Abstract
Prostaglandin E2 (PGE2) is produced in the airway during allergic lung inflammation and both promotes and inhibits features of asthma pathology. These mixed effects relate to 4 E-prostanoid (EP) receptor subtypes (EP1, 2, 3 and 4) expressed at different levels on different resident and infiltrating airway cells. Although studies have asserted both EP2 and EP4 expression in human airway smooth muscle (HASM), a recent study asserted EP4 to be the functionally dominant EP subtype in HASM. Herein, we employ recently-developed subtype-selective ligands to investigate singular or combined EP2 and EP4 receptor activation in regulating HASM signaling and proliferation. The subtype specificity of ONO-AE1-259-01 (EP2 agonist) and ONO-AE1-329 (EP4 agonist) was first demonstrated in human embryonic kidney 293 cells stably expressing different EP receptor subtypes. EP receptor knockdown and subtype-selective antagonists demonstrated EP2 and EP4 receptor responsiveness in HASM cells to the specific ONO compounds, whereas PGE2 appeared to preferentially signal via the EP4 receptor. Both singular EP2 and EP4 receptor agonists inhibited HASM proliferation, and combined EP2 and EP4 receptor agonism exhibited positive cooperativity in both chronic Gs-mediated signaling and inhibiting HASM proliferation. These findings suggest both EP2 and EP4 are functionally important in HASM, and their combined targeting optimally inhibits airway smooth muscle proliferation.—Michael, J. V. Gavrila, A., Nayak, A. P., Pera, T., Liberato, J. R., Polischak, S. R., Shah, S. D., Deshpande, D. A., Penn, R. B. Cooperativity of E-prostanoid receptor subtypes in regulating signaling and growth inhibition in human airway smooth muscle.
Keywords: airway remodeling, prostaglandin, asthma, EP receptor
Prostaglandin E2 (PGE2) exerts multiple effects in the lung by regulating the function of multiple airway cell types. During the allergic lung inflammation that occurs with asthma, PGE2 levels are increased and regulate both pro- and antiasthmatic features (1–4). In animal models and cell-based assays, PGE2 has been demonstrated to inhibit multiple indices of allergic inflammation (1, 5–8), inhibit proliferation of cultured human airway smooth muscle (HASM) cells (9–14), relax contracted airways ex vivo (15–18), and promote bronchoprotection and bronchorelaxation in vivo (16, 18). However, PGE2 has also been implicated as a proinflammatory mediator and is involved in inflammatory diseases (19, 20). Further, the net effect of PGE2 on antigen-presenting cells favors a type 2 T-helper cell response, which enhances B cell IgE production and allergic inflammation (21).
The diverse effects of PGE2 can be attributed to the existence of 4 different [E-prostanoid (EP)1–4] G protein–coupled receptors responsive to PGE2, which are expressed at different levels on various cell types. Because EP2 and EP4 are Gs-coupled receptors—whereas EP1 and EP3 receptors couple to Gq and Gi, respectively—cellular responses to PGE2 are highly dependent on the EP receptor subtypes expressed. In cells expressing more than 1 EP receptor subtype, PGE2 has the potential to stimulate cooperative signaling (e.g., by Gs-coupled EP2 and EP4 receptors) or competitive signaling (e.g., between Gi-coupled EP3 and Gs-coupled EP2/EP4 receptors).
The short half-life of PGE2 and its promiscuity for multiple receptors on multiple cell types has limited the utility of PGE2 as a therapeutic (22). Indeed, the potential of PGE2 as an asthma therapeutic, at least with respect to its bronchorelaxant property, has been recognized for years. The bronchodilator effect of PGE2 has been demonstrated in healthy and asthmatic subjects (23–25). However, side effects of inhaled PGE2 have been problematic, consisting of both cough and substernal burning (26, 27).
Gs-coupled EP receptors on HASM are particularly attractive targets for asthma treatment, given we and others have demonstrated an ability (superior to that of β-agonists) of PGE2 to both relax contracted HASM and inhibit mitogen-stimulated HASM proliferation (14). These Gs-, cAMP-, and PKA-dependent effects occur despite a demonstrated counteracting effect of EP3 receptor signaling in HASM (14, 28). Efforts to limit the side effects of PGE2 by developing subtype-selective EP ligands have, until recently, been disappointing. Hampered by either a lack of sufficient specificity or a lack of efficacy (29), no EP subtype–selective ligands have advanced to the clinic (30). A purported EP2-specific ligand, AH13205, was shown to relax contracted guinea pig tracheae ex vivo and induce bronchodilation in a guinea pig model of asthma (31). However, in a subsequent clinical trial, AH13205 failed to exhibit bronchodilation activity in humans, while promoting wheezing or cough (31).
More recently, ONO-AE1-259-01 and ONO-AE1-329 have been developed as subtype-selective EP2 and EP4 receptor agonists, respectively (32). Although an early study by our group examining desensitization of PGE2-mediated signaling asserted EP2 as the predominant Gs-coupled EP receptor in HASM, a subsequent study by Buckley et al. (16) demonstrated the EP4-selective ONO-AE1-329, but not ONO-AE1-259-01, potently relaxed contracted human airways ex vivo. To clarify the expression and functional utility of EP receptor subtypes in HASM, the present study characterizes the time- and dose- dependent signaling of subtype-selective EP agonists in HASM cultures and links such signaling to the regulation of HASM proliferation.
MATERIALS AND METHODS
Materials
Antibodies against phosphorylated ERK1/2 (threonine 202, tyrosine 204; 4370), hemagglutinin (HA) tag conjugated to Alexa-488 (2350), RIPA cell lysis buffer (9806), and platelet-derived growth factor (PDGF)-BB (8912) were from Cell Signaling Technology (Danvers, MA, USA). Antibodies against β-actin (A5441) and PGE2 were from MilliporeSigma (Burlington, MA, USA). Protease and phosphatase inhibitors were from Bimake (Houston, TX, USA). IRDye 680 or 800 secondary antibodies were from Rockland Immunochemicals (Limerick, PA, USA). Sulprostone and PF-04418948 were obtained from Cayman Chemical (Ann Arbor, MI, USA). ONO-AE1-259-01, ONO-AE-248, ONO-AE1-329, and ONO-AE3-208 were from Ono Pharmaceutical (Osaka, Japan). Insulin-transferrin-selenium was from Thermo Fisher Scientific (41400045; Waltham, MA, USA). All other materials were obtained from MilliporeSigma or from previously identified sources (33–35).
Cell culture
Human embryonic kidney 293 (HEK-293) cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics and maintained in an incubator at 37°C supplemented with 5% CO2, as previously described (36). Sequences encoding human EP2, EP3, and EP4 receptors were subcloned into pcDNA3.1 possessing an HA cassette, creating constructs encoding each receptor with an N-terminal HA tag. HEK-293 cells stably expressing HA-EP2, HA-EP3, HA-EP4, or a vector control were generated by transfection of constructs using Lipofectamine 3000 Reagent (Thermo Fisher Scientific), followed by selection with 250 µg/ml G418, as previously described (37). Primary HASM cultures were established from human tracheae and cultured in Ham’s F12 medium supplemented with 10% FBS as previously described (12). Culture passages 2–5 were used in the present study. In addition, senescence-resistant HASM cultures stably expressing hTERT (38) were also used. Data from the hTERT and the primary HASM cultures were similar and were, therefore, analyzed collectively. As previously described (39), cells were maintained in medium containing 10% FBS and then switched to serum-free medium 24 h prior to stimulation.
Cellular cAMP accumulation
For assay of cAMP accumulation, cells plated in 24-well plates were grown to near confluence in Ham’s F12 medium supplemented with 10% FBS, washed, and replaced with serum-free media for 24 h, then stimulated with plain Ham’s F12 medium containing a final concentration of 1 mM isobutylmethylxanthine, with vehicle (0.1% DMSO) or indicated agonist. To minimize the effects of within-group variance of absolute agonist-stimulated cAMP accumulation, values were calculated and reported as a percentage of cAMP accumulation stimulated by 100 μM forskolin (FSK) for each group, as previously described (35).
Intracellular calcium measurements
Cells were plated onto collagen-coated 96-well plates, grown to confluence, and loaded with 2 µM Fluo-4 acetoxymethyl ester (BD Biosciences, Franklin Lakes, NJ, USA) and probenecid for 1 h. Indicated final concentrations of agonists were added by an automated pipetting system in duplicate, and the 525 nm signals were generated by excitation at 485 nm with a Flex Station II (Molecular Devices, San Jose, CA, USA) as previously described (40). The net peak calcium (Ca2+) response was calculated as [(agonist-induced fluorescence units) − (basal fluorescence units)]. Maximal Ca2+ response was determined by stimulating the cells with ionomycin (1 µM).
Immunoblotting
Cultured HEK-293 or HASM cells were grown to near confluence in 12-well plates and switched to serum-free medium 24 h prior to stimulation. Cells were stimulated as indicated, lysed, sonicated briefly, electrophoresed on an 8 or 10% SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and subsequently probed with the indicated antibodies (10). Bands were visualized and signals (infrared emission) quantified directly using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA), as previously described (41).
Small interfering RNA–mediated knockdown of EP receptors in HASM cells
For knockdown of EP2, EP3, or EP4 receptors (On-Targetplus Smartpool PTGER2- L-005712-00, PTGER3- L-005713-02, PTGER4- L-005714-00; Horizon Discovery, Cambridge, United Kingdom), 2 µg of small interfering RNA (siRNA) duplexes or scrambled (Scr; control) sequence (5′-GCGCGCUUUGUAGGAUUCGdTdT-3′) were mixed in 1× siRNA buffer, and HASM cultures (plated 24 h previously at a density of 104 cells/cm−2) were transfected using Dharmafect transfection reagent (Thermo Fisher Scientific) as per the manufacturer’s instructions. After 24 h, cells were passaged onto 12-well plates for immunoblot analysis and into a matched 6-well plate for quantitative PCR analysis, followed by the indicated stimulation 72 h later corresponding to the period of knockdown. Because of the lack of sufficiently sensitive EP receptor antibodies, specific EP receptor subtype knockdown was assessed by quantitative PCR as per Saxena et al. (37).
Confocal microscopy
HEK-293 cells stably expressing indicated EP receptor or empty vector were fixed on collagen-coated coverslips with ice-cold methanol. Coverslips were permeabilized with 0.01% Triton X-100 and stained with FITC-conjugated anti-HA antibody. Confocal images were obtained on an Eclipse TI2 microscope (Nikon, Tokyo, Japan) using multitrack sequential excitation (408 for DAPI, 488 for FITC-conjugated antibody) and emission filter sets.
Retroviral expression of PKA inhibitory peptide
Stable expression of green fluorescent protein (GFP) or the GFP-chimera of the PKA inhibitory peptide (PKI) [PKI-GFP, the utility of which in HASM was previously established (11, 12, 14,17)] was achieved by retroviral infection, as previously described (11, 14). Briefly, retrovirus for the expression of each was produced by cotransfecting GP2-293 cells with p-vesicular stomatitis virus (VSV)-G vector (encoding the pantropic VSV-G envelope protein) and either pLPCX-GFP or pLPCX-PKI-GFP. At 48 h after transfection, supernatants were harvested and used to infect primary HASM cultures, and cultures were subsequently selected to homogeneity with 5 μg/ml puromycin (Tocris Bioscience, Bristol, United Kingdom).
HASM growth assays
CyQuant
Cells were plated in a 96-well plate and maintained in complete Ham’s F12 medium supplemented with 10% FBS until 90% confluent. Cells were then switched to serum-free Ham’s F12 medium, and 24 h later pretreated with indicated agonists or vehicle 30 min before addition of 10 ng/ml PDGF as per (42, 43). After 72 h treatment of PDGF with vehicle or agonists, medium was changed to assay buffer containing CyQuant dye, and fluorescence intensity was measured as per the manufacturer’s instructions. Increasing HASM cell numbers tested using CyQuant dye showed a direct linear correlation of cell number to increase in fluorescence value (r2 = 0.97, data not shown).
Cell counts
A total of 48,000 cells were seeded into a 6-well plate and maintained in complete Ham’s F12 medium supplemented with 10% FBS. After 48 h, cells were switched to serum-free Ham’s F12 medium with insulin-transferrin-selenium. Cells were starved for 48 h before addition of indicated conditions, with or without the presence of 10 ng/ml PDGF. After 72 h treatment of PDGF with vehicle or agonists, cells were counted using a hemocytometer.
Statistical analyses
Data are presented as mean ± sem values. Experiments employing HASM cells were repeated with cultures from different donors to create the indicated n values. Statistically significant differences among vehicle and treatment groups were assessed by 2-way ANOVA with Bonferroni post hoc analysis or Student’s t test where appropriate, with values of P < 0.05 sufficient to reject the null hypothesis using Prism software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Specificity of EP receptor subtype–specific ligands assessed in HEK-293 cells expressing recombinant EP receptors
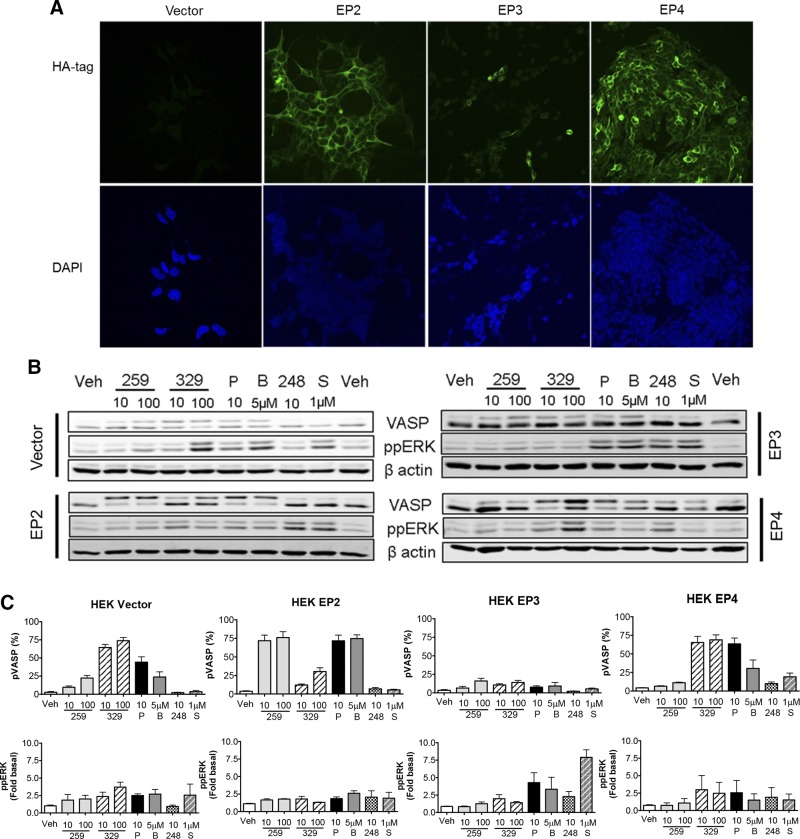
To establish the specificity of the EP subtype–specific ligands employed in the present study, we generated HEK-293 cells overexpressing HA-tagged human EP2, EP3, and EP4 receptors and a vector-transfected control line, which were confirmed by immunocytochemistry (Fig. 1A). Because we previously established that HASM cells do not express appreciable levels of EP1 receptor (44), we focused on characterization of EP2, EP3, and EP4 agonists. We first assessed the ability of agonists to stimulate phosphorylation of the PKA substrate vasodilator-stimulated phosphoprotein (VASP) as a readout for Gs-coupled receptor signaling (45, 46) using 10 and 100 nM concentrations of ligands. VASP phosphorylation by PKA at serine 157 causes a mobility shift when run on an SDS-PAGE gel (46–50 kDa), on which the top band depicts phospho-VASP (p-VASP) and culmination of both bands represents total VASP (17). In vector-expressing HEK-293 cells, ONO-AE1-259-01 and butaprost stimulated a low level of VASP phosphorylation, whereas ONO-AE1-329 and PGE2 stimulated VASP more robustly, suggesting low levels of endogenous EP2 receptor expression and slightly higher levels of endogenous EP4 receptors in this particular HEK-293 cell line (Fig. 1B, C). In HA-EP2–expresing cells, but not HA-EP3– or HA-EP4–expressing cells, both ONO-AE1-259-01 and butaprost significantly promoted VASP phosphorylation to a greater extent than that observed in vector control cells. Conversely, in HA-EP4–expressing cells, but not HA-EP2– or HA-EP3–expressing cells, only ONO-AE1-329 promoted VASP phosphorylation beyond that observed in vector control cells. PGE2 induced p-VASP levels in both HA-EP2– and HA-EP4–expressing cells, but not HA-EP3–expressing cells, beyond that induced in vector control cells (Fig. 1B, C). In the HA-EP2–, HA-EP4–, and HA-EP3–expressing lines, phosphorylation of ERK 1/2 was not increased by EP2- and EP4-specific agonists. ERK 1/2 phosphorylation was observed in all lines stimulated with PGE2 and in HA-EP3–expressing cells stimulated with ONO-AE-248 and sulprostone (EP3 ligands). These results suggest that ONO-AE1-259-01 and ONO-AE1-329 possess a high level of specificity for the EP2 and EP4 receptors, respectively.
Figure 1.
Characterization and signaling of novel EP subtype–selective agonists in HEK-293 cells expressing recombinant EP receptor subtypes. Stable lines of HEK-293 were selected after transfection of vector or indicated HA-tagged EP receptor, as described in Materials and Methods. A) Cells were plated onto collagen-coated glass coverslips, fixed, and stained for the HA tag; original magnification, ×40. B) Cells were stimulated for 10 min with indicated concentrations of agonists. All concentrations are given in nM unless otherwise indicated. C) Graphic representation of mean ± sem values from 3 to 4 experiments. p-VASP values induced by 10 and 100 nM ONO-AE1-259-01 and by 5 µM butaprost were significantly greater (P < 0.05) in HA-EP2-expressing cells relative to vector control cells (vector cells: 10 nM 10 ± 2% and 100 nM 22 ± 4%; HA-EP2 cells: 72 ± 8 and 76 ± 8%; vector cells and 5 µM butaprost 24 ± 7%, HA-EP2 cells 73 ± 5%, respectively). 248, ONO-AE-248; 259, ONO-AE1-259-01; 329, ONO-AE1-329; B, butaprost; P, PGE2; S, sulprostone.
Signaling induced by EP-specific ligands in HASM cells
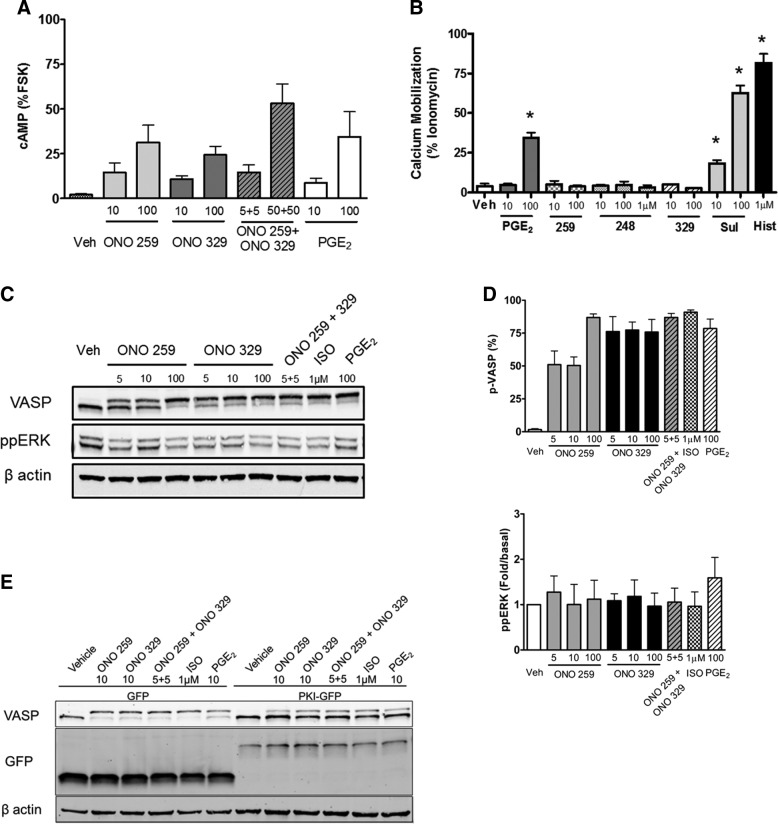
Next, we characterized EP receptor subtype signaling in HASM cells. We first assessed acute signaling indicated by either intracellular cAMP accumulation (Gs-mediated signaling readout) (Fig. 2A) or calcium mobilization (Gq-mediated signaling readout) (Fig. 2B). Accumulation of cAMP (represented as percentage of the FSK-stimulated value) response in primary HASM cells stimulated with ONO-AE1-259-01 (10 nM 14 ± 5%, 100 nM 31 ± 9%) or ONO-AE1-329 (10 nM 10 ± 2%, 100 nM 24 ± 4%) was similar. Accumulation of cAMP induced by ONO-AE1-259-01 and ONO-AE1-329 was consistent with the ability of each drug to promote VASP phosphorylation at 10 min (Fig. 2C, D). In Flexstation analysis of agonist-stimulated calcium mobilization, among the EP receptor agonists, only PGE2 and sulprostone significantly induced a calcium signal (100 nM PGE2 34 ± 3%, 10 nM sulprostone 18 ± 2%, and 100 nM sulprostone 63 ± 5% maximum response), whereas ONO-AE1-259-01 and ONO-AE1-329 had no detectable effect (Fig. 2B). Interestingly, all doses of ONO-AE-248 tested (10 nM–1 µM) failed to promote calcium mobilization.
Figure 2.
Effects on second messenger generation and signaling upon acute EP-specific stimulation in HASM cells. A) HASM cultures were grown to near confluence in 24-well plates and treated with indicated agonists for 10 min. cAMP generation was isolated, quantified, and represented as a percentage of 100 µM FSK as described in Materials and Methods. B) Calcium mobilization of primary HASM cells stimulated with indicated agonists measured using Flexstation analysis. C) Representative Western blot analysis of HASM cells stimulated for 10 min with indicated agonist. D) Graphic representation of data from Western blot analyses. 248, ONO-AE-248; 259, ONO-AE1-259-01; 329, ONO-AE1-329; Hist, histamine; Sul, sulprostone. E) Representative Western blot of HASM cultures infected with retrovirus enabling expression of GFP (control) or PKI-GFP selected with puromycin, as described in Materials and Methods. Cultures were plated into 12-well plates and stimulated with indicated agonist for 10 min. All concentrations are given in nM, unless otherwise indicated. Graphical data represent mean ± sem values from 3 to 6 experiments, 2-way ANOVA. *P < 0.05.
We next compared the effects of ONO-AE1-259-01 and ONO-AE1-329 with those of PGE2 and isoproterenol (ISO) on acute (10 min) additional Gs- (p-VASP) and Gq- or Gi- dependent (ppERK1/2) signaling outcomes (Fig. 2C, D). p-VASP levels were induced by ONO-AE1-259-01, ONO-AE1-329 (alone or in conjunction with ONO-AE1-259-01), PGE2, and ISO (Fig. 2C, D). Acute treatment with 100 nM ONO-AE1-259-01 and ONO-AE1-329 induced nearly complete VASP phosphorylation (87 ± 3 and 76 ± 10%, respectively) similar to that induced by 1 µM ISO (91 ± 2%) and 100 nM PGE2 (79 ± 8%). A lesser, yet significant, induction of p-VASP levels was stimulated with both 10 nM ONO-AE1-259-01 (50 ± 7%) and ONO-AE1-329 (77 ± 6%) (Fig. 2C, D). ERK 1/2 phosphorylation was not significantly induced by ONO-AE1-259-01, ONO-AE1-329 (alone or in conjunction with ONO-AE1-259), or ISO, and only weakly by PGE2 (Fig. 2C, D). Consistent with our previous studies assessing PKA-dependent PGE2 signaling in HASM (12, 14, 17), the EP2 (ONO-AE1-259-01)- and EP4 (ONO-AE1-329)-mediated phosphorylation of VASP was reversed by heterologous expression of PKI-GFP (Fig. 2E).
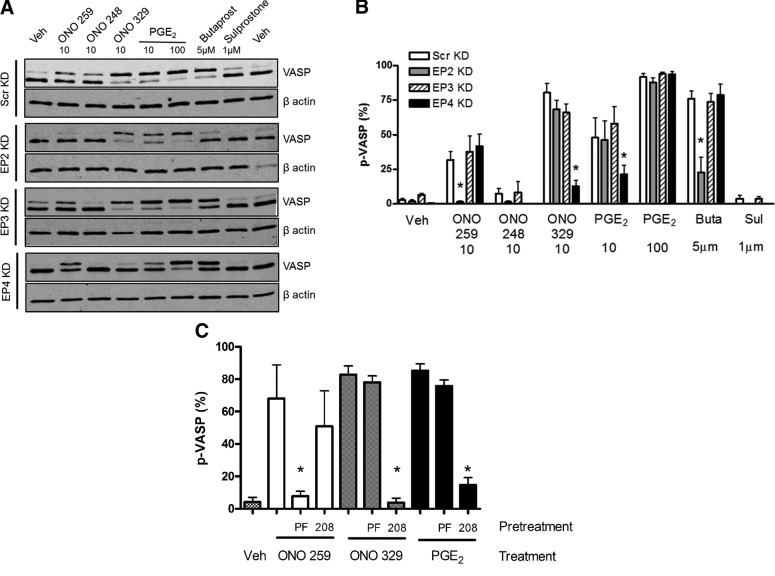
To further confirm ligand specificity and assess the dominant EP receptor in PGE2-mediated Gs signaling in HASM, we performed siRNA-mediated knockdown of EP2, EP3, and EP4 receptors. We achieved a range of 73–92% knockdown of the targeted EP receptor subtype, with specificity achieved except with Smartpool EP2 siRNA, which produced a mean 91% knockdown of EP2 receptor but a concomitant 50% knockdown of EP4 receptor (Table 1). Experiments employing alternative EP2-targeting siRNA failed to achieve superior specificity (data not shown). Knockdown of EP2 receptor, but not of EP3 or EP4 receptors, significantly (P < 0.05) inhibited the induction of p-VASP stimulated by ONO-AE1-259-01 (Scr knockdown (KD) 32 ± 6% to EP2 KD 2 ± 0.4%) or butaprost (Scr KD 76 ± 12% to EP2 KD 23 ± 24%) (Fig. 3A, B). EP4 receptor KD, but not EP2 or EP3 receptor KD, significantly (P < 0.05) inhibited the induction of p-VASP stimulated by ONO-AE1-329 (Scr KD 80 ± 7% to EP4 KD 13 ± 4%) (Fig. 3A, B). Despite the established ability for PGE2 to signal through EP2, KD of the EP2 receptor had no significant effect on the (10 or 100 nM) PGE2-mediated VASP phosphorylation (Scr KD 10 nM PGE2 48 ± 12% and 100 nM PGE2 88 ± 3% to EP2 KD 10 nM PGE2 46 ± 13% and 100 nM PGE2 88 ± 4%). Alternatively, knockdown of EP4 had a more profound effect on VASP phosphorylation after treatment with a low-dose (but not high-dose) PGE2 (Scr KD 10 nM PGE2 48 ± 12% and 100 nM PGE2 88 ± 3% to EP4 KD 10 nM PGE2 21 ± 6% and 100 nM PGE2 94 ± 2%).
TABLE 1.
EP receptor knockdown efficacy
| Knockdown efficacy (%) |
|||
|---|---|---|---|
| EP receptor | EP2 | EP3 | EP4 |
| EP2 | 91 ± 8 | 1 ± 1 | 50 ± 7 |
| EP3 | 29 ± 10 | 95 ± 2 | 42 ± 5 |
| EP4 | 20 ± 7 | 0 ± 0 | 73 ± 4 |
Percent reduction in EP receptor subtype mRNA abundance assessed by
quantitative PCR, as described in Materials and Methods.
Figure 3.
Genetic and pharmacological modulation of EP2 or EP4 receptors in HASM reverses PKA-mediated signaling. A) Representative blots probing for VASP and β actin in Scr or indicated EP receptor knockdown. B) Graphic representation of data presented in A. C) HASM pretreated for 10 min with vehicle, PF-04418948 (1 µM), or ONO-AE3-208 (1 µM) (EP2 and EP4 antagonist, respectively) before treatment with indicated agonist (50 nM) for 10 min. Graph depicts mean ± sem values from 3 to 6 experiments. Buta, butaprost; Sul, sulprostone; Veh, vehicle. All concentrations are given in nanomolar units, unless otherwise indicated. Scr vs. specific EP KD (B), or vehicle vs. indicated antagonist (C). *P < 0.05.
To further assess the relative role of EP receptor subtypes in PGE2-mediated signaling in HASM, we employed subtype-selective antagonists. HASM cells were pretreated with antagonists specific to EP2 (PF-04418948) or EP4 (ONO-AE3-208), and induction of p-VASP by stimulation with EP subtype–selective agonists or PGE2 was assessed. Pretreatment of HASM cells with PF-04418948 (1 µM) for 10 min inhibited acute p-VASP induction stimulated by ONO-AE1-259-01, but it did not inhibit induction stimulated by ONO-AE1-329 or PGE2. Interestingly, ONO-AE3-208 (1 µM) pretreatment inhibited p-VASP induction stimulated by both ONO-AE1-329 and PGE2 (vehicle/PGE2 85 ± 4% to ONO-AE-208/PGE2 15 ± 5%), but not by stimulation by ONO-AE1-259-01 (Fig. 3C). Taken together, these data implicate EP4 as the predominant effector of PGE2-mediated Gs signaling.
Cooperativity of EP2 and EP4 in time-dependent signaling and inhibition of mitogen-stimulated HASM proliferation
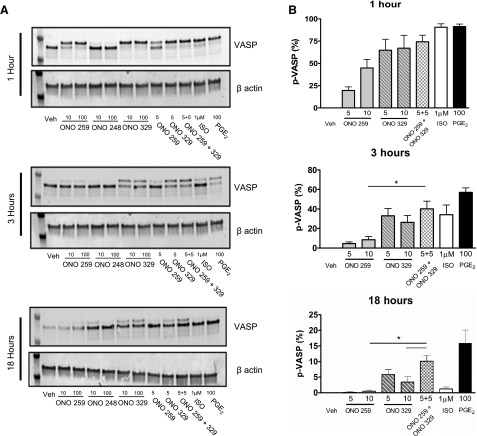
To further characterize the effect of EP-subtype signaling, we investigated the kinetics of prolonged ONO-AE1-259-01 or ONO-AE1-329 signaling. Because specific activation of either EP2 or EP4 can promote VASP phosphorylation in HASM (Figs. 2C, D and 3), we investigated whether we could detect cooperative signaling by treating HASM with both EP2 and EP4 agonists. In HASM cells stimulated for 1 h, 100 nM PGE2 consistently induced higher p-VASP levels than 5–10 nM of either ONO-AE1-259-01 or ONO-AE1-329 alone. Stimulation with ONO-AE1-259-01 and ONO-AE1-329 (5 nM each) for 3 h induced VASP phosphorylation that was greater than that induced by ONO-AE1-259-01 (10 nM) (P < 0.05), but only modestly (P > 0.05) greater than that stimulated by ONO-AE1-329 (10 nM) alone. After 18 h stimulation, p-VASP levels induced by combined stimulation with ONO-AE1-259-01 and ONO-AE1-329 (5 nM each) were significantly (P < 0.05) greater than those stimulated by either agonist alone (10 nM) and were comparable to that stimulated by 100 nM PGE2 (Fig. 4).
Figure 4.
Time-dependent regulation of VASP levels by subtype-selective EP agonists alone or combined. A) Representative blots probing for VASP or β-actin of HASM cells grown to near confluence in 12-well plates and stimulated with indicated agonist and time. B) Graphic representation of data presented in A. All concentrations are given in nanomolar units, unless otherwise indicated. Graph depicts mean ± sem values from 6 experiments. *P < 0.05.
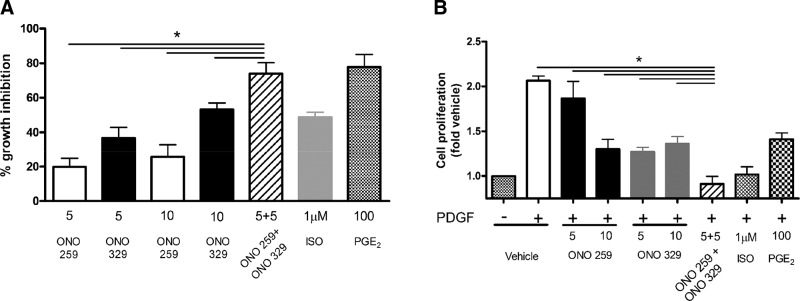
We have previously reported the PKA dependence of the antimitogenic effect of both β-agonists and PGE2 on HASM and have noted a correlation of the antimitogenic effect of these agents with their ability to sustain PKA activity (12, 14). Consistent with the ability of combined EP2 and EP4 agonists to signal chronically in an additive or greater manner, combined treatment of ONO-AE1-259-01 and ONO-AE1-329 (5 nM each) caused an inhibition of PDGF-stimulated proliferation significantly greater than that caused by either agonist alone (10 nM). Using CyQuant analysis, the percent inhibition elicited by combined (5 nM each) ONO-AE1-259-01 and ONO-AE1-329 treatment approached that elicited by 100 nM PGE2 (74 ± 6 and 78 ± 3%, respectively), and was significantly (P < 0.05) greater than that elicited by the β-agonist ISO (49%) (Fig. 5A). Similarly, using cell counts, the percent inhibition elicited by combined (5 nM each) ONO-AE1-259-01 and ONO-AE1-329 treatment approached that elicited by 100 nM PGE2 (100 ± 8 and 98 ± 8%, respectively), and was significantly (P < 0.05) greater than that elicited by either ONO-AE1-259-01 and ONO-AE1-329 at 5 or 10 nM (72 ± 11 and 66 ± 8%, at 10 nM concentrations respectively) or the β-agonist ISO (61 ± 7%) (Fig. 5B).
Figure 5.
Cooperative growth inhibition by EP2 and EP4 receptor agonists in HASM. HASM cultures were pretreated with indicated agonists prior to stimulation with 10 ng/ml PDGF for 72 h, and cell proliferation was assessed by CyQuant (A) or cell counts (B). All concentrations are given in nanomolar units, unless otherwise indicated. Graph represents mean ± sem values from 7 experiments. *P < 0.05.
DISCUSSION
Findings from the present study provide the first comprehensive assessment of HASM EP receptor subtype signaling and regulation of proliferation, enabled by the recent development of subtype-specific agonists of the EP2 and EP4 receptors. Both EP2 and EP4 receptor subtype–specific agonists demonstrated activity and specificity in HASM cells, mediating cAMP generation and prolonging downstream Gs signaling. We also observed positive cooperativity upon treatment with EP2 and EP4 agonists with respect to prolonged downstream Gs signaling, which was associated with a significantly greater inhibition of HASM proliferation than that mediated by either agonist alone. Interestingly, despite a clear and strong ability of the EP2 receptor agonist to signal in HASM, EP2 receptors appeared dispensable in signaling by PGE2 in the cells. Collectively, these findings advance our understanding of EP receptor biology and pharmacology and suggest efficacious strategies for the application of EP ligands as asthma therapies.
Based on our previous study that used primarily artificial expression systems to demonstrate that the EP2 receptor, but not the EP4 receptor, was resistant to G protein-coupled receptor kinase/arrestin-mediated desensitization, we assumed that the pronounced ability (relative to that of β-agonist) of PGE2 to sustain signaling in HASM was explained by HASM preferentially expressing the EP2 receptor (35). We further postulated that such sustained signaling contributed to the greater ability of PGE2 to inhibit HASM proliferation. However, the recent study by Buckley et al. (16) demonstrated that ONO-AE1-329, but not ONO-AE1-259-01, relaxed contracted human airways, suggesting that EP4 was the predominant EP receptor subtype expressed in HASM. In the present study, we found that EP2 knockdown had no significant effect on PGE2-mediated VASP signal, whereas EP4 knockdown had a robust attenuation of PGE2-mediated VASP phosphorylation (Fig. 3). Furthermore, pretreatment with an EP4 antagonist (but not EP2 antagonist) attenuated PGE2-mediated VASP signaling. These data support EP4 as the physiologically predominant EP receptor subtype driving PGE2-mediated Gs signaling and bronchodilation.
Curiously, in the current study, we demonstrate the ability of a specific EP2 receptor agonist to acutely induce cAMP generation and VASP phosphorylation in HASM cells in a manner comparable to that induced by specific EP4 receptor agonism. Moreover, our studies of recombinant EP receptor subtypes in HEK-293 cells demonstrate a clear ability of PGE2 to stimulate EP2 receptor signaling. Why EP2 receptor signaling in HASM appears dissociated from the functional effect of HASM relaxation and bronchodilation is unclear. One possible explanation relates to compartmentalized signaling; an interesting study by Bogard et al. (47) provides clear evidence of compartmentalized signaling distinguishing disparate functional effects EP2 receptor and β-2-adrenoceptor agonism in HASM. Whether EP2 and EP4 receptors signal in distinct compartments remains to established, but with respect to the regulation of HASM proliferation, unlike in the regulation of HASM contraction EP2 agonism is clearly impactful and can cooperate in a positive manner with EP4 agonism.
The extent to which the use of dual EP2 and EP4 receptor agonist therapy can be employed in the management of obstructive lung disease remains to be established. However, the current study strongly suggests that, at least for 1 important pathogenic feature of asthma (increased airway smooth muscle mass contributing to airway remodeling), EP2 and EP4 receptor signaling is therapeutically cooperative. Moreover, the approach of combined EP2 and EP4 receptor agonist dual therapy represents a logical strategy to avoid the problematic side effects of inhaled PGE2 (cough and substernal burning), which appear to be mediated by the EP3 receptor (48). Future studies examining the efficacy and safety of this dual therapy in in vivo models of asthma are the next important step in advancing the clinical utility of targeting EP receptor subtypes.
ACKNOWLEDGMENTS
This study was funded by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL136209 (to R.B.P.), HL58506 (to R.B.P.), and HL139035 (to J.V.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- EP
E-prostanoid
- FBS
fetal bovine serum
- FSK
forskolin
- GFP
green fluorescent protein
- HA
hemagglutinin
- HASM
human airway smooth muscle
- HEK-293
human embryonic kidney 293
- ISO
isoproterenol
- KD
knockdown
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PKI
PKA inhibitor peptide
- p-VASP
phosphovasodilator-stimulated phosphoprotein
- Scr
scramble
- siRNA
small interfering RNA
- VASP
vasodilator-stimulated phosphoprotein
AUTHOR CONTRIBUTIONS
J. V. Michael and A. Gavrila performed experiments, analyzed and interpreted data, and wrote the manuscript; D. A. Deshpande and R. B. Penn designed the studies, analyzed and interpreted the data, and wrote the manuscript; and A. P. Nayak, T. Pera, J. R. Liberato, S. R. Polischak, and S. D. Shah performed experiments, analyzed data, and provided comments contributing to the final content of the manuscript.
REFERENCES
- 1.Birrell M. A., Maher S. A., Dekkak B., Jones V., Wong S., Brook P., Belvisi M. G. (2015) Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax 70, 740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sastre B., del Pozo V. (2012) Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm. 2012, 645383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claar D., Hartert T. V., Peebles R. S., Jr (2015) The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 9, 55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 5.Feng C., Beller E. M., Bagga S., Boyce J. A. (2006) Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood 107, 3243–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazzeri N., Belvisi M. G., Patel H. J., Yacoub M. H., Chung K. F., Mitchell J. A. (2001) Effects of prostaglandin E2 and cAMP elevating drugs on GM-CSF release by cultured human airway smooth muscle cells. Relevance to asthma therapy. Am. J. Respir. Cell Mol. Biol. 24, 44–48 [DOI] [PubMed] [Google Scholar]
- 7.Liu T., Laidlaw T. M., Katz H. R., Boyce J. A. (2013) Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc. Natl. Acad. Sci. USA 110, 16987–16992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundequist A., Nallamshetty S. N., Xing W., Feng C., Laidlaw T. M., Uematsu S., Akira S., Boyce J. A. (2010) Prostaglandin E(2) exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J. Immunol. 184, 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belvisi M. G., Saunders M., Yacoub M., Mitchell J. A. (1998) Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound reductions in cell growth. Br. J. Pharmacol. 125, 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billington C. K., Kong K. C., Bhattacharyya R., Wedegaertner P. B., Panettieri R. A., Jr., Chan T. O., Penn R. B. (2005) Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry 44, 14595–14605 [DOI] [PubMed] [Google Scholar]
- 11.Guo M., Pascual R. M., Wang S., Fontana M. F., Valancius C. A., Panettieri R. A., Jr., Tilley S. L., Penn R. B. (2005) Cytokines regulate beta-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44, 13771–13782 [DOI] [PubMed] [Google Scholar]
- 12.Misior A. M., Yan H., Pascual R. M., Deshpande D. A., Panettieri R. A., Penn R. B. (2008) Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A and are unmasked by steroids and cyclooxygenase inhibitors. Mol. Pharmacol. 73, 566–574 [DOI] [PubMed] [Google Scholar]
- 13.Pascual R. M., Billington C. K., Hall I. P., Panettieri R. A., Jr., Fish J. E., Peters S. P., Penn R. B. (2001) Mechanisms of cytokine effects on G protein-coupled receptor-mediated signaling in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1425–L1435 [DOI] [PubMed] [Google Scholar]
- 14.Yan H., Deshpande D. A., Misior A. M., Miles M. C., Saxena H., Riemer E. C., Pascual R. M., Panettieri R. A., Penn R. B. (2011) Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J. 25, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birrell M. A., Maher S. A., Buckley J., Dale N., Bonvini S., Raemdonck K., Pullen N., Giembycz M. A., Belvisi M. G. (2013) Selectivity profiling of the novel EP2 receptor antagonist, PF-04418948, in functional bioassay systems: atypical affinity at the guinea pig EP2 receptor. Br. J. Pharmacol. 168, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley J., Birrell M. A., Maher S. A., Nials A. T., Clarke D. L., Belvisi M. G. (2011) EP4 receptor as a new target for bronchodilator therapy. Thorax 66, 1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan S. J., Deshpande D. A., Tiegs B. C., Misior A. M., Yan H., Hershfeld A. V., Rich T. C., Panettieri R. A., An S. S., Penn R. B. (2014) β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 289, 23065–23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilley S. L., Hartney J. M., Erikson C. J., Jania C., Nguyen M., Stock J., McNeisch J., Valancius C., Panettieri R. A., Jr., Penn R. B., Koller B. H. (2003) Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L599–L606 [DOI] [PubMed] [Google Scholar]
- 19.McCoy J. M., Wicks J. R., Audoly L. P. (2002) The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Invest. 110, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuchiwal S. K., Balestrieri B., Raff H., Boyce J. A. (2017) Endogenous prostaglandin E2 amplifies IL-33 production by macrophages through an E prostanoid (EP)2/EP4-cAMP-EPAC-dependent pathway. J. Biol. Chem. 292, 8195–8206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca B., FitzGerald G. A. (2002) Cyclooxygenases and prostaglandins: shaping up the immune response. Int. Immunopharmacol. 2, 603–630 [DOI] [PubMed] [Google Scholar]
- 22.Bygdeman M. (2003) Pharmacokinetics of prostaglandins. Best Pract. Res. Clin. Obstet. Gynaecol. 17, 707–716 [DOI] [PubMed] [Google Scholar]
- 23.Melillo E., Woolley K. L., Manning P. J., Watson R. M., O’Byrne P. M. (1994) Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am. J. Respir. Crit. Care Med. 149, 1138–1141 [DOI] [PubMed] [Google Scholar]
- 24.Walters E. H., Bevan C., Parrish R. W., Davies B. H., Smith A. P. (1982) Time-dependent effect of prostaglandin E2 inhalation on airway responses to bronchoconstrictor agents in normal subjects. Thorax 37, 438–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami Y., Uchiyama K., Irie T., Murao M. (1973) Evaluation of aerosols of prostaglandins E1 and E2 as bronchodilators. Eur. J. Clin. Pharmacol. 6, 127–132 [DOI] [PubMed] [Google Scholar]
- 26.Walters E. H., Davies B. H. (1982) Dual effect of prostaglandin E2 on normal airways smooth muscle in vivo. Thorax 37, 918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka H., Kanako S., Abe S. (2005) Prostaglandin E2 receptor selective agonists E-prostanoid 2 and E-prostanoid 4 may have therapeutic effects on ovalbumin-induced bronchoconstriction. Chest 128, 3717–3723 [DOI] [PubMed] [Google Scholar]
- 28.Aso H., Ito S., Mori A., Suganuma N., Morioka M., Takahara N., Kondo M., Hasegawa Y. (2013) Differential regulation of airway smooth muscle cell migration by E-prostanoid receptor subtypes. Am. J. Respir. Cell Mol. Biol. 48, 322–329 [DOI] [PubMed] [Google Scholar]
- 29.Markovič T., Jakopin Ž., Dolenc M. S., Mlinarič-Raščan I. (2017) Structural features of subtype-selective EP receptor modulators. Drug Discov. Today 22, 57–71 [DOI] [PubMed] [Google Scholar]
- 30.Jones R. L., Giembycz M. A., Woodward D. F. (2009) Prostanoid receptor antagonists: development strategies and therapeutic applications. Br. J. Pharmacol. 158, 104–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nials A. T., Vardey C. J., Denyer L. H., Thomas M., Sparrow S. J., Shepherd G. D., Coleman R. A. (1993) AH13205, a selective prostanoid EP2-receptor agonist. Cardiovasc. Drug Rev. 11, 165–179 [Google Scholar]
- 32.Sugimoto Y., Narumiya S. (2007) Prostaglandin E receptors. J. Biol. Chem. 282, 11613–11617 [DOI] [PubMed] [Google Scholar]
- 33.Pera T., Deshpande D. A., Ippolito M., Wang B., Gavrila A., Michael J. V., Nayak A. P., Tompkins E., Farrell E., Kroeze W. K., Roth B. L., Panettieri R. A., Jr., Benovic J. L., An S. S., Dulin N. O., Penn R. B. (2018) Biased signaling of the proton-sensing receptor OGR1 by benzodiazepines. FASEB J. 32, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshpande D. A., Theriot B. S., Penn R. B., Walker J. K. (2008) Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 22, 2134–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong K. C., Gandhi U., Martin T. J., Anz C. B., Yan H., Misior A. M., Pascual R. M., Deshpande D. A., Penn R. B. (2008) Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 47, 9279–9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik S., Billington C. K., Pascual R. M., Deshpande D. A., Stefano F. P., Kohout T. A., Eckman D. M., Benovic J. L., Penn R. B. (2005) Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J. Biol. Chem. 280, 8722–8732 [DOI] [PubMed] [Google Scholar]
- 37.Saxena H., Deshpande D. A., Tiegs B. C., Yan H., Battafarano R. J., Burrows W. M., Damera G., Panettieri R. A., Dubose T. D., Jr., An S. S., Penn R. B. (2012) The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br. J. Pharmacol. 166, 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gosens R., Stelmack G. L., Dueck G., McNeill K. D., Yamasaki A., Gerthoffer W. T., Unruh H., Gounni A. S., Zaagsma J., Halayko A. J. (2006) Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L523–L534 [DOI] [PubMed] [Google Scholar]
- 39.Penn R. B., Panettieri R. A., Jr., Benovic J. L. (1998) Mechanisms of acute desensitization of the beta2AR-adenylyl cyclase pathway in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 19, 338–348 [DOI] [PubMed] [Google Scholar]
- 40.Deshpande D. A., Wang W. C., McIlmoyle E. L., Robinett K. S., Schillinger R. M., An S. S., Sham J. S., Liggett S. B. (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pera T., Hegde A., Deshpande D. A., Morgan S. J., Tiegs B. C., Theriot B. S., Choi Y. H., Walker J. K., Penn R. B. (2015) Specificity of arrestin subtypes in regulating airway smooth muscle G protein-coupled receptor signaling and function. FASEB J. 29, 4227–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan S., Sharma P., Shah S. D., Deshpande D. A. (2017) Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L154–L165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma P., Panebra A., Pera T., Tiegs B. C., Hershfeld A., Kenyon L. C., Deshpande D. A. (2016) Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L365–L376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess J. K., Ge Q., Boustany S., Black J. L., Johnson P. R. (2004) Increased sensitivity of asthmatic airway smooth muscle cells to prostaglandin E2 might be mediated by increased numbers of E-prostanoid receptors. J. Allergy Clin. Immunol. 113, 876–881 [DOI] [PubMed] [Google Scholar]
- 45.Halbrügge M., Walter U. (1989) Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur. J. Biochem. 185, 41–50 [DOI] [PubMed] [Google Scholar]
- 46.Butt E., Abel K., Krieger M., Palm D., Hoppe V., Hoppe J., Walter U. (1994) cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J. Biol. Chem. 269, 14509–14517 [PubMed] [Google Scholar]
- 47.Bogard A. S., Adris P., Ostrom R. S. (2012) Adenylyl cyclase 2 selectively couples to E prostanoid type 2 receptors, whereas adenylyl cyclase 3 is not receptor-regulated in airway smooth muscle. J. Pharmacol. Exp. Ther. 342, 586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maher S. A., Birrell M. A., Belvisi M. G. (2009) Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am. J. Respir. Crit. Care Med. 180, 923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]