Abstract
Human skeletal muscle fibers exist across a continuum of slow → fast-twitch. The amount of each fiber type (FT) influences muscle performance but remains largely unexplored in elite athletes, particularly from strength/power sports. To address this nescience, vastus lateralis (VL) biopsies were performed on World/Olympic (female, n = 6, “WCF”) and National-caliber (female, n = 9, “NCF”; and male, n = 6, “NCM”) American weightlifters. Participant accolades included 3 Olympic Games, 19 World Championships, 25 National records, and >170 National/International medals. Samples were analyzed for myosin heavy chain (MHC) content via SDS-PAGE using two distinct techniques: single fiber (SF) distribution (%) and homogenate (HG) composition. The main finding was that these athletes displayed the highest pure MHC IIa concentrations ever reported in healthy VL (23±9% I, 5±3% I/IIa, 67±13% IIa, and 6±10% IIa/IIx), with WCF expressing a notable 71±17% (NCF = 67±8%, NCM = 63±16%). No pure MHC IIx were found with SF. Secondary analysis revealed the heavyweights accounted for 91% of the MHC IIa/IIx fibers, which caused a correlation between this FT and body mass. Additionally, when compared to SF, HG overestimated MHC I (23±9 vs. 31±9%) and IIx (0±0 vs. 3±6%) by misclassifying I/IIa fibers as I and IIa/IIx fibers as IIx, highlighting the limitation of HG as a measure of isoform distribution. These results collectively suggest that athlete caliber (World vs. National) and/or years competing in the sport determine FT% more than sex, particularly for MHC IIa. The extreme fast-twitch myofiber abundance likely explains how elite weightlifters generate high forces in rapid time-frames.
Introduction
Italian physician Stefano Lorenzini made the first distinction of “red” and “white” muscle fibers (myofibers) in 1678, and almost 200 years later (1873) French histologist Louis-Antoine Ranvier confirmed the existence of two distinct myofiber types in vertebrate skeletal muscle. Reintroduction of the skeletal muscle biopsy procedure in 1962 [1] allowed scientists to begin exploring the topic in athletes and resulted in the discovery that each FT is comprised of a unique MHC isoform signature. Human skeletal muscle therefore contains three pure (MHC I, IIa, and IIx) and several hybrid (single myofibers that co-expresses multiple MHC isoforms) FTs [2]. The pure and hybrid FTs combine to form a robust slow → fast continuum (MHC I → I/IIa → IIa → IIa/IIx → IIx) with each displaying specific morphological, metabolic, and contractile properties [3–6]. FT% (the relative quantity of each FT in a given muscle) influences whole muscle function [7] and is often highly correlated with athletic performance [3, 7–13].
Extensive evidence indicates endurance athletes possess a slow-twitch myofiber majority [9, 10, 12, 14, 15], yet comparatively, far few investigations have explored FT in speed, power, or strength athletes. Initial research in the 1970–80’s found that resistance-trained men expressed high quantities (~60–65%) of fast-twitch fibers [11, 12, 15, 16], which was substantiated by later studies on elite powerlifters [17] and national-caliber (‘Olympic’) weightlifters [8]. This work provided an important foundation, but used sub-elite participants [18] and/or laboratory methods that failed to accurately resolve the highly prevalent hybrids [19–21]—which compromises measurement fidelity and produces erroneous FT% conclusions [19, 22–25]. More precise techniques were developed in the early 1990’s that allowed proper quantification of FT% by analyzing each SF.
Since this time only 13 studies (Table 1) implemented SF in young speed, power, or strength-trained individuals [5, 13, 19, 20, 22, 23, 25–30], and only 3 included females (n = 13, total). Only 5/13 included athletes: unknown-caliber male sprinters (n = 6) [25], male soccer players (n = 8) [24], elite female track and field athletes from a combination of pole vault, heptathlon, 100 and 400 m hurdles, and long jump events (n = 6) [20], National-caliber male bodybuilders (n = 8) [19], and a former World-champion male sprinter (n = 1) [13]. Accurately accounting for the full FT spectrum resulted in all five studies finding far lower MHC IIa concentrations than expected (52%, 30%, 16%, 39%, and 34%, respectively). The extremely low 16% found by Parcell et al. (2003) [20] is possibly explained by sex as females are often purported to possess more slow-twitch fibers than men [31, 32]. Such sex-specific phenotypes are often the case in murine models [31], but the topic remains unexplored in athletes. Moreover, these data are difficult to interpret as the athletes sampled were from a combination of several dissimilar events.
Table 1. Summary of literature reporting SF MHC FT% from the VL in young speed, power, or strength-trained individuals.
| Reference | Subjects | Condition | MHC Distribution (%) | |||||
|---|---|---|---|---|---|---|---|---|
| I | I/IIa | IIa | IIa/IIx | IIx | I/IIa/IIx | |||
| Andersen (1994) | Sprinting; 6M (23y) | Post 12-week RE & Interval Training | 41 | 1 | 52 | 5 | 0 | 0 |
| Andersen (1994) | Soccer; 8M (23y) | National Players Post 12-week RE Training | 59 | 3 | 30 | 9 | 0 | <1 |
| Williamson (2001) | Non Ath; 6M (25y) 6F (21 y) |
Post 12-week RE Training | 30 35 | 5 3 | 59 52 | 5 12 | 0 0 | 0 0 |
| Parcell (2003) | Track & Field; 6F (23y) | Division I / Interntional—Caliber | 57 | 9 | 16 | 14 | 1 | 1 |
| Raue (2005) | Non Ath; 6M (24y) 6M (24y) | Post-Con RE Post-Ecc RE | 38 25 | 1 7 | 34 39 | 27 25 | 0 2 | <1 <1 |
| Parcell (2005) | Non Ath; 10M (22y) | Post 8-week Sprint Cycle Training | 34 | 8 | 44 | 12 | 0 | 2 |
| Malisoux (2006) | Non Ath; 8M (23) | Post 8-week Plyometric Training | 28 | 5 | 42 | 26 | 2 | 0 |
| Kesidis (2008) | Bodybuilding; 8M (26 y) | National-Caliber | 35 | 19 | 39 | 7 | 0 | 0 |
| Trappe (2015) | Sprinting; 1M (? y) | Previously World Champion | 24 | 5 | 34 | 9 | 24 | 0 |
| Murach (2016) | Non Ath; 9M (25y) | Resistance Trained | 17 | 10 | 60 | 11 | <1 | <1 |
| Bagley (2017) | Non Ath; 15M (25y) | Resistance Trained | 20 | 10 | 58 | 11 | 1 | 1 |
| Arevalo (2017) | Non Ath; 13M (24y) | Resistance Trained | 28 | 9 | 60 | 3 | <1 | <1 |
| Tobias (2017) | Non Ath; 1F (32y) | Concurrently Trained | 45 | 13 | 31 | 9 | 0 | 2 |
M = Male; F = Female; y = Year; RE = Resistance exercise; Non Ath = Not a competitive athlete; Track & Field = athletes from a combination of pole vault, heptathlon, 100 and 400 m hurdles, and long jump events; Con = Concentric, Ecc = Eccentric; Concurrently Trained = combined endurance and resistance training
Numerous other knowledge gaps persist because in over 50 years of human muscle FT research only two studies have utilized SF with elite (i.e., world or international) athletes (one male sprinter and six female track and field) and no research has done so with any strength or power athletes. Thus, the purpose of this study was to examine the FT% of elite weightlifters to provide novel insight into the phenotype of competitive female and male strength and power athletes.
Methods
Experimental approach to the problem
Twenty-one elite (‘Olympic’) Weightlifters (15 female, 6 male) underwent resting VL biopsies between 2–96 hours after competing in either the International Weightlifting Federation World Championships or the USA Weightlifting American Open Finals (2017). All procedures and risks were explained to the athletes prior to obtaining written consent and completing medical and exercise history questionnaires. Performance records (taken from this event) in the snatch and clean and jerk (1RM), competition medals, and other accolades were gathered from personal interviews and publically available records from these or other sanctioned meets. Each muscle sample was analyzed for MHC content using two distinct FT techniques: SF and HG. The California State University, Fullerton Institutional Review Board approved all experimental procedures prior to any testing and consent was received in oral and written format.
Participants
Participants were subdivided into three categories; WCF (n = 6 female), NCF (n = 9), and NCM (n = 6). Athletes were considered “World-caliber” if they were on the most recent Olympic or World team and competed at the most recent national event. Athletes were considered “National-caliber” if they were top 5 placers at the 2017 American Open Finals meet but had never been on a World or Olympic team. Athletes spanned multiple weight categories, had a minimum of two years of national competition experience, had competed exclusively for the United States of America, and were otherwise eligible for all American national meets (Table 2). Athlete accolades at the time of data collection included participation in 3 Olympic Games, 19 World Championships, 11 Pan American Championships, 49 National Championships, 32 American Opens, 8 University National Championships, and 25 Junior World/Pan American/National Championships. Participants also held 25 national records and >170 national/international medals either at the time of the study or in the past. One athlete had tested positive for substances prohibited by the World Anti-Doping Agency and was suspended from the sport for two years prior to participating in the study.
Table 2. Descriptive information of elite female and male American weightlifters.
| Age (y) | Body Mass (kg) | Height (cm) | Years Competing | Snatch Relative 1RM | Clean & Jerk Relative 1RM | |
| WCF | 28.2 ± 3.6* | 81.2 ± 36.0 | 164.0 ± 11.1 | 7.7 ± 4.7*ǂ | 1.32 ± 0.31* | 1.69 ± 0.40* |
| NCF | 23.6 ± 3.9 | 66.6 ± 11.0 | 164.8 ± 7.0 | 3.8 ± 0.8 | 1.29 ± 0.18ǂ | 1.68 ± 0.19ǂ |
| NCM | 26.0 ± 2.4 | 85.3 ± 26.9 | 169.0 ± 9.0 | 3.3 ± .08 | 1.64 ± 0.25 | 2.04 ± 0.29 |
| Average | 25.6 ± 3.8 | 76.1 ± 25.0 | 165.8 ± 8.7 | 4.8 ± 3.1 | 1.40 ± 0.28 | 1.79 ± .032 |
Data are described as mean ± standard deviation. y = years. Relative 1RM = competition record one repetition maximum divided by body mass. Years competing = number of years competing in USA Weightlifting sanctioned meets.
* = significantly different than NCF.
ǂ = significantly different than NCM. Significant = p < 0.05 .
Procedures
Muscle biopsies
Following 30 minutes of supine rest, athletes underwent a mid-muscle belly (approximately halfway between the greater trochanter and patella) biopsy of the VL. A detailed description of the biopsy procedure has been previously described by our lab [9, 22, 23, 33]. Briefly, a small area of the thigh was numbed by injection of a local anesthetic (Xylocaine/Lidocaine without epinephrine). An approximately ¼ inch incision was made in the superficial cutaneous tissues. Muscle samples were obtained using the Bergström technique with suction [1], immediately cleansed of excess blood and connective tissue, divided into approximately 10–15 mg strips, placed into cold skinning solution (125 mM K propionate, 2.0 mM EGTA, 4.0 mM ATP, 1.0 mM MgCl2, 20.0 mM imidazole [pH 7.0], and 50% [vol ml/vol ml] glycerol), and stored at -20° C for at least one week. Each sample was split such that a portion (~5 mg) could be used for SF or HG. The incision site was cleaned, pulled closed with a sterile Band-Aid, and covered with sterile gauze and cohesive bandage tape.
MHC FT identification
All biopsy samples were analyzed for MHC via SDS-PAGE using two distinct techniques: SF and HG. For SF, individual fibers (N = 2,147; 102 ± 3 fibers per athlete) were mechanically isolated with fine tweezers under a light microscope and placed in 80 μl of sodium dodecyl sulfate (SDS) buffer (1% SDS, 23 mM EDTA, 0.008% bromophenol blue, 15% glycerol, and 715 mM b-mercaptoethanol [pH 6.8]). HG samples (~5 mg) were hand homogenized and then diluted between 1:10 to 1:50 based on sample amount and protein quantity. As described in detail elsewhere [5, 9, 22, 23, 27], 1–2 μl aliquots of both SF or HG (run separately) were then loaded into individual wells in a 3.5% loading and 5% separating gel (SDS-PAGE), run at 5°C for 15.5 hours (SE 600 Series; Hoefer, San Francisco, CA, USA), and silver stained for MHC identification. The SF approach used known molecular weights and standards to identify the MHC isoform (MHC I, I/IIa, IIa, IIa/IIx, and IIx) of each individual myofiber. This enabled the most accurate calculation of the FT% within the muscle sample [21]. HG utilized densitometry (ImageJ, National Institutes of Health, Bethesda, MD) to quantify the relative MHC protein composition (i.e., percent area occupied by each pure isoform; MHC I, IIa, and IIx) of each sample, which is highly correlated with FT area [34]. Thus, SF indicates how frequently each isoform exists but cannot address how much area each FT occupies within the muscle. HG addresses the latter, but cannot delineate hybrids, therefore inaccurately quantifying FT% [9, 21–25, 27].
Statistical analysis
Potential differences between groups in descriptive information were examined via ANOVA. For SF, potential differences in FT% between groups were assessed via a 3 (group: WCF, NCF, NCM) x 4 (fiber type: MHC I, I/IIa, IIa, IIa/IIx) ANOVA. For HG, potential differences in FT composition between groups were examined via a 3 (group: WCF, NCF, NCM) x 3 (fiber type: MHC I, IIa, IIx) ANOVA. Comparison of SF vs. HG was accomplished by a 2 (group: SF, HG) x 3 (fiber type: MHC I, IIa, IIx) ANOVA. Effect size was calculated with Cohen’s D (0.2 = small difference, 0.5 = medium difference, and 0.8 = large difference) to identify the magnitude of difference between two groups. Pearson Product Moment Correlations (r) were assessed for WCF, NCF, and NCM between 1RM, body mass, and SF FT%. All individual FT data are reported in Table 3. Data are reported as mean ± standard deviation (SD), unless otherwise noted. Significance was established a priori at an alpha level of p < 0.05. All analyses were performed with SPSS (SPSS Statistics Version 24, IBM).
Table 3. Individual FT% of elite female and male American weightlifters.
Data are reported as a percentage.
| MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC IIx | ||||
|---|---|---|---|---|---|---|---|---|
| Athlete | SF | HG | SF | SF | HG | SF | SF | HG |
| WCF | ||||||||
| 1 | 13 | 31 | 7 | 74 | 69 | 7 | 0 | 0 |
| 2 | 39 | 34 | 13 | 48 | 66 | 0 | 0 | 0 |
| 3 | 18 | 21 | 2 | 79 | 79 | 1 | 0 | 0 |
| 4 | 9 | 19 | 2 | 89 | 81 | 0 | 0 | 0 |
| 5 | 12 | 22 | 4 | 85 | 78 | 0 | 0 | 0 |
| 6* | 10 | 17 | 9 | 52 | 70 | 28 | 0 | 13 |
| NCF | ||||||||
| 7 | 23 | 38 | 1 | 76 | 62 | 0 | 0 | 0 |
| 8* | 14 | 18 | 1 | 63 | 68 | 22 | 0 | 15 |
| 9 | 32 | 43 | 6 | 62 | 57 | 0 | 0 | 0 |
| 10 | 29 | 39 | 8 | 58 | 61 | 4 | 0 | 0 |
| 11 | 20 | 26 | 2 | 78 | 74 | 0 | 0 | 0 |
| 12 | 29 | 46 | 5 | 66 | 54 | 0 | 0 | 0 |
| 13 | 25 | 30 | 2 | 73 | 70 | 0 | 0 | 0 |
| 14 | 19 | 25 | 6 | 74 | 75 | 0 | 0 | 0 |
| 15 | 34 | 35 | 6 | 56 | 65 | 4 | 0 | 0 |
| NCM | ||||||||
| 16 | 29 | 40 | 8 | 63 | 60 | 0 | 0 | 0 |
| 17 | 26 | 37 | 1 | 73 | 63 | 0 | 0 | 0 |
| 18 | 32 | 44 | 3 | 65 | 56 | 0 | 0 | 0 |
| 19 | 7 | 18 | 3 | 84 | 82 | 6 | 0 | 0 |
| 20* | 26 | 36 | 3 | 54 | 57 | 17 | 0 | 7 |
| 21* | 29 | 33 | 2 | 37 | 49 | 32 | 0 | 18 |
* Denotes athlete in the heavyweight (or super) (>90 kg for women and >105 kg for men) category.
Results
Descriptive
WCF were significantly older than NCF, but not NCM (Table 2). WCF also had significantly more years of sport competition experience than NCF and NCM. NCM exceeded both WCF and NCF in relative strength in both the snatch 1RM and clean and jerk 1RM.
SF distribution
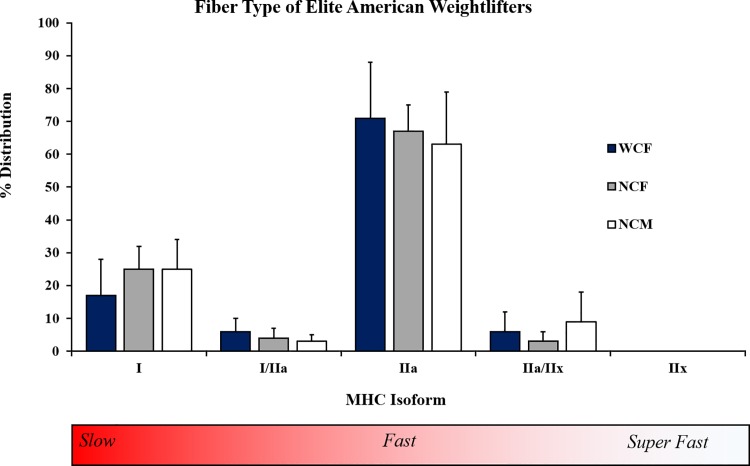
FT% for all lifters combined was 23 ± 9% I, 5 ± 3% I/IIa, 67 ± 13% IIa, and 6 ± 10% IIa/IIx. No MHC IIx or I/IIa/IIx fibers were identified. No significant differences existed between groups, despite WCF possessing 8% (absolute, not percent difference) less MHC I than NCF (d = 0.88) and NCM (d = 0.78) (Fig 1). The difference in MHC IIa between WCF and NCM (also 8%) was also not statistically significant, but had a moderate effect size (d = 0.50). The vast majority of the MHC IIa/IIx fibers (91%) belonged to just five lifters, all of whom competed in the heavyweight or super heavyweight categories (≥ 90 kg for women and ≥105 kg for men). This produced significant correlations between body mass and MHC IIa/IIx frequency for WCF (r = 0.919, p = 0.010) and NCF (r = 0.826, p = 0.006) and a trend for NCM (r = 0.757, p = 0.080).
Fig 1. MHC FT% of elite American weightlifters.
Data are reported as a percentage ± standard deviation.
HG composition
FT composition for all lifters combined was 31 ± 9% I, 67 ± 9% IIa, and 3 ± 6% IIx. MHC I tended (p = 0.08) to be lower in WCF (24 ± 7%) than NCF (33 ± 9%, p = 0.125, d = 1.14) and NCM (35 ± 9%, p = 0.106, d = 1.33), yet MHC IIa was significantly higher (p = 0.046) in WCF (74 ± 6%) than NCM (61 ± 11%, p = 0.043, d = 1.39), but not NCF (65 ± 7%, p = 0.145, d = 1.28) and. FT was significantly different (p < 0.001) between SF and HG for MHC I (p = 0.005) and MHC IIx (p = 0.046), but not MHC IIa. SF MHC IIa/IIx and HG MHC IIx were highly correlated (r = 0.96, p < 0. 001). No correlations existed for SF or HG between FT% and snatch or clean and jerk relative 1RM when analyzed as subgroups or when combined together.
Discussion
The current study resulted in the most detailed investigation of muscle phenotype in Olympic and World-caliber anaerobic athletes published to date. These data are the first comparison of World vs. National-caliber athletes at the SF level. Additionally, they enabled the most precise description of FT% in strength or power sport competitors, and the first ever in females. The primary finding was that the pure MHC IIa abundance was the highest in healthy muscle (VL) ever reported, especially for females. This finding suggests athlete caliber and/or years competing in the sport influence FT% more than sex per se and also questions the pronouncement that male athletes possess more fast-twitch myofibers than females. Secondary findings revealed that our utilization of two different typing methods confirmed the limitations of HG for FT% (inappropriately categorizes MHC I/IIa as MHC I and MHC IIa/IIx as MHC IIx) and also allowed identification of a previously undocumented relationship between body mass and MHC IIa/IIx concentrations. The unique morphology and phenotypes in our participants highlight the need to further study elite anaerobic athletes, particularly females.
WCF contained the highest concentration of MHC IIa (71%) reported in the literature to our knowledge. NCF (67%) and NCM (63%) also possessed more MHC IIa than previous research in competitive male bodybuilders (40%) [16, 19] as well as male power/weightlifters [8, 11, 12, 15, 16, 18], elite female pole vaulters, heptathletes, 100 and 400 m hurdlers, and long jumpers (20), elite male hammer throwers [35], and resistance-trained men [18, 19, 22, 23, 27, 29, 36], which all ranged from 50–60%. Only six previous studies using SF have found pure MHC IIa concentrations of >50%, with just two reporting 60% (Table 1). The resulting minimal MHC I (~17–25%) in our athletes was strikingly lower than the previously described track and field athletes (57%) [20] and National-caliber bodybuilders (35%) [19]. These pronounced differences are likely explained by the substantial dissimilarities in training styles (e.g., external loading strategies, contraction type and velocity, training frequency, etc.) between the various sports. More research is therefore needed to continue delineating the subtle but significant differences in FT% between athletes from a range of anaerobic sports and the specific role each training approach might play in altering MHC I and IIa distribution.
Although the differences in FT% between our three groups did not reach statistical significance, large effect sizes were evident and MHC IIa frequencies of 74%-89% occurred in 66% of WCF but only in 44% and 33% of NCF and NCM, respectively. Thus, scientists should further examine how FT% may separate World from National-level athletes as it would enhance our understanding of the physiological factors determining maximal human performance. For example, the only published report on a world-record holding anaerobic athlete found a FT profile remarkably different from our study or any other previous research in elite sprinters [13]. The minimal exploration in this area makes it difficult to determine if such a separation in FT profile between elite subgroups is a true and consistent phenomenon or merely an artifact of too little research.
Our groups differed in two other important characteristics; sex and years competing in the sport. Sex comparisons between athletes remain tenuous [31, 37] because nearly all investigations utilize non-gold standard FT% methods [21] and sedentary [38, 39] or “recreationally active” individuals [32]. Not only do our findings contradict the claim that women possess more slow-twitch myofibers than men [40], they illustrate the opposite when accounting for talent level (WCF < NCF = NCM). WCF had also been competing in the sport for ~5 years longer than both NCF and NCM. The current cross-sectional study-design precludes direct analysis, but extensive research affords strong support for exercise history as a critical determinate of FT% [2, 9, 26, 28, 30, 41–44]. Chronic exercise generally decreases hybrids [30, 42] and induces style-specific shifts in FT% such as increases in MHC I with endurance [9, 43, 45] or MHC IIa with sprint [28], plyometric [26], or strength training [36, 43, 44, 46]. For example, one study reported an increase in MHC IIa from 46% to 60% following 19 weeks of resistance training [36]. MHC IIa/IIx fibers appear particularly responsible for exercise-induced increases in MHC IIa and are thus uncommon in exercise-trained individuals [9, 20, 22–25, 29, 43]. A reduction of MHC IIx in favor of IIa following chronic resistance exercise is also purported extensively in the literature [28, 34], yet the overwhelming majority of this evidence comes from experiments with methodologies (e.g. HG) directly shown here and elsewhere [9, 24, 25, 47] to produce erroneous FT% conclusions.
Most research from the 1970’s– 2000’s utilized either ATPase histochemistry or HG SDS-PAGE to determine FT% [8, 14–17, 24, 25, 34, 36]. Similar to SF, histochemistry allows assessment of individual fibers for calculation of percent distribution, yet it does not enable simultaneous delineate of hybrids [36]. HG suffers the same drawback and actually indicates FT area/composition [34] more so than distribution making it greatly influenced by the size of each fiber; which is not uniform across all FTs (particularly in resistance trained individuals) [48]. All three approaches hold strong merit and are often correlated to each other [34, 49] and performance [8], but are clearly not interchangeable for maximally precise FT% assessment. In the current study, HG accurately quantified MHC IIa (within 0–4%), but not I or IIx. MHC I was overestimated by 8% percent (23 vs. 31%), which is largely explained by the non-differentiated MHC I/IIa fibers (5%). HG also greatly exaggerated MHC IIx, particularly in individuals with >4% MHC IIa/IIx. The inability of HG to account for MHC IIa/IIx explains why MHC IIx appear common in some studies [50] even though the actual abundance of pure MHC IIx fibers in healthy human skeletal muscle is extraordinarily rare; typically <0.1% [9, 22–25, 27] and 0 of the >2,100 isolated fibers from the current sample. Thus, the seeming conversion of MHC IIx to IIa with exercise is more precisely IIa/IIx changing to IIa.
MHC IIa/IIx hybrids are typically inversely associated with muscle health and physical activity [2, 9, 30, 43, 47, 51–54]. Yet, the heavyweights (male and female) expressed irregularly high concentrations (24%) and accounted for 91% of all MHC IIa/IIx myofibers, explaining the correlations between body mass and MHC IIa/IIx quantities. Terzis and colleagues (2010) noted a similar abnormal abundance of MHC IIx (typed via HG, so likely IIa/IIx) in six large (116 kg, body fat composition >22%), but presumably highly strength-trained throwers [35]. Body composition was not assessed in the current study and little research exists on well-trained, but high body mass individuals. Not knowing the amount of muscle vs. fat on these larger participants limits the ability to speculate on potential mechanisms. Thus, additional studies with a larger sample size across a broader spectrum of physical size are required to truly interpret the correlations between body mass and MHC IIa/IIx prevalence and to explore possible mechanisms.
Another juxtaposition was that of FT% and performance. Previous work in 94 kg male competitive weightlifters found strong correlations between FT composition (via HG) and the percentage of total area in a muscle that each FT occupies to both snatch 1RM and vertical jump height [8], but not clean and jerk 1RM. We failed to identify any such correlations (when all subjects were combined or sub-grouped), but also utilized multiple sexes and weight classes. Thus, while FT% differed between our groups, that factor alone did not predict performance among our lifters. Several possible explanations exist for this discrepancy. First, FT area may determine whole muscle strength more than FT%. Second, neither studies found correlations to the clean and jerk, which is heavier and slower than the snatch or vertical jump. This compliments previous isokinetic research [23] and indicates FT% does not predict performance on strength tasks among strength-trained individuals. FT% probably determines movement speed more than force production [7]. Further speculation on this point is unwarranted as limitations prohibited the ability to assess FT-specific size or contractile properties, which likely differed significantly across our groups [55] and are known to changes with training [3, 48].
Conclusion
This study provides novel insight into the muscle phenotype of elite competitive strength and power athletes and highlights the need for more research in this area. The extreme fast-twitch abundance partially explains how elite weightlifters are able to generate high forces in short time-frames. Our data also indicate that athlete caliber and years competing in the sport dictate FT% more than sex per se, but more work is needed to draw firm conclusions as a single biopsy may not perfectly represent the entire muscle [56]. Most athletes contained few hybrids and no MHC IIx or I/IIa/IIx, except the heavyweights who possessed atypically high quantities of IIa/IIx. Future research should use high fidelity techniques to explore FT-specific distribution, size, and contractile properties in female and male athletes of various caliber, sports, and body size; ideally across several years of competition. The resulting data could have practical significance if it enabled experimentation of differing training volumes or recovery protocols based on athlete-specific FT properties [57]. Scientifically, our findings importantly contribute to the knowledge-base of fiber type-specific physiology.
Acknowledgments
The authors would like to thank Irene S. Tobias and Cameron Yen for their help with this project.
Data Availability
All relevant data are within the manuscript.
Funding Statement
Funding for this project was provided by Renaissance Periodization. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bergstrom J. Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. The Scandinavian Journal of Clinical and Laboratory Investigations. 1962;14 (Suppl. 68). [Google Scholar]
- 2.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50(6):500–9. Epub 2000/09/22. . [DOI] [PubMed] [Google Scholar]
- 3.Methenitis S, Karandreas N, Spengos K, Zaras N, Stasinaki AN, Terzis G. Muscle Fiber Conduction Velocity, Muscle Fiber Composition, and Power Performance. Med Sci Sports Exerc. 2016;48(9):1761–71. Epub 2016/04/30. 10.1249/MSS.0000000000000954 . [DOI] [PubMed] [Google Scholar]
- 4.Galpin AJ, Raue U, Jemiolo B, Trappe TA, Harber MP, Minchev K, et al. Human skeletal muscle fiber type specific protein content. Anal Biochem. 2012;425(2):175–82. Epub 2012/04/04. 10.1016/j.ab.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias IS, Lazauskas KK, Arevalo JA, Bagley JR, Brown LE, Galpin AJ. Fiber type-specific analysis of AMPK isoforms in human skeletal muscle: advancement in methods via capillary nano-immunoassay. J Appl Physiol (1985). 2017. Epub 2018/01/24. 10.1152/japplphysiol.00894.2017 . [DOI] [PubMed] [Google Scholar]
- 6.Bagley JR. Fibre type-specific hypertrophy mechanisms in human skeletal muscle: potential role of myonuclear addition. J Physiol. 2014;592(23):5147–8. Epub 2014/12/03. 10.1113/jphysiol.2014.282574 592/23/5147 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol. 1999;9(2):87–95. Epub 1999/03/31. S1050-6411(98)00040-6 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8.Fry AC, Schilling BK, Staron RS, Hagerman FC, Hikida RS, Thrush JT. Muscle fiber characteristics and performance correlates of male Olympic-style weightlifters. J Strength Cond Res. 2003;17(4):746–54. Epub 2003/12/12. . [DOI] [PubMed] [Google Scholar]
- 9.Bathgate KE, Bagley JR, Jo E, Talmadge RJ, Tobias IS, Brown LE, et al. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur J Appl Physiol. 2018. Epub 2018/07/15. 10.1007/s00421-018-3943-7 . [DOI] [PubMed] [Google Scholar]
- 10.Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol. 1976;40(2):149–54. Epub 1976/02/01. 10.1152/jappl.1976.40.2.149 . [DOI] [PubMed] [Google Scholar]
- 11.Tesch PA, Karlsson J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J Appl Physiol (1985). 1985;59(6):1716–20. Epub 1985/12/01. 10.1152/jappl.1985.59.6.1716 . [DOI] [PubMed] [Google Scholar]
- 12.Prince FP, Hikida RS, Hagerman FC. Human muscle fiber types in power lifters, distance runners and untrained subjects. Pflugers Arch. 1976;363(1):19–26. Epub 1976/05/06. . [DOI] [PubMed] [Google Scholar]
- 13.Trappe S, Luden N, Minchev K, Raue U, Jemiolo B, Trappe TA. Skeletal muscle signature of a champion sprint runner. J Appl Physiol (1985). 2015;118(12):1460–6. Epub 2015/03/10. 10.1152/japplphysiol.00037.2015 japplphysiol.00037.2015 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollnick PD, Armstrong RB, Saubert CWt, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972;33(3):312–9. Epub 1972/09/01. 10.1152/jappl.1972.33.3.312 . [DOI] [PubMed] [Google Scholar]
- 15.Tesch PA, Thorsson A, Kaiser P. Muscle capillary supply and fiber type characteristics in weight and power lifters. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(1):35–8. Epub 1984/01/01. 10.1152/jappl.1984.56.1.35 . [DOI] [PubMed] [Google Scholar]
- 16.Tesch PA, Larsson L. Muscle hypertrophy in bodybuilders. Eur J Appl Physiol Occup Physiol. 1982;49(3):301–6. Epub 1982/01/01. . [DOI] [PubMed] [Google Scholar]
- 17.Fry AC, Webber JM, Weiss LW, Harber MP, Vaczi M, Pattison NA. Muscle fiber characteristics of competitive power lifters. J Strength Cond Res. 2003;17(2):402–10. Epub 2003/05/14. . [DOI] [PubMed] [Google Scholar]
- 18.Galpin AJ, Fry AC, Chiu LZ, Thomason DB, Schilling BK. High-power resistance exercise induces MAPK phosphorylation in weightlifting trained men. Appl Physiol Nutr Metab. 2012;37(1):80–7. Epub 2012/01/10. 10.1139/h11-131 . [DOI] [PubMed] [Google Scholar]
- 19.Kesidis N, Metaxas TI, Vrabas IS, Stefanidis P, Vamvakoudis E, Christoulas K, et al. Myosin heavy chain isoform distribution in single fibres of bodybuilders. Eur J Appl Physiol. 2008;103(5):579–83. Epub 2008/05/08. 10.1007/s00421-008-0751-5 . [DOI] [PubMed] [Google Scholar]
- 20.Parcell AC, Sawyer RD, Craig Poole R. Single muscle fiber myosin heavy chain distribution in elite female track athletes. Med Sci Sports Exerc. 2003;35(3):434–8. Epub 2003/03/06. 10.1249/01.MSS.0000053735.99344.C0 . [DOI] [PubMed] [Google Scholar]
- 21.Pandorf CE, Caiozzo VJ, Haddad F, Baldwin KM. A rationale for SDS-PAGE of MHC isoforms as a gold standard for determining contractile phenotype. J Appl Physiol (1985). 2010;108(1):222–2; author reply 6. Epub 2010/01/08. 10.1152/japplphysiol.01233.2009 . [DOI] [PubMed] [Google Scholar]
- 22.Arevalo JA, Lynn SK, Bagley JR, Brown LE, Costa PB, Galpin AJ. Lower-Limb Dominance, Performance, and Fiber Type in Resistance-trained Men. Med Sci Sports Exerc. 2017. Epub 2017/12/23. 10.1249/MSS.0000000000001533 . [DOI] [PubMed] [Google Scholar]
- 23.Bagley JR, McLeland KA, Arevalo JA, Brown LE, Coburn JW, Galpin AJ. Skeletal Muscle Fatigability and Myosin Heavy Chain Fiber Type in Resistance Trained Men. J Strength Cond Res. 2017;31(3):602–7. 10.1519/JSC.0000000000001759 . [DOI] [PubMed] [Google Scholar]
- 24.Andersen JL, Klitgaard H, Bangsbo J, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of soccer players: effects of strength-training. Acta Physiol Scand. 1994;150(1):21–6. Epub 1994/01/01. 10.1111/j.1748-1716.1994.tb09655.x . [DOI] [PubMed] [Google Scholar]
- 25.Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand. 1994;151(2):135–42. Epub 1994/06/01. 10.1111/j.1748-1716.1994.tb09730.x . [DOI] [PubMed] [Google Scholar]
- 26.Malisoux L, Francaux M, Nielens H, Renard P, Lebacq J, Theisen D. Calcium sensitivity of human single muscle fibers following plyometric training. Med Sci Sports Exerc. 2006;38(11):1901–8. Epub 2006/11/11. 10.1249/01.mss.0000232022.21361.47 . [DOI] [PubMed] [Google Scholar]
- 27.Murach KA, Bagley JR, McLeland KA, Arevalo JA, Ciccone AB, Malyszek KK, et al. Improving human skeletal muscle myosin heavy chain fiber typing efficiency. J Muscle Res Cell Motil. 2016;37(1–2):1–5. 10.1007/s10974-016-9441-9 . [DOI] [PubMed] [Google Scholar]
- 28.Parcell AC, Sawyer RD, Drummond MJ, O'Neil B, Miller N, Woolstenhulme MT. Single-fiber MHC polymorphic expression is unaffected by sprint cycle training. Med Sci Sports Exerc. 2005;37(7):1133–7. Epub 2005/07/15. . [DOI] [PubMed] [Google Scholar]
- 29.Raue U, Terpstra B, Williamson DL, Gallagher PM, Trappe SW. Effects of short-term concentric vs. eccentric resistance training on single muscle fiber MHC distribution in humans. Int J Sports Med. 2005;26(5):339–43. Epub 2005/05/17. 10.1055/s-2004-821041 . [DOI] [PubMed] [Google Scholar]
- 30.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol (1985). 2001;91(5):1955–61. 10.1152/jappl.2001.91.5.1955 . [DOI] [PubMed] [Google Scholar]
- 31.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30(1):30–9. Epub 2015/01/07. 10.1152/physiol.00024.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norman B, Esbjornsson M, Rundqvist H, Osterlund T, von Walden F, Tesch PA. Strength, power, fiber types, and mRNA expression in trained men and women with different ACTN3 R577X genotypes. J Appl Physiol (1985). 2009;106(3):959–65. Epub 2009/01/20. 10.1152/japplphysiol.91435.2008 91435.2008 [pii]. . [DOI] [PubMed] [Google Scholar]
- 33.Bagley JR, Galpin AJ. Three-dimensional printing of human skeletal muscle cells: An interdisciplinary approach for studying biological systems. Biochem Mol Biol Educ. 2015;43(6):403–7. 10.1002/bmb.20891 . [DOI] [PubMed] [Google Scholar]
- 34.Fry AC, Allemeier CA, Staron RS. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994;68(3):246–51. Epub 1994/01/01. . [DOI] [PubMed] [Google Scholar]
- 35.Terzis G, Spengos K, Kavouras S, Manta P, Georgiadis G. Muscle fibre type composition and body composition in hammer throwers. J Sports Sci Med. 2010;9(1):104–9. Epub 2010/01/01. [PMC free article] [PubMed] [Google Scholar]
- 36.Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol (1985). 1993;74(2):911–5. Epub 1993/02/01. 10.1152/jappl.1993.74.2.911 . [DOI] [PubMed] [Google Scholar]
- 37.Prince FP, Hikida RS, Hagerman FC. Muscle fiber types in women athletes and non-athletes. Pflugers Arch. 1977;371(1–2):161–5. Epub 1977/10/19. . [DOI] [PubMed] [Google Scholar]
- 38.Miller MS, Callahan DM, Tourville TW, Slauterbeck JR, Kaplan A, Fiske BR, et al. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive older adults with knee osteoarthritis. J Appl Physiol (1985). 2017;122(4):775–87. Epub 2017/01/14. 10.1152/japplphysiol.00830.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257(4 Pt 1):E567–72. Epub 1989/10/01. 10.1152/ajpendo.1989.257.4.E567 . [DOI] [PubMed] [Google Scholar]
- 40.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3(1):e1385 Epub 2008/01/03. 10.1371/journal.pone.0001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 42.Pette D. The adaptive potential of skeletal muscle fibers. Can J Appl Physiol. 2002;27(4):423–48. Epub 2002/11/22. . [DOI] [PubMed] [Google Scholar]
- 43.Bagley JR, Murach KA, Trappe S. Microgravity-Induced Fiber Type Shift in Human Skeletal Muscle Gravitational and Space Biology. 2012;26(1):34–40. [Google Scholar]
- 44.Liu Y, Schlumberger A, Wirth K, Schmidtbleicher D, Steinacker JM. Different effects on human skeletal myosin heavy chain isoform expression: strength vs. combination training. J Appl Physiol (1985). 2003;94(6):2282–8. Epub 2003/05/09. 10.1152/japplphysiol.00830.2002 . [DOI] [PubMed] [Google Scholar]
- 45.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, et al. Single muscle fiber adaptations with marathon training. J Appl Physiol (1985). 2006;101(3):721–7. Epub 2006/04/15. 10.1152/japplphysiol.01595.2005 . [DOI] [PubMed] [Google Scholar]
- 46.Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports. 2007;17(4):422–30. Epub 2007/05/11. 10.1111/j.1600-0838.2006.00575.x . [DOI] [PubMed] [Google Scholar]
- 47.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev. 2014;42(2):45–52. Epub 2014/02/11. 10.1249/JES.0000000000000012 . [DOI] [PubMed] [Google Scholar]
- 48.Grgic J, Schoenfeld BJ. Are the Hypertrophic Adaptations to High and Low-Load Resistance Training Muscle Fiber Type Specific? Front Physiol. 2018;9:402 Epub 2018/05/04. 10.3389/fphys.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staron RS. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in single human muscle fibers. Histochemistry. 1991;96(1):21–4. Epub 1991/01/01. . [DOI] [PubMed] [Google Scholar]
- 50.Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39(11):1989–96. Epub 2007/11/08. 10.1249/mss.0b013e31814fb402 . [DOI] [PubMed] [Google Scholar]
- 51.Borina E, Pellegrino MA, D'Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports. 2010;20(1):65–73. Epub 2009/11/04. 10.1111/j.1600-0838.2009.01029.x . [DOI] [PubMed] [Google Scholar]
- 52.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol (1985). 2007;102(1):340–9. Epub 2006/10/14. 10.1152/japplphysiol.00609.2006 . [DOI] [PubMed] [Google Scholar]
- 53.Klitgaard H, Zhou M, Richter EA. Myosin heavy chain composition of single fibres from m. biceps brachii of male body builders. Acta Physiol Scand. 1990;140(2):175–80. Epub 1990/10/01. 10.1111/j.1748-1716.1990.tb08989.x . [DOI] [PubMed] [Google Scholar]
- 54.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140(1):55–62. Epub 1990/09/01. 10.1111/j.1748-1716.1990.tb08975.x . [DOI] [PubMed] [Google Scholar]
- 55.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48(5):623–9. Epub 2000/04/18. 10.1177/002215540004800506 . [DOI] [PubMed] [Google Scholar]
- 56.Lexell J, Henriksson-Larsen K, Sjostrom M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol Scand. 1983;117(1):115–22. Epub 1983/01/01. 10.1111/j.1748-1716.1983.tb07185.x . [DOI] [PubMed] [Google Scholar]
- 57.Jones N, Kiely J, Suraci B, Collins DJ, de Lorenzo D, Pickering C, et al. A genetic-based algorithm for personalized resistance training. Biol Sport. 2016;33(2):117–26. Epub 2016/06/09. 10.5604/20831862.1198210 1198210 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.