Abstract
Human leukocyte antigen G (HLA-G), a nonclassic HLA class Ib molecule involved in the maintenance of maternal tolerance to semiallogeneic fetal tissues during pregnancy, has emerged as a potential therapeutic target to control allograft rejection. We demonstrate here that the level of soluble HLA-G dimer was higher in a group of 90 patients with a functioning renal allograft compared with 40 patients who rejected (RJ) their transplants. The HLA-G dimer level was not affected by demographic status. One of the potential mechanisms in tissue-organ allograft rejection involves the induction of granzymes and perforin, which are the main effector molecules expressed by CD8+ cytotoxic T lymphocytes and function to destroy allogeneic transplants. Using genomics and molecular and cellular analyses of cells from T-cell–mediated RJ and nonrejected kidney transplant patients, cells from leukocyte Ig-like receptor B1 (LILRB1) transgenic mice, humanized mice, and genetically engineered HLA-G dimer, we demonstrated a novel mechanism by which HLA-G dimer inhibits activation and cytotoxic capabilities of human CD8+ T cells. This mechanism implicated the down-regulation of Granzyme B expression and the essential involvement of LILRB1. Thus, HLA-G dimer has the potential to be a specific and effective therapy for prevention of allograft rejection and prolongation of graft survival.—Ajith, A., Portik-Dobos, V., Nguyen-Lefebvre, A. T., Callaway, C., Horuzsko, D. D., Kapoor, R., Zayas, C., Maenaka, K., Mulloy, L. L., Horuzsko, A. HLA-G dimer targets Granzyme B pathway to prolong human renal allograft survival.
Keywords: HLA-G, human kidney transplantation, humanized mouse
Kidney transplantation is the most optimal treatment for patients facing end-stage renal disease. However, up to 15% of transplant patients undergo acute T cell–mediated rejection (TCMR) in the first 5 yr after kidney transplantation (1, 2). Although advances in potent immunosuppressive agents as part of the maintenance regimen for kidney transplantation have led to markedly decreased rejection rates, this treatment is associated with adverse side effects, including increased risk for cancer and opportunistic infections (3–6). Therefore, there is a growing interest for the development of alternative therapeutic approaches for the management of solid-organ transplants (4, 5, 7, 8). Among them, the induction of transplantation tolerance is especially attractive because it facilitates modulation of the host immune system to specifically prevent targeting of the transplanted graft tissue. Recently, human leukocyte antigen G (HLA-G), a nonclassic HLA class Ib molecule involved in the maintenance of maternal tolerance to semiallogeneic fetal tissues during pregnancy, has emerged as a potential therapeutic target to control allograft rejection (9–15). HLA-G has highly restricted tissue expression and is found only in trophoblastic cells of fetal tissues, thymic medulla, cornea, and pancreatic islet cells. It is found in either a membrane-bound form on specific immune cells or as a soluble isoform in the blood [soluble HLA-G (sHLA-G)], where it can undergo dimerization to form HLA-G dimers (sHLA-G dimers) that have a more potent signaling capacity than their monomeric variants. It has been well established that HLA-G via its receptors leukocyte Ig-like receptor B1 (LILRB1) (also called LIR1, ILT2, or CD85j), LILRB2 (LIR2, ILT4, or CD85d), and killer cell Ig-like receptor 2DL4 can inhibit immune responses by targeting the maturation and function of dendritic cells, allo-proliferation of CD4+ T cells, and the cytotoxicity of natural killer cells and virus-specific CD8+ T cells (16–18). In addition, HLA-G stimulates the development of immunosuppressive myeloid-derived suppressor cells and regulatory T cells (19, 20).
We had previously reported a positive correlation between high levels of sHLA-G dimers in plasma of patients and the prolongation of kidney allograft survival (15). In the present study, with an expanded sample number, we were able to demonstrate that the level of sHLA-G dimer is not affected by demographic status such as age, gender, or race of the transplant recipients. However, the level of sHLA-G dimer differed significantly between patients who accepted or rejected (RJ) a kidney transplant. Here, we demonstrate that patients with successful kidney allograft survival had an elevated number of circulating CD8+ T cells expressing HLA-G in contrast to patients who had RJ their transplants. In addition, patients with prolongation of allograft survival had decreased numbers of CD8+ T cells expressing Granzyme B (GZMB). Kidney transplant graft tissue destruction is critically mediated by infiltrating CD8+ T cells (21–23). These cells differentiate to form cytotoxic T lymphocytes, which undergo granule exocytosis and release the potent mediators of apoptosis, granzymes, and perforin (24–26). In addition to the well-established cytotoxicity of granzymes, it has been demonstrated that granzymes trigger proinflammatory cytokine responses (27, 28). Moreover, Granzyme-mediated extracellular matrix degradation further contributes to inflammation, one of the crucial factors in graft rejection (29–31). Histologic studies have shown the abundance of GZMB in RJ kidney graft tissues and numerous animal model studies have elegantly established the critical necessity of these GZMB-dependent apoptotic pathways to facilitate graft tissue destruction (32, 33). It has been well established that HLA-G can inhibit dendritic cell function and expand myeloid-derived suppressor cells in LILRB2 and LILRB1 transgenic mice, respectively, but little is known about the effect of HLA-G dimer on CD8+ T cells. Using genomics and molecular and cellular analyses of human CD8+ T cells, cells from LILRB1 transgenic mice, humanized mice, and genetically engineered HLA-G dimer, we demonstrated a novel mechanism by which HLA-G dimer inhibits activation and cytotoxic capabilities of human CD8+ T cells. This mechanism implicated the down-regulation of GZMB expression and the essential involvement of LILRB1. Because sHLA-G dimer is augmented in the circulation in patients with prolongation of kidney allograft survival, the potential of HLA-G dimer may indeed be an important therapeutic tool to limit rejection episodes and improve long-term outcomes following tissue-organ transplantation.
MATERIALS AND METHODS
Enrolled cohort and study design
Kidney transplant recipients (KTRs) were enrolled for the study as per protocol 611136, approved by the Augusta University Institutional Review Board. The blood samples from healthy volunteers (HVs) were obtained from the Shepeard Community Blood Center, Augusta, GA, USA. Written informed consent was obtained from all subjects participating in the study. A total of 130 KTRs were enrolled in the study, including 64 males and 66 females with a median age of >40 yr. TCMR was confirmed from a renal allograft biopsy by a pathologist and was selected as criteria for the RJ group. Forty patients had graft failure as a result of TCMR after a mean of 1863 d. The control nonrejected (NR) group was selected from among 90 patients who showed no history of rejection (after using the same immunosuppressive and therapeutic regimen) and retained a functional kidney allograft for >5 yr. The majority of the kidney transplant patients had suffered end-stage renal disease due to complications associated with diabetes (17.4%), glomerular disease (42.42%), polycystic kidney disease (33.33%), hypertension (21.87%), and other causes (18.18%).
Animals and generation of humanized mouse model
The LILRB1 transgenic mouse model was generated in our laboratory as previously described (34). For development of a humanized mouse model, we used NOD-scid γ (NSG) mice from The Jackson Laboratory (Bar Harbor, ME, USA). NSG mice (5–12 wk old) were given a single intravenous lateral tail injection of 8.0 × 106 human peripheral blood mononuclear cells (PBMCs) from HVs. The samples were taken with signed informed consent with approval from the Institutional Review Board of Augusta University. PBMCs were collected in EDTA and purified by Histopaque 1077 (MilliporeSigma, Burlington, MA, USA) density gradient. To assess the rate of human cell engraftment in the humanized mice, flow cytometry analysis was conducted using blood samples from the humanized mice and control nonengrafted mice. Red blood cells were lysed using ammonium-chloride-potassium lysis buffer (A1049201; Thermo Fisher Scientific, Waltham, MA, USA) followed by staining of PBMCs with anti-human CD45 (368531,1:400) and anti-mouse CD45 (109823,1:400) mAb (all from BioLegend, San Diego, CA, USA). For studies on human allogeneic responses in vivo, a humanized mouse received 2.0 × 106 PBMCs from unrelated donors with 25 U/ml recombinant human IL-2 (rhIL-2) (202-IL-010; R&D Systems, Minneapolis, MN, USA). In some experiments, mice were treated with 30 ng of HLA-G dimer on d 0. HLA-G dimer was prepared and purified as previously described in refs 35 and 36. On d 5, mice were euthanized, and human cells were analyzed for the activation of T cells and intracellular expression of GZMB. All animals were housed in pathogen-free facilities and maintained in accordance with Augusta University Institutional Animal Care and Use Committee guidelines. These studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA].
Human plasma separation, depletion of albumin and IgG, immunoprecipitation, and immunoblotting
Depletion of unwanted proteins that could interfere with the immunoprecipitation of HLA-G from plasma was performed using ProteoPrep Immunoaffinity Albumin and IgG Depletion Kit (Protia-1KT; MilliporeSigma) in accordance with the manufacturer’s recommendations. Total protein concentration was determined by the Bradford Protein Assay (500-0006; Bio-Rad, Hercules, CA, USA). Immunoprecipitation was performed as previously described in Ezeakile et al. (15). Briefly, proteins from all depleted human samples were resuspended at the same concentration. Equal amounts of protein from each sample were mixed with cold RIPA buffer at 1:1 ratio and incubated on ice for 15 min. Protein G bead slurry (161-4021; Bio-Rad) was then added to precipitate the remaining Ig, and the mixture was incubated at 4°C for 1 h and centrifuged at 8000 g for 1 min. Two microliters of HLA-G antibody (MEM-G/9), anti-HLA-G mAb (7758; Abcam, Cambridge, United Kingdom) was added to the supernatant, and the mixture was incubated at 4°C overnight. After incubation, protein G bead slurry was again added to the plasma lysate, which was then incubated at 4°C for 1 h and centrifuged at 8000 g for 30 s. Samples were run under both reduced and nonreduced conditions. For reduced conditions, the mixture was denatured at 95°C for 5 min and centrifuged at 8000 g for 5 min. The supernatant was loaded onto 10% resolving gels for electrophoresis. Proteins were transferred onto a PVDF membrane (1PFL00010; MilliporeSigma). The membrane was blocked with 5% bovine serum albumin (Thermo Fisher Scientific) and incubated with MEM-G/9 mAb (1:2000; Abcam), followed by goat anti-mouse IgG-horseradish peroxidase–conjugated antibody (sc-516102, 1:2000; Santa Cruz Biotechnology, Dallas, TX, USA). Chemiluminescent horseradish peroxidase–conjugated detection reagent (E2500; Denville Scientific, Holliston, MA, USA) was used to detect the HLA-G–specific bands. Quantitation of blotted proteins was determined by densitometry analysis of scanned films using ImageJ software (NIH).
Human T-cell activation, HLA-G dimer treatment, and LILRB1 receptor–blocking studies
1 × 107 human PBMCs were incubated for 3 d in Roswell Park Memorial Institute (RPMI) 1640 medium containing 25 U/ml rhIL-2 and 2.5 µg/ml of concanavalin A (ConA) (C2010; MilliporeSigma) for adequate stimulation (23). For inhibition studies, cells were treated with 10 ng/ml of HLA-G dimer for up to 4 h prior to stimulation. In addition, LILRB1 receptor–blocking studies were performed with 20 µg/ml of anti-LILRB1 mAb (333702; BioLegend) prior to HLA-G dimer pretreatment and stimulation. Cell membrane–bound HLA-G was blocked using 50 µg/ml of anti-HLA-G mAb (130-111-848; Miltenyi Biotech, Bergisch Gladbach, Germany).
Human transplant rejection PCR arrays and real-time quantitative PCR
Cells from patients of both NR and RJ groups were harvested, and total RNA was isolated using Trizol reagent (15-596-026; Thermo Fisher Scientific) and purified using RNEasy mini kit (74104; Qiagen, Venlo, The Netherlands). A total of 1 µg high-quality total RNA was then reverse transcribed using the First Strand Synthesis Kit (330404; Qiagen) and subsequently analyzed by the Human Transplant Rejection RT2 Profiler PCR Array (PAHS-166Z; Qiagen) in accordance with the manufacturer’s instructions. Qiagen’s online analysis tool (https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/) was used to formulate the comparative heat maps, and fold change was determined by calculating the ratio of mRNA levels to control values using the Δ cycle threshold (Ct) method (2−ΔΔCt). All data were normalized based on the mean of the following 3 housekeeping genes: ACTB, GAPDH, and HPRT1. PCR conditions used for the Applied Biosystems Step One Plus Real Time PCR System (Applied Biosystems, Foster City, CA, USA) involved holding for 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. For real-time quantitative PCR (qPCR) analysis, a total of 1 µg of total RNA was isolated and then reverse transcribed using the first strand synthesis kit (Qiagen). One nanogram of cDNA was then amplified by real-time PCR using primers. Specific primer sequences and expected product sizes are listed in Table 1. Quantification was performed by normalizing the Ct values of each sample to rRNA, ACTB, GAPDH, and HPRT1. Values are expressed as fold induction in comparison with the NR group. Real-time qPCR was performed for 40 cycles of 20 s at 95°C and 30 s at different temperatures for an annealing or extension step using an ABI StepOnePlus Detection System (Applied Biosystems).
TABLE 1.
Primer sequences used for real-time qPCR and the expected product size primer
| Sequence, 5′–3′ |
Product size (bp) | ||
|---|---|---|---|
| Gene | Forward | Reverse | |
| CD14 | CTGGAACAGGTGCCTAAAGGAC | GTCCAGTGTCAGGTTATCCACC | 120 |
| B2M | CCACTGAAAAAGATGAGTATGCCT | CCAATCCAAATGCGGCATCTTCA | 126 |
| CXCR4 | CTCCTCTTTGTCATCACGCTTCC | GGATGAGGACACTGCTGTAGAG | 127 |
| TGFB1 | TACCTGAACCCGTGTTGCTCTC | GTTGCTGAGGTATCGCCAGGAA | 122 |
| ACTB | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT | 135 |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA | 131 |
| IL2 | AGAACTCAAACCTCTGGAGGAAG | GCTGTCTCATCAGCATATTCACAC | 153 |
| GZMB | CGACAGTACCATTGAGTTGTGCG | TTCGTCCATAGGAGACAATGCCC | 122 |
| CCL4 | GGTCATACACGTACTCCTGGAC | GCTTCCTCGCAACTTTGTGGTAG | 124 |
| CXCL11 | AAGGACAACGATGCCTAAATCCC | CAGATGCCCTTTTCCAGGACTTC | 112 |
| GZMA | CCACACGCGAAGGTGACCTTAA | CCTGCAACTTGGCACATGGTTC | 130 |
| VCAM1 | GATTCTGTGCCCACAGTAAGGC | TGGTCACAGAGCCACCTTCTTG | 118 |
| PRF1 | CAGTACAGCTTCAGCACTGAC | ATGAAGTGGGTGCCGTAGTTG | 176 |
| LILRB1 | CTCCCTATGAGTGGTCTCTACC | CTGTTGTAGCCAGCATCAGAGC | 151 |
Human gene 2.0 Sense Target array and data analysis
Cells from HVs were harvested and total RNA isolated as previously described. RNA purity and concentration were evaluated by spectrophotometry using NanoDrop ND-1000 (Thermo Fisher Scientific). RNA quality was assessed by the Agilent 2200 TapeStation (Agilent Technologies, Santa Clara, CA, USA) and assured of an RNA integrity number ≥7. The Human Gene 2.0 Sense Target (ST) Array (Thermo Fisher Scientific), which covers 24,838 genes, was used for the gene expression profiling. Total RNA samples were processed using the GeneChip Whole Transcript Plus Reagent Kit (Thermo Fisher Scientific). Briefly, the kit was used to generate sense strand cDNAs using 250 ng of starting RNA material. The synthesized sense strand cDNAs (5.5 µg) were fragmented, biotin labeled, and hybridized onto the arrays according to the manufacturer’s protocol. After 16 h of hybridization, the arrays were washed and stained using Affymetrix GeneChip Fluidics Station 450 systems (Thermo Fisher Scientific). The stained arrays were scanned on an Affymetrix GeneChip Scanner 3000 (Thermo Fisher Scientific). Data were obtained in the form of .cel files, which were imported into Partek Genomic Suites v.6.6 (Partek, St. Louis, MO, USA) using the standard import tool with a Robust Multi-array Average normalization. The differential expressions were calculated using ANOVA of the Partek package and filtered with a cutoff value of P < 0.05 and fold-change cutoffs to screen out the differentially expressed genes in each comparison. The significant gene list was used to generate a hierarchical clustering plot by the standardized expression values. Further analysis for the Venn diagram and signaling pathway was carried out using the Transcriptome Analysis Console 4.0 (Applied Biosystems). Additional real-time qPCR was performed as previously described.
Murine T-cell activation, HLA-G dimer treatment, and LILRB1 blocking
We stimulated 2.0 × 106 T cells isolated from splenocytes of LILRB1 transgenic and control wild-type (WT) mice in 6-well plates with anti-mouse CD3 mAb (100313; BioLegend). Plates were precoated with anti-CD3 mAb at 10 µg/ml. Anti-mouse CD28 mAb (14-0281-82; Thermo Fisher Scientific) at a concentration of 2 µg/ml was added, and cells were incubated at 37°C for 72 h. HLA-G dimer treatment was performed as previously described for human T-cell activation. LILRB1 receptor–blocking studies were performed with 20 µg/ml of anti-LILRB1 mAb (333704; BioLegend) prior to HLA-G dimer treatment and T-cell stimulation.
Antibodies and flow cytometry analysis
For each experimental condition, cells from human PBMCs and mouse PBMCs or splenocytes were isolated and labeled with the following antibodies at 4°C for 45 min in the dark: anti-human CD3 (300412, clone UCHT1, 1:300), CD4 (17-0049-73, clone RPA-T4, 1:300), CD8 (301008, clone RPA-T8, 1:300), CD25 (302606, clone BC96, 1:200), and FOXP3 (32007, clone 150D, 1:300); anti-human/mouse GZMB (515403, clone GB11, 1:300); anti-mouse CD3 (100321, clone 145-2C11, 1:300), CD4 (100407, clone GK1,5, 1:200), CD8 (100713, clone 53-6.7, 1:300), and CD25 (10211, clone PC61, 1:200) (all from BioLegend). All samples were preincubated with TruStain fcX (101320, clone 93, 1:100) (BioLegend) to block the Fc receptors. Intracellular staining was carried out per the manufacturer’s instructions described in the True Nuclear Transcription Factor Kit (BioLegend). Samples were acquired on the FACS Canto (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (FlowJo; BD Biosciences, San Jose, CA, USA). Dead cells were excluded from analysis based on the forward and side scatter characteristics.
Cell-based flow cytometry assay to measure cytotoxic activity of human CD8+ T cells
A flow cytometry–based killing assay was used to compare the in vitro cytolytic activity of CD8+ T cells isolated from HVs under stimulated vs. HLA-G dimer–treated conditions as previously described. K562 target cells were washed with PBS, resuspended at 1 × 106 cells/ml and labeled with 120 mM of 5 (6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (65-0850-84; Thermo Fisher Scientific). These labeled target cells were plated onto 6-well tissue culture plates along with the indicated effector cells (stimulated or treated PBMCs) in complete RPMI medium containing 25 U/ml of rhIL-2 (23). The effector:target cell ratio was maintained at 10:1 for all time points. Immediately prior to analysis, 1 µl of 7-amino-actinomycin D (7-AAD) (420403; BioLegend) was added to each sample. We used 7-AAD incorporation as a surrogate marker for late cell death or apoptosis. When excited by 488 nm laser light, 7-AAD fluorescence is detected in the far-red range of the spectrum (650 nm long-pass filter). All cytotoxicity assays were performed in duplicate. Data are representative of 3 or more individual cytotoxicity experiments.
Statistical analysis
All data are expressed as means ± sd. Differences between experimental and control groups were assessed by a 2-tailed, unpaired Student’s t test using Prism (GraphPad Software, La Jolla, CA, USA). ANOVA was used for demographic analysis for comparison of multiple parameters. A value of P < 0.05 was considered to be statistically significant.
RESULTS
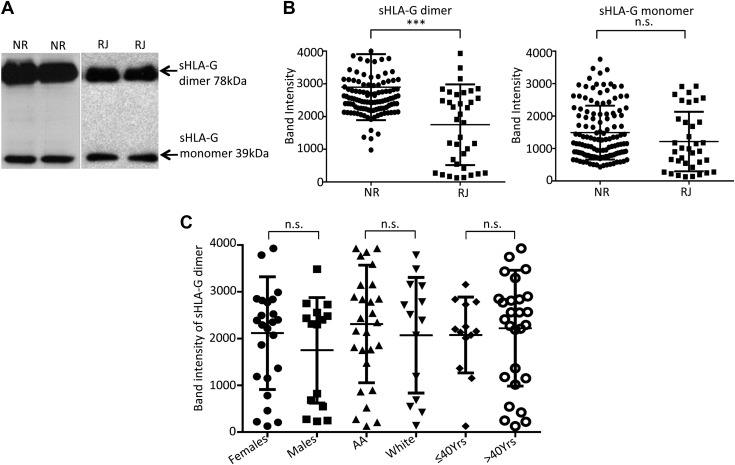
High level of sHLA-G dimer is associated with prolongation of kidney allograft survival and is not dependent on demographic status of patients
In order to identify potential factors involved in the beneficial role of sHLA-G dimer in allograft survival, we performed a comprehensive analysis of plasma levels of sHLA-G monomer and dimer in expanded groups of NR and RJ kidney transplant patients with different demographic status such as age, race, and gender. sHLA-G was immunoprecipitated from plasma, followed by immunoblotting analysis and quantification. We observed 2 bands in the immunoblot: the sHLA-G monomer with a molecular mass of 39 kDa and the dimer with molecular mass at 78 kDa (Fig. 1A). The levels of sHLA-G dimer were significantly higher in the NR transplant group compared with the RJ group, whereas the monomeric form showed no difference between the 2 groups (Fig. 1B). Further, we investigated the possibility of an inherent disparity in sHLA-G dimer levels due to demographic status such as age, gender, or race. With regard to racial background, we observed a greater incidence of TCMR among the African American population; however, their sHLA-G dimer levels were comparable to the white group (Fig. 1C and Table 2). This is perhaps indicative of a predisposed condition of strong TCMR among the African American demographic. With respect to gender, we noted that women had a significantly higher level of sHLA-G dimer in comparison with men (Fig. 1C and Table 2). However, in the present cohort, women also had a higher rate of TCMR for the transplants. In the case of age, we observed that the sHLA-G dimer level gradually increased with age. Interestingly, patients younger than 40 yr had a greater rate of TCMR, which was significantly reduced in patients older than 40 yr. Meanwhile, the sHLA-G dimer level increased with age, suggesting their levels might be beneficial for the establishment of prolongation of allograft survival.
Figure 1.
sHLA-G dimer levels are higher in NR than RJ kidney transplant patients and are not affected by demographic status. A) Representative immunoblot showing sHLA-G monomer and sHLA-G dimer levels from plasma of KTRs (n = 40/group). Each lane represents 1 patient. B) Dot plots show pooled data as mean band densities of sHLA-G dimer and sHLA-G monomer in NR and RJ kidney transplant patients. Data presented as means ± sd. N.s., not significant. ***P < 0.001. C) Data represent the level of sHLA-G dimer among the various demographic categories of KTRs. AA, African American; n.s., not significant.
TABLE 2.
Demographic characteristics of NR and RJ kidney transplant patients and sHLA-G dimer
| Demographic | Transplant status | n | sHLA-G dimer (mean ± sd) |
|---|---|---|---|
| Total patients | NR | 90 | 2850 ± 73.0 |
| RJ | 40 | 1950 ± 202.5 | |
| Gender | |||
| Male | NR | 49 | 2708 ± 94.2 |
| RJ | 15 | 1751 ± 291.5 | |
| Female | NR | 41 | 3020 ± 68.9 |
| RJ | 25 | 2117 ± 241.2 | |
| Age | |||
| ≤40 | NR | 8 | 2822 ± 80.8 |
| RJ | 12 | 2076 ± 224.9 | |
| >40 | NR | 82 | 2853 ± 169.2 |
| RJ | 28 | 2223 ± 233.9 | |
| Race | |||
| African-American | NR | 56 | 2512 ± 105.2 |
| RJ | 26 | 2313 ± 237.9 | |
| White | NR | 34 | 2128 ± 134.1 |
| RJ | 14 | 2071 ± 330.8 |
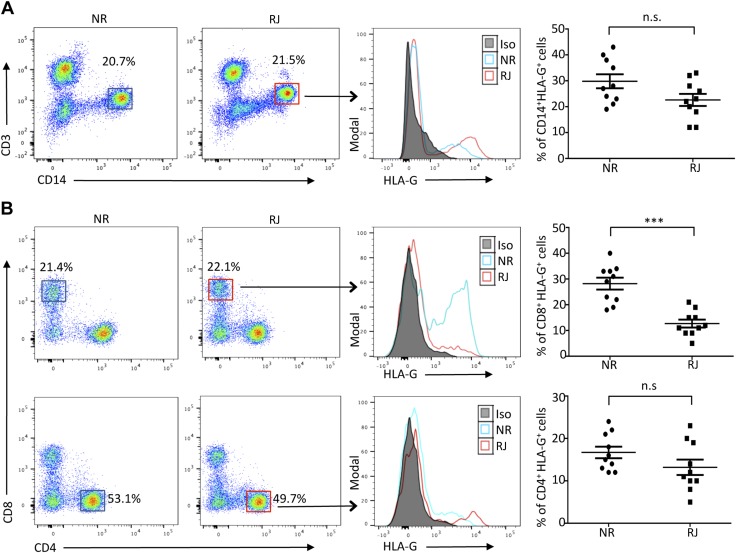
sHLA-G is generated through 2 mechanisms: alternative splicing and proteolytic shedding from the membrane-bound form that is mediated by metalloproteinases. sHLA-G dimer mainly accumulates in the blood plasma after shedding from its membrane-bound form that is expressed in immune cells such as CD4, CD8, and peripheral monocytes. The overall number of each population of cells did not show any significant difference between the groups. To identify the potential source of elevated sHLA-G dimer in plasma of NR patients, we analyzed expression levels of the corresponding membrane-bound HLA-G among its target cells. Peripheral monocytes expressing HLA-G were slightly greater in number in the NR group (Fig. 2A, right panels). However, among the T-cell populations, CD8+ T cells expressing membrane-bound HLA-G made up to 30% of the total in the NR group, whereas only 10% of the CD8+ T cells in the RJ group were HLA-G positive (Fig. 2B, right panels). Moreover, the significant increase was restricted to the CD8+ T cells alone, whereas HLA-G–expressing CD4+ T-cell numbers were similar between the 2 groups (Fig. 2B, right panels). Analysis of the HLA-G expression pattern in the various mononuclear cells from HVs demonstrated minimal levels of HLA-G and only 10% of circulating CD8+ T cells expressing membrane-bound HLA-G (Supplemental Fig. S1). In addition, despite the differences in the number of HLA-G–expressing cells, the overall percent of CD4+ and CD8+ T cells between the transplant groups remain unchanged (Supplemental Fig. S2A). Therefore, these findings may suggest a significant contribution by CD8+ T cells expressing membrane-bound HLA-G to the increasing levels of sHLA-G in the plasma of patients. The high level of sHLA-G dimer in serum and increased expression of the membrane-bound form of HLA-G on CD8+ T cells in the NR group of KTRs may affect the function of immune cells and make them less potent to reject an organ-tissue allograft. This is especially relevant as the cytotoxic responses of CD8+ T cell are responsible for the majority of graft tissue destruction.
Figure 2.
Increased expression of HLA-G on CD8+ T cells of NR patients. A) Representative flow cytometry plots from NR and RJ kidney transplant patients (n = 10/group), showing the gating strategy used to determine HLA-G expression on CD14+ CD3− cells (monocytes). Histogram depicts HLA-G expression in RJ KTRs (red lines) and NR patients (blue lines). Filled histogram represents isotype control (Iso). Graphical summary shows the frequency (%) of HLA-G–expressing monocytes within each group. B) Representative flow cytometry dot plots from NR and RJ transplant patients (n = 10/group) gated on CD8+ and CD4+ populations of T cells. Histograms depict HLA-G expression of the NR (blue line) and RJ (red line) groups. Graphical summary shows the frequency (%) of HLA-G–expressing CD4 and CD8+ T cells within each group. Representative of at least 2 separate experiments (A, B). N.s., not significant. Data are shown as means ± sd. ***P < 0.001.
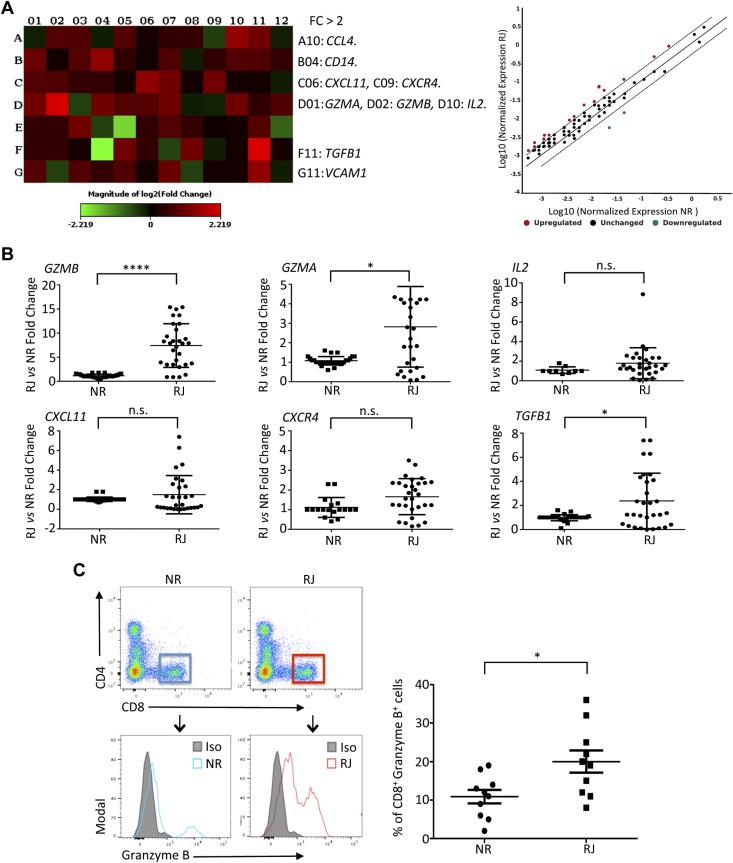
Transcription level and protein expression of GZMB are elevated in peripheral CD8+ T cells in patients with rejection of kidney transplant and low level of sHLA-G dimer
To determine the potential effect of sHLA-G dimer on the function of human immune cells, especially on modulation of functions of CD8+ T cells, we performed transcriptional analyses of genes isolated from PBMCs of transplant recipients using the RT2 Human Transplantation Rejection Array. Screening of the expression profile of 84 key genes implicated in transplantation rejection demonstrated a significant increase in the RJ group (>2-fold) of several genes, including Chemokine (C-C motif) ligand 4 (CCL4), CD14, CXCL11, CXCR4, IL2, TGFB1, VCAM1, Granzyme A (GZMA), and GZMB in comparison with the NR group (Fig. 3A). However, only the GZMA and GZMB transcript increases were statistically confirmed in the RJ group by using additional real-time qPCR analyses and custom primers (Fig. 3B and Table 1). Flow cytometry analysis of the protein expression of GZMB in patient PBMCs revealed that peripheral monocytes showed slight expression for GZMB; however, the number of positive cells was nonsignificant between the RJ and NR groups (unpublished results). We observed a significantly increased number of GZMB-expressing CD8+ T cells in the RJ group compared with the NR group (20.0 ± 0.2.87% compared with 10.9 ± 1.7%, P < 0.05, Fig. 3C). Moreover, as shown in Fig. 2B, the RJ patients had lower numbers of HLA-G–positive CD8+ T cells. This expression pattern of low levels of sHLA-G dimer and high levels of GZMB in CD8+ T cells of RJ patients, in contrast with high levels of sHLA-G dimer and low levels of GZMB expression in NR CD8+ T cells, led us to investigate the broad effects of sHLA-G dimer on gene expression in immune cells.
Figure 3.
Increased transcript and protein levels of GZMB associated with human kidney allograft rejection. RNA was extracted from PBMCs of both RJ and NR groups of KTRs. Individual samples were processed using Human Transplant Rejection RT2 Profiler PCR Array and analyzed using the Qiagen online web analysis tool. The gene expression was normalized to the average of 3 housekeeping genes (ACTB, GAPDH, and HPRT1), and the expression of each gene relative to the NR group is depicted. A) Heat map and associated scatter plot showing fold change of transplant rejection–specific genes comparing RJ vs. NR (n = 4/group). Red indicates increased expression, and green indicates decreased expression. B) The pattern of transplant rejection gene expression was confirmed by custom real-time qPCR (primers in Table 1) using RNA isolated from PBMCs of both RJ (n = 30) and NR (n = 15) KTRs. The gene expression was normalized to GAPDH levels, and the fold change in mRNA levels of each gene in the RJ group compared with the NR group is shown. Only the GZMA and GZMB gene expression increases (>5-fold for GZMB) were statistically confirmed in the RJ group in comparison with the NR group. C) Representative flow cytometry dot plots from NR and RJ transplant patients (n = 10/group) gated on CD8+ T cells. Histograms depict GZMB expression in NR (blue line) and RJ (red line) groups. Filled histogram represents isotype control (Iso). Graphical summary shows the frequency (%) of GZMB-expressing CD8+ T cells in each group. Data are representative of 2 separate experiments. Data are as shown as means ± sd. *P < 0.05, ****P < 0.0001.
Transcription profile analysis identifies potential targets of sHLA-G dimer
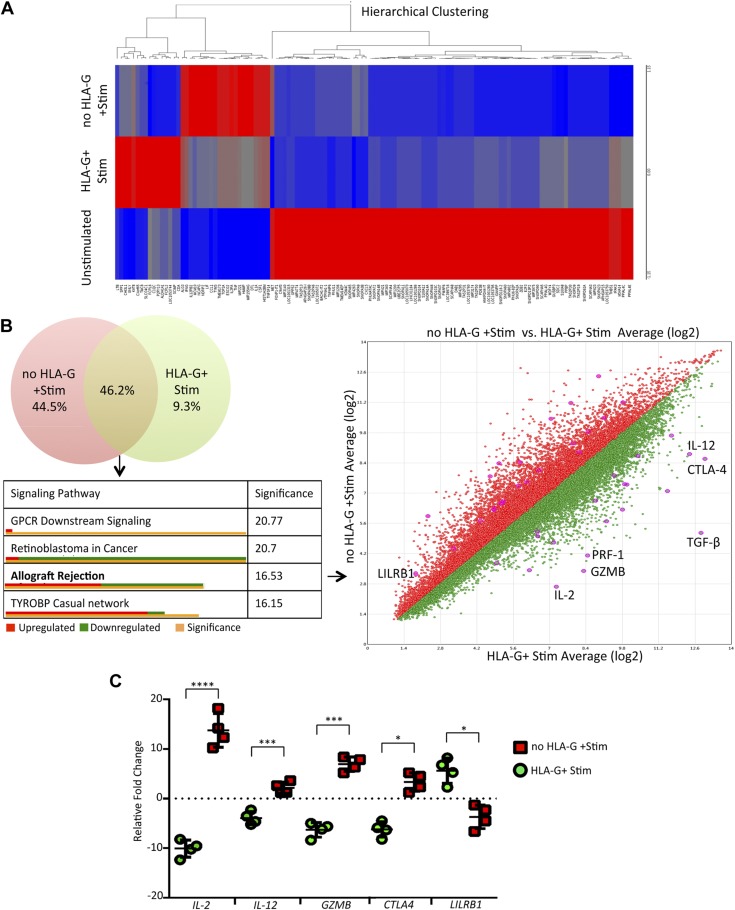
To determine the global effects of sHLA-G dimer on immune cell genes, we used PBMCs from HVs to avoid the influence of medication regimens taken by our transplant patient cohorts. We performed gene array analysis using the GeneChip Human Gene 2.0 ST Array to assay the differential expression of 24,838 genes in PBMCs from these HVs. PBMCs were pretreated with HLA-G dimer followed by activation with ConA and IL-2. The main aim of this experiment was to identify potential genes whose expression was significantly affected by HLA-G pretreatment followed by activation. We used unstimulated PBMCs to serve as control for hierarchical clustering to sort out only those genes affected by HLA-G treatment (Fig. 4A). We further enriched this set of genes using the pathway analysis and observed that the genes differentially expressed between the HLA-G–treated and untreated groups had a significant effect in allograft rejection, being down-regulated or up-regulated in their respective signaling pathway (Fig. 4B and Supplemental Fig. S3). Moreover, HLA-G treatment induced down-regulation in expression of GZMB, a key molecule responsible for graft destruction. Interestingly, HLA-G treatment induced significant up-regulation of its receptor LILRB1, which is expressed mainly on CD8+ T cells (Fig. 4C). Activation of resting PBMCs showed a >10-fold induction in IL-2 expression. However, HLA-G pretreatment completely abrogated this and, moreover, induced a 5-fold down-regulation of the same cytokine (Fig. 4C). These observations point toward an HLA-G/LILRB1–based interaction signaling via IL-2 to downregulate GZMB in CD8+ T cells. This signaling network is in line with prior established data showing the crucial role of IL-2 in GZMB induction in CD8+ T cells (37).
Figure 4.
Transcription profile analysis identifying potential targets of HLA-G. A) The Human Gene 2.0 ST array was used to plot the heat map representing the differential expression of genes between unstimulated and stimulated HV PBMCs with or without HLA-G pretreatment. A hierarchical clustering model was used to segregate individual gene expression in each group into reduced expression (blue) and overexpressed (red) conditions. B) The Transcriptome Analysis Console 4.0 (Applied Biosystems) was used to make a Venn diagram, sorting genes specifically changed by the HLA-G treatment (green) vs. untreated (red). Those genes showing differential expression common to both groups were analyzed to obtain a histogram of affected signaling pathways in order of significance. The allograft rejection pathway had 17 genes up-regulated (shown in orange) and 18 genes down-regulated (shown in green), with specific genes of interest depicted in the scatter plot. Statistical significance for the histogram was calculated using a 2-sided Fisher’s exact test. C) Custom real-time qPCR was carried out to confirm the fold change of target genes playing an integral role in T-cell activation and allograft rejection comparing HLA-G–treated vs. untreated samples (n = 4/group). N.s., not significant. Data are as shown as means ± sd. *P < 0.05, ***P < 0.001, ****P < 0.0001.
HLA-G dimer modulates expression of GZMB on CD8+ T cells via its receptor LILRB1
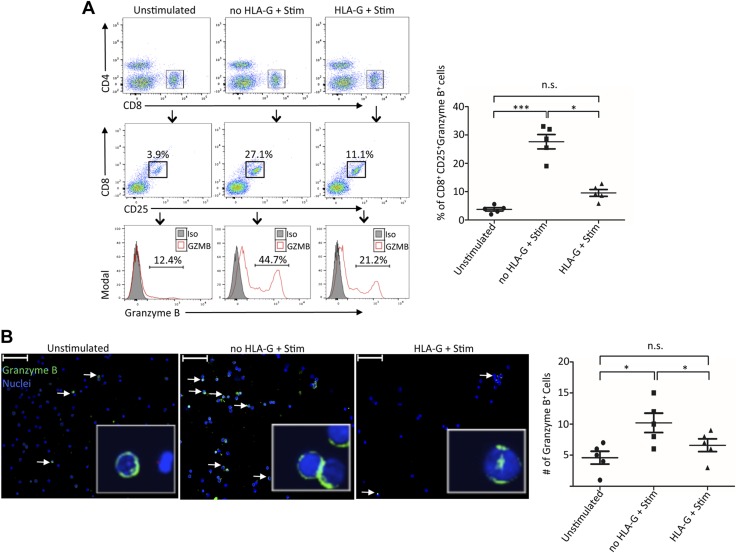
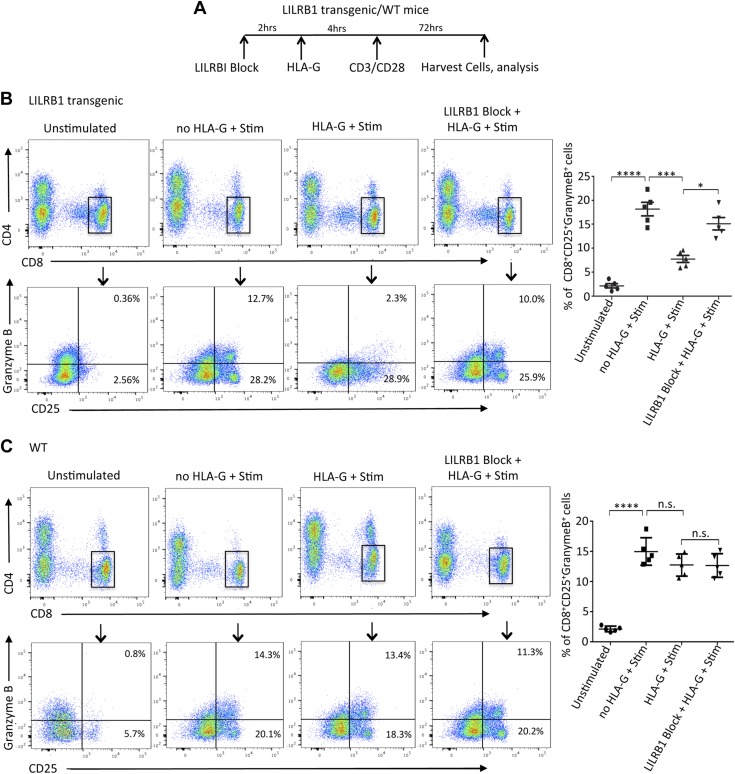
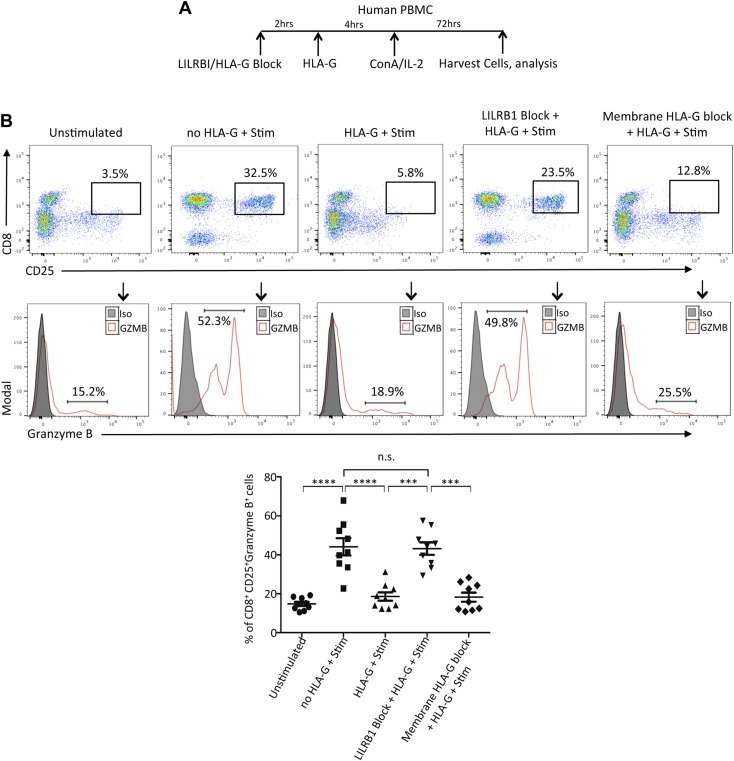
Our observations from kidney transplant patients have suggested a regulatory signaling between HLA-G dimer and GZMB specifically targeted to CD8+ T cells. To further dissect this relationship, we designed an in vitro model wherein PBMCs isolated from HVs were pretreated with HLA-G dimers followed by activation with ConA-IL-2. Subsequently, CD8+ T cells were analyzed for their GZMB expression, cytotoxicity, and surface marker profile for confirmation of their cellular phenotype. We observed that almost 45% of activated CD25+ CD8+ T cells were GZMB-positive postactivation, suggesting their expression is associated with the activation pathway (Fig. 5). However, HLA-G dimer treatment of PBMCs prior to stimulation resulted in significant inhibition of CD8+ T-cell activation and GZMB expression (Fig. 5A). To further understand how HLA-G dimer inhibits CD8+ T-cell activation and GZMB expression, we investigated the involvement of its primary receptor LILRB1. Our analysis on transplant cohorts had shown that despite the rejection status, all patients had comparatively similar levels of CD8+ LILRB1+ HLA-G- T cells (Supplemental Fig. S2B). In addition, HLA-G and LILRB1–expressing double-positive CD8+ T cells were not significant as well (Supplemental Fig. S2B). Furthermore, we determined that LILRB1, which is the dominant HLA-G receptor expressed in CD8+ T cells, was up-regulated in our transcription profile studies (Fig. 4C). To dissect the involvement of LILRB1 on the inhibitory effect of HLA-G in CD8+ cells, we used transgenic mice expressing human LILRB1 on T cells. Immune cells were isolated from both LILRB1 transgenic and WT mice and were pretreated with HLA-G dimers followed by activation with anti-CD3/CD28 mAb (Fig. 6A). Our results demonstrated that the activation of CD8+ T cells and GZMB expression of cells isolated from LILRB1 transgenic mice were significantly inhibited with HLA-G dimer treatment, whereas cells from WT mice lacking LILRB1 receptors were not affected by HLA-G (Fig. 6B, C). The data from these experiments suggested that LILRB1 is the major receptor through which HLA-G dimer can mediate its inhibitory effects on the activation of CD8+ T cells and its expression of GZMB. To further establish LILRB1 as the main axis for HLA-G to exert its immune-modulatory effects, we performed experiments where LILRB1 was blocked by specific mAbs (Fig. 7A). Under these experimental conditions, the inhibitory properties of HLA-G were completely reversed; stimulation alone yielded 52.3% CD8+CD25+ GZMB-expressing cells, which was reduced to 18.2% with HLA-G pretreatment. However, abrogating the HLA-G-LILRB1 interaction reversed the HLA-G inhibitory effect and returned it to the normal stimulated levels of 47.8% CD8+CD25+ GZMB-expressing cells (Fig. 7B). Moreover, blocking the membrane-bound HLA-G on the PBMCs with anti-HLA-G mAb had minimal effect on inhibition, showing that the tolerogenic effect of sHLA-G dimer requires an active interaction with LILRB1, rather than a cis interaction between the membrane-bound form of HLA-G interacting with its receptor. Together, we have effectively proven that LILRB1 is the primary HLA-G receptor on CD8+ T cells and that the HLA-G-LILRB1 interaction is required for its inhibitory properties.
Figure 5.
HLA-G dimer inhibits activation of CD8+ T cells and GZMB expression in vitro. A) Representative flow cytometry plots from HVs, showing gating strategy used to identify CD25+-expressing activated CD8+ T cells for indicated groups (n = 5/group). Histograms depict GZMB expression of CD8+ CD25+ cells (red line). Filled histogram shows the isotype control (Iso). The flow cytometry dot plots shown are from 1 of 2 separate experiments. Graphical summary shows the frequency (%) of CD8+ CD25+ GZMB+ cells per group. B) Immunofluorescence microscopy showing unstimulated and stimulated PBMCs from HVs with or without HLA-G dimer pretreatment (n = 5/group). Number of GZMB-positive cells was determined by immunofluorescence staining with anti-GZMB mAb conjugated with FITC; nuclear staining is shown by DAPI (Original magnification, ×20. Scale bars, 50 μm). Graphical summary shows the number of GZMB-positive cells counted per group. Data are representative of 2 separate experiments. N.s., not significant. Data are shown as means ± sd. *P < 0.05, ***P < 0.001.
Figure 6.
LILRB1 receptor is essential for HLA-G dimer–mediated inhibition of CD8+ T-cell activation and GZMB expression in LILRB1 transgenic mice. A) Experimental design schematic showing time course for LILRB1 receptor blocking, HLA-G treatment, CD3/CD28 activation, and cell isolation using LILRB1 transgenic and WT mice. B, C) Representative flow cytometry dot plots from LILRB1 transgenic (B) and WT mice (C) gated on CD8+ T cells depicting their GZMB expression and activation status based on CD25 expression in the indicated groups (n = 5/group). Graphical summary shows the frequency (%) of CD8+ CD25+ GZMB+ cells per group. Data from 1 of 2 separate experiments are shown. N.s., not significant. Data presented as means ± sd. *P < 0.05, ***P < 0.001, ****P < 0.0001.
Figure 7.
LILRB1 receptor is essential for HLA-G dimer–mediated inhibition of CD8+ T-cell activation and GZMB expression in human PBMCs. A) Experimental design schematic showing time course for LILRB1 or HLA-G blocking, HLA-G treatment, ConA-IL-2 activation, and cell isolation using PBMCs from HVs. B) Representative flow cytometry dot plots from HVs were gated on activated double-positive CD8+ CD25+ T cells in the indicated groups (n = 10/group). Histograms depict GZMB expression of activated CD8+T cells (red line). Filled histogram shows the isotype control (Iso). Graphical summary shows the frequency (%) of CD8+ CD25+ GZMB+ cells per group. N.s., not significant. Data presented as means ± sd. ***P < 0.001, ****P < 0.0001.
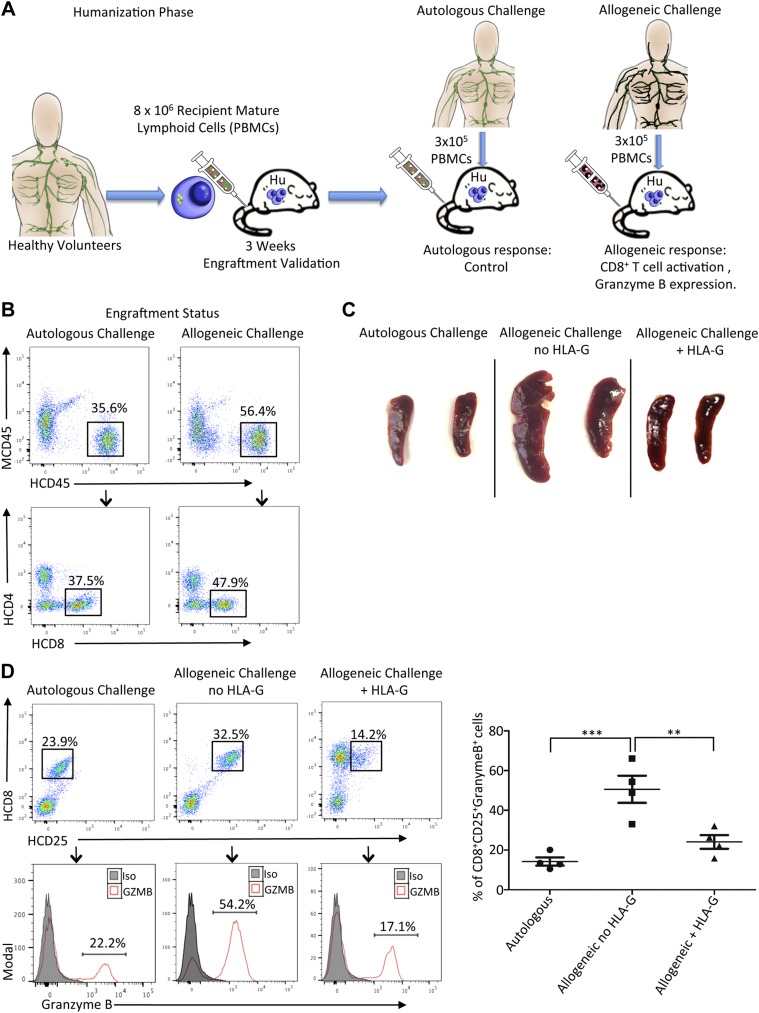
HLA-G dimer pretreatment inhibits allogeneic recognition in a humanized mouse model
The inhibitory effect of HLA-G has been successfully demonstrated in our in vitro models using human PBMCs and cells from LILRB1 transgenic mice. However, for effective translation into the clinical transplantation field, the immunomodulatory effect of HLA-G on allogeneic recognition and allograft survival must be demonstrated in a suitable in vivo model. Thus, we designed a novel in vivo humanized mouse model to assess the tolerogenic property of HLA-G to minimize allogeneic recognition (Fig. 8A). During the humanization phase, ∼8 × 106 PBMCs from an HV were injected into an NSG mouse via tail vein. Three weeks postinjection we observed successful engraftment with up to 40% of human CD45+ cells in the spleen of the NSG mouse for both autologous and allogeneic challenge groups (Fig. 8B). Posthumanization, the allogeneic challenge with unmatched donor PBMCs served to elicit an allogeneic response, whereas the autologous challenge with the volunteer PBMCs served as the control. In the allogeneic group, we observed significant splenomegaly due to massive cell infiltration within 5 d of the challenge; however, this was effectively minimized with HLA-G pretreatment, whereas the autologous challenge group showed negligible effects (Fig. 8C). Flow cytometry analyses confirmed that during allogeneic recognition in vivo, donor-activated human CD8+ T cells became activated with markedly up-regulated GZMB expression. Remarkably, HLA-G dimer pretreatment during this challenge significantly down-regulated the activation of recipient CD8+ T cells (from 32.5 to 14.2%) and the expression of GZMB (from 66.1 ± 4.1% to 17.1 ± 5.2%, Fig. 8D). In addition, HLA-G dimer treatment facilitated significantly increased expression of T regulatory cells (Supplemental Fig. S4). Overall HLA-G dimer pretreatment during the allogeneic challenge in vivo effectively abrogated CD8+ T cell–mediated allogeneic recognition.
Figure 8.
HLA-G dimer inhibits activation of CD8+ T cells and GZMB expression in a humanized mouse model of allogeneic recognition. A) Experimental design schematic showing the humanization of NSG mouse with HV PBMCs followed by the challenge phase with autologous or allogeneic PBMCs. B) The phenotype of engrafted human T cells in the humanized mouse generated for the challenge conditions was determined on the basis of human CD45 expression (HCD45) and negative staining with anti-murine CD45 mAb (MCD45). Representative flow cytometry dot plots from the humanized mouse for the indicated groups (n = 4 mice/group) showing a population of human CD45 cells with further gating to show HCD8+ T cells at the third week of engraftment. C) Spleens isolated from the different groups show significant splenomegaly with allogeneic challenge, which is minimized with HLA-G treatment. D) Representative flow cytometry dot plots from spleen of humanized mouse shows a population of human activated CD8+ CD25+ T cells after allogeneic challenge with or without HLA-G treatment. Autologous challenge serves as control. Histograms depict GZMB expression of activated CD8+ CD25+ T cells (red line). Filled histogram shows the isotype control (Iso). Graphical summary shows the frequency (%) of CD8+ CD25+ GZMB+ cells per group. Data presented as means ± sd. **P < 0.01, ***P < 0.001.
HLA-G dimer inhibits cytotoxic capacity of CD8+ T cells
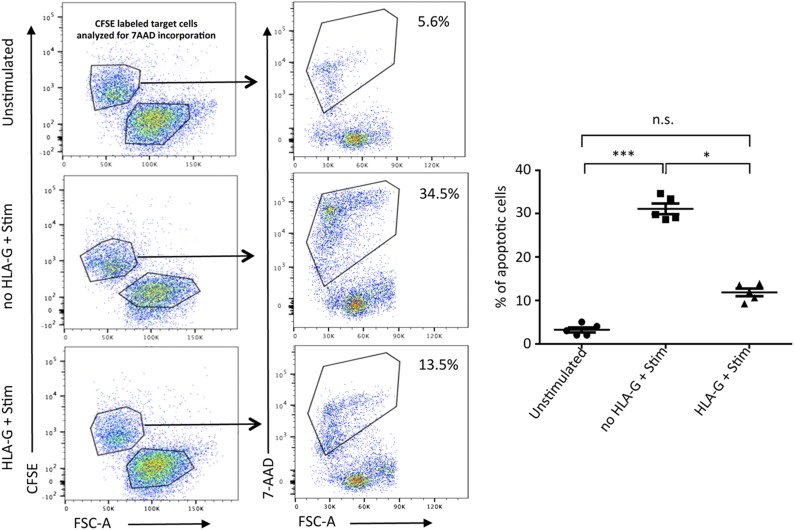
Our in vitro and in vivo models together established the inhibitory effect of HLA-G dimer treatment on CD8+ T cells. We further investigated whether HLA-G dimer could inhibit the functional cytotoxic capacity of CD8+ T cells. We used a flow cytometry–based killing assay in which stimulated PBMCs with or without HLA-G pretreatment were incubated with CFSE-labeled K562 target cells (Fig. 9). The killing capacity of the stimulated cells was assayed by measuring the extent of cell death among K562 cells as indicated by the incorporation of 7-AAD, an early marker of cell death. The amount of target cell killing was associated with GZMB expression in the CD8+ T cells among the different groups. We determined that HLA-G dimer pretreatment significantly inhibited target cell killing (Fig. 7), indicating decreased cytotoxicity of CD8+ T cells. In contrast, resting PBMCs showed the least killing capacity due to negligible levels of activated CD8+ T cells (Supplemental Fig. S5). This experiment demonstrates that HLA-G dimer treatment can abrogate the cytotoxicity of CD8+ T cells, which is one of the main pathways for graft tissue destruction in allograft rejection.
Figure 9.
HLA-G dimer inhibits cytotoxicity of CD8+ T cells. PBMCs from HVs (n = 5/group) were stimulated with ConA-IL-2 as described in Materials and Methods. Data from flow cytometry–based killing assay are shown. In the representative flow cytometry dot plots (left panels) K562 target cells are shown as CFSE-positive cells and the effector PBMCs are CFSE-negative populations. CFSE-positive target cells were gated and analyzed for 7-AAD incorporation as an early indicator of cell death (right panels). Graphical representation indicating percent of apoptotic cells per group. Representative from 1 of 3 separate experiments is shown. N.s., not significant. Data presented as means ± sd. *P < 0.05, ***P < 0.001.
DISCUSSION
The data presented here show an unknown mechanism of HLA-G–mediated down-regulation of the cytotoxic function of CD8+ T cells and GZMB expression for prolongation of kidney allograft survival. HLA-G is a powerful molecule associated with immune tolerance and immunosuppression. HLA-G preferentially binds to LILR receptors, killer cell inhibitory receptor (killer cell Ig-like receptor 2DL4), and CD8 molecules. The LILR receptor family includes activating (LILRA1, LILRA2, LILRA3) and inhibitory (LILRB1, LILRB2, LILRB4) receptors. Protein sequence motifs known as immunoreceptor tyrosine-based activating motifs and immunoreceptor tyrosine-based inhibitory motifs are responsible for the activating or inhibitory signals transmitted by LILRs (38). The mechanisms of binding and engagement of the specific activating or inhibitory receptors by HLA-G are still the subject of debate (35, 38, 39). Discovery of the HLA-G dimer form significantly improves our knowledge in controlling the balance of the activating or inhibitory signals. The crystal structure of the HLA-G dimer demonstrated the importance of the intermolecular Cys42-Cys42 disulfide bond for dimer formation (35). In addition, the proper structural configuration of the dimer makes it more efficient to bind inhibitory LILRB receptors and CD8 molecules to induce selective dominant inhibitory signaling pathways.
Here we demonstrate that a high level of sHLA-G dimer in plasma of KTRs is associated with functioning kidney transplants. Moreover, we show that this association was not dependent on age, gender, or race, but higher sHLA-G dimer levels correlated most strongly with graft survival and could be a potential marker for prediction of allograft survival. Particular sources of elevated HLA-G dimer in plasma of transplant patients includes the secretion of sHLA-G or shedding from the cell surface of the membrane-bound form of HLA-G by metalloproteinases and formation of disulfide-linked dimers by natural oxidation. It was previously demonstrated that a disulfide-linked HLA-G dimer mediates an inhibitory signal through LILRB1 100 times more than that of HLA-G monomer (35). Moreover, HLA-G dimer provides much more efficient signals than HLA-G monomer by binding both LILRB1 and LILRB2 at the same time (40). It is interesting that the membrane-bound form of HLA-G can also form a disulfide-linked dimer on the cell surface of the human choriocarcinoma cell line Jeg3, which endogenously expresses HLA-G (41) as well as HLA-G transfectants (42, 43). In the future, it will be important to determine if this form of HLA-G dimer exists on the cell surface of HLA-G–expressing cells in kidney transplant patients and, if so, its potential role in modulation of the function of immune cells. Moreover, Gonen-Gross et al. (41) discovered that the β2microglobulin-free form of HLA-G also forms disulfide-linked dimers and multimers on the cell surface similar to the dimer form of normal β2microglobulin-associated HLA-G protein, which we demonstrated above in our study. Because β2microglobulin forms a major contact site between LILRB1 and HLA-G that is specific for LILRB1 only, the β2microglobulin-free form may not provide inhibitory signaling through LILRB1. The role of the β2microglobulin-free form of HLA-G remains elusive, and more studies are warranted in the future, especially in patients with organ transplants. Our data demonstrated that the number of CD8+ T cells expressing the membrane-bound form of HLA-G significantly increased in patients with functioning kidney transplants. This finding indicates that CD8+ T cells might be an important source of HLA-G dimer. In addition, the expression of HLA-G on CD8+ T cells could directly compromise the function of these cells. LILRB receptor has potent immunosuppressive effects capable of suppressing alloantigen-activated T cells and inhibiting TCMR. Recent studies have identified CD8+ LILRB1+ T cells in the peripheral blood as a possible biomarker for predicting recurrence in bladder cancer (44). However, in our study, we could not determine a significant difference in CD8+ LILRB1+ T cells between RJ and NR groups that excludes these cells as potential marker for kidney graft survival.
Rejection of solid allografts by the recipient immune system is mediated by several mechanisms, a major one of which is the T-cell effector mechanism (37, 45, 46). Cytotoxic lymphocytes, which include CD8+ T cells, some CD4+ T cells, and natural killer cells, are directly involved in the elimination of cells with different Major Histocompatibility Complex (MHC) backgrounds. CD8+ T cells are the best representative cytotoxic T lymphocyte population. Granule-mediated cytotoxicity is one of the major mechanisms used by CD8+ T cells to eliminate allografts. After antigen recognition, activated CD8+ T cells release the contents of their cytotoxic granules into the extracellular space, where they are taken up by the target cell, and apoptosis is initiated (37, 46, 47). One of the major components of cytotoxic granules is serine proteases, known as granzymes, of which GZMB is a potent proapoptotic molecule. A gene expression profile of PBMCs from kidney transplant patients demonstrated a significant increase of several genes involved in transplantation rejection in patients with RJ grafts compared with patients with functioning grafts. The increase in GZMA and GZMB transcripts was statistically confirmed by additional real-time qPCR analyses. Moreover, protein expression of GZMB was found to be significantly increased in CD8+ T cells from the RJ group compared with patients with functioning grafts. In addition, the level of expression of the membrane-bound form of HLA-G on CD8+ T cells and the plasma levels of HLA-G dimer were significantly less in RJ patients, which could indicate a negative association between expression of HLA-G and GZMB. Interestingly, gene array analysis to assay the differential expression of 24,838 genes affected by HLA-G dimer treatment of human PBMCs showed a significant effect of HLA-G in the allograft rejection pathway (Fig. 4A, B). The most affected representative genes included IL-2, IL-12, GZMB, CTLA4, and LILRB1 (Fig. 4C). The Granzyme genes are known to be inducible because T cells must be activated before the cytolytic molecule is expressed at the mRNA or protein levels (31, 46). IL-2 is a master regulator of perforin and Granzyme gene expression in CD8+ T cells (48). Moreover, the binding sites for the IL-2–induced transcription factor STAT5 have been located in the perforin promoter region (49, 50). It is possible that the response of granzymes to IL-2 is due to enhanced cell viability or proliferation and not to direct induction. However, Janas et al. (37) clearly demonstrated that IL-2 directly regulates Granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation.
Our study has demonstrated that the inhibitory effect of HLA-G dimer on activation and cytotoxic capacity of CD8+ T cells is mediated by decreased GZMB expression. It is well established that T-cell–mediated GZMB-dependent cytotoxicity is initiated by the specific recognition of antigenic peptides presented on class I MHC molecules (in addition to costimulatory and cytokine signaling) by the T-cell receptor, which involves the recruitment and activation of the tyrosine kinases Fyn, Lck, and ZAP70. This early signaling cascade is crucial for T-cell activation. HLA-G–based inhibitory receptors such as LILRB1 have immunoreceptor tyrosine-based inhibitory motifs in their cytoplasmic tail to recruit the Src homology 2 domain-containing protein tyrosine phosphatase 1, which can dephosphorylate molecules involved in the early activation pathway for T cells, resulting in inhibitory signaling (36, 51, 52). Our in vitro and in vivo studies using a humanized mouse model demonstrated that HLA-G dimer can prevent activation of human CD8+ T cells in allogeneic stimulation. Moreover, even when PBMCs are treated with more potent ConA-IL-2 stimulation, HLA-G dimer can inhibit GZMB expression and decrease the cytotoxic potential of CD8+ T cells. The experiments with LILRB1 transgenic mice and LILRB1 blocking studies demonstrated the requirement of this HLA-G–specific inhibitory receptor in the novel mechanism of HLA-G dimer–mediated inhibition of GZMB expression and diminished the cytotoxic capacities of human CD8+ T cells. We do not exclude the possibility that other inhibitory receptors, especially LILRB2, which is primarily expressed on monocytes, B cells, and dendritic cells, contribute indirectly to this inhibitory signaling mechanism and may have an additional separate role in allograft survival. Overall, our findings support that HLA-G dimer plays an important role in the prolongation of allograft survival, as observed in kidney transplant patients and provides a novel mechanism by which HLA-G dimer inhibits the cytotoxic capabilities of CD8+ T cells via down-regulation of GZMB expression and the essential involvement of the inhibitory receptor LILRB1. Therefore, HLA-G with its specific inhibitory axis emerges as an effective therapeutic target for prolonging graft survival in transplant patients.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. R. B. Markowitz (Georgia Cancer Center, Augusta University) for critically reading the manuscript. The authors thank Peggy Best, Walidah Walker, Joan Holloway, and physicians at the Medical College of Georgia for support of collection materials from kidney transplant recipients. The authors thank Eiko Kitamura (Georgia Cancer Center Integrated Genomics Shared Resource) for technical support. The authors are grateful to the Department of Medicine and the Georgia Cancer Center at the Augusta University community for fruitful discussion and support. These research results were supported by the Carlos and Marguerite Mason Trust, and by U.S. National Institutes of Health, National Cancer Institute Grant CA 172230 (to A.H.). The authors declare no conflicts of interest.
Glossary
- 7-AAD
7-amino-actinomycin D
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- ConA
concanavalin A
- GZMB
Granzyme B
- HLA-G
human leukocyte antigen G
- HV
healthy volunteers
- KTR
kidney transplant recipient
- LILRB1
leukocyte Ig-like receptor B1
- NR
nonrejected
- NSG
NOD-scid γ
- PBMC
peripheral blood mononuclear cell
- qPCR
quantitative PCR
- RJ
rejected
- rhIL-2
recombinant human IL-2
- sHLA-G
soluble HLA-G
- TCMR
T-cell–mediated rejection
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
V. Portik-Dobos, A. T. Nguyen-Lefebvre, C. Callaway, and D. D. Horuzsko designed and conducted experiments and analyzed data; R. Kapoor and C. Zayas analyzed data; K. Maenaka contributed new reagents; and A. Ajith, L. L. Mulloy, and A. Horuzsko designed and conducted experiments, analyzed results, and wrote the manuscript.
REFERENCES
- 1.Matas A. J., Smith J. M., Skeans M. A., Thompson B., Gustafson S. K., Stewart D. E., Cherikh W. S., Wainright J. L., Boyle G., Snyder J. J., Israni A. K., Kasiske B. L. (2015) OPTN/SRTR 2013 annual data report: kidney. Am. J. Transplant. 15 (Suppl 2), 1–34 [DOI] [PubMed] [Google Scholar]
- 2.Leblanc J., Subrt P., Paré M., Hartell D., Sénécal L., Blydt-Hansen T., Cardinal H. (2018) Practice patterns in the treatment and monitoring of acute T cell-mediated kidney graft rejection in Canada. Can. J. Kidney Health Dis. 5, 2054358117753616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran P. F. (2010) Commemorating the 10th anniversary of the launch of the AJT project. Am. J. Transplant. 10, 1111; erratum: 1496 [DOI] [PubMed] [Google Scholar]
- 4.Wood K. J., Goto R. (2012) Mechanisms of rejection: current perspectives. Transplantation 93, 1–10 [DOI] [PubMed] [Google Scholar]
- 5.Chapman J. R. (2017) Progress in transplantation: will it be achieved in big steps or by marginal gains? Am. J. Kidney Dis. 69, 287–295 [DOI] [PubMed] [Google Scholar]
- 6.Riella L. V., Djamali A., Pascual J. (2017) Chronic allograft injury: mechanisms and potential treatment targets. Transplant. Rev. (Orlando) 31, 1–9 [DOI] [PubMed] [Google Scholar]
- 7.Carosella E. D., Horuzsko A. (2007) HLA-G and cancer. Semin. Cancer Biol. 17, 411–412 [DOI] [PubMed] [Google Scholar]
- 8.Esensten J. H., Bluestone J. A., Lim W. A. (2017) Engineering therapeutic T cells: from synthetic biology to clinical trials. Annu. Rev. Pathol. 12, 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu J., Terasaki P. I., Miller J., Mizutani K., Cai J., Carosella E. D. (2006) Soluble HLA-G expression and renal graft acceptance. Am. J. Transplant. 6, 2152–2156 [DOI] [PubMed] [Google Scholar]
- 10.Ristich V., Zhang W., Liang S., Horuzsko A. (2007) Mechanisms of prolongation of allograft survival by HLA-G/ILT4-modified dendritic cells. Hum. Immunol. 68, 264–271 [DOI] [PubMed] [Google Scholar]
- 11.Rouas-Freiss N., Naji A., Durrbach A., Carosella E. D. (2007) Tolerogenic functions of human leukocyte antigen G: from pregnancy to organ and cell transplantation. Transplantation 84(Suppl), S21–S25 [DOI] [PubMed] [Google Scholar]
- 12.Brugière O., Thabut G., Pretolani M., Krawice-Radanne I., Dill C., Herbreteau A., Poras I., Moreau P., Colombat M., Danel C., Dehoux M., Fournier M., Carosella E. D., Rouas-Freiss N. (2009) Immunohistochemical study of HLA-G expression in lung transplant recipients. Am. J. Transplant. 9, 1427–1438 [DOI] [PubMed] [Google Scholar]
- 13.Favier B., HoWangYin K. Y., Wu J., Caumartin J., Daouya M., Horuzsko A., Carosella E. D., LeMaoult J. (2011) Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PLoS One 6, e21011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeMaoult J., Daouya M., Wu J., Loustau M., Horuzsko A., Carosella E. D. (2013) Synthetic HLA-G proteins for therapeutic use in transplantation. FASEB J. 27, 3643–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezeakile M., Portik-Dobos V., Wu J., Horuzsko D. D., Kapoor R., Jagadeesan M., Mulloy L. L., Horuzsko A. (2014) HLA-G dimers in the prolongation of kidney allograft survival. J. Immunol. Res. 2014, 153981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouas-Freiss N., Gonçalves R. M., Menier C., Dausset J., Carosella E. D. (1997) Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 94, 11520–11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeMaoult J., Krawice-Radanne I., Dausset J., Carosella E. D. (2004) HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc. Natl. Acad. Sci. USA 101, 7064–7069: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S., Ristich V., Arase H., Dausset J., Carosella E. D., Horuzsko A. (2008) Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6--STAT3 signaling pathway. Proc. Natl. Acad. Sci. USA 105, 8357–8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., Liang S., Wu J., Horuzsko A. (2008) Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation 86, 1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carosella E. D., Rouas-Freiss N., Tronik-Le Roux D., Moreau P., LeMaoult J. (2015) HLA-G: an immune checkpoint molecule. Adv. Immunol. 127, 33–144 [DOI] [PubMed] [Google Scholar]
- 21.Kummer J. A., Kamp A. M., Tadema T. M., Vos W., Meijer C. J., Hack C. E. (1995) Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin. Exp. Immunol. 100, 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelso A., Costelloe E. O., Johnson B. J., Groves P., Buttigieg K., Fitzpatrick D. R. (2002) The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int. Immunol. 14, 605–613 [DOI] [PubMed] [Google Scholar]
- 23.Cai Q., Dierich A., Oulad-Abdelghani M., Chan S., Kastner P. (2009) Helios deficiency has minimal impact on T cell development and function. J. Immunol. 183, 2303–2311 [DOI] [PubMed] [Google Scholar]
- 24.Wagner C., Iking-Konert C., Denefleh B., Stegmaier S., Hug F., Hänsch G. M. (2004) Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood 103, 1099–1104 [DOI] [PubMed] [Google Scholar]
- 25.Anthony D. A., Andrews D. M., Watt S. V., Trapani J. A., Smyth M. J. (2010) Functional dissection of the granzyme family: cell death and inflammation. Immunol. Rev. 235, 73–92 [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Fueyo A., Strom T. B. (2011) Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 140, 51–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metkar S. S., Menaa C., Pardo J., Wang B., Wallich R., Freudenberg M., Kim S., Raja S. M., Shi L., Simon M. M., Froelich C. J. (2008) Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 29, 720–733 [DOI] [PubMed] [Google Scholar]
- 28.Wensink A. C., Kemp V., Fermie J., García Laorden M. I., van der Poll T., Hack C. E., Bovenschen N. (2014) Granzyme K synergistically potentiates LPS-induced cytokine responses in human monocytes. Proc. Natl. Acad. Sci. USA 111, 5974–5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiebert P. R., Granville D. J. (2012) Granzyme B in injury, inflammation, and repair. Trends Mol. Med. 18, 732–741 [DOI] [PubMed] [Google Scholar]
- 30.Sorokin L. (2010) The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 [DOI] [PubMed] [Google Scholar]
- 31.Wensink A. C., Hack C. E., Bovenschen N. (2015) Granzymes regulate proinflammatory cytokine responses. J. Immunol. 194, 491–497 [DOI] [PubMed] [Google Scholar]
- 32.Revell P. A., Grossman W. J., Thomas D. A., Cao X., Behl R., Ratner J. A., Lu Z. H., Ley T. J. (2005) Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J. Immunol. 174, 2124–2131 [DOI] [PubMed] [Google Scholar]
- 33.Altimari A., Gruppioni E., Capizzi E., Bagni A., Corti B., Fiorentino M., Lazzarotto T., Lauro A., Pinna A. D., Ridolfi L., Grigioni W. F., D’errico-Grigioni A. (2008) Blood monitoring of granzyme B and perforin expression after intestinal transplantation: considerations on clinical relevance. Transplantation 85, 1778–1783 [DOI] [PubMed] [Google Scholar]
- 34.Liang S., Zhang W., Horuzsko A. (2006) Human ILT2 receptor associates with murine MHC class I molecules in vivo and impairs T cell function. Eur. J. Immunol. 36, 2457–2471 [DOI] [PubMed] [Google Scholar]
- 35.Shiroishi M., Kuroki K., Ose T., Rasubala L., Shiratori I., Arase H., Tsumoto K., Kumagai I., Kohda D., Maenaka K. (2006) Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 281, 10439–10447 [DOI] [PubMed] [Google Scholar]
- 36.Shiroishi M., Kuroki K., Rasubala L., Tsumoto K., Kumagai I., Kurimoto E., Kato K., Kohda D., Maenaka K. (2006) Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc. Natl. Acad. Sci. USA 103, 16412–16417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janas M. L., Groves P., Kienzle N., Kelso A. (2005) IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J. Immunol. 175, 8003–8010 [DOI] [PubMed] [Google Scholar]
- 38.Brown D., Trowsdale J., Allen R. (2004) The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 64, 215–225 [DOI] [PubMed] [Google Scholar]
- 39.López-Álvarez M. R., Jones D. C., Jiang W., Traherne J. A., Trowsdale J. (2014) Copy number and nucleotide variation of the LILR family of myelomonocytic cell activating and inhibitory receptors. Immunogenetics 66, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroki K., Hirose K., Okabe Y., Fukunaga Y., Takahashi A., Shiroishi M., Kajikawa M., Tabata S., Nakamura S., Takai T., Koyanagi S., Ohdo S., Maenaka K. (2013) The long-term immunosuppressive effects of disulfide-linked HLA-G dimer in mice with collagen-induced arthritis. Hum. Immunol. 74, 433–438 [DOI] [PubMed] [Google Scholar]
- 41.Gonen-Gross T., Achdout H., Arnon T. I., Gazit R., Stern N., Horejsí V., Goldman-Wohl D., Yagel S., Mandelboim O. (2005) The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J. Immunol. 175, 4866–4874 [DOI] [PubMed] [Google Scholar]
- 42.Boyson J. E., Erskine R., Whitman M. C., Chiu M., Lau J. M., Koopman L. A., Valter M. M., Angelisova P., Horejsi V., Strominger J. L. (2002) Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc. Natl. Acad. Sci. USA 99, 16180–16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonen-Gross T., Achdout H., Gazit R., Hanna J., Mizrahi S., Markel G., Goldman-Wohl D., Yagel S., Horejsí V., Levy O., Baniyash M., Mandelboim O. (2003) Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J. Immunol. 171, 1343–1351 [DOI] [PubMed] [Google Scholar]
- 44.Desgrandchamps F., LeMaoult J., Goujon A., Riviere A., Rivero-Juarez A., Djouadou M., de Gouvello A., Dumont C., Wu C. L., Culine S., Verine J., Rouas-Freiss N., Hennequin C., Masson-Lecomte A., Carosella E. D. (2018) Prediction of non-muscle-invasive bladder cancer recurrence by measurement of checkpoint HLAG’s receptor ILT2 on peripheral CD8+ T cells. Oncotarget 9, 33160–33169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choy J. C. (2010) Granzymes and perforin in solid organ transplant rejection. Cell Death Differ. 17, 567–576 [DOI] [PubMed] [Google Scholar]
- 46.Voskoboinik I., Whisstock J. C., Trapani J. A. (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 15, 388–400 [DOI] [PubMed] [Google Scholar]
- 47.Lieberman J. (2003) The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3, 361–370 [DOI] [PubMed] [Google Scholar]
- 48.Liu C. C., Rafii S., Granelli-Piperno A., Trapani J. A., Young J. D. (1989) Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J. Exp. Med. 170, 2105–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu C. R., Ortaldo J. R., Curiel R. E., Young H. A., Anderson S. K., Gosselin P. (1999) Role of a STAT binding site in the regulation of the human perforin promoter. J. Immunol. 162, 2785–2790 [PubMed] [Google Scholar]
- 50.Zhang J., Scordi I., Smyth M. J., Lichtenheld M. G. (1999) Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J. Exp. Med. 190, 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colonna M., Navarro F., Bellón T., Llano M., García P., Samaridis J., Angman L., Cella M., López-Botet M. (1997) A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravetch J. V., Lanier L. L. (2000) Immune inhibitory receptors. Science 290, 84–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.