Abstract
Impairment of adult neurogenesis in the hippocampus causes cognitive deficits; however, the underlying molecular mechanisms have not been fully elucidated. microRNAs (miRNAs) regulate neural stem cell (NSC) function. With the use of a transgenic mouse line with conditional ablation of the miR-17-92 cluster in nestin lineage NSCs, we tested the hypothesis that the miR-17-92 cluster regulates adult neurogenesis and cognitive function in vivo. Compared with wild-type mice, ablation of the miR-17-92 cluster significantly reduced the number of proliferating NSCs and neuroblasts and neuronal differentiation in the dentate gyrus (DG) of the hippocampus and significantly impaired hippocampal-dependent learning and memory, as assayed by social recognition memory, novel object recognition, and Morris water-maze tests. Statistical analysis showed a highly significant correlation between newly generated neuroblasts in the DG and cognition deficits in miR-17-92 knockout (KO) mice. Western blot analysis showed that conditional KO of the miR-17-92 cluster significantly increased and reduced a cytoskeleton-associated protein, Enigma homolog 1 (ENH1), and its downstream transcription factor, inhibitor of differentiation 1 (ID1), respectively, as well as increased phosphatase and tensin homolog gene. These proteins are related to neuronal differentiation. Our study demonstrates that the miR-17-92 cluster in NSCs is critical for cognitive and behavioral function and regulates neurogenesis and that the miR-17-92 cluster may target ENH1/ID1 signaling.—Pan, W. L., Chopp, M., Fan, B., Zhang, R., Wang, X., Hu, J., Zhang, X. M., Zhang, Z. G., Liu, X. S. Ablation of the microRNA-17-92 cluster in neural stem cells diminishes adult hippocampal neurogenesis and cognitive function.
Keywords: cognition, hippocampus, small RNA, neuronal differentiation, adult NSC
The hippocampus is 1 of the neurogenic regions of the adult brain where new neurons are continuously generated throughout life (1–3). Neural stem cells (NSCs) are born in the subgranular zone (SGZ) of the dentate gyrus (DG) and have the capacity to self renew and differentiate, giving rise to both neurons and glia. These adult-born neurons in the hippocampus migrate tangentially along the SGZ and develop into immature neurons, which then migrate radially into the granule cell layer to differentiate into dentate granule neurons that integrate into the local neural network to become functionally active within 4–6 wk. Adult hippocampal neurogenesis is a source of robust plasticity in the brain and is thought to contribute to learning and memory (4, 5). Defects in hippocampal neurogenesis have been associated with human neurologic and psychiatric diseases (5).
microRNAs (miRNAs) are small, noncoding RNAs and play an important role in NSC function (6). miRNAs are associated with the learning and memory processes of mammals by regulating dendritogenesis and synapse remodeling (6–8). Whether miRNA-regulated neurogenesis contributes to cognition remains poorly understood. NSCs express miRNAs, and the functions of miRNAs in neurogenesis are just beginning to be revealed (6, 9, 10). We previously discovered that stroke substantially changes mature miRNA profiles in adult NSCs, which affects the proliferation, survival, and differentiation of NSCs (11–13). It remains elusive if altered miRNAs in NSCs have an impact on cognitive dysfunction. Our previous in vitro data have demonstrated that the miR-17-92 cluster regulates adult neurogenesis in the subventricular zone (SVZ) (12). However, the in vivo effect of the miR-17-92 cluster on adult neurogenesis in the hippocampus and consequent neurobehavioral function, especially learning and memory, has not been investigated. In the present study, by specific ablation of this cluster in NSCs in a conditional transgenic mouse line, we tested the hypothesis that the miR-17-92 cluster regulates neurogenesis in adult brain, which is highly related to cognition function.
MATERIALS AND METHODS
All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA), and were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Generation of miR-17-92 cluster knockout mice
To generate the miR-17-92 cluster knockout (KO) mouse line in NSCs, we crossed miR-17-92flox/flox mice (Mirc1tm1.1Tyj/J; 008458; The Jackson Laboratory, Bar Harbor, ME, USA) with nestin-CreERT2 mice [C57BL/6-Tg(Nes-cre/ERT2)KEisc/J; 016261; The Jackson Laboratory] to generate nestin-CreERT2;miR-17-92flox/flox mice, leading to the ablation of the miR-17-92 cluster in nestin-expressing NSCs. To trace these cells, we then crossed nestin-CreERT2;miR-17-92flox/flox mice with Rosa-TdTomato reporter mice [B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, 007909; The Jackson Laboratory] to generate a nestin-CreERT2;miR-17-92flox/flox;Rosa-TdTomato triple-transgenic mouse line (nestin-Cre;miR-17-92−/−;Tomato), in which nestin-expressing cells with ablation of miR-17-92 can be traced by means of TdTomato as a marker detected by antibody against DsRed. Male mice (3–4 mo) were used in this study. Primers for genotyping PCR are as follows: nestin-Cre (mutant 450 bp; forward): 5′-GGAACACCCAGTGAAGCT-3′, nestin (reverse): 5′-TAGGCGGTGGGCGTCCG-3′; miR-17-92 loxp [mutant 289 bp; wild-type (WT); 255 bp; forward]: 5′-TCGAGTATCTGACAATGTGG-3′, miR-17-92 (reverse): 5′-TAGCCAGAAGTTCCAAATTGG-3′; tomato (mutant 197 bp; WT; 296 bp; forward): 5′-AAGGGAGCTGCAGTGGAGTA-3′, tomato (reverse): 5′-CCGAAAATCTGTGGGAAGTC-3′. The total 6 mice in WT and miR-17-92 KO groups were used for cognitive assays, and 3–6 brain samples from all mice were fixed for immunostaining analyses. The extra 3 animals in each group were used for gene expression and in vitro NSC culture, respectively.
Tamoxifen administration
To obtain miR-17-92 cluster KO mice, 2- to 3-mo-old nestin-CreERT2;miR-17-92flox/flox;Rosa-TdTomato mice were intraperitoneally administered with tamoxifen (TAM) or vehicle for 2 rounds (180 mg/kg WT once per day for 5 consecutive days per round). The mice were euthanized 28 d later after initial TAM administration. TAM was prepared as a 10-mg/ml solution in seed oil.
Learning and memory assays
Social recognition memory test
The experimental procedure consisted of 3 consecutive parts: 1) habituation (5 min)—the test mouse was placed in the middle chamber, sliding doors were opened, and the mice were allowed to explore all 3 chambers. The 2 side chambers contained an empty cylinder. The empty cylinder presented a novel inanimate object without social value. General activity, possible preference for a certain part of the apparatus (middle or one of the side chambers), and exploration of the cylinder were measured. 2) Sociability (10 min)—after the habituation period, the sliding doors were shut, and the test mouse was enclosed in the middle chamber. An unfamiliar mouse (stranger) was put into one of the cylinders and placed in one of the side chambers. The location for stranger was alternated, either to the left or right chamber of the social test box. The cylinder in the other chamber was empty. Following placement of stranger, the doors were opened, and the test mouse will have access to all 3 chambers. Increased time spent in the chamber and in the perimeter around the cylinder with the stranger indicates preference for the social stimulus compared with the empty cage. For extra analysis, this 10 min period was also subdivided into 2 blocks of 5 min. 3) Social discrimination (5 min)—again, the test mouse was confined to the middle chamber. Another unfamiliar mouse (new stranger) was placed in the cylinder that was empty during the sociability test. The “old” stranger will remain in position in its cylinder and chamber. The sliding doors were opened, and the test mouse got access to the side chambers. It was expected that the test mouse spent more time with the new stranger than the old stranger. Increased time spent with the new stranger was a measure for the discriminative ability of the test mouse, also indicative for intact working memory.
Novel object recognition test
The test included 3 steps: 1) prehabituation procedures—animals were removed from group-housing cages and rehoused singly in identical cages with sawdust bedding and removable wire tops. Once singly housed, animals remained in these test cages for the duration of the experiment. During the initial 24 h familiarization period, four, 20-mm round, wooden beads, each with a small hole that bore through its diameter, was introduced into the test cages to acquire the odor of the animal and to serve as familiar odors for subsequent use in the experiment. The housing of the animals in the test cages with the beads for 24 h allowed for familiarization to both the testing environment and the presence of the beads. Several beads were also introduced into the cages of 3 selected odor-donor groups (housed 3 mice/cage), for which cages were not changed for 1 wk to allow for a build-up of animal-specific, novel odors. The cages designated to provide donor odor beads were counterbalanced so that any one odor served as either a recently novel odor (N1) or a brand new novel odor (N2) during memory assessment for different experimental mice. 2) Habituation to the first novel odor—after 24 h of familiarization to the presence of 4 beads in the testing environment, the 4 now-familiar beads were removed for 1 h. After this 1-h period, a novel odor wood bead (N1), taken from an odor-donor cage, and 3 familiar beads that were taken from their own cages, 1 h previously, were introduced into the cage. They were exposed to these 4 beads for three, 1-min trials with 1-min intervals, during which the beads were removed from the testing enclosure. For each 1-min trial, the 3 familiar odor beads and the N1 bead were placed in the middle of the testing cage, and the mice were allowed 1 min to explore the beads actively. The first approach to a bead made during this period initiated the timing of the 1 min trial. Exploration time for each of the 4 beads was recorded. 3) Odor-recognition memory assessment—24 h after the novel odor habituation phase, the odor-recognition test was conducted. For this phase of the task, mice were presented with the odor N1 bead in the presence of 1 unfamiliar novel odor bead (N2) taken from a different odor-donor cage and 2 familiar (own cage) odor beads, following the same procedure outlined for the habituation phase. The N2 ratio was calculated as time spent exploring the novel wood bead (N2) relative to the total time spent exploring all objects.
Morris water-maze test
The mouse was placed in a swimming pool with water of a comfortable temperature (22–25°C), which has been made opaque. The swimming pool was subdivided into 4 equal quadrants formed by imaging lines. At the start of each trial, the mouse was placed at 1 of 4 fixed starting points, randomly facing toward a wall (designated North, South, East, and West), and allowed to swim for 90 s or until it finds the platform, which is transparent and invisible to the animals. If the animal finds the platform by spatial navigation, it was allowed to remain on it for 10 s. If the animal fails to find the platform within 90 s, it was placed on the platform for 10 s. Throughout the test period, the platform was located in the Northeast quadrant, 2 cm below water, in a randomly changing position, including locations against the wall, toward the middle of the pool, or off center, but always within the target quadrant. If the animal is unable to locate the platform within 90 s, then the trial was terminated, and a maximum score of 90 s was assigned. If the animal reaches the platform within 90 s, then the percentage of time traveled within the Northeast (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform and was used for statistical analysis. The latency to find the hidden escape platform was also recorded and analyzed.
Neural stem cell cultures
Adult mice were anesthetized with isoflurane and cervically dislocated. Animals were decapitated, and the meninges were removed. For hippocampal dissections, sagittal slices were made using a scalpel blade, and the DG was dissected under a microscope and collected. The tissue was enzymatically and mechanically dissociated into a single-cell suspension, as previously described (14). The cells were plated at a density of 2 × 104 cells/ml in growth medium. Growth medium contains DMEM/F-12 medium (Thermo Fisher Scientific, Waltham, MA, USA), 20 ng/ml epidermal growth factor (EGF; R&D Systems, Minneapolis, MN, USA), and basic fibroblast growth factor (bFGF; R&D Systems). DMEM/F-12 medium contains l-glutamine (2 mM; Thermo Fisher Scientific), glucose (0.6%; MilliporeSigma, Burlington, MA, USA), putrescine (9.6 mg/ml; MilliporeSigma), insulin (0.025 mg/ml; MilliporeSigma), progesterone (6.3 ng/ml; MilliporeSigma), apo-transferrin (0.1 mg/ml; MilliporeSigma), and sodium selenite (5.2 ng/ml; MilliporeSigma). The generated neurospheres (primary spheres) were passaged by mechanical dissociation and reseeded as single cells at a density of 20 cells/µl. NSCs used in all experiments were from passages 2 to 5.
Neurosphere assay
A neurosphere assay was used to investigate the effect of miRNAs on NSCs. The assay used has been widely used by us (11, 15, 16) and others (17) to investigate the biology of NSCs. The NSCs isolated from WT or miR-17-92 KO mice were incubated in growth medium in the presence of EGF and bFGF for 24 h to allow the cells to recover. Then, single cells at a density of 10 cells/μl were plated directly onto laminin-coated glass coverslips and incubated for an additional 48 h to allow cell growth. To analyze cell proliferation, bromodeoxyuridine (BrdU; 30 μg/ml; MilliporeSigma), the thymidine analog that is incorporated into the DNA of dividing cells during S-phase, was added, 18 h before the termination of incubation. BrdU-positive cells were measured.
To examine the NSC differentiation, neurospheres were plated directly onto laminin-coated glass coverslips in the growth medium containing 2% fetal bovine serum, but growth factors, including EGF and bFGF, were withdrawn, which is referred to as differentiation medium. Every 4 d, half of the medium was replaced with fresh medium. Incubation was terminated 10 d after plating, and immunostaining for neuronal markers was performed for evaluation of cell differentiation.
Small interfering RNA electroporation in vitro
Small interfering RNA (siRNA) against mouse Enigma homolog 1 (ENH1) was purchased from Dharmacon (Chicago, IL, USA; M-047511-01-0005), and siGlo (D-001630-02; Dharmacon) was used as the negative control. siRNAs were introduced into cells using a Nucleofector Kit (Amaxa; Lonza, Cologne, Germany). In brief, 200 pmol/well siRNAs were mixed with 100 μl Nucleofector solution, and cell–DNA mixtures were transferred into a cuvette and electroporated using program A33. Total proteins were extracted at 72 h after nucleofection for the following experiment.
Quantification of mature miRNAs by real-time quantitative RT-PCR
For in vivo miRNA analysis, we manually dissected DG tissues from frozen coronal brain sections of WT and miR-17-92 cluster KO mice (n = 3 mice/group), as previously described (14). Total RNAs from tissues or cells were isolated using the miRNeasy Kit (Qiagen, Germantown, MD, USA), followed by reverse transcription (RT). Individual RT and TaqMan miRNA assays were performed on an Applied Biosystems 7000 instrument (Thermo Fisher Scientific). RT reactions (15 μl) consisted of 1–10 ng total RNA isolated with Trizol (Qiagen), 5 U MultiScribe RT, 0.5 mM each deoxynucleotides, 1 × RT buffer, 4 U RNase inhibitor, and nuclease-free water. RT reactions were incubated at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min and then stored at 4°C until use in TaqMan assays. TaqMan real-time PCR reactions (20 μl) consisted of 1× TaqMan Universal PCR Master Mix No AmpErase uracil N-glycosylase, 1× TaqMan miRNA assay, 1.33 μl undiluted cDNA, and nuclease-free water. Each TaqMan assay was done in triplicate for each sample tested. Relative quantities were calculated using the 2−ΔΔCt method with the U6 small nuclear RNA TaqMan miRNA control assay (Thermo Fisher Scientific) as the endogenous control and calibrated to the WT samples. Three independent experiments were performed. Reactions were run with the standard 7000 default cycling protocol without the 50°C incubation stage, with reactions incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescence readings were collected during the 60°C step.
Histologic and immunohistochemical assessment
Under deep anesthesia with ketamine (50 mg/kg body weight; MilliporeSigma), animals were perfused transcardially with normal saline and 4% paraformaldehyde (PFA; Merck, Darmstadt, Germany) in 0.1 M phosphate buffer, pH 7.4. After having been removed from the skulls, postfixed in 4% PFA overnight and transferred into a 10, 20, and 30% sucrose solutions (in 1× PBS) for 12–24 h each, brains were cut in 20-mm-thick coronal sections on a dry-ice-cooled copper block on a sliding microtome (SM 200R; Leica, Bensheim, Germany) and cryoprotected.
Immunofluorescent staining was performed on brain tissues and cultured cells, according to our published protocols (18). The following primary antibodies were used in the present study: mouse anti-β-tubulin III (Tuj1; 1:500; Covance, Princeton, NJ, USA), rabbit anti-glial fibrillary acidic protein (anti-GFAP; 1:500; Dako Cytomation, Carpinteria, CA, USA), goat anti-doublecortin (anti-DCX; 1:200; Santa Cruz Biotechnology, Dallas, TX, USA), sex-determining region Y box 2 (SOX2; 1:200, Santa Cruz Biotechnology), mouse anti-BrdU (1:100; Boehringer Mannheim, Indianapolis, IN, USA), calretinin (1:500; Swant, Marly, Switzerland), and chicken anti-red fluorescent protein (anti-RFP; 1:500; Rockland Antibodies & Assays, Limerick, PA, USA). Tissues and cultured cells were fixed in 4% PFA for 20 min at room temperature. Nonspecific binding sites were blocked with PBS, with 1% bovine serum albumin for 1 h at room temperature. The tissues and cultured cells were then incubated with the primary antibodies listed above and were visualized by FITC- and Cy3-conjugated secondary antibodies (The Jackson Laboratory). Nuclei were counterstained with DAPI (1:10,000; Vector Laboratories, Burlingame, CA, USA).
Quantification analysis
The number of colabeled cells was counted in the SGZ of DG. The fields of interest were digitized under a light microscope (Eclipse 80i; Nikon, Tokyo, Japan) at a magnification of either ×20 or 40 objective (BX40; Olympus, Tokyo, Japan) using the CoolSnap color camera (Photometrics, Tucson, AZ, USA) interfaced with the microcomputer imaging device (MCID) Image Analysis System (Molecular Devices, Sunnyvale, CA, USA), as previously described in detail in Zhang et al. (18). The immunopositive cells or area of positive staining were calculated and divided by the measured areas and presented as numbers per square millimeter or percentage of area. Cell counting and area measurements were performed by observers blinded to the individual treatment status of the animals.
SDS-PAGE and Western blot
Tissues or cells were lysed in RIPA buffer, and lysate was sonicated and then centrifuged for 10 min at 12,000 rpm to remove cell debris. Protein concentrations were determined using a bicinchoninic acid assay (Thermo Fisher Scientific). Equal amounts of proteins were then separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with an appropriate primary antibody and a secondary antibody conjugated to horseradish peroxidase. The following antibodies were used: β-actin (1:10,000 dilution; Abcam, Cambridge, MA, USA), ENH1 (1:500 dilution; Abcam), inhibitor of differentiation 1 (ID1; 1:2000 dilution; Abcam), and phosphatase and tensin homolog (PTEN) deleted on chromosome 10 (1:1000 dilution; Abcam). Proteins were visualized by ECL (Thermo Fisher Scientific).
Statistical analysis
Data were evaluated for normality. Data transformation would be considered if data were abnormal. Independent sample Student’s t test was used for 2-group comparisons from the WT vs. KO samples. We determined Pearson correlation coefficients among neurogenesis variables/measurements and cognition of the novel object recognition test, Morris water-maze test, and social recognition memory test, stratified by animal type of WT and miR-17-92 KO using SAS software (SAS Institute, Cary, NC, USA). The data are presented as means ± se. A value of P < 0.05 was taken as significant.
RESULTS
Conditional KO of the miR-17-92 cluster in NSCs impairs adult neurogenesis
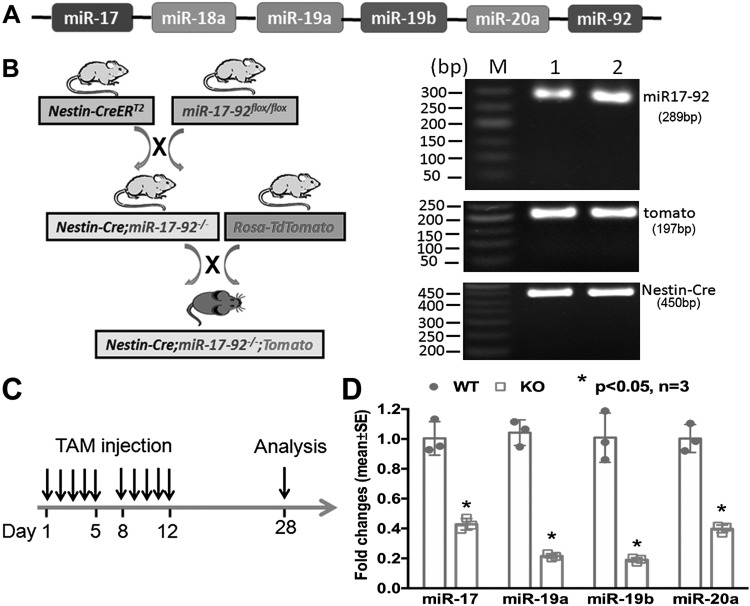
The miR-17-92 cluster incorporates a family composed of 6 miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a), which is highly conserved in humans and rodents (Fig. 1A). Our previous in vitro work has demonstrated that the miR-17-92 cluster affects adult neurogenesis (12). However, the role of this cluster in vivo, in regulating adult neurogenesis, remains unknown. Thus, we ablated the cluster in nestin lineage NSCs in adult nestin-CreERT2;miR-17-92flox/flox mice by means of administration of TAM (Fig. 1B). We dissected DG tissues from frozen coronal brain sections of WT and miR-17-92 cluster KO mice (n = 3 mice/group). Quantitative RT-PCR analysis showed that levels of individual members of the miR-17-92 cluster were significantly down-regulated in NSCs isolated from the DG after TAM treatment (Fig. 1C) compared with that in WT mice (Fig. 1D), indicating that the miR-17-92 cluster was knocked out in NSCs.
Figure 1.
Generation of miR-17-92 cluster conditional KO mouse line in NSCs. A) Schematic genomic organization of miRNAs in the miR-17-92 cluster on mouse chromosome 14. The color code represents miRNAs with the conserved seed sequence. B) The strategy to generate miR-17-92 KO mice using the Nestin-CreERT2;miR-17-92flox/flox and Rosa-TdTomato reporter mice lines and mouse genotyping. M: 50bp DNA marker. 1, 2: different mice of nestin-CreERT2;miR-17-92flox/flox transgenic line. C) Scheme of NSC analysis in the TAM-treated nestin-CreERT2;miR-17-92flox/flox;Rosa-TdTomato mice. D) Expression levels of miR-17, miR-18, miR-19a, and miR-92 were significantly reduced in NSCs of the miR-17-92 cluster KO compared with those in WT mice; n = 3/group. *P < 0.05.
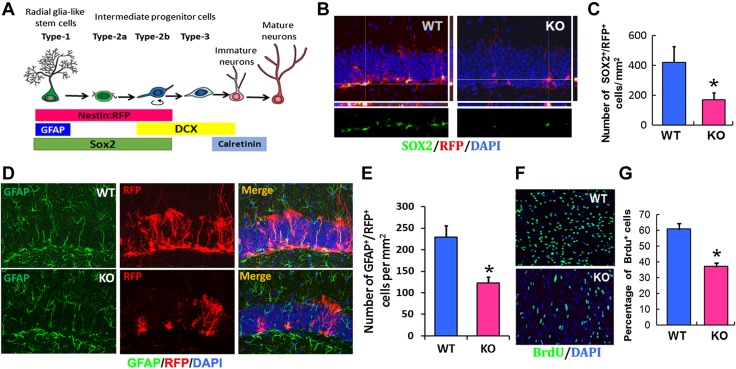
Double-immunofluorescent staining analysis showed that the conditional KO of the miR-17-92 cluster in NSCs significantly reduced the number of SOX2+ proliferating progenitor cells, nestin+/RFP+ NSCs, and GFAP+/RFP+ type IA NSCs in the SGZ of DG compared with WT mice (Fig. 2A–E). Consistent with in vivo data, deletion of the miR-17-92 cluster significantly reduced the proliferation of NSCs, as measured by reduction of the number of BrdU+ cells in primary cultured NSCs isolated from miR-17-92 KO mice compared with WT mice (Fig. 2F, G).
Figure 2.
Ablation of miR-17-92 cluster in NSCs reduces the proliferation. A) Schematic illustration showing the markers used to evaluate adult neurogenesis in hippocampus DG. B) Confocal images showed that deletion of miR-17-92 significantly reduced the number of SOX2+ and nestin/RFP+ NSCs in DG compared with WT mice. C) Quantification of SOX2+/RFP+ NSCs in miR-17-92 KO mice and WT mice; n = 3/group. D) Immunostaining demonstrated that deletion of miR-17-92 significantly decreased the number of GFAP+/RFP+ NSCs in DG compared with WT mice. E) Quantification of GFAP+/RFP+ NSCs in miR-17-92 KO mice and WT mice; n = 6/group. F, G) In vitro data showed that knockdown of miR-17-92 significantly reduced the number of BrdU+ cells in primary cultured NSCs. Quantification of BrdU+ proliferating progenitors in NSCs isolated from miR-17-92 KO mice and WT mice; n = 3/group. *P < 0.05 vs. WT group.
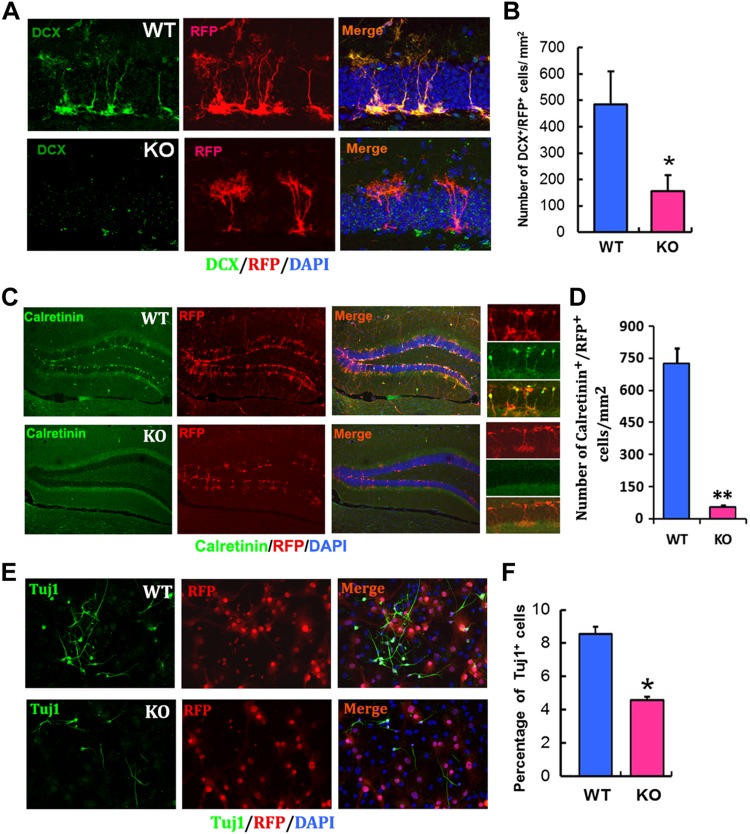
In addition to NSCs, KO of the miR-17-92 cluster substantially reduced the number of DCX+ neuroblasts in the DG compared with WT mice (Fig. 3A, B). Morphologically, process length and branching points of DCX+ neuroblasts in the KO mice were reduced (Fig. 3A), which was associated with significant reduction of newly generated calretinin+ neurons (Fig. 3C, D). Furthermore, the number of RFP+/DCX+ neuroblasts in the SVZ was decreased in miR-17-92 cluster KO mice (Supplemental Fig. S1). In line with the in vivo data, a significant reduction of Tuj1+ neuroblasts was also detected in cultured miR-17-92 KO NSCs (Fig. 3E, F). Collectively, these in vivo data indicate that ablation of the miR-17-92 cluster in nestin lineage cells reduces neurogenesis in 2 neurogenic regions: the SVZ and the DG.
Figure 3.
Ablation of miR-17-92 cluster in NSCs reduces the neurogenesis in the hippocampus. A) miR-17-92 cluster KO mice displayed significantly reduced DCX+ neuroblasts in the DG of hippocampus compared with WT mice. B) Quantification of DCX+/RFP+ neuroblasts in miR-17-92 KO and WT mice; n = 3 mice/group. C) miR-17-92 KO mice displayed a significant reduction of calretinin+/RFP+ neuronal differentiation compared with WT mice. D) Quantification of calretinin+/RFP+ postmitotic immature neurons in miR-17-92 KO mice and WT mice. E) A significant reduction of Tuj1+ neuroblasts was also detected in cultured miR-17-92 KO NSCs. F) Quantification of Tuj1+ neuroblasts in primary-cultured NSCs isolated from miR-17-92 KO and WT mice; n = 3/group. *P < 0.05, **P < 0.01.
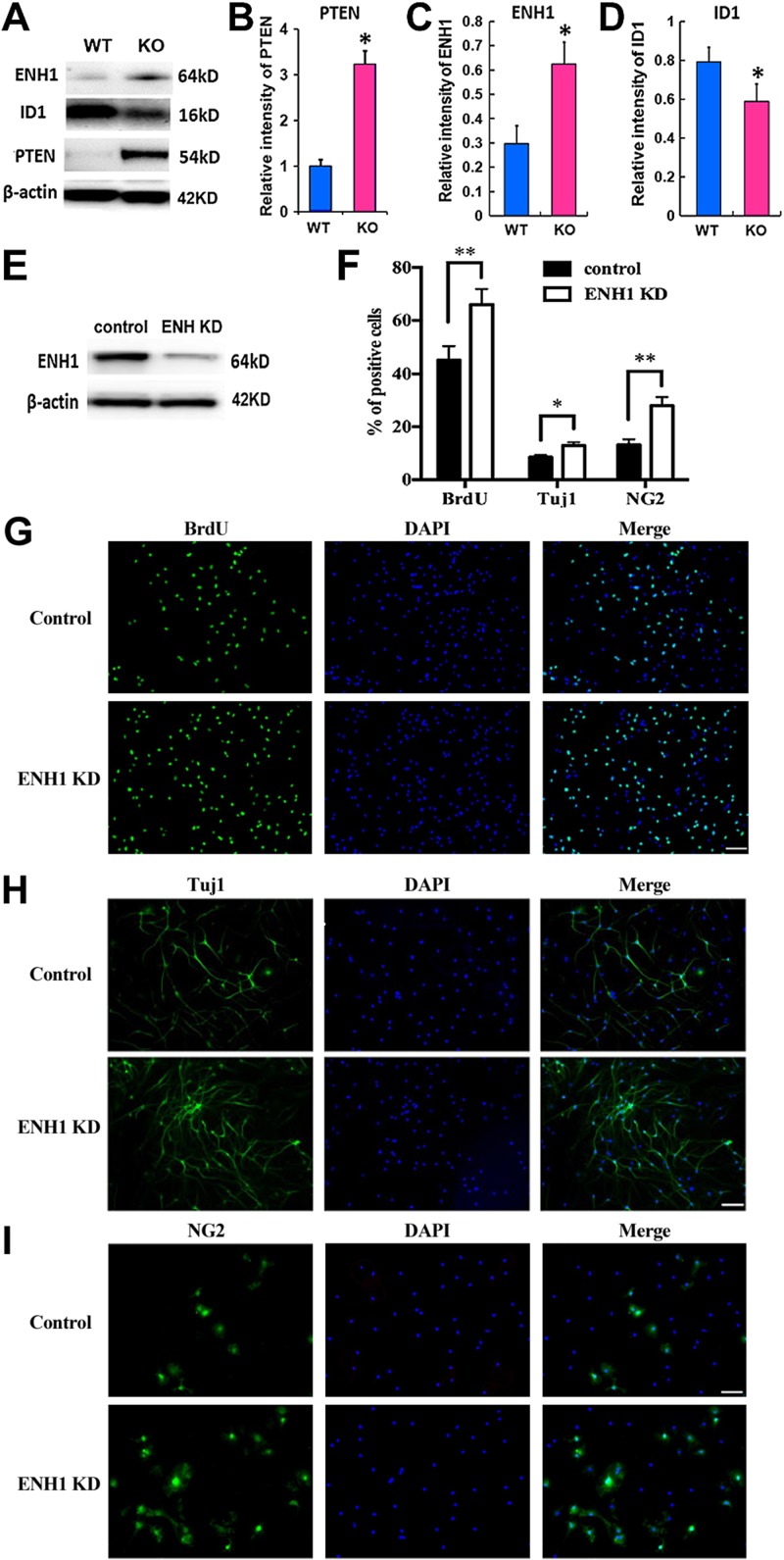
We next investigated genes that are putatively targeted by the miR-17-92 cluster. Consistent with our previous in vitro data (12), Western blot analysis showed increased PTEN in the hippocampal NSCs of miR-17-92 KO mice (Fig. 4A, B). It was previously reported that multiple members of the miR-17-92 cluster, including miR-17 and miR-20, potentially target a cytoskeleton-associated protein, ENH1 (also called PDLIM5), in the myoblasts (19). However, the function of ENH1 in the NSCs remains unknown. Our Western blot analysis showed that ENH1 levels were significantly increased, whereas the ID1 protein, a natural inhibitor of the basic helix–loop–helix transcription factors, was substantially decreased in the hippocampal NSCs of miR-17-92 KO mice (Fig. 4A, C, D). In addition, knockdown of ENH1 by delivery of siRNA against ENH1 (Fig. 4E) significantly increased the percentage of BrdU+ under the proliferating conditions (Fig. 4F, G), as well as elevated the number of Tuj1+ neuroblasts (Fig. 4H) and neural/glial antigen 2 (NG2)+ oligodendrocyte progenitor cells (Fig. 4I). Our data demonstrated that ID1 levels were positively correlated with the miR-17-92 cluster but inversely correlated with ENH1 expression. ENH binds to ID1 with consequent inactivation of transcriptional functions of ID2. ID proteins increase self-renewal and proliferation potential in cortical NSCs (20). In concert, these data suggest that miR-17-92 KO results in the reduction of NSC proliferation by the increase of ENH1, which inversely decreases ID1 protein.
Figure 4.
ENH1 is a potential target of the miR-17-92 cluster in NSCs. A) Western blot analysis of ENH1 protein, its downstream gene ID1, and PTEN expression in the hippocampal NSCs isolated from miR-17-92 KO and WT mice. β-Actin served as the loading control. B–D) Bar graph of relative protein level of PTEN (B), ENH1 (C), and ID1 (D). *P < 0.05 vs. WT; n = 3/group. E) Protein levels of ENH1 knockdown (KD) were decreased in NSCs transfected with siRNA against ENH1 compared with those transfected with scrambled siRNAs. F) Quantitative data of the percentage of BrdU-, Tuj1-, and NG2-positive cells from ENH1 knockdown and control NSCs. *P < 0.05, **P < 0.01 vs. control group. G–I) Representative immunofluorescent images of BrdU (G)-, Tuj1 (H)-, and NG2 (I)-positive cells; n = 3/group. Original scale bars, 20 µm.
KO of the miR-17-92 in NSCs induces learning and memory deficits
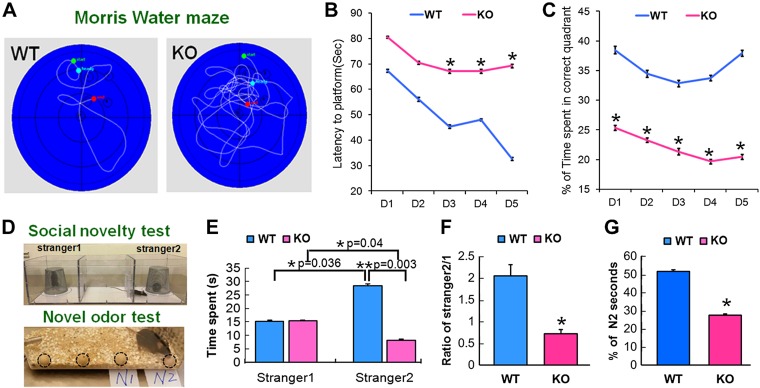
Neurogenesis in the hippocampus is closely related to learning and memory (5, 21). With the use of a Morris water-maze assay that detects hippocampal-related spatial learning and memory, we found that mice with KO of the miR-17-92 cluster in NSCs spent significantly more time attempting to locate the hidden platform in the correct quadrant, as indicated by quantitative escape latency compared with WT mice (Fig. 5A–C), and significantly spent less time in the correct quadrant (Fig. 5A–C).
Figure 5.
KO of miR-17-92 causes cognition deficits. The Morris water maze was used to test the spatial learning and memory. A) Representative swimming paths, on d 5 of the place navigation trial, were recorded with a video-tracking system. B) miR-17-92 KO mice spent significant time to locate the hidden platform in the correct quadrant, as indicated by quantitative latency compared with WT mice. C) miR-17-92 KO mice also spent much less time in the correct quadrant. D) Schematic diagrams of 3-chambered box for social novelty test and wooden beads for odor-based novelty recognition test. Circles indicate the wooden beads in the cage. E–G) WT mice spent a lot of time exploring a stranger mouse (stranger 2; E, F) and a new, novel bead (N2 bead; G). Compared with WT mice, miR-17-92 KO mice spent significantly less time in recognizing a stranger (E, F) and a new bead (G). Data are presented as the percentage of time spent on N2 relative to the total amount of time spent with all beads (G). *P < 0.05, **P < 0.01 (n = 6/group).
Moreover, the social odor-based recognition task to detect nonspatial memory deficits that examined sociability of animals was performed using the 3-chambered social approach test, which was sensitive to detect order-related memory (22). In this assay, sociability and preference for social novelty were investigated (Fig. 5D). We found that the WT and the KO mice showed different preferences; the WT mice spent a significantly longer time exploring the cage with a stranger mouse than with a familiar mouse, but the KO mice spent an equivalent amount of time between the 2 cages (Fig. 5D–F). These results suggest that social interaction with a novel mouse was normal but that there was a lack of preference for a novel mouse over a familiar mouse in miR-17-92 KO mice.
With the use of a novel object recognition assay, we found that WT mice spent significantly more time exploring a novel bead than a familiar bead (Fig. 5D, G). However, compared with WT mice, KO mice significantly lost their preferential attention to a new bead (Fig. 5D, G and Supplemental Videos S1 and S2), indicating that KO of the miR-17-92 cluster in NSCs significantly impaired the animals’ ability to discriminate the novel from the familiar object. These data indicate that KO of the miR-17-92 cluster in NSCs impairs hippocampal-related learning and memory.
To address if the miR-17-92-induced cognition impairment was mediated through the decrease of neurogenesis, a correlational analysis was performed to determine their relationship for miR-17-92 KO mice. Pearson correlation analyses demonstrated a highly significantly negative correlation of DCX+, DCX+/RFP+ cells with the latency to platform in the water-maze test (r = −1.00, P = 0.010; Table 1), as well as a significantly positive correlation of nestin+/RFP+ with time spent with stranger 2 mouse in a social recognition memory test (r = −0.997, P = 0.048; Table 1) and time in correct quadrant (r = −1.00, P = 0.014; Table 1) in the miR-17-92 KO mouse group, which were not apparent in the WT group (P > 0.05; Table 2). We also observed that ratio of stranger 2:1 time was significantly correlated with the percentage of calretinin+/RFP+ cells in miR-17-92 KO (r = −0.851, P = 0.032; Table 3) and WT (r = −0.825, P = 0.043; Table 4) mouse groups. In addition, we detected a significantly positive correlation of SOX2+/RFP+ cells with latency to platform in the water-maze test (r = −0.999, P = 0.021; Table 1) in the miR-17-92 KO mouse group but not in the WT group.
TABLE 1.
Correlation of neurogenesis and cognition in miR-17-92 KO mice
| Variable | DCX | DCX/RFP | SOX2 | RFP | SOX2/RFP |
|---|---|---|---|---|---|
| Percent of N2 novel odor | |||||
| Pearson r | 0.482 | 0.482 | 0.800 | −0.998 | −0.467 |
| P | 0.680 | 0.680 | 0.410 | 0.039* | 0.691 |
| Latency (WM) | |||||
| Pearson r | −1.000 | −1.000 | 0.125 | 0.547 | 0.999 |
| P | 0.010* | 0.010* | 0.920 | 0.632 | 0.021* |
| Correct quadrant (WM) | |||||
| Pearson r | −0.553 | −0.553 | −0.748 | 1.000 | 0.538 |
| P | 0.627 | 0.627 | 0.462 | 0.014* | 0.639 |
| Test (WM) | |||||
| Pearson r | 0.849 | 0.849 | 0.404 | −0.900 | −0.840 |
| P | 0.354 | 0.354 | 0.735 | 0.287 | 0.365 |
| Time in stranger 1 | |||||
| Pearson r | 0.973 | 0.973 | −0.366 | −0.324 | −0.977 |
| P | 0.149 | 0.149 | 0.762 | 0.790 | 0.138 |
| Time in stranger 2 | |||||
| Pearson r | −0.596 | −0.596 | −0.711 | 0.997 | 0.582 |
| P | 0.593 | 0.593 | 0.497 | 0.048* | 0.604 |
| Ratio of stranger 2:1 | |||||
| Pearson r | −0.962 | −0.962 | −0.135 | 0.744 | 0.957 |
| P | 0.176 | 0.176 | 0.914 | 0.466 | 0.187 |
n = 3/group. WM, water maze.
P < 0.05.
TABLE 2.
Correlation of neurogenesis and cognition in WT mice
| Variable | DCX | DCX/RFP | SOX2 | RFP | SOX2/RFP |
|---|---|---|---|---|---|
| Percent of N2 novel odor | |||||
| Pearson r | −0.427 | −0.208 | −0.656 | −0.860 | −0.656 |
| P | 0.719 | 0.867 | 0.544 | 0.341 | 0.544 |
| Latency (WM) | |||||
| Pearson r | −0.850 | −0.949 | −0.676 | −0.410 | −0.676 |
| P | 0.353 | 0.205 | 0.528 | 0.731 | 0.528 |
| Correct quadrant (WM) | |||||
| Pearson r | 0.510 | 0.298 | 0.724 | 0.904 | 0.724 |
| P | 0.659 | 0.807 | 0.485 | 0.281 | 0.485 |
| Test (WM) | |||||
| Pearson r | −0.658 | −0.4673 | −0.837 | −0.967 | −0.837 |
| P | 0.543 | 0.6904 | 0.368 | 0.1643 | 0.368 |
| Time in stranger 1 | |||||
| Pearson r | −0.982 | −0.912 | −0.996 | −0.919 | −0.996 |
| P | 0.121 | 0.269 | 0.054 | 0.257 | 0.054 |
| Time in stranger 2 | |||||
| Pearson r | 0.715 | 0.856 | 0.498 | 0.200 | 0.498 |
| P | 0.493 | 0.345 | 0.668 | 0.872 | 0.668 |
| Ratio of stranger 2:1 | |||||
| Pearson r | 0.826 | 0.933 | 0.642 | 0.368 | 0.642 |
| P | 0.382 | 0.234 | 0.556 | 0.760 | 0.556 |
n = 3/group. WM, water maze.
TABLE 3.
Correlation of neurogenesis and cognition in miR-17-92 KO mice
| Variable | GFAP/RFP | Calretinin/RFP |
|---|---|---|
| Percent of N2 novel odor | ||
| Pearson r | 0.765 | 0.341 |
| P | 0.076 | 0.508 |
| Latency (WM) | ||
| Pearson r | −0.782 | −0.096 |
| P | 0.066 | 0.857 |
| Correct quadrant (WM) | ||
| Pearson r | −0.684 | 0.577 |
| P | 0.134 | 0.230 |
| Test (WM) | ||
| Pearson r | 0.830 | −0.012 |
| P | 0.041* | 0.982 |
| Time in stranger 1 | ||
| Pearson r | 0.225 | −0.691 |
| P | 0.668 | 0.128 |
| Time in stranger 2 | ||
| Pearson r | −0.503 | 0.784 |
| P | 0.309 | 0.065 |
| Ratio of stranger 2:1 | ||
| Pearson r | −0.425 | 0.851 |
| P | 0.401 | 0.032* |
n = 6/group. WM, water maze.
P < 0.05.
TABLE 4.
Correlation of neurogenesis and cognition in WT mice
| Variable | GFAP/RFP | Calretinin/RFP |
|---|---|---|
| Percent of N2 novel odor | ||
| Pearson r | 0.946 | −0.102 |
| P | 0.004* | 0.848 |
| Latency (WM) | ||
| Pearson r | 0.196 | −0.734 |
| P | 0.710 | 0.097 |
| Correct quadrant (WM) | ||
| Pearson r | −0.542 | −0.138 |
| P | 0.266 | 0.794 |
| Test (WM) | ||
| Pearson r | 0.156 | −0.023 |
| P | 0.768 | 0.965 |
| Time in stranger 1 | ||
| Pearson r | 0.437 | −0.893 |
| P | 0.386 | 0.017* |
| Time in stranger 2 | ||
| Pearson r | 0.195 | 0.582 |
| P | 0.712 | 0.226 |
| Ratio of stranger 2:1 | ||
| Pearson r | 0.016 | 0.825 |
| P | 0.976 | 0.043* |
n = 6/group. WM, water maze.
P < 0.05.
DISCUSSION
In this study, we investigated the pathophysiologic roles of the NSC-specific miR-17-92 cluster in adult neurogenesis and neurobehavioral function using a genetic approach. Our data demonstrated that specific deletion of the miR-17-92 cluster in nestin lineage NSCs in the SGZ of hippocampus substantially reduces the proliferation and differentiation of NSCs, leading to cognitive impairment. Our results indicate that the miR-17-92 cluster in adult nestin lineage NSCs contributes to learning and memory.
Adult hippocampal neurogenesis involves multiple steps, including proliferation of neural progenitors, differentiation to neurons, and survival of newborn neurons (23). Our results have shown that miR-17-92 plays a role in the maintenance of proliferative neural progenitors and the generation of newborn neurons. The role of miR-17-92 in the proliferation of neural progenitors in the adult hippocampus is consistent with its demonstrated function in the maintenance of embryonic NSCs and the proliferation of various cancer cells (24). We have found that selective NSC miR-17-92 KO causes a reduction of neurogenesis, indicating that miR-17-92 regulates hippocampal neurogenesis and that miR-17-92 is required to maintain physiologic proliferative neural progenitors and to generate new neurons in the adult hippocampus.
Moreover, in our study, we have identified that the expression levels of 2 genes, including PTEN and ENH1, known to be involved in cell proliferation and differentiation (12, 25, 26), were altered in the hippocampus of miR-17-92 KO mice. We validated PTEN as a target for the miR-17-92 cluster in adult neurogenesis, suggesting a conserved role of miR-17-92 in regulating progenitor expansion. In particular, we have identified a cytoskeleton-associated protein, ENH1, a target of the miR-17-92, which was observed to be increased in the hippocampus of miR-17-92 KO mice (25). We further performed additional experiments in which a cause–effect of ENH1 on neurogenesis was examined. We found that knockdown of ENH1 in NSCs by an siRNA approach significantly increases the number of Tuj1- and BrdU-positive NSCs, indicating that ENH1 regulates neurogenesis. Thus, neurogenesis reduced by ablation of the miR-17-92 cluster could be, at least in part, by the up-regulation of its target gene, ENH1. ENH1 has been linked to the cytoplasmic localization of ID proteins in neuronal cells and consequently, inhibits transcriptional function of the ID protein (25). Our data demonstrated that KO of miR-17-92 significantly increased levels of ENH1 and inversely reduced ID1 protein in NSCs, which provides a novel molecular mechanism for the miR-17-92/ENH1/ID1 regulatory axis in adult neurogenesis.
Growing evidence indicates that neurogenic deficits are paralleled by learning and memory deficits and that associative cognition depends on the generation of new neurons in the DG (27–29). Our data showed that reduction of neurogenesis was associated with decreases in generation of calretinin neurons in the hippocampus. In addition, statistical analysis revealed that reduced neuroblasts were significantly correlated with the cognitive behavioral deficits in miR-17-92 cluster KO mice. However, statistical analysis did not confirm a significant correlation between the numbers of SOX2/RFP-positive proliferating NSCs and cognitive impairment, indicating that the proliferation of NSCs may not be responsible for miR-17-92 KO reduced memory performance. Together, these data provide additional insights into the role of the miR-17-92 cluster in mediating adult hippocampal neurogenesis related to cognitive function. Additionally, given that the neurogenic deficit of the miR-17-92 KO mouse is not unique to the SGZ of the DG, we have detected a comparable deficit in proliferative capacity of the miR-17-92 KO in SVZ so that we do not exclude the impact of SVZ neurogenesis on the cognition deficit.
Recent studies have demonstrated that miRNA–miRNA networks have synergistic roles in the regulation of pathologic conditions (30, 31). If 2 miRNAs cross talk with each other in a network, then they are more likely to target genes and regulate the pathways with similar functions. Multiple miRNAs, such as miR-9, miR-124, and miR-34a, have been revealed to modulate adult neurogenesis (11, 17, 32–34). The caveat of the current study is that we did not examine whether the miR17-92 cluster KO affects these miRNAs. Further investigation of the interactions between the miR17-92 cluster and neurogenesis-related miRNAs in the network might provide significant insights to explain the regulatory mechanisms in neurogenesis and cognition.
In summary, our data show that the absence of miR-17-92 in NSCs suppressed hippocampal neurogenesis and spatial memory, which is consistent with previous studies showing that increased hippocampal neurogenesis is associated with improved cognition (5, 21, 35, 36). Therefore, the observed decrease in SGZ neurogenesis could be a secondary contributor to the behavioral impairment observed with miR-17-92 KO. Our data suggest that the miR-17-92 cluster could potentially be a promising therapeutic target in the treatment of cognitive impairment in neurologic diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK102861 (to X.S.L.); American Heart/Stroke Association Grant 18IPA34170331 (to X.S.L.); and NIH National Institute of Neurological Disorders and Stroke Grants RO1 NS075156 (to Z.G.Z.) and RO1 NS088656 (to M.C.). The authors declare no conflicts of interest.
Glossary
- bFGF
basic fibroblast growth factor
- BrdU
bromodeoxyuridine
- DCX
doublecortin
- DG
dentate gyrus
- EGF
epidermal growth factor
- ENH1
Enigma homolog 1
- GFAP
glial fibrillary acidic protein
- ID1
inhibitor of differentiation 1
- KO
knockout
- miRNA
microRNA
- N1/2
novel odor 1/2
- NG2
neural/glial antigen 2
- NSC
neural stem cell
- PFA
paraformaldehyde
- PTEN
phosphatase and tensin homolog
- RFP
red fluorescent protein
- SGZ
subgranular zone
- siRNA
small interfering RNA
- SOX2
sex-determining region Y box 2
- SVZ
subventricular zone
- TAM
tamoxifen
- Tuj1
anti-β-tubulin III
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Z. G. Zhang and X. S. Liu designed research; W. L. Pan, B. Fan, and R. Zhang performed animal and primary cell experiments; X. Wang generated transgenic mice and conducted genotyping; W. L. Pan and X. S. Liu analyzed the data and wrote the manuscript; and M. Chopp, J. Hu, X. M. Zhang, Z. G. Zhang, and X. S. Liu supervised the project and edited the manuscript.
REFERENCES
- 1.Alvarez-Buylla A., Herrera D. G., Wichterle H. (2000) The subventricular zone: source of neuronal precursors for brain repair. Prog. Brain Res. 127, 1–11 [DOI] [PubMed] [Google Scholar]
- 2.Gage F. H., Ray J., Fisher L. J. (1995) Isolation, characterization, and use of stem cells from the CNS. Annu. Rev. Neurosci. 18, 159–192 [DOI] [PubMed] [Google Scholar]
- 3.Kirschenbaum B., Doetsch F., Lois C., Alvarez-Buylla A. (1999) Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J. Neurosci. 19, 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonçalves J. T., Schafer S. T., Gage F. H. (2016) Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914 [DOI] [PubMed] [Google Scholar]
- 5.Deng W., Aimone J. B., Gage F. H. (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Jin P. (2010) Roles of small regulatory RNAs in determining neuronal identity. Nat. Rev. Neurosci. 11, 329–338; erratum: 449 [DOI] [PubMed] [Google Scholar]
- 7.Schratt G. (2009) microRNAs at the synapse. Nat. Rev. Neurosci. 10, 842–849 [DOI] [PubMed] [Google Scholar]
- 8.Wang W., Kwon E. J., Tsai L. H. (2012) microRNAs in learning, memory, and neurological diseases. Learn. Mem. 19, 359–368 [DOI] [PubMed] [Google Scholar]
- 9.Lang M. F., Shi Y. (2012) Dynamic roles of microRNAs in neurogenesis. Front. Neurosci. 6, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y., Zhao X., Hsieh J., Wichterle H., Impey S., Banerjee S., Neveu P., Kosik K. S. (2010) microRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 30, 14931–14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X. S., Chopp M., Zhang R. L., Tao T., Wang X. L., Kassis H., Hozeska-Solgot A., Zhang L., Chen C., Zhang Z. G. (2011) microRNA profiling in subventricular zone after stroke: miR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6, e23461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X. S., Chopp M., Wang X. L., Zhang L., Hozeska-Solgot A., Tang T., Kassis H., Zhang R. L., Chen C., Xu J., Zhang Z. G. (2013) microRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J. Biol. Chem. 288, 12478–12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X. S., Chopp M., Pan W. L., Wang X. L., Fan B. Y., Zhang Y., Kassis H., Zhang R. L., Zhang X. M., Zhang Z. G. (2017) microRNA-146a promotes oligodendrogenesis in stroke. Mol. Neurobiol. 54, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W., Patzlaff N. E., Jobe E. M., Zhao X. (2012) Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc. 7, 2005–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X. S., Chopp M., Zhang R. L., Hozeska-Solgot A., Gregg S. C., Buller B., Lu M., Zhang Z. G. (2009) Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J. Biol. Chem. 284, 22680–22689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Zhang Z. G., Gregg S. R., Zhang R. L., Jiao Z., LeTourneau Y., Liu X., Feng Y., Gerwien J., Torup L., Leist M., Noguchi C. T., Chen Z. Y., Chopp M. (2007) The sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J. Biol. Chem. 282, 32462–32470 [DOI] [PubMed] [Google Scholar]
- 17.Cheng L. C., Pastrana E., Tavazoie M., Doetsch F. (2009) miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 12, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R. L., Chopp M., Roberts C., Wei M., Wang X., Liu X., Lu M., Zhang Z. G. (2012) Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One 7, e48141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu H., Liu N., Luo L., Zhong J., Tang Z., Kang K., Qu J., Peng W., Liu L., Li L., Gou D. (2016) microRNA-17-92 regulates myoblast proliferation and differentiation by targeting the ENH1/Id1 signaling axis. Cell Death Differ. 23, 1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niola F., Zhao X., Singh D., Castano A., Sullivan R., Lauria M., Nam H. S., Zhuang Y., Benezra R., Di Bernardo D., Iavarone A., Lasorella A. (2012) Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat. Cell Biol. 14, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian K. M., Song H., Ming G. L. (2014) Functions and dysfunctions of adult hippocampal neurogenesis. Annu. Rev. Neurosci. 37, 243–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaidanovich-Beilin O., Lipina T., Vukobradovic I., Roder J., Woodgett J. R. (2011) Assessment of social interaction behaviors. J. Vis. Exp. 2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin K., Minami M., Lan J. Q., Mao X. O., Batteur S., Simon R. P., Greenberg D. A. (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 98, 4710–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian S., Hong J., Li Q., Schebelle L., Pollock A., Knauss J. L., Garg V., Sun T. (2013) microRNA cluster miR-17-92 regulates neural stem cell expansion and transition to intermediate progenitors in the developing mouse neocortex. Cell Rep. 3, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasorella A., Iavarone A. (2006) The protein ENH is a cytoplasmic sequestration factor for Id2 in normal and tumor cells from the nervous system. Proc. Natl. Acad. Sci. USA 103, 4976–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiri A., Cho W., Zhou J., Birnbaum S. G., Sinton C. M., McKay R. M., Parada L. F. (2012) Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J. Neurosci. 32, 5880–5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coras R., Siebzehnrubl F. A., Pauli E., Huttner H. B., Njunting M., Kobow K., Villmann C., Hahnen E., Neuhuber W., Weigel D., Buchfelder M., Stefan H., Beck H., Steindler D. A., Blümcke I. (2010) Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain 133, 3359–3372 [DOI] [PubMed] [Google Scholar]
- 28.Marschallinger J., Schäffner I., Klein B., Gelfert R., Rivera F. J., Illes S., Grassner L., Janssen M., Rotheneichner P., Schmuckermair C., Coras R., Boccazzi M., Chishty M., Lagler F. B., Renic M., Bauer H. C., Singewald N., Blümcke I., Bogdahn U., Couillard-Despres S., Lie D. C., Abbracchio M. P., Aigner L. (2015) Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 6, 8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janus C. (2004) Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn. Mem. 11, 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Li C. X., Li Y. S., Lv J. Y., Ma Y., Shao T. T., Xu L. D., Wang Y. Y., Du L., Zhang Y. P., Jiang W., Li C. Q., Xiao Y., Li X. (2011) miRNA-miRNA synergistic network: construction via co-regulating functional modules and disease miRNA topological features. Nucleic Acids Res. 39, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L., Sun B., Wu Q., Yang S., Chen F. (2012) miRNA-miRNA interaction implicates for potential mutual regulatory pattern. Gene 511, 187–194 [DOI] [PubMed] [Google Scholar]
- 32.Fineberg S. K., Datta P., Stein C. S., Davidson B. L. (2012) miR-34a represses Numbl in murine neural progenitor cells and antagonizes neuronal differentiation. PLoS One 7, e38562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao C., Sun G., Li S., Shi Y. (2009) A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 16, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coolen M., Katz S., Bally-Cuif L. (2013) miR-9: a versatile regulator of neurogenesis. Front. Cell. Neurosci. 7, 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahay A., Scobie K. N., Hill A. S., O’Carroll C. M., Kheirbek M. A., Burghardt N. S., Fenton A. A., Dranovsky A., Hen R. (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin K., Peel A. L., Mao X. O., Xie L., Cottrell B. A., Henshall D. C., Greenberg D. A. (2004) Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 101, 343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.