Abstract
We quantitatively determined the relation between the decrease in orbital fat and enophthalmos due to bimatoprost using magnetic resonance imaging (MRI). Nine orbits in nine patients were treated unilaterally with bimatoprost for glaucoma or ocular hypertension. The contralateral orbits were used as controls. The volumes of the orbital tissues and the enophthalmos were measured using MRI. The mean volumes on the treated and untreated sides were, respectively, 14.6 ± 2.1 and 17.0 ± 4.3 cm3 for orbital fat (P = 0.04) and 3.4 ± 0.5 and 3.3 ± 0.5 cm3 for total extraocular muscles (P = 0.85). The mean enophthalmos values were 14.7 ± 2.5 and 16.0 ± 2.3 mm on the treated and untreated sides, respectively (P = 0.002). The data acquired by quantitatively measuring the volumes of orbital fat and enophthalmos on MRI showed that each might be reduced by bimatoprost administration. The enophthalmos could be caused by the bimatoprost-induced decrease in orbital fat.
Introduction
Glaucoma is a disease with chronic progression of visual field defects [1]. Previous studies reported that intraocular pressure (IOP) reduction helps prevent the progression of glaucoma [2–6]. Prostaglandin (PG) F2α analogs have been effective and widely used in patients with glaucoma and ocular hypertension [7–9]. These PGF2α analogs have been approved as first-line treatment for glaucoma because of their potent IOP reduction activity, which, when administered in the morning, is maintained throughout the day [10–12].
Clinically, PGF2α analogs have few negative systemic side effects [13]. They do have some local side effects, however, such as causing prostaglandin-associated periorbitopathy (PAP), including periorbital fat atrophy, enophthalmos, deepening of the upper eyelid sulcus (DUES), and upper eyelid ptosis [14–19]. The mechanism of the symptoms has not been clearly identified but is thought to be related to the effect of PGF2α on adipocytes [20]. PAP has been reported to be more frequently associated with bimatoprost administration than with that of other PGF2α analogs, such as latanoprost, travoprost, and tafluprost [21, 22].
Although enophthalmos seems to be caused by the PGF2α analog-associated decrease in orbital fat, the mechanism has not been shown quantitatively by measuring the orbital fat volume. The purpose of this study was to use magnetic resonance imaging (MRI) to reveal quantitatively the relation between the bimatoprost-associated decrease in orbital fat and the appearance of enophthalmos.
Materials and methods
Subjects
This observational cross-sectional study was approved by the institutional review board of Shiga University of Medical Science. The study adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each patient in the study.
We studied nine orbits of nine patients (mean ± SD age 62.9 ± 16.0, range 34–80 years) who were treated unilaterally with bimatoprost (Allergan, Inc., Irvine, CA, USA) for glaucoma or ocular hypertension for more than 6 months (mean 29.0 ± 14.2 months, range 11–56 months) in our Department of Ophthalmology between November 2015 and March 2018. The patients’ nine contralateral orbits were used as controls. The characteristics of the patients are shown in Table 1.
Table 1. Characteristics of patients.
| Case | Age | Sex | Disease | Eye with disease | Duration of the bimatoprost treatment (months) |
|---|---|---|---|---|---|
| 1 | 75 | M | Primary angle-closure glaucoma | Right | 11 |
| 2 | 56 | M | Exfoliation glaucoma | Right | 32 |
| 3 | 57 | M | Exfoliation glaucoma | Left | 14 |
| 4 | 80 | F | Secondary open angle glaucoma due to uveitis | Right | 28 |
| 5 | 34 | F | Steroid-induced glaucoma | Right | 28 |
| 6 | 75 | M | Neovascular glaucoma | Right | 56 |
| 7 | 68 | M | Neovascular glaucoma | Right | 28 |
| 8 | 41 | M | Exfoliation glaucoma | Left | 48 |
| 9 | 80 | M | Exfoliation glaucoma | Right | 16 |
F-female, M-male
None of the patients had a history of treatment with PGF2α analogs for the contralateral eye, prior extraocular surgery including scleral buckling and encircling procedures, an orbital disease such as trauma, an inflammatory disease of unknown origin in the orbit, or for whom MRI examination posed a risk.
Volume measurements
All patients were examined using a 3.0-tesla MRI unit (Signa HDxt 3.0 T; GE Healthcare, Little Chalfont, UK) at Shiga University of Medical Science Hospital. Coronal, axial, and sagittal MRI images using T2-weighted spin echo were used to measure the volumes of each tissue in the orbit in a manner similar to that described in previous studies [23, 24]. The slice thickness of the images was 1.5 mm.
The cross-sectional areas (CSAs) of the orbital fat, extraocular muscles, optic nerve, and eyeball were measured by tracing outlines of the tissue on the images using Aquarius iNtuition software (TeraRecon, San Mateo, Foster City, CA, USA) (Fig 1). CSAs on axial images were used for the orbital fat, lateral rectus, medial rectus, superior oblique, eyeball, and optic nerve measurements. CSAs on sagittal images were used for the superior rectus and inferior rectus measurements. CSAs on coronal images were used for the inferior oblique measurements. The orbital fat was traced together with the lacrimal gland because it was difficult to separate them on the MRI images. The superior rectus was traced along with the levator palpebrae muscle for the same reason. The volumes of the tissues were calculated by multiplying the sum of the CSAs × the slice increment. The volumes of the images were measured in a masked fashion by one technician (F.K.).
Fig 1. Volume measurements.
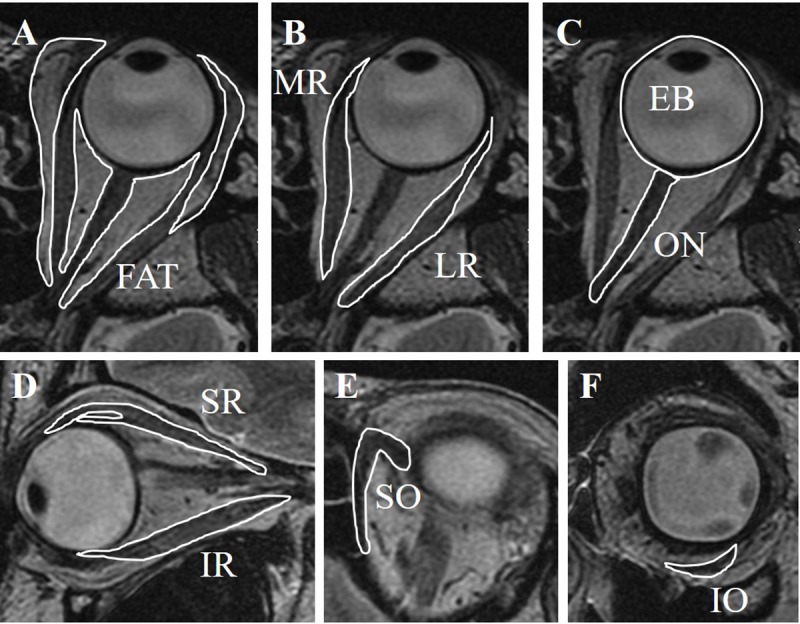
Cross-sectional areas of each tissue were measured. The volumes of the tissues were calculated by multiplying the sum of the cross-sectional areas by the slice increment (1.5 mm). A, Orbital fat. B, Lateral rectus (LR) and medial rectus (MR). C, Optic nerve (ON) and eyeball (EB). D, Superior rectus (SR) and inferior rectus (IR). E, Superior oblique. F, Inferior oblique (IO).
Enophthalmos measurements
The enophthalmos was measured on axial images using Aquarius iNtuition software (TeraRecon: https://www.terarecon.com) in a manner similar to that used in previous studies [24]. A baseline was demarcated between the bilateral frontal processes of the zygomatic bones. The perpendicular distance from the top point of the corneal surface to the baseline was defined as the enophthalmos value (Fig 2). Enophthalmos values were also measured in a masked fashion by one technician (F.K.).
Fig 2. Enophthalmos measurements.
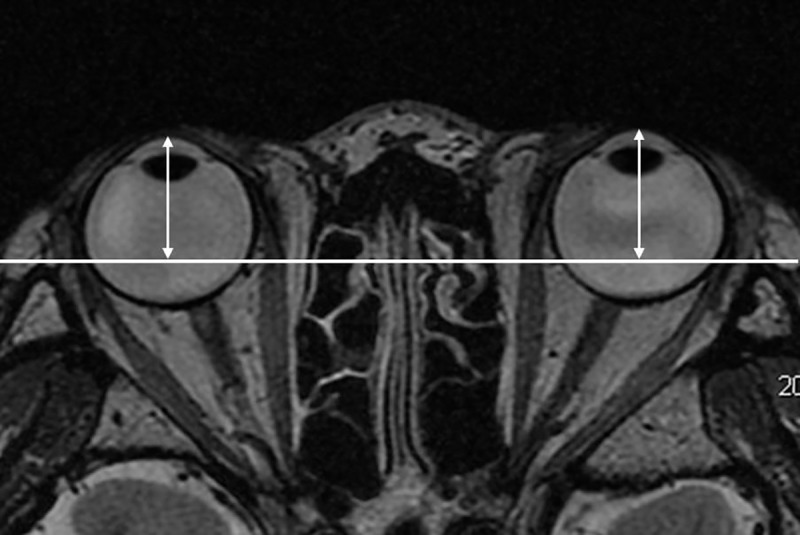
A baseline was demarcated between the bilateral frontal processes of the zygomatic bones. The perpendicular distance from the top point of the corneal surface to the baseline was defined as the enophthalmos value.
Statistical analysis
All statistical analyses were performed using SPSS Statistics 22 software (IBM, Armonk, NY, USA). The normality of the numerical variables was evaluated using the Shapiro–Wilk test. A paired t-test was used to compare volumes and values between the orbits treated with bimatoprost and their untreated contralateral orbits. Pearson’s product–moment correlation coefficient was used to analyze the correlations. The values are expressed as means ± SD. A value of P < 0.05 indicated statistical significance.
Results
Volumes
The mean volumes on the treated and untreated sides are shown in Table 2.
Table 2. Mean orbital tissue volumes and enophthalmos values.
| The treated side | The untreated side | P-value | |
|---|---|---|---|
| Orbital fat (cm3) | 14.6 ± 2.1 | 17.0 ± 4.3 | 0.04 |
| Total extraocular muscles (cm3) | 3.4 ± 0.5 | 3.3 ± 0.5 | 0.85 |
| Eyeball (cm3) | 9.3 ± 1.2 | 9.3 ± 1.0 | 0.85 |
| Optic nerve (cm3) | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.10 |
| Enophthalmos (mm) | 14.7 ± 2.5 | 16.0 ± 2.3 | 0.002 |
All values are shown as means ± standard deviation.
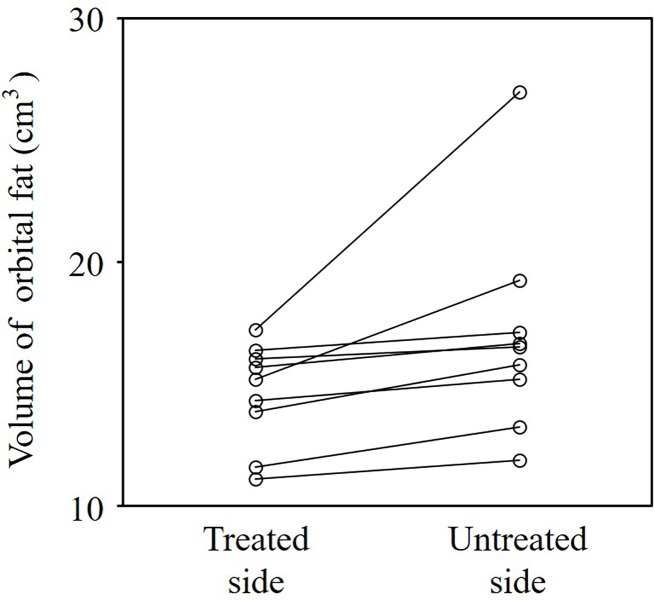
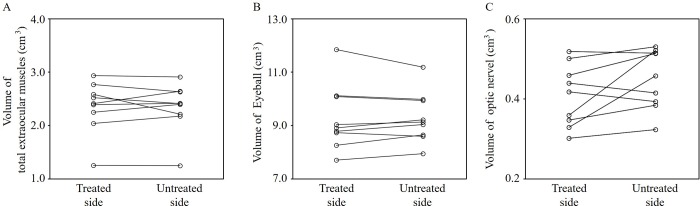
The mean volume of orbital fat on the treated side was significantly smaller than that on the untreated side (P = 0.04) (Fig 3). In all patients, the volume of orbital fat was smaller on the treated side than on the untreated side, although the differences were minimal in some patients. There were no significant differences in the mean volumes between the two orbits for the extraocular muscles, eyeballs, or optic nerve (total extraocular muscles P = 0.85; eyeball P = 0.85; optic nerve P = 0.10) (Fig 4).
Fig 3. Orbital fat volumes on the treated and untreated sides.
The mean orbital fat volume was significantly smaller on the treated side than on the untreated side (P = 0.04). The volumes of the orbital fat on the treated side were smaller than those on the untreated side in all patients, although the differences were slight in some patients.
Fig 4. Orbital tissues’ volumes on the treated and untreated sides.
A, Total extraocular muscles. B, Eyeball. C, Optic nerve. There were no significant differences in the mean volumes between the two orbits (total extraocular muscles P = 0.85; eyeball P = 0.85; optic nerve P = 0.10).
Enophthalmos
The mean enophthalmos values were 14.7 ± 2.5 and 16.0 ± 2.3 mm on the treated and untreated sides, respectively (Table 2). The mean value on the treated side was significantly smaller than that on the untreated side (P = 0.002) (Fig 5). In all patients, the enophthalmos value was smaller on the treated side than on the untreated side, although the differences were minimal in some patients.
Fig 5. Enophthalmos values on the treated and untreated sides.
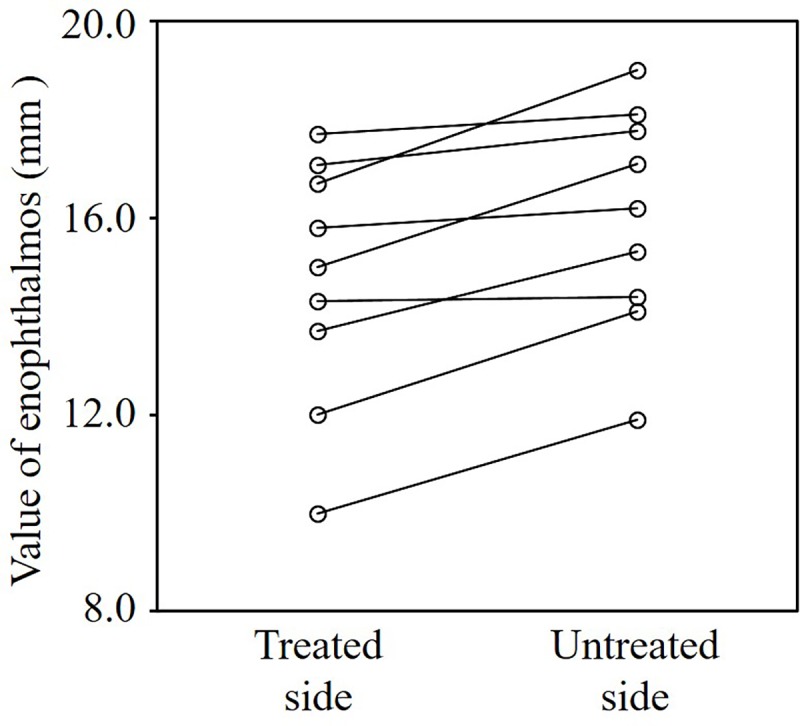
The mean value on the treated side was significantly smaller than that on the untreated side (P = 0.002), although the differences were slight in some patients.
Relation between the orbital fat volume and enophthalmos value
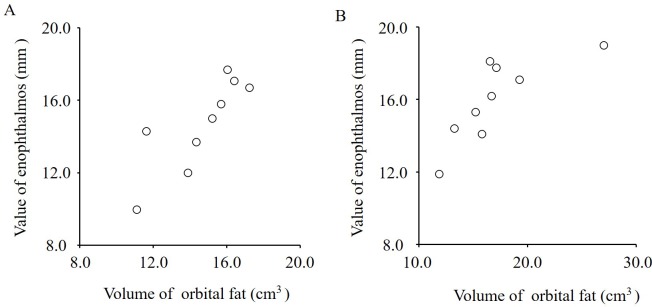
The orbital fat volumes showed statistically significantly positive correlations with the enophthalmos values on both the treated side (r = 0.83, P = 0.006) and the untreated side (r = 0.79, P = 0.01) (Fig 6).
Fig 6. Correlation between the orbital fat volumes and the enophthalmos values.
A, Treated side. B, Untreated side. The orbital fat volumes showed statistically significantly positive correlations with the enophthalmos values on both the treated (r = 0.83, P = 0.006) and untreated (r = 0.79, P = 0.01) sides.
Discussion
The mean volume of orbital fat and the mean enophthalmos value on the treated side were significantly smaller than those on the untreated side, whereas there were no significant differences in the mean orbital fat volumes of the other measured tissues (i.e., extraocular muscles, eyeball, optic nerve). Hence, this study quantitatively revealed that enophthalmos could be caused by the bimatoprost-induced decrease in orbital fat. In addition, the orbital fat volumes showed statistically significant positive correlations with the enophthalmos values on both the treated and untreated sides. These correlations support our hypothesized mechanism. Jayaprakasam and Ghazi-Nouri reported the MRI features of orbital fat atrophy in one patient using bimatoprost [25], but they did not show quantitatively that the decrease in orbital fat was due to bimatoprost. Our current study quantitatively revealed not only the decrease in orbital fat but also its relation to enophthalmos.
The mechanism of PAP (e.g., enophthalmos) due to PGF2α analogs remains unclear. The important regulators of fat cell lipolysis are hormones—mainly insulin, catecholamines, natriuretic peptides—and paracrine factors such as prostaglandins, cytokines, and adenosine [26]. Some reports stated that activation of PGF2α receptors could inhibit adipocyte differentiation [27–29]. Serrero and Lepak investigated the effect of receptor agonists on the differentiation of newborn rat adipocytes in primary cultures and reported that PGF2α receptor agonists were indeed potent inhibitors of adipocyte differentiation [28]. Thus, PAPs due to PGF2α analogs could be caused by that mechanism.
Enophthalmos values were smaller on the treated side than on the untreated side in all patients, and the mean enophthalmos value of the treated eyes was statistically significantly smaller than that in their contralateral eyes. A previous study investigated the frequency of enophthalmos due to bimatoprost using Hertel exophthalmometry measurements [22]. They found that a difference of ≥2 mm between the two eyes was indicative of enophthalmos, which was seen in 80% of cases. However, the accuracy of Hertel exophthalmometric measurements is questionable because the value is determined visually by the examiner. In addition, these measurements could be biased because patients treated with bimatoprost may have periorbital changes, such as pigmentation of the eyelid and iris, increased eyelash growth, and conjunctival hyphemia [10, 11, 14–19, 30]. In contrast, in the current study, the enophthalmos was measured on MRI axial images in a masked fashion, thereby minimizing measurement errors by avoiding possible bias, resulting in more accurate measurements than those obtained using Hertel exophthalmometry.
The volumes of the orbital tissues in the present study were measured using MRI. Tian et al. also reported orbital tissues volumes in 21 normal subjects [31] that were measured using MRI in a manner similar to that described herein. Their mean eyeball volume was 9.047 cm3, which is well matched by the mean volume we found.
The present study has some limitations. First, the study group was small, with only nine patients. The results, however, showed a similar tendency in all patients—that is, the orbital fat volumes and exophthalmos values were smaller on the treated side than on the untreated side in all patients. Unfortunately, we were unable to perform a subgroup analysis because of the small sample size. Thus, it will be necessary to confirm the study findings using a subgroup analysis and prediction modeling in a future study with a larger sample size. Second, the study was designed to be an observational, cross-sectional trial. Thus, the bimatoprost-induced decrease in orbital fat over time must be confirmed in future studies. Third, this study did not investigate whether the enophthalmos continued to recover after stopping bimatoprost. Sakata et al. reported recovery from DUES after switching from bimatoprost to latanoprost [12].At 2 months after switching analogs, the DUES had either decreased or disappeared in 11 of their 13 patients. It is also necessary in future MRI studies to confirm the recovery from decreased orbital fat and enophthalmos after stopping bimatoprost or switching from bimatoprost to another PGF2α analog.
In conclusion, using MRI, we quantitatively showed that bimatoprost lowered the orbital fat volume and reduced the enophthalmos value. The enophthalmos could be caused by the bimatoprost-induced decrease in orbital fat.
Supporting information
(XLSX)
Acknowledgments
We thank Ms. Fumiko Kimura for measuring the volumes and enophthalmos in this study. This work was supported by JSPS KAKENHI Grant Number JP17K16966. We also thank Nancy Schatken, BS, MT(ASCP), from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
TH: This work was supported by JSPS KAKENHI Grant Number JP17K16966, Funder: Japan Society for the Promotion of Science, https://www.jsps.go.jp/english/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Inoue K, Shiokawa M, Fujimoto T, Tomita G. Effects of treatment with bimatoprost 0.03% for 3 years in patients with normal-tension glaucoma. Clin Ophthalmol. 2014; 8: 1179–1183. 10.2147/OPTH.S60538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003; 121: 48–56. [DOI] [PubMed] [Google Scholar]
- 5.Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, et al. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004; 111: 651–664. 10.1016/j.ophtha.2003.09.025 [DOI] [PubMed] [Google Scholar]
- 6.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R; CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011; 118: 1766–1773. 10.1016/j.ophtha.2011.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Xu L, Yuan Z, Yao K, Zhao J, Xu L, et al. Intraocular pressure-lowering efficacy and safety of bimatoprost 0.03% therapy for primary open-angle glaucoma and ocular hypertension patients in China. BMC Ophthalmol. 2014; 14: 21 10.1186/1471-2415-14-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, et al. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology. 2016; 123: 129–140. 10.1016/j.ophtha.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan N, Dehabadi MH, Nair S, Quartilho A, Bunce C, Reekie I, et al. Efficacy and safety of bimatoprost in glaucoma and ocular hypertension in non-responder patients. Int J Ophthalmol. 2017; 10: 1251–1254. 10.18240/ijo.2017.08.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008; 24: 302–307. 10.1097/IOP.0b013e31817d81df [DOI] [PubMed] [Google Scholar]
- 11.Maruyama K, Tsuchisaka A, Sakamoto J, Shirato S, Goto H. Incidence of deepening of upper eyelid sulcus after topical use of tafluprost ophthalmic solution in Japanese patients. Clin Ophthalmol. 2013; 7: 1441–1446. 10.2147/OPTH.S47783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013; 57: 179–184. 10.1007/s10384-012-0219-3 [DOI] [PubMed] [Google Scholar]
- 13.Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014; 8: 1967–1985. 10.2147/OPTH.S59162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol. 2009; 53: 176–179. 10.1007/s10384-008-0623-x [DOI] [PubMed] [Google Scholar]
- 15.Yoshino T, Fukuchi T, Togano T, Seki M, Ikegaki H, Abe H. Eyelid and eyelash changes due to prostaglandin analog therapy in unilateral treatment cases. Jpn J Ophthalmol. 213; 57: 172–178. 10.1007/s10384-012-0199-3 [DOI] [PubMed] [Google Scholar]
- 16.Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn J Ophthalmol. 2014; 58: 212–217. 10.1007/s10384-013-0299-8 [DOI] [PubMed] [Google Scholar]
- 17.Nakakura S, Yamamoto M, Terao E, Nagatomi N, Matsuo N, Fujisawa Y, et al. Prostaglandin-associated periorbitopathy in latanoprost users. Clin Ophthalmol. 2014; 9: 51–56. 10.2147/OPTH.S75651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowitz MP, Katz LJ, Moster MR, Myers JS, Pro MJ, Spaeth GL, et al. Unilateral Prostaglandin-Associated Periorbitopathy: A Syndrome Involving Upper Eyelid Retraction Distinguishable From the Aging Sunken Eyelid. Ophthal Plast Reconstr Surg. 2015; 31: 373–378. 10.1097/IOP.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 19.Shrirao N, Khurana M, Mukherjee B. Prostaglandin-associated periorbitopathy. Indian J Ophthalmol. 2016; 64: 459 10.4103/0301-4738.187676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HW, Choi YJ, Lee KW, Lee MJ. Periorbital changes associated with prostaglandin analogs in Korean patients. BMC Ophthalmol. 2017; 17: 126 10.1186/s12886-017-0521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013; 22: 626–631. 10.1097/IJG.0b013e31824d8d7c [DOI] [PubMed] [Google Scholar]
- 22.Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014; 42: 126–131. 10.1111/ceo.12163 [DOI] [PubMed] [Google Scholar]
- 23.Nishida Y, Tian S, Isberg B, Tallstedt L, Lennerstrand G. MRI measurements of orbital tissues in dysthyroid ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2001; 239: 824–831. [DOI] [PubMed] [Google Scholar]
- 24.Higashiyama T, Nishida Y, Ohji M. Changes of orbital tissue volumes and proptosis in patients with thyroid extraocular muscle swelling after methylprednisolone pulse therapy. Jpn J Ophthalmol. 2015; 59: 430–435. 10.1007/s10384-015-0410-4 [DOI] [PubMed] [Google Scholar]
- 25.Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy—an unfamiliar side effect of prostaglandin analogues. Orbit. 2010; 29: 357–359. 10.3109/01676830.2010.527028 [DOI] [PubMed] [Google Scholar]
- 26.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005; 19: 471–482. 10.1016/j.beem.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology. 1996; 137: 5641–5650. 10.1210/endo.137.12.8940395 [DOI] [PubMed] [Google Scholar]
- 28.Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997; 233: 200–202. 10.1006/bbrc.1997.6433 [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Clipstone NA. Prostaglandin F2alpha inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J Cell Biochem. 2007; 100: 161–173. 10.1002/jcb.21044 [DOI] [PubMed] [Google Scholar]
- 30.Aihara M, Shirato S, Sakata R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn J Ophthalmol. 2011; 55: 600–604. 10.1007/s10384-011-0075-6 [DOI] [PubMed] [Google Scholar]
- 31.Tian S, Nishida Y, Isberg B, Lennerstrand G. MRI measurements of normal extraocular muscles and other orbital structures. Graefes Arch Clin Exp Ophthalmol. 2000; 238: 393–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.