Abstract
Neurodegeneration has been reported in young animals after exposure to all commonly used general anesthetic agents. The brain may be particularly vulnerable to anesthetic toxicity during peak synaptogenesis (in gestation and infancy). Human studies of long-term neurodevelopmental outcome following general anesthesia in early childhood report contradictory findings. This review assesses the strengths and deficiencies in human research methodologies to inform future studies. We identified 76 studies, published between 1990 and 2017, of long-term neurodevelopmental outcome following early childhood or in utero general anesthesia exposure: 49 retrospective, 9 ambidirectional, 17 prospective cohort studies, and 1 randomized controlled trial. Forty-nine studies were explicitly concerned with anesthetic-induced neurotoxicity. Full texts were appraised for methodological challenges and possible solutions. Major challenges identified included delineating effects of anesthesia from surgery, defining the timing and duration of exposure, selection of a surgical cohort and intervention, addressing multiple confounding life course factors, detecting modest neurotoxic effects with small sample sizes (median, 131 children; interquartile range, 50–372), selection of sensitive neurodevelopmental outcomes at appropriate ages for different developmental domains, insufficient length of follow-up (median age, 6 years; interquartile range, 2–12 years), and sample attrition. We discuss potential solutions to these challenges. Further adequately powered, multicenter, prospective randomized controlled trials of anesthetic-induced neurotoxicity in children are required. However, we believe that the inherent methodological challenges of studying anesthetic-induced neurotoxicity necessitate the parallel use of well-designed observational cohort studies.
General anesthesia has long been considered a safe means of enabling pediatric surgery, unpleasant procedures, or medical imaging. However, concerns have accumulated that fetuses, babies, and young children exposed to general anesthesia may experience long-lasting neurotoxic effects.1 Approximately 200,0002 of the 4 million3 children below 6 years of age in the United Kingdom undergo general anesthesia annually (5%), making the risk of anesthetic-induced neurotoxicity a critical public health issue.
Preclinical studies demonstrate that exposure to all commonly used IV and inhalational anesthetic agents is associated with altered brain development in immature animals including nonhuman primates.4,5 Single long exposures6 and multiple exposures7 adversely affect neurodevelopment. The duration and timing of exposure influence the neurotoxic potential of general anesthetic agents. The brain is thought to be particularly vulnerable during the period of synaptogenesis.4 In humans, this “vulnerable time window” is reportedly between the third trimester and 2–3 years of age.6,8–11
Human observational studies of anesthetic-induced neurotoxicity are heterogeneous in their methodologies and offer contrary conclusions. Studies of single brief general anesthesia for minor procedures are generally reassuring, but worse long-term neurodevelopmental outcome has been reported following prolonged/repeated exposure.1 Pooled effect estimates from observational studies indicate at least a modest risk of impaired neurodevelopment following general anesthesia for surgery in childhood.12,13 To date, only 1 ongoing randomized controlled trial of awake-spinal versus sevoflurane general anesthesia for herniorrhaphy before 60 weeks postmenstrual age has reported secondary outcomes.14 The General Anesthesia compared to Spinal trial reassuringly finds equivalent cognitive scores between groups at 2 years of age. However, more comprehensive cognitive assessment in later childhood could still detect anesthetic-induced neurotoxicity.
Increasing numbers of original studies and an exponential increase in review articles on pediatric anesthetic neurotoxicity over the past 10 years (Figure 1) have prompted regulatory and professional bodies to release precautionary statements concerning pediatric general anesthesia. The US Food and Drug Administration cautions against lengthy/repeated general anesthesia or sedation in the third trimester and in children younger than 3 years old.17 Guidance from the United Kingdom and Ireland20 and a statement from European bodies19 advocate avoiding unnecessary general anesthesia but recommend no changes to clinical practice.
Figure 1.
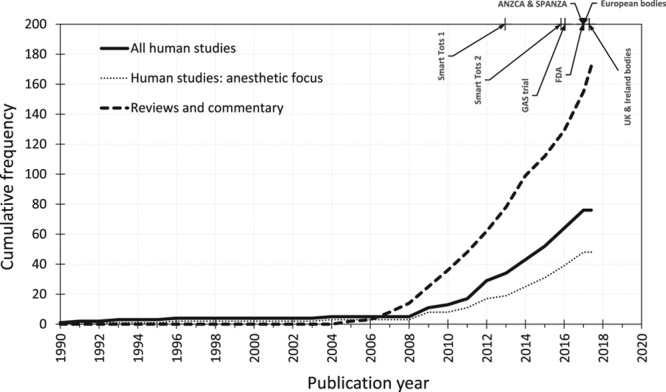
Cumulative number of human observational studies and randomized controlled trials of neurodevelopment following general anesthesia exposure at age <6 years (thick black line) and those specifically designed to study anesthetic-induced neurotoxicity (dotted line). We place this in the context of the number of commentaries and review articles (dashed line) and milestone statements and publications concerning anesthetic-induced neurotoxicity. Smart Tots 1: Smart Tots consensus statement on the use of anesthetics and sedatives in children 201215; Smart Tots 2: consensus statement on the use of anesthetic and sedative drugs in infants and toddlers 201516; GAS trial: General Anesthesia compared to Spinal randomized controlled trial secondary outcomes published 201614; FDA: US Food and Drug Administration safety communication 201617; ANZCA and SPANZA: joint warning from the Australian and New Zealand College of Anaesthetists and the Society for Paediatric Anaesthesia in New Zealand and Australia 201618; European bodies: consensus statement of the European Society of Anaesthesiology, the European Society for Paediatric Anaesthesiology, the European Association of Cardiothoracic Anaesthesiology and the European Safe Tots Anaesthesia Research Initiative 201719; UK and Ireland bodies: joint professional guidance on the use of general anesthesia in young children 2017.20 ANZCA indicates Australian and New Zealand College of Anaesthetists; FDA, Food and Drug Administration; GAS, General Anesthesia compared to Spinal; SPANZA, Society for Paediatric Anaesthesia in New Zealand and Australia.
There has been much discussion of the limitations of the existing human evidence base for anesthetic-induced neurotoxicity. Therefore, to inform the design of future clinical studies, we identified and reviewed the 76 clinical studies of long-term neurodevelopmental outcome following early childhood or in utero general anesthesia exposure that were published between 1990 and April 2018 (Supplemental Digital Content, Appendix 1, http://links.lww.com/AA/C723) to identify particular challenges encountered in performing these types of studies, as well as feasible pragmatic methodological solutions. We sought methods used to isolate the effects of general anesthesia from surgery/disease, characterize anesthetic exposure and surgical intervention, address confounding, detect marginal neurotoxic effects, and define what the implications of the research are for clinical practice. These are summarized in Table 1.
Table 1.
Challenges and Potential Solutions in Human Studies of Anesthetic-Induced Neurotoxicity

DELINEATING THE NEUROTOXIC EFFECT OF ANESTHESIA
Perhaps the greatest challenge to studying anesthetic-induced neurotoxicity is in separating direct toxic effects of general anesthesia on the brain from indirect effects of anesthesia (disturbance of normal physiology, eg, hypoxia, hyperoxia, hypotension, and hypothermia),30 surgery (stress response31 and systemic inflammation), and the perioperative course (complications, pain,27 artificial or inadequate nutrition52). We illustrate this concept in Figure 2A.
Figure 2.
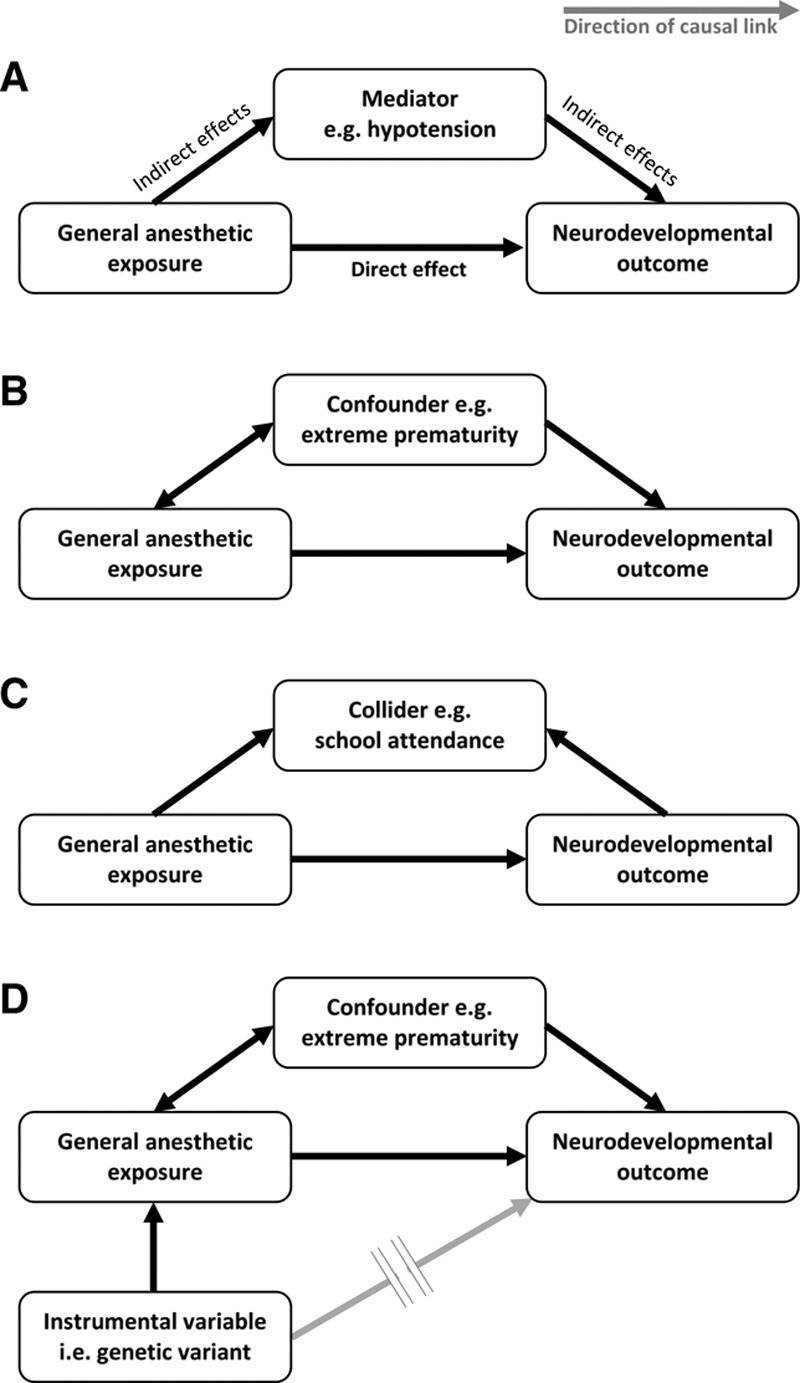
Key concepts in the epidemiology of anesthetic-induced neurotoxicity (see text for detailed explanation). Arrows represent the direction of causality between variables. A, Impaired neurodevelopmental outcome may result from direct neurotoxic effects of general anesthesia (the effect of interest) and/or indirect effects, which lie on different causal pathways that operate through mediator variables. B, Confounding variables are associated with the anesthetic exposure and also influence neurodevelopmental outcome, but do not lie on a causal pathway between anesthesia and neurodevelopment. If confounders are not balanced through randomized study design or accounted for in statistical analyses, then the estimated direct neurotoxic effect of general anesthesia is biased. C, Collider variables are a common effect of general anesthesia exposure and neurodevelopmental outcome. Statistical adjustment for a collider variable that has been mistaken for a confounder can introduce collider-stratification bias. D, Mendelian randomization is a novel study design for unbiased causal inference in observational studies, which exploits the random allocation of genetic material during human reproduction to set up a natural analogy to a randomized controlled trial. It utilizes genetic variants that are selected to be associated with general anesthetic exposure (but importantly, not directly with impaired neurodevelopment) as instrumental variables.
All but 2 studies14,53 make comparisons between general anesthesia and surgery groups, with or without control and therefore cannot distinguish anesthesia-induced effects from surgery-induced effects. Although methodologically ideal, a 2 × 2 factorial design (anesthesia yes/no × surgery yes/no) to determine the effect of anesthesia on neurodevelopment would be logistically and ethically challenging in children or animals and arguably not possible.
A pragmatic nonrandomized study might compare (a) general anesthesia without surgery, eg, undergoing imaging, endoscopic, or interventional procedures; (b) general anesthesia with surgery, and (c) no general anesthesia or surgery.21 Careful choice of the category (a) children would be required. For example, children undergoing neuroimaging may have comorbidities that are independent risk factors for poor neurodevelopmental outcome.21 Category (c) controls could be nonhospitalized siblings/classmates or hospitalized nonsurgical children. It is important that children who undergo additional surgeries in later childhood are not excluded from either the intervention or control groups to avoid selection biases.32
Spinal anesthesia in immature rats has been shown to not accelerate neuronal apoptosis or cause neurobehavioral abnormality.54 An ideal randomized study, therefore, could compare (a) general anesthesia for surgery, (b) awake-regional anesthesia for surgery, and (c) no anesthesia or surgery controls. The General Anesthesia compared to Spinal trial14 adopted a similar strategy in children undergoing general anesthesia/surgery or intended to undergo awake-spinal anesthesia for inguinal herniorrhaphy. In reality, this approach restricts the sample to children undergoing infraumbilical procedures for which awake-neuraxial anesthesia is a feasible alternative to general anesthesia and may therefore limit external generalizability to other patient groups. Careful control or adjustment for differential incidence of deranged physiology between general anesthesia and awake-regional anesthesia groups (eg, significant hypotension more common in the former55) is required to avoid biasing results. Furthermore, children with inadequate blocks or who do not tolerate awake-regional anesthesia may require sedation or conversion to general anesthesia (18% in the General Anesthesia compared to Spinal trial but may be up to 80%56), which may defeat the purpose of the study design. However, per protocol analyses of noninferiority or equivalence trials where there is a crossover of patients between exposure categories would still test whether general anesthesia was harmful to child neurodevelopment.
THE TOXIC EXPOSURE TO GENERAL ANESTHESIA
Although brain structure and function develop throughout childhood, a period of peak synaptogenesis in early childhood has strong implications for later cognition, language, and social behavior.6,39 Exposure during this “vulnerable time window” of brain development ought to be the focus of anesthetic research. Although its timing is well defined in animal species, with the overwhelming majority of studies performed on postnatal day 7 in rats,11,32 human anesthetic-induced neurotoxicity studies have quoted a heterogeneous range of definitions, eg, “third trimester to 2 years,”8 “third trimester to 6 weeks,”9 “0–36 months,”33 “early gestation through to infancy,”10 or “birth to 2–3 years.”57 The concept of a single vulnerable time window may be an oversimplification since there are significant regional differences in the timing and pace of peak synaptogenesis,32,58 which are reflected in discordant results for different domains of neurodevelopment.34,59–61 Furthermore, the age of the neuron as opposed to the age of the child can determine vulnerability to anesthetics.46,62 At present, it seems pragmatic to investigate general anesthesia exposures up to 3 years of age.
Since most of the studies (n = 49; 64.5%) employ retrospective observational designs and many were not designed to investigate anesthetic-induced neurotoxicity per se (n=27, 35.5%),35,63–72 data concerning anesthetic exposure are often limited. Some investigators make assumptions that, if incorrect, could undermine their studies, eg, babies are presumed to undergo general anesthesia for minor procedures that may have been conducted under regional anesthesia10; or circumcision is presumed to be performed without general anesthesia in the perinatal period but under general anesthesia for older children in another study.46 Whether randomized or nonrandomized prospective or retrospective designs, anesthetic-induced neurotoxicity studies need to strive to accurately ascertain the exposure of each child to avoid underestimating the true effect of general anesthesia (false-negative results).
A dose-response relationship has been detected with increasing numbers of coadministered anesthetic agents57 and been sought by comparing single versus multiple anesthetic exposures.40,41 However, because dose and duration of general anesthesia vary widely between procedures, these are poor surrogates for cumulative dose of anesthetic drug exposure.34 Furthermore, inaccurate reporting of composite procedures, eg, adenoidectomy/tonsillectomy/myringotomy, may lead to misclassification of children to the multiple-exposure group.73 Children requiring repeated procedures may have confounding reasons for poor neurodevelopmental outcome, which may not be captured in the study data set. Ideally, dose–response analyses ought to use a prospectively determined duration of anesthesia in minutes for specified drugs or dose in age-adjusted minimum alveolar concentration-hours for inhalational agents22–24 or cumulative milligram per kilogram for IV anesthesia.25 This level of detail may be more achievable with electronic anesthetic record-keeping systems.
CHOICE OF INTERVENTION
In observational studies, selection of participants in terms of their diagnosis/disease and surgical procedure ought to minimize “confounding by indication”—a scenario in which the disease or the surgery itself is an independent risk factor for poor neurodevelopmental outcome. Studies of neurosurgical and cardiothoracic surgical cohorts,35,74 as well as children operated on with major congenital or chromosomal abnormalities52,75 are classically affected. However, studies of general anesthesia for neuroimaging,21 some otorhinolaryngology procedures (eg, adenotonsillectomy for obstructive sleep apnea associated with learning difficulty40,76 or myringotomy and grommet insertion associated with speech/language delay77), pyloromyotomy associated with significant hyperbilirubinemia32 or nutritional inadequacy,72 gastroschisis,60 craniosynostosis,30 and cancer surgery47 may be similarly compromised.
When selecting study participants, a balance ought to be struck between the risk of confounding by indication and being as inclusive as possible to maximize external validity. A healthy, elective surgical cohort undergoing relatively minor surgery would be ideal.26 Inguinal herniorrhaphy14,27,28 or surgery for solitary urogenital problems29 (eg, circumcision or hypospadias repair) is common and has no known independent association with poor neurodevelopmental outcome. Particular care should be exercised if it is necessary to pool multiple surgical procedures to increase statistical power.36
Anesthetic agents readily cross the placental barrier, which has previously permitted studies in children born to occupationally exposed mothers78 and children born by cesarean delivery under general anesthesia.53,79,80 These studies may not demonstrate anesthetic-induced neurotoxicity because of the poorly defined, chronic low-dose occupational exposure or the relatively brief exposure at cesarean delivery. Studying anesthetic-induced neurotoxicity in the context of (a) general anesthesia cesarean delivery versus (b) neuraxial anesthetic cesarean delivery and (c) spontaneous vertex delivery is also fraught with difficulty. Results may be confounded by opioids used for labor analgesia, which may cause neonatal respiratory depression; or the use of labor epidural analgesia, which may reduce stress response in the control group.53 The indication for cesarean delivery intervention, as well as an increased frequency of prematurity, complications of pregnancy, and perinatal insults in the intervention groups may also confound results. Studying intrauterine surgery to correct fetal abnormalities would offer a longer well-defined general anesthetic drug exposure, but no such work has been published.
ADDRESSING CONFOUNDING
The association between general anesthesia and neurodevelopmental outcome is heavily confounded by factors throughout the life course (Figure 2B; Table 2). Properly conducted randomized controlled trials should evenly distribute known/measured and unknown/unmeasured confounders across groups at randomization, thereby overcoming confounder bias.
Table 2.
Potential Confounders of the Association Between Anesthesia and Neurodevelopment, Which Have Been Measured in Human Anesthetic-Induced Neurotoxicity Studies

Observational studies of anesthetic-induced neurotoxicity must control (via restriction, stratification, or regression adjustment) for differences in known/measured confounders between groups to avoid extensive bias. However, data concerning pregnancy/peripartum factors (eg, prematurity, fetal acidosis, birth asphyxia) and perioperative factors (eg, temperature, hypoxia/hyperoxia, hemodynamics, adverse events) are often unknown, especially in retrospective studies. Some factors that ought to be adjusted for, eg, American Society of Anesthesiologists physical status, are not routinely recorded for nonexposed children, and smaller studies may make no attempt to adjust for confounders at all.72,91,100,102–104 By definition, unknown/unmeasured confounders cannot be controlled for, but their potential impacts on the results of observational studies can be simulated statistically.43
Adjustment for multiple potential confounders in observational studies is performed with the intention of reducing confounder bias. However, care must be exercised to avoid “overadjustment”105—whereby this very process decreases precision or paradoxically increases net bias though several mechanisms. First, attempting to control for increasing numbers of variables reduces the precision of the neurotoxic effect estimates generated by statistical models. Wide (imprecise) CIs around the effect estimates may mask any evidence of anesthetic-induced neurotoxicity, leading to false-negative conclusions. The second mechanism concerns “intermediate variables,” which are distinguished from confounders by lying on the causal pathway between exposure and outcome. For example, we might speculate that anesthetic-induced neurotoxicity is mediated via hypotension (Figure 2A). In the case of multiple causal pathways between exposure and outcome, then mistakenly controlling for hypotension (or some descending proxy thereof such as volume of crystalloid or amount of vasoactive drug administered) would produce a null-biased result, ie, falsely reducing the apparent strength of any neurotoxic effect estimate. Worse still, if the only causal path between general anesthesia exposure and impaired neurodevelopment were mediated through hypotension, then mistakenly controlling for this intermediate variable (or its proxies) ought to entirely nullify any neurotoxic effect estimate, again producing falsely reassuring conclusions. The third mechanism involves “collider variables,” which are defined as a common effect of the exposure and outcome (Figure 2C). Mistaken control for this common effect induces a spurious (noncausal) association between general anesthesia and neurodevelopmental outcome through which confounding can flow, paradoxically inducing bias (termed “collider-stratification bias”) into the neurotoxic effect estimate where none previously existed. An illustrative example comes from studies of prenatal pollutant exposure and long-term child neurodevelopment in which the pollutants also cause fetal loss.106 Since outcome can only be determined in live-born children, if investigators condition on live birth status (in this case by restriction to live-born children as is typical in pediatric cohort studies), bias arising from common causes of fetal death and long-term neurodevelopmental outcome (ie, confounders of the association between fetal death and neurodevelopment) is induced.
Collectively, the pitfalls of multivariable analysis necessitate thoughtful selection of potential confounders, which may be assisted by drawing a “directed acyclic graph”107—a visual representation of the assumed associations among exposure, outcome, and other measured/unmeasured variables using unidirectional arrows to represent the direction of causality (and temporality). These graphs distill the causal model underlying the epidemiological problem, informing the choice of confounding, intermediate and collider variables, which would be required to build a statistical model to test for an unbiased relationship between general anesthesia and neurodevelopmental outcome. The aforementioned pitfalls of multiple confounder adjustment also necessitate cautious “stepwise” modeling whereby potential confounders are sequentially added to the developing statistical model and its output scrutinized at each step for paradoxical effects. A sudden reversal of the effect estimate following the stepwise incorporation of the latest potential confounder, for example, may prompt a reevaluation of the causal assumptions regarding that variable and whether it may operate as a collider as opposed to a confounder in the causal model. It would be dangerous to simply attempt to simultaneously adjust for all measured child characteristics in a nonrandomized anesthetic-induced neurotoxicity study.
Conventional techniques for confounder adjustment include various regression models (eg, linear, logistic, Poisson or Cox proportional hazards modeling)9,25,30–38 and matching techniques. Group/frequency matching ensures that the proportions of subjects with given characteristics are the same in each group.41,42 Individual/pair matching ensures that pairs of children, 1 from each group, share similar characteristics.39,40 Results from matched pairs are less confounded but require larger sample sizes to achieve the same precision.
More innovative approaches may help uncover associations. Propensity score analysis is a pragmatic choice of method to reduce the complexity and computational burden of statistical models, which attempt to control for a multitude of potential confounding variables in a nonrandomized study. It reduces the dimensionality of the data set from a large collection of variables to a single propensity score, which is generated by a regression model from those variables that are thought to influence membership to the general anesthesia group in the study. The propensity score assigned to each child would take a value between 0 and 1 and represent the estimated probability of general anesthesia group membership, conditional on the values of those variables thought to influence general anesthesia versus nongeneral anesthesia group membership. The propensity score can then be adjusted for as an independent variable in a regression model (as opposed to entering the collection of known/measured confounders). Alternatively, one can match individual children between general anesthesia and nongeneral anesthesia groups who have similar likelihoods of general anesthesia group membership (ie, similar propensity scores), such that known/measured confounders are balanced across the 2 groups.10,23,27,38,46,74 These “propensity-adjusted” or “propensity-matched” estimates of neurotoxic effect on neurodevelopment ought to be unbiased by known/measured confounders.
Mendelian randomization is an advance in observational epidemiology, which overcomes confounding by both known/measured and unknown/unmeasured factors. It can provide unbiased evidence for causal relations between a modifiable exposure and patient outcome.44,45 Instead of the traditional exposure variable (ie, general anesthesia/surgery), it considers “instrumental variables” (Figure 2D). These are either one or a combination of multiple genetic variants (ie, alleles or single nucleotide polymorphisms) that are randomly allocated to children at meiosis in human reproduction and are selected on the basis that they robustly predict general anesthesia exposure without directly influencing neurodevelopmental outcome (except via the general anesthesia exposure itself). Candidate genetic variants are typically identified from large genome-wide association studies but could conceivably be associated with certain disease states (increasing the propensity for general anesthesia to facilitate procedures, medical imaging, or surgery) or with suxamethonium apnea or malignant hyperpyrexia (reducing the propensity for general anesthesia where there is an established child or family history). Random natural assortment of genetic material ensures that instrumental variable status is independent of factors that confound the association between the traditional exposure variable (general anesthesia/surgery) and the neurodevelopmental outcome. Once child outcomes are compared based on the instrumental variable (rather than general anesthesia exposure), then intergroup differences in general anesthesia exposure and neurodevelopment ought to reflect true, unconfounded causal relationships between general anesthesia/surgery and neurodevelopmental outcome (Figure 3). We believe that the Mendelian randomization approach to detecting anesthetic-induced neurotoxicity may be especially feasible using a “2-sample” Mendelian randomization in which data linking the chosen genetic variants to general anesthesia exposure need not come from the same sample as data that link general anesthesia exposure to neurodevelopment. No observational studies of anesthetic-induced neurotoxicity published to date have used Mendelian randomization. However, it offers the potential to elucidate an unconfounded link between anesthesia and neurodevelopment using what is an efficient natural analogy to a randomized controlled trial.
Figure 3.

Contrasting the conduct of (A) randomized controlled trials and (B) Mendelian randomization studies. See text for full explanation.
As an illustrative example, the effect of prenatal alcohol exposure on child academic achievement has been studied recently using the Mendelian randomization approach.108,109 Here, researchers have exploited genetic variation in the alcohol dehydrogenase gene as an instrument for in utero alcohol exposure. Mothers with the rare allele metabolize alcohol faster, resulting in more rapid production of ethanol metabolites that cause unpleasant symptoms. These mothers are shown to consume less alcohol. Investigators demonstrate that the instrumental variable, unlike alcohol consumption, is unrelated to potential confounders of the association between prenatal alcohol exposure and academic achievement such as socioeconomic status. While traditional regression analyses based on the alcohol consumption exposure variable have returned ambiguous results, presumably due to residual confounding (eg, maternal wine consumption being protective for child educational attainment), the instrumental variable analyses demonstrate robust positive effects on child educational achievement in children whose mothers were induced by their genotype to abstinence or lower alcohol consumption in pregnancy.
Twin or sibling studies attempt to eliminate confounding by genetic and environmental factors, eg, uterine environment, parental education, parenting style, home/family environment, neighborhood, educational, and socioeconomic factors.28,33,40,75 In a monozygotic concordant-discordant design, participants in each group share the same genetics and family-level environmental factors.75 Differences in neurodevelopmental outcome across groups would then reflect the toxic effect of general anesthesia/surgery.
Longitudinal study designs, where neurodevelopment is repeatedly assessed over time, allow children to serve as their own controls.66,68,71 This approach mitigates confounding by static confounders, eg, genetics and socioeconomic status.
Finally, other approaches may dispense with control groups altogether. One could focus on the interaction between general anesthesia and age at exposure, ie, compare children who undergo early versus late surgery.9,29,39,46,81,94 Associations would not be confounded by diagnosis and surgery/anesthetic factors since all subjects could be similarly exposed. However, this approach mandates that surgery can be postponed, which is not always feasible.
DETECTING MODEST NEUROTOXIC EFFECTS
In utero or early childhood exposure to a range of neurotoxicants (eg, metals, organic solvents, pesticides) can adversely affect neurobehavioral development.110,111 Ethanol, like anesthetic agents, acts at γ-aminobutyric acid and N-methyl-d-aspartic acid receptors and causes neuronal apoptosis in the developing brain.92 Robust detrimental associations between heavy and binge prenatal alcohol exposure and adverse child neurodevelopment are established.112,113 However, studies of light-to-moderate prenatal alcohol exposure have suffered from residual confounding and have reported inconsistent conclusions even with sample sizes in the order of 10,000 children. We can presume that large samples will similarly be required to reliably detect any long-term neurotoxic effects following childhood general anesthesia—an effect that may also be comparable or small relative to the effects of confounding factors.9,25,39,47,73,82 Large samples are also required to permit adjustment or matching techniques to account for confounding. Existing anesthetic-induced neurotoxicity studies vary in size between 15 and 125,000 subjects with a median 131 children (interquartile range, 50–372), so are often likely to be underpowered and potentially falsely reassuring.
Besides pursuing larger sample sizes, comparing exposed children with unexposed children in 1:4 ratio to maximize statistical power,38,47,48 avoiding short-duration interventions (eg, maternal general anesthesia for cesarean delivery or myringotomy and grommet insertion), studying exposure during the “vulnerable time window” of brain development, and using sensitive outcome measures are strategies that may increase the likelihood of detecting the neurotoxic effects of general anesthesia.
NEURODEVELOPMENTAL OUTCOME
The neurodevelopmental outcome measures reported in the literature vary and encompass (a) intelligence/cognition, (b) academic achievement, (c) development/behavior, and (d) neuropsychiatric diagnoses, ie, attention-deficit/hyperactivity disorder, autism spectrum disorder, and learning disability.49 Prospective evaluation in multiple domains of development using a battery of sensitive, validated outcomes and trained, blinded assessors is the gold standard. However, the risk of detecting spurious associations increases with multiple outcomes. Therefore, it is wise to caution against the overinterpretation of solitary detrimental associations in the context of a panel of otherwise reassuring results.
Measures of intelligence/cognition are thought to remain stable throughout the life course unless disrupted by severe disease.49,114 However, assessment is not feasible until basic cognitive skills are achieved by 4–6 years of age.49,50 Age-normalized intelligence scores permit comparisons of outcome at different ages and enable referencing to population scores.104
Academic achievement in standardized national tests reflects intelligence/cognition,115 but is muddied by multiple external factors, eg, self-esteem and lifestyle factors.49 School grade performance in children with dyslexia or dyspraxia may be boosted by extra help in school, mitigating any negative effect on academic achievement.26 Although standardized national tests are administered at population level, which makes them a feasible outcome for large population studies, not all children participate, eg, private schools or nonentry due to learning difficulty.8 However, investigating academic achievement does confer the pragmatic advantage that parents/guardians are likely to be highly invested in their child’s school performance.27
Child development evolves in surges and plateaus, referenced to well-defined developmental milestones expected at certain ages, which permits outcome assessment even at the youngest ages.49 The reliability of subjective developmental/behavioral data collected through parental survey is questionable: developmental delay in language/speech, mathematics, and reading domains may not be noticed until challenged in school; behavioral problems may not manifest until children communicate and interact with their peers in school.34,38,48,89 An ideal anesthetic-induced neurotoxicity study should use trained, blinded assessors (eg, pediatric neuropsychologists) to measure outcome using a comprehensive battery of developmental assessments. Scores generated by this method of outcome assessment are objective and highly sensitive to subtle neurotoxic effects that may be difficult to detect clinically.34 The use of such comprehensive neurodevelopmental assessments is most feasible in smaller studies, which prospectively assess outcome,27 but it is also available in some retrospective data sets.73 The Bayley Scales of Infant Development28,34 is the most extensively used example,116 but the latest third version may overestimate development in certain groups,117,118 and caution is required if comparisons are made with scores from previous iterations.119
Neuropsychiatric diagnoses for developmental/behavioral disorders are multifactorial in origin (including genetic predisposition), with a heterogeneous and changing clinical presentation over time.49 Children may spontaneously “catch-up”40 or benefit from supportive interventions in childhood.49,81 Neuropsychiatric diagnoses are almost exclusively parameterized as binary outcomes (eg, from International Classification of Diseases, Ninth Revision diagnosis codes, school or health care records) as opposed to “risk scores.” These binary outcomes are likely to be too crude/insensitive to detect any subtle effects of anesthetic exposure.21 Nondiagnosis (especially before the group communication/interaction and higher cognitive demands placed on schoolchildren34,48), underreporting, and incorrect diagnosis coding in databases is likely to introduce misclassification bias. Studying learning disability confers particular advantages though: a high incidence (5%–10%) and recording in large educational databases.49
POSTOPERATIVE FOLLOW-UP AND SAMPLE ATTRITION
The time interval between anesthesia and first neurodevelopmental assessment must be sufficiently long to distinguish long-term neurotoxic effects from short-term postoperative cognitive-behavioral changes (ie, ≥6 months46). It must also allow sufficient latency for marginal neurodevelopmental deficits to manifest in domains of development, which emerge, differentiate, and are amenable to thorough neuropsychological testing at older ages, eg, cognitive skills such as language/speech/reading, mathematics, memory, and executive functioning from late childhood.14,24,33 Furthermore, neurodevelopmental evaluation in school children is known to be more robust and predictive for adulthood than when measured in preschool children because of the variability in young children’s developmental trajectories.14,22,24,52,60 There has been concern that multiple life course factors may dilute any differences in outcome between exposed and unexposed children after such long follow-up. However, subtle associations between starting school in January versus December and educational achievement and intelligence quotient scores have been detected in large cohorts as late as 18 years old.47 Existing studies of anesthetic-induced neurotoxicity follow up children until a median age of 6 years (interquartile range, 2–12 years).
Prolonged follow-up makes retrospective or ambidirectional (meaning retrospective ascertainment of exposure but prospective measurement of outcome) studies28,33,93,96 efficient compared to prospective randomized and nonrandomized designs. But it also makes sample attrition (eg, due to withdrawal, death, migration, moving schools, or health care provider) a significant problem, eg, 50% of initially enrolled children completing assessment at 2 years in 1 study.66 Most observational studies report a “complete case analysis,” in which any children with missing data are disregarded.8,24,27,30,52,89,93 The amount of missing data and reasons for this are frequently omitted. As well as suffering a reduction in precision, their results may be biased when neurodevelopmental outcome data are missing nonrandomly.51,73 For example, if general anesthesia slowed child neurodevelopment, then exposed children may be lost to follow-up if they were unable or reluctant to engage in intelligence testing. Effect estimates would then underestimate the true effect of general anesthesia in the complete case analysis.
Even research funded to intensively follow up children in prospective randomized or nonrandomized studies will have missing data. Statistical methods can be used to permit unbiased analyses without excluding affected cases.51 Choice of method depends on the probable mechanism of data loss. Multiple imputation is a popular technique used when data are believed to be missing at random. Missing data are inferred from a rich observed data set to construct multiple plausible data sets, which are pooled to produce a result that reflects the uncertainty in the imputed data. Data that are missing not at random can only be addressed through experiments that test the sensitivity of results to different mechanisms of data loss.
INTERPRETING RESULTS IN CLINICAL PRACTICE
Despite considerable interest and anxiety, there is at present no conclusive evidence or consensus that general anesthesia harms the developing brain. Childhood general anesthesia typically comprises single short exposures and is likely to carry low risk.14,33,120 However, if general anesthesia is thought to pose long-term neurodevelopmental risks, then the impacts on clinical practice could be far reaching.
In considering the current clinical implications, it should be noted that the evidence base is comprised mainly of retrospective observational studies, whose subjects were anesthetized in the 1970s–1990s, since when there have been widespread changes in practice. Pediatric anesthesia may have become safer32 as isoflurane/sevoflurane and IV anesthesia have replaced the “Liverpool technique” (muscle relaxation and nitrous oxide for neonatal procedures), halothane, enflurane, and methoxyflurane53; and our profession became more conscious of optimal fluid management, adopted obligatory multiparameter monitoring incorporating pulse oximetry and capnography; and there have been changes in who is delivering anesthetic care to children.74
Nonetheless, if the evidence base becomes stronger, then surgeons, physicians, and general practitioners will require a new appreciation of the neurotoxic risks of anesthesia to inform clinical decision making and the consent process. Important topics for discussion with children, parents, or guardians would include which elective procedures could be deferred, the associated risks of delay, alternative anesthetic management (eg, alternative anesthetic agents or regional techniques), and possible mitigating or protective strategies.28
Withholding general anesthetic drugs during neonatal surgery (eg, the “Liverpool technique”) may not be an option today and is certainly unethical in later childhood. Painful stimulation and the associated strong stress response are also thought to impair neurodevelopment.27,121
Modifiable factors certainly include optimizing perioperative physiology, good perioperative analgesia, psychosocial support, and avoidance of unpleasant experiences or prolonged hospitalization. Determining which general anesthetic drugs and techniques might carry the lowest risk will require researchers to accurately quantify the duration, cumulative dose, and interactions of specific agents.33 Whether time to allow remodeling/repair between sequential general anesthesia can mitigate neurotoxic damage could be investigated.9 Neuroprotection afforded by strict maintenance of physiological parameters, pharmacotherapies, preconditioning, and novel neurogenesis techniques are being researched.63,88 Maintaining cerebral glucose and oxygen delivery by minimizing cardiopulmonary bypass and deep hypothermic circulatory arrest times may play a role in pediatric cardiac surgery.63,68
Most general anesthesia is provided for healthy elective cases. Here, the physical or psychosocial harms of deferring or cancelling surgery or procedures would need careful weighing against the risk and impact of potential neurodevelopmental impairment on the individual, especially for repeated or prolonged anesthesia. For example, impaired wound healing and cosmesis, concerns about impaired speech/language development, and social stigma may preclude deferral of surgery in cleft lip and palate.94 The current level of concern about neurotoxicity would not preclude the provision of general anesthesia for emergency surgery or cesarean delivery.
High-risk groups for poor developmental outcome (eg, multiple prolonged general anesthesia) may require follow-up neurodevelopmental screening with the option of referral for early school intervention programs to attempt to mitigate any harms and improve developmental acquisition and school performance.122
CONCLUSIONS
Despite growing international concern that general anesthesia in childhood leads to long-term neurodevelopmental impairment, delineating general anesthesia–induced effects from those of surgery remains a significant challenge in the study of anesthetic-induced neurotoxicity. Deficiencies of existing research also include inconsistent exposure definitions, selection of cohorts with independent risk factors for impaired neurodevelopment, extensive confounding, the need to detect subtle neurotoxic effects, blunt neurodevelopmental assessment tools, and sample attrition over the long-term follow-up required.
Randomized controlled trials represent the gold standard tool in the present climate of clinical equipoise.14 However, randomly assigning children to general anesthesia-surgery versus regional anesthesia-surgery versus no anesthesia-no surgery poses significant ethical and logistical challenges, particularly if prolonged or repeated general anesthesia is to be studied. This coupled with the large sample sizes and prolonged follow-up required to detect neurotoxic effects necessitates the design of more efficient, sophisticated observational studies1,33,123 and has driven calls for the adoption of surrogate indices such as neuroimaging and biomarker techniques to evaluate neuronal inflammation and apoptosis.124
Large observational studies can produce more precise, more timely results that are not constrained to studying single short general anesthesia exposures. We advocate prospective or ambidirectional cohort studies that accurately ascertain general anesthesia exposure, rigorously control for confounders, and prospectively follow up neurodevelopment into adolescence. They will also permit researchers to elucidate the role of potential mediators and effect modifiers of any neurotoxic effect to inform strategies to mitigate the potential neurotoxic risks of general anesthesia in early childhood.
In parallel, there is a need for ongoing animal work to characterize the mechanisms of anesthetic-induced neurotoxicity, the relative neurotoxic potentials of different anesthetic agents at different stages of development, and modifiable factors to reduce anesthetic-induced neurotoxicity. These animal studies will need to more carefully control physiological parameters and anesthetic dosing and more closely mimic the surgical insult if their findings are to be generalizable to human pediatric anesthesia.
Given the inherent challenges of studying anesthetic-induced neurotoxicity, we must acknowledge that it may never be possible to demonstrate anesthetic-induced neurotoxicity in conventional clinical trials. Ultimately, multiple complementary approaches are required to accumulate sufficient evidence to inform a consensus opinion on the neurotoxic potential of general anesthesia—currently, the single greatest issue in modern pediatric anesthetic practice.
DISCLOSURES
Name:Graham J. Walkden, MBChB.
Contribution:This author helped plan the study, the literature search, review process, and prepare the manuscript.
Name:Anthony E. Pickering, FRCA, PhD.
Contribution:This author helped plan the study and prepare the manuscript.
Name:Hannah Gill, FRCA, PhD.
Contribution:This author helped plan the study and prepare the manuscript.
This manuscript was handled by: Gregory J. Crosby, MD.
Supplementary Material
Footnotes
Published ahead of print 31 December 2018.
Funding: G.J.W. and H.G. were supported by NIHR funds.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med. 2017;376:905–907. [DOI] [PubMed] [Google Scholar]
- 2.Sury MR, Palmer JH, Cook TM, Pandit JJ. The state of UK anaesthesia: a survey of National Health Service activity in 2013. Br J Anaesth. 2014;113:575–584. [DOI] [PubMed] [Google Scholar]
- 3.Humby P. Overview of the UK population: February 2016. Overview of the UK population, its size, characteristics and the causes of population change including national and regional variation: Office for National Statistics; 2016.
- 4.Jevtovic-Todorovic V. Exposure of developing brain to general anesthesia what is the animal evidence? Anesthesiology. 2017;128:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. [DOI] [PubMed] [Google Scholar]
- 7.Zou X, Patterson TA, Sadovova N, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausen NG, Pedersen DA, Pedersen JK, et al. Oral clefts and academic performance in adolescence: the impact of anesthesia-related neurotoxicity, timing of surgery, and type of oral clefts. Cleft Palate Craniofac J. 2017;54:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology. 2016;125:667–677. [DOI] [PubMed] [Google Scholar]
- 10.Morriss FH, Jr, Saha S, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr. 2014;168:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol. 2017;60:2–23. [DOI] [PubMed] [Google Scholar]
- 12.DiMaggio C, Sun LS, Ing C, Li G. Pediatric anesthesia and neurodevelopmental impairments: a Bayesian meta-analysis. J Neurosurg Anesthesiol. 2012;24:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Du L, Du Z, Jiang H, Han D, Li Q. Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth. 2015;29:749–757. [DOI] [PubMed] [Google Scholar]
- 14.Davidson A, Disma N, Graaff J, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet (London, England). 2016;387:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smart Tots. Consensus statement on the use of anesthetics and sedatives in children. 2012.
- 16.Smart Tots. Consensus statement on the use of anesthetic and sedative drugs in infants and toddlers. 2015. [DOI] [PubMed]
- 17.US Food and Drug Administration. Drug Safety Communication: FDA Review Results in New Warnings About Using General Anesthetics and Sedation Drugs in Young Children and Pregnant Women.2016. [Google Scholar]
- 18.Australian and New Zealand College of Anaesthetists, Society for Paediatric Anaesthesia in New Zealand and Australia. Warnings: young children, pregnant women. 2016.
- 19.Hansen TG. Use of anesthetics in young children Consensus statement of the European Society of Anaesthesiology (ESA), the European Society for Paediatric Anaesthesiology (ESPA), the European Association of Cardiothoracic Anaesthesiology (EACTA), and the European Safe Tots Anaesthesia Research Initiative (EuroSTAR). Paediatr Anaesth. 2017;27:558–559. [DOI] [PubMed] [Google Scholar]
- 20.Association of Paediatric Anaesthetists of Great Britain and Ireland, Royal College of Anaesthetists, Association of Anaesthetists of Great Britain and Ireland, The College of Anaesthetists of Ireland. Joint Professional Guidance on the Use of General Anaesthesia in Young Children. 2017. [Google Scholar]
- 21.Nestor KA, Zeidan M, Boncore E, et al. Neurodevelopmental outcomes in infants undergoing general anesthesia. J Pediatr Surg. 2017;52:895–900. [DOI] [PubMed] [Google Scholar]
- 22.Diaz LK, Gaynor JW, Koh SJ, et al. Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2016;152:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia Guerra G, Robertson CM, Alton GY, et al. ; Western Canadian Complex Pediatric Therapies Follow-up Group. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Paediatr Anaesth. 2014;24:257–265. [DOI] [PubMed] [Google Scholar]
- 26.Bong CL, Allen JC, Kim JT. The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesth Analg. 2013;117:1419–1428. [DOI] [PubMed] [Google Scholar]
- 27.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. [DOI] [PubMed] [Google Scholar]
- 28.Sun LS, Li G, DiMaggio CJ, et al. Feasibility and pilot study of the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) project. J Neurosurg Anesthesiol. 2012;24:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–812. [DOI] [PubMed] [Google Scholar]
- 30.Naumann HL, Haberkern CM, Pietila KE, et al. Duration of exposure to cranial vault surgery: associations with neurodevelopment among children with single-suture craniosynostosis. Paediatr Anaesth. 2012;22:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naguib AN, Winch PD, Tobias JD, et al. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi J Anaesth. 2015;9:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Educational outcome in adolescence following pyloric stenosis repair before 3 months of age: a nationwide cohort study. Paediatr Anaesth. 2013;23:883–890. [DOI] [PubMed] [Google Scholar]
- 33.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. [DOI] [PubMed] [Google Scholar]
- 35.Hansen JH, Rotermann I, Logoteta J, et al. Neurodevelopmental outcome in hypoplastic left heart syndrome: impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J Thorac Cardiovasc Surg. 2016;151:1358–1366. [DOI] [PubMed] [Google Scholar]
- 36.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Neurosurgical conditions and procedures in infancy are associated with mortality and academic performances in adolescence: a nationwide cohort study. Paediatr Anaesth. 2015;25:186–192. [DOI] [PubMed] [Google Scholar]
- 37.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012;160:409–414. [DOI] [PubMed] [Google Scholar]
- 38.Ko WR, Liaw YP, Huang JY, et al. Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Paediatr Anaesth. 2014;24:741–748. [DOI] [PubMed] [Google Scholar]
- 39.O’Leary JD, Janus M, Duku E, et al. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125:272–279. [DOI] [PubMed] [Google Scholar]
- 40.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery [Erratum appears in Pediatrics. 2012 Mar;129(3):595]. Pediatrics. 2011;128:e1053–e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnegie NB, Harada M, Hill JL. Assessing sensitivity to unmeasured confounding using a simulated potential confounder. J Res Educ Effect. 2016;9:395–420. [Google Scholar]
- 44.Gupta V, Walia GK, Sachdeva MP. ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public Health. 2017;145:113–119. [DOI] [PubMed] [Google Scholar]
- 45.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16:309–330. [DOI] [PubMed] [Google Scholar]
- 46.Ing C, Sun M, Olfson M, et al. Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg. 2017;125:1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171:e163470. [DOI] [PubMed] [Google Scholar]
- 48.Ko WR, Huang JY, Chiang YC, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. Eur J Anaesthesiol. 2015;32:303–310. [DOI] [PubMed] [Google Scholar]
- 49.Clausen NG, Kähler S, Hansen TG. Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. Br J Anaesth. 2018;120:1255–1273. [DOI] [PubMed] [Google Scholar]
- 50.Beers SR, Rofey DL, McIntyre KA. Neurodevelopmental assessment after anesthesia in childhood: review of the literature and recommendations. Anesth Analg. 2014;119:661–669. [DOI] [PubMed] [Google Scholar]
- 51.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsinga RM, Roze E, Van Braeckel KN, Hulscher JB, Bos AF. Motor and cognitive outcome at school age of children with surgically treated intestinal obstructions in the neonatal period. Early Hum Dev. 2013;89:181–185. [DOI] [PubMed] [Google Scholar]
- 53.Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yahalom B, Athiraman U, Soriano SG, et al. Spinal anesthesia in infant rats: development of a model and assessment of neurologic outcomes. Anesthesiology. 2011;114:1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCann ME, Withington DE, Arnup SJ, et al. ; GAS Consortium. Differences in blood pressure in infants after general anesthesia compared to awake regional anesthesia (GAS Study-A Prospective Randomized Trial). Anesth Analg. 2017;125:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somri M, Tome R, Yanovski B, et al. Combined spinal-epidural anesthesia in major abdominal surgery in high-risk neonates and infants. Paediatr Anaesth. 2007;17:1059–1065. [DOI] [PubMed] [Google Scholar]
- 57.Chemaly M, El-Rajab MA, Ziade FM, Naja ZM. Effect of one anesthetic exposure on long-term behavioral changes in children. J Clin Anesth. 2014;26:551–556. [DOI] [PubMed] [Google Scholar]
- 58.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. [DOI] [PubMed] [Google Scholar]
- 59.Ing CH, DiMaggio CJ, Whitehouse AJ, et al. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. J Neurosurg Anesthesiol. 2014;26:377–386. [DOI] [PubMed] [Google Scholar]
- 60.Lap CC, Bolhuis SW, Van Braeckel KN, et al. Functional outcome at school age of children born with gastroschisis. Early Hum Dev. 2017;106–107:47–52. [DOI] [PubMed] [Google Scholar]
- 61.Ludman L, Spitz L, Lansdown R. Developmental progress of newborns undergoing neonatal surgery. J Pediatr Surg. 1990;25:469–471. [DOI] [PubMed] [Google Scholar]
- 62.Hofacer RD, Deng M, Ward CG, et al. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 2013;73:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman GM, Brosig CL, Bear LM, Tweddell JS, Mussatto KA. Effect of intercurrent operation and cerebral oxygenation on developmental trajectory in congenital heart disease. Ann Thorac Surg. 2016;101:708–716. [DOI] [PubMed] [Google Scholar]
- 64.Gaynor JW, Ittenbach RF, Gerdes M, et al. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng HH, Wypij D, Laussen PC, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. Ann Thorac Surg. 2014;98:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sananes R, Manlhiot C, Kelly E, et al. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93:1577–1583. [DOI] [PubMed] [Google Scholar]
- 67.Long SH, Galea MP, Eldridge BJ, Harris SR. Performance of 2-year-old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development, Third Edition. Early Hum Dev. 2012;88:603–607. [DOI] [PubMed] [Google Scholar]
- 68.Long SH, Harris SR, Eldridge BJ, Galea MP. Gross motor development is delayed following early cardiac surgery. Cardiol Young. 2012;22:574–582. [DOI] [PubMed] [Google Scholar]
- 69.Minutillo C, Rao SC, Pirie S, McMichael J, Dickinson JE. Growth and developmental outcomes of infants with gastroschisis at one year of age: a retrospective study. J Pediatr Surg. 2013;48:1688–1696. [DOI] [PubMed] [Google Scholar]
- 70.Rocha G, Azevedo I, Pinto JC, Guimarães H. Follow-up of the survivors of congenital diaphragmatic hernia. Early Hum Dev. 2012;88:255–258. [DOI] [PubMed] [Google Scholar]
- 71.Fan XC, Ye M, Li DZ, Shi Y, Xu Y. Cognitive function in congenital heart disease after cardiac surgery with extracorporeal circulation. World J Pediatr. 2010;6:268–270. [DOI] [PubMed] [Google Scholar]
- 72.Walker K, Halliday R, Holland AJ, Karskens C, Badawi N. Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2010;45:2369–2372. [DOI] [PubMed] [Google Scholar]
- 73.de Heer IJ, Tiemeier H, Hoeks SE, Weber F. Intelligence quotient scores at the age of 6 years in children anaesthetised before the age of 5 years. Anaesthesia. 2017;72:57–62. [DOI] [PubMed] [Google Scholar]
- 74.Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253. [DOI] [PubMed] [Google Scholar]
- 76.Kurnatowski P, Putyński L, Lapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70:419–424. [DOI] [PubMed] [Google Scholar]
- 77.Kacmarynski DS, Levine SC, Pearson SE, Maisel RH. Complications of otitis media before placement of tympanostomy tubes in children. Arch Otolaryngol Head Neck Surg. 2004;130:289–292. [DOI] [PubMed] [Google Scholar]
- 78.Ratzon NZ, Ornoy A, Pardo A, Rachel M, Hatch M. Developmental evaluation of children born to mothers occupationally exposed to waste anesthetic gases. Birth Defects Res A Clin Mol Teratol. 2004;70:476–482. [DOI] [PubMed] [Google Scholar]
- 79.Hattori R, Desimaru M, Nagayama I, Inoue K. Autistic and developmental disorders after general anaesthetic delivery. Lancet. 1991;337:1357–1358. [DOI] [PubMed] [Google Scholar]
- 80.Chien LN, Lin HC, Shao YH, Chiou ST, Chiou HY. Risk of autism associated with general anesthesia during cesarean delivery: a population-based birth-cohort analysis. J Autism Dev Disord. 2015;45:932–942. [DOI] [PubMed] [Google Scholar]
- 81.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrad AL, Goodwin JW, Choi J, Block RI, Nopoulos P. The relationship of exposure to anesthesia on outcomes in children with isolated oral clefts. J Child Neurol. 2017;32:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poor Zamany Nejat Kermany M, Roodneshin F, Ahmadi Dizgah N, Gerami E, Riahi E. Early childhood exposure to short periods of sevoflurane is not associated with later, lasting cognitive deficits. Paediatr Anaesth. 2016;26:1018–1025. [DOI] [PubMed] [Google Scholar]
- 84.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stratmann G, Lee J, Sall JW, et al. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39:2275–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ing C, Hegarty MK, Perkins JW, et al. Duration of general anaesthetic exposure in early childhood and long-term language and cognitive ability. Br J Anaesth. 2017;119:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ludman L, Spitz L, Lansdown R. Intellectual development at 3 years of age of children who underwent major neonatal surgery. J Pediatr Surg. 1993;28:130–134. [DOI] [PubMed] [Google Scholar]
- 88.Majnemer A, Limperopoulos C, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C. A new look at outcomes of infants with congenital heart disease. Pediatr Neurol. 2009;40:197–204. [DOI] [PubMed] [Google Scholar]
- 89.Fan Q, Cai Y, Chen K, Li W. Prognost0ic study of sevoflurane-based general anesthesia on cognitive function in children. J Anesth. 2013;27:493–499. [DOI] [PubMed] [Google Scholar]
- 90.Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012;94:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walker K, Badawi N, Halliday R, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. J Pediatr. 2012;161:748–752.e1. [DOI] [PubMed] [Google Scholar]
- 92.Taghon TA, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015;25:239–246. [DOI] [PubMed] [Google Scholar]
- 93.Harmsen WJ, Aarsen FJ, van der Cammen-van Zijp MHM, et al. Developmental problems in patients with oesophageal atresia: a longitudinal follow-up study. Arch Dis Child Fetal Neonatal E. 2017;102:F214–F219. [DOI] [PubMed] [Google Scholar]
- 94.Petráčková I, Zach J, Borský J, et al. Early and late operation of cleft lip and intelligence quotient and psychosocial development in 3-7 years. Early Hum Dev. 2015;91:149–152. [DOI] [PubMed] [Google Scholar]
- 95.Gano D, Andersen SK, Glass HC, et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr Res. 2015;78:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doberschuetz N, Dewitz R, Rolle U, Schlösser R, Allendorf A. Follow-up of children with gastrointestinal malformations and postnatal surgery and anesthesia: evaluation at two years of age. Neonatology. 2016;110:8–13. [DOI] [PubMed] [Google Scholar]
- 97.Seltzer L, Swartz MF, Kwon J, et al. Neurodevelopmental outcomes after neonatal cardiac surgery: role of cortical isoelectric activity. J Thorac Cardiovasc Surg. 2016;151:1137–1142. [DOI] [PubMed] [Google Scholar]
- 98.Birajdar S, Rao S, McMichael J. Neurodevelopmental outcomes of neonates undergoing surgery under general anesthesia for malrotation of intestines. Early Hum Dev. 2017;109:32–36. [DOI] [PubMed] [Google Scholar]
- 99.Guerra GG, Robertson CM, Alton GY, et al. ; Western Canadian Complex Pediatric Therapies Follow-up Group. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011;21:932–941. [DOI] [PubMed] [Google Scholar]
- 100.Bakri MH, Ismail EA, Ali MS, Elsedfy GO, Sayed TA, Ibrahim A. Behavioral and emotional effects of repeated general anesthesia in young children. Saudi J Anaesth. 2015;9:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terushkin V, Brauer J, Bernstein L, Geronemus R. Effect of general anesthesia on neurodevelopmental abnormalities in children undergoing treatment of vascular anomalies with laser surgery: a retrospective review. Dermatol Surg. 2017;43:534–540. [DOI] [PubMed] [Google Scholar]
- 102.Yazar S, Hewitt AW, Forward H, et al. Early anesthesia exposure and the effect on visual acuity, refractive error, and retinal nerve fiber layer thickness of young adults. J Pediatr. 2016;169:256–259.e1. [DOI] [PubMed] [Google Scholar]
- 103.Yan J, Li YR, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol. 2014;29:1333–1338. [DOI] [PubMed] [Google Scholar]
- 104.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. [DOI] [PubMed] [Google Scholar]
- 105.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015;44:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von Hinke Kessler Scholder S, Wehby GL, Lewis S, Zuccolo L. Alcohol exposure in utero and child academic achievement. Econ J (London). 2014;124:634–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuccolo L, Lewis SJ, Smith GD, et al. Prenatal alcohol exposure and offspring cognition and school performance. A ‘Mendelian randomization’ natural experiment. Int J Epidemiol. 2013;42:1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. [DOI] [PubMed] [Google Scholar]
- 112.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res. 2014;38:214–226. [DOI] [PubMed] [Google Scholar]
- 113.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol Alcohol. 2003;38:295–304. [DOI] [PubMed] [Google Scholar]
- 114.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. [DOI] [PubMed] [Google Scholar]
- 115.Naglieri JA, Bornstein BT. Intelligence and achievement: just how correlated are they? J Psychoeduc Assess. 2003;21:244–260. [Google Scholar]
- 116.Albers CA, Grieve AJ. Test review: Bayley, N.(2006). Bayley scales of infant and toddler development–third edition. San Antonio, TX: Harcourt assessment. J Psychoeduc Assess. 2007;25:180–190. [Google Scholar]
- 117.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW; Victorian Infant Collaborative Group. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164:352–356. [DOI] [PubMed] [Google Scholar]
- 118.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–341. [DOI] [PubMed] [Google Scholar]
- 119.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012;160:553–558. [DOI] [PubMed] [Google Scholar]
- 120.Davidson AJ, Morton NS, Arnup SJ, et al. ; General Anesthesia Compared to Spinal Anesthesia (GAS) Consortium. Apnea after awake regional and general anesthesia in infants: the general anesthesia compared to spinal anesthesia study—comparing apnea and neurodevelopmental outcomes, a randomized controlled trial. Anesthesiology. 2015;123:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anand KJ; International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180. [DOI] [PubMed] [Google Scholar]
- 122.Anderson LM, Shinn C, Fullilove MT, et al. ; Task Force on Community Preventive Services. The effectiveness of early childhood development programs. A systematic review. Am J Prev Med. 2003;24:32–46. [DOI] [PubMed] [Google Scholar]
- 123.Gleich SJ, Flick R, Hu D, et al. Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp Clin Trials. 2015;41:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bilotta F, Evered LA, Gruenbaum SE. Neurotoxicity of anesthetic drugs: an update. Curr Opin Anaesthesiol. 2017;30:452–457. [DOI] [PubMed] [Google Scholar]


