Chérasse Y., Chaveroux C., Lefloch R., Lenormand P., Bruhat A., Fafournoux P. (2007) TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J. Biol. Chem. 282, 15851–15861 [DOI] [PubMed] [Google Scholar]
- 26.Ord D., Ord T. (2003) Mouse NIPK interacts with ATF4 and affects its transcriptional activity. Exp. Cell Res. 286, 308–320 [DOI] [PubMed] [Google Scholar]
- 27.Ord D., Ord T. (2005) Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem. Biophys. Res. Commun. 330, 210–218 [DOI] [PubMed] [Google Scholar]
- 28.Walter P., Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 29.Goodman C. A., Mabrey D. M., Frey J. W., Miu M. H., Schmidt E. K., Pierre P., Hornberger T. A. (2011) Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 25, 1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacheck J. M., Ohtsuka A., McLary S. C., Goldberg A. L. (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 287, E591–E601 [DOI] [PubMed] [Google Scholar]
- 31.Ogata T., Oishi Y., Higuchi M., Muraoka I. (2010) Fasting-related autophagic response in slow- and fast-twitch skeletal muscle. Biochem. Biophys. Res. Commun. 394, 136–140 [DOI] [PubMed] [Google Scholar]
- 32.Hua F., Li K., Yu J. J., Lv X. X., Yan J., Zhang X. W., Sun W., Lin H., Shang S., Wang F., Cui B., Mu R., Huang B., Jiang J. D., Hu Z. W. (2015) TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat. Commun. 6, 7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine B., Kroemer G. (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 35.Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323 [DOI] [PubMed] [Google Scholar]
- 36.Koh H. J., Arnolds D. E., Fujii N., Tran T. T., Rogers M. J., Jessen N., Li Y., Liew C. W., Ho R. C., Hirshman M. F., Kulkarni R. N., Kahn C. R., Goodyear L. J. (2006) Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol. Cell. Biol. 26, 8217–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi L., Heredia J. E., Altarejos J. Y., Screaton R., Goebel N., Niessen S., Macleod I. X., Liew C. W., Kulkarni R. N., Bain J., Newgard C., Nelson M., Evans R. M., Yates J., Montminy M. (2006) TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312, 1763–1766 [DOI] [PubMed] [Google Scholar]
- 38.Avery J., Etzion S., DeBosch B. J., Jin X., Lupu T. S., Beitinjaneh B., Grand J., Kovacs A., Sambandam N., Muslin A. J. (2010) TRB3 function in cardiac endoplasmic reticulum stress. Circ. Res. 106, 1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An D., Lessard S. J., Toyoda T., Lee M. Y., Koh H. J., Qi L., Hirshman M. F., Goodyear L. J. (2014) Overexpression of TRB3 in muscle alters muscle fiber type and improves exercise capacity in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R925–R933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W., Wu M., Kim T., Jariwala R. H., Garvey W. J., Luo N., Kang M., Ma E., Tian L., Steverson D., Yang Q., Fu Y., Garvey W. T. (2016) Skeletal muscle TRIB3 mediates glucose toxicity in diabetes and high- fat diet-induced insulin resistance. Diabetes 65, 2380–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsushima R., Harada N., Webster N. J., Tsutsumi Y. M., Nakaya Y. (2006) Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J. Biol. Chem. 281, 29719–29729 [DOI] [PubMed] [Google Scholar]
- 42.Kammoun M., Cassar-Malek I., Meunier B., Picard B. (2014) A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur. J. Histochem. 58, 2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J. B., Goldberg A. L. (1976) Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am. J. Physiol. 231, 441–448 [DOI] [PubMed] [Google Scholar]
- 44.Ciciliot S., Rossi A. C., Dyar K. A., Blaauw B., Schiaffino S. (2013) Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 45, 2191–2199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.