Abstract
Context
In adults, noninvasive follicular variant of papillary thyroid carcinoma (FVPTC) is considered a low risk for metastasis and persistent/recurrent disease.
Objective
The goal of this study was to assess the clinical, sonographic, and histopathologic features of FVPTC in a pediatric cohort.
Design
A retrospective review of subjects <19 years of age with papillary thyroid carcinoma (PTC) who underwent thyroidectomy between January 2010 and July 2015.
Setting
Multidisciplinary academic referral center.
Patients
Patients with FVPTC, defined as a tumor ≥1 cm in the largest dimension with predominant follicular growth, complete lack of well-formed papillae, and nuclear features of PTC.
Main Outcome Measures
Tumor size and location, presence of a tumor capsule, capsule and vascular invasion, lymph node invasion, and distant metastasis.
Results
Eighteen patients with FVPTC were identified from a case cohort of 110 patients with PTC. On histopathology, 13 (72%) had unifocal nodules and 14 (78%) had completely encapsulated FVPTC. Capsule invasion was frequent (nine of 14; 64%), and vascular invasion was found in one-third of patients (six of 18; 33%). No lymph node metastases were found in the 13 patients (72%) who had a central neck lymph node dissection. One patient with vascular invasion had distant metastases.
Conclusion
When strictly defined, FVPTC in pediatric patients has a low risk for bilateral disease and metastasis. Prospective studies are needed to confirm whether lobectomy with surveillance is sufficient to achieve remission in pediatric patients with low-risk FVPTC.
We assessed the clinical, sonographic, and histologic features of FVPTC in a pediatric cohort and identified criteria to select patients for lobectomy with surveillance rather than total thyroidectomy.
The incidence of thyroid nodules and thyroid cancer in pediatric patients has increased over the last several decades (1–4). Differentiated thyroid carcinoma (DTC) is now the second-most common malignancy in 15- to 19-year-old females (3). Papillary thyroid carcinoma (PTC) accounts for at least 90% of all childhood cases of DTC (5–8). Despite higher rates of multifocality and metastasis at diagnosis than in adults, pediatric patients respond more rapidly to therapy and demonstrate excellent prognosis (9).
In 2015, the American Thyroid Association (ATA) published inaugural guidelines for children with thyroid nodules and DTC. These guidelines emphasize the need for accurate and complete preoperative evaluation as well as stratification of medical and surgical care in an effort to reduce complications while maintaining excellent prognosis. Within this context, pediatric patients are stratified into postsurgical risk levels addressing the potential need for radioiodine treatment based on the American Joint Commission on Cancer (AJCC) tumor node metastasis classification system (8).
However, these pediatric guidelines do not provide stratification for surgical management, recommending total thyroidectomy as the favored surgical approach for nearly all pediatric patients with PTC. This recommendation was based on previous data showing that more extensive surgery was associated with a lower risk of recurrence (10–12). However, these studies were limited by small numbers of patients who underwent lobectomy, as complete removal of the thyroid has been the accepted standard of care for pediatric PTC. For example, one retrospective review of 215 patients showed that compared with lobectomy, total thyroidectomy decreased the incidence of local recurrence from 35% to 6% after a median surveillance of 29 years; 25 patients in this study underwent lobectomy (13). In contrast to the pediatric guidelines, the 2015 ATA adult guidelines suggest consideration of lobectomy as the initial treatment of patients with PTC variants with low-risk features, including tumor size <4 cm; no clinical evidence of lymph node metastases; and no evidence of extrathyroidal extension (ETE) (14).
In the adult population, noninvasive subtypes of follicular variant of PTC (FVPTC) present with a lower risk of aggressive histopathologic characteristics and metastasis (locoregional and distant) (15). These tumors are most frequently divided into two groups according to the presence or absence of a tumor capsule, with noninvasive encapsulated FVPTC (E-FVPTC) displaying significantly lower rates of cervical neck lymph node metastases and recurrence than invasive FVPTC (16–18). Additional data suggest that the degree of vascular and/or capsular invasion is more predictive of FVPTC progression than encapsulation status alone and that individualization of treatment on the basis of invasion is a more effective approach to stratification of care (19–21). This is highlighted by one case series in which five patients with E-FVPTC and extensive vascular invasion had distant bone metastases (22).
Data to guide the best approach to surgical treatment of pediatric patients with PTC are limited, particularly for lower-risk variants including FVPTC. The primary aim of this study was to assess the clinical, sonographic, and histopathologic features of FVPTC in a pediatric cohort.
Materials and Methods
Patients
A retrospective medical record review was performed using our institutional database of 110 children aged 0 to 19 years with PTC who were evaluated at the Children’s Hospital of Philadelphia (CHOP) for DTC between January 2010 and July 2015. Cases were selected for complete medical record, ultrasonography (US), and histopathology review when the surgical pathology report indicated FVPTC architecture within the primary tumor. Patients were divided into two groups on the basis of histologic findings of unilateral vs bilateral disease after total or completion thyroidectomy.
Methods
Data extracted from the medical record included demographic information, medical history, fine-needle aspiration (FNA) biopsy findings, US and surgical pathology findings, and treatment history. FNA results were categorized using the Bethesda classification (23).
Preoperative US images were retrospectively reviewed by one radiologist (J.E.L.) with expertise in thyroid US. The number of nodules, the location of each nodule, and the presence of unilateral vs bilateral nodules were recorded. US images were assessed for the following characteristics according to categories established by the American College of Radiology Thyroid Imaging, Reporting, and Data System: (1) composition (cystic, spongiform, mixed cystic and solid, or solid); (2) echogenicity (anechoic, hypoechoic or isoechoic, hypoechoic, or very hypoechoic); (3) shape on transverse imaging (wider-than-tall or taller-than-wide); (4) margin (smooth, ill-defined, lobulated or irregular, or extrathyroidal extension); and (5) echogenic foci (none or large comet-tail artifacts, macrocalcifications, peripheral calcifications, or punctate echogenic foci) (24). When lateral neck US was performed, lymph nodes were assessed for abnormality, including (1) shape; (2) presence or absence of echogenic foci; (3) composition; and (4) blood flow (hilar/central vs peripheral) on Doppler imaging (25).
Histopathology review was performed by four pathologists, two with particular expertise in thyroid neoplasia (V.A.L. and Z.B.) and two with advanced training in pediatric pathology (L.F.S. and T.B.), to confirm the diagnosis, extent of disease, and tumor characteristics. Each specimen was reviewed by all four pathologists. Thyroid carcinomas were classified according to the World Health Organization’s published recommendations, with modifications based on recent literature (26–28). FVPTC was defined as a tumor ≥1 cm in the largest dimension with predominant follicular growth, complete lack of well-formed papillae, and nuclear features of PTC. Nuclear features of PTC included (1) crowding/overlapping, (2) elongation, (3) irregular contour, (4) grooves, (5) pseudo inclusion, and (6) chromatin clearing. Histology for each FVPTC was described as uniform (only follicular architecture) or mixed (containing a solid component <50% of overall cellularity). For this study, all tumors measuring <1 cm were excluded from the case cohort.
The size of the carcinoma was determined by its largest dimension on the basis of review of the gross pathology for large nodules (≥1 cm) and direct microscopic measurements on the slides for small nodules (<1 cm). Encapsulation was described as present or absent and when present was further characterized as complete or partial. Of those cases with complete encapsulation, capsule invasion was defined as none, partial (spreads into but not through the capsule), or complete (capsule transgression). When vascular invasion was present, the number of vessels involved was recorded. When mitotic activity was present, it was classified as low or high (≥3 mitoses/400× power field). When lymph nodes were sampled during surgery, they were histologically examined and recorded for metastatic disease.
Patients were evaluated for evidence of persistent disease 4 to 6 weeks after surgery using thyroid hormone withdrawal‒stimulated thyroglobulin (Tg) and a 123I diagnostic whole-body scan. Radioiodine treatment with I-131 was administered using common empiric adult radioactive iodine (RAI) ablation activities (uptake limited to the thyroid bed, 30 to 100 mCi; presence of neck or distant metastasis, 100 to 150 mCi), scaled down according to the pediatric patient’s weight using the following formula: pediatric dose = adult dose × patient’s body weight (kg)/70 kg (8). Seventeen of 18 patients underwent postoperative evaluation for persistent disease, and 14 patients received RAI therapy (range, 45 to 128 mCi; median, 81 mCi). All patients who received RAI had a post-RAI whole-body scan 5 days after therapy.
Patients subsequently received thyrotropin (TSH)-suppressive doses (TSH target of <0.1 mIU/L) of l-thyroxine. Follow-up included physical examination, testing of thyroid function and determination of levels of serum Tg and Tg antibodies while receiving TSH-suppressive therapy, and radiologic imaging (neck US for all, with the addition of a noncontrast chest computed tomography 6 months after initial treatment. The frequency of laboratory and radiologic surveillance was decreased to every 6 and 12 months, respectively, when the surveillance data were consistent with remission, defined as a TSH-suppressed Tg value below the level of detection of the assay (biochemical remission) and no evidence of persistent disease on radiologic imaging (anatomic remission) (8). A TSH-stimulated Tg level and RAI whole-body scan was pursued for any patient with an increasing trend in TSH-suppressed Tg value or a single Tg value >10 ng/mL and negative results on neck US and chest computed tomography.
Risk levels for persistent postsurgical disease were determined on the basis of the 2015 pediatric ATA guidelines (8). This classification system utilizes the AJCC 7th edition tumor node metastasis classification system, specifically regional lymph node and distant metastasis staging, to categorize pediatric patients into one of three risk groups: low, intermediate, and high. Pediatric ATA low-risk criteria include (1) disease grossly confined to the thyroid with (2) N0 (no positive lymph nodes after central neck dissection), Nx (no central neck dissection performed), or incidental microscopic metastasis to the central neck (N1a) lymph nodes (29).
Study data were collected and managed using the REDCap electronic data capture tools hosted at CHOP (30). Standard descriptive summaries such as mean ± standard deviation or median (range) were used as appropriate for baseline demographic information and core retrospective data. This study was approved by the CHOP institutional review board.
Results
Patient demographic and treatment characteristics
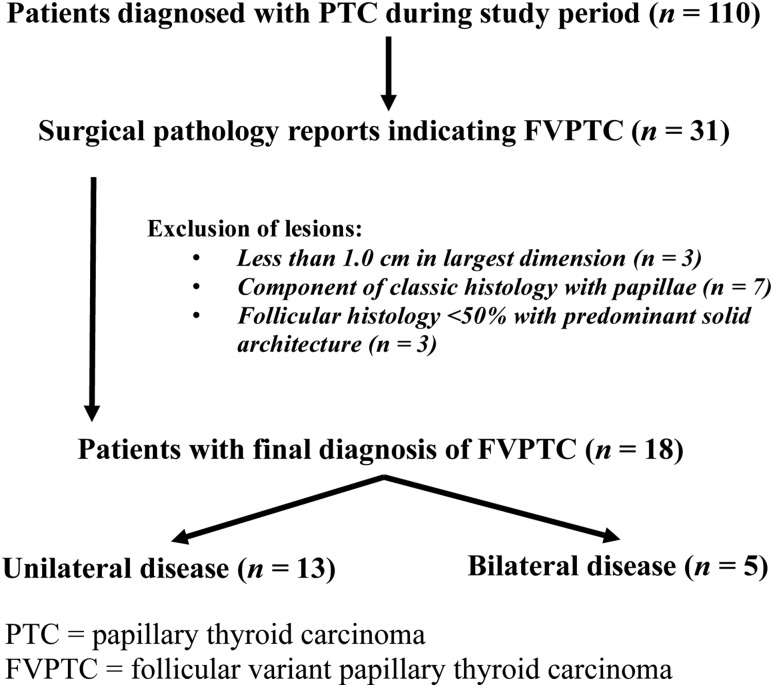
Thirty-one cases for which the pathology report indicated follicular variant architecture within the primary tumor were reviewed. After additional histopathology review, 13 patients did not meet the criteria for FVPTC and were excluded from the analysis: three had tumors <1.0 cm, seven had components of classic PTC with papillae, and three had mixed histology with predominant (>50%) solid PTC architecture (Fig. 1). Patients with FVPTC were divided into two groups according to histologic findings of unilateral (13 of 18; 72%) vs bilateral (five of 18; 28%) disease.
Figure 1.
Sample selection criteria.
The median (range) age at the time of initial surgery was 14 years (range, 10 to 18 years) for the 18 patients (12 female, 67%) with confirmed histologic diagnosis of FVPTC. Patient demographics and treatment characteristics are shown in Table 1. All patients underwent total thyroidectomy (13 of 18; 72%) or lobectomy followed by completion thyroidectomy (five of 18; 28%). Most patients (17 of 18; 94%) had their initial surgery at CHOP, where two surgeons (K.K. and N.S.A.) performed all surgeries.
Table 1.
Patient Demographics and Treatment Characteristics
| Characteristic | n = 18 |
|---|---|
| Female | 12 (67%) |
| Age at time of initial surgery | |
| Median (range), y | 14 (10–18) |
| Race | |
| Black | 1 (6%) |
| White | 13 (72%) |
| Other/not specified | 4 (22%) |
| Surgery | |
| Total thyroidectomy | 13 (72%) |
| Lobectomy + completion thyroidectomy | 5 (28%) |
| Radioiodine treatment | 14 (78%) |
RAI was administered to all but two patients (16 of 18; 89%) after surgery. Two patients who had undetectable postsurgical TSH-stimulated Tg levels and negative RAI diagnostic whole-body scans did not receive RAI.
Presurgical characteristics
Presurgical characteristics for the total cohort and for those with unilateral vs bilateral histologic disease are shown in Table 2. Three patients (17%) had a predisposing risk factor for thyroid cancer: One patient had a history of phosphatase and tensin homolog hamartoma tumor syndrome, and two patients had a history of malignancy and radiation exposure. Preoperative US images were available for review for most patients (15 of 18; 83%), and US reports from the medical records were reviewed for the remaining three patients. Most patients (12 of 18; 67%) had unilateral nodules on preoperative US. Both patients with a history of malignancy had bilateral nodules noted on US and subsequent bilateral findings of papillary thyroid microcarcinoma (PTMC) on histology. Two patients with bilateral nodules on presurgical US were ultimately diagnosed with benign nodules in the contralateral lobe; one of these patients had phosphatase and tensin homolog hamartoma tumor syndrome, and the other had benign cytology on FNA in the contralateral lobe before surgery. Preoperative US at our institution did not identify bilateral nodules in two patients who were incidentally found to have bilateral disease after surgical resection; both of these patients had bilateral multifocal PTMC.
Table 2.
Comparison of Presurgical Characteristics by Histologic Laterality
| Characteristic | Total Cohort (n = 18) | Unilateral Disease (n = 13) | Bilateral Disease (n = 5) |
|---|---|---|---|
| Median age at initial surgery, y | 14.4 | 13.8 | 15.8 |
| Predisposing risk factor | |||
| History of malignancy/radiation | 2 (11%) | 0 | 2 (40%) |
| History of familial tumor predisposition syndrome | 1 (6%) | 1 (7%) | 0 |
| History of autoimmune (Hashimoto) thyroiditis | 4 (22%) | 3 (23%) | 1 (20%) |
| Presurgical USa | (n = 15) | (n = 10) | (n = 5) |
|---|---|---|---|
| Total no. of nodules | |||
| 1 | 10 (66%) | 8 (80%) | 2 (40%) |
| 2 | 1 (7%) | 1 (10%) | 0 |
| 3 or more | 4 (26%) | 1 (10%) | 3 (60%) |
| Laterality of nodules on US | |||
| Unilateral | 10 (67%) | 8 (80%) | 2 (40%) |
| Bilateral | 5 (33%) | 2 (20%) | 3 (60%) |
| Characteristics of nodule with most concerning features | |||
| Composition | |||
| Mixed cystic and solid | 2 (13%) | 1 (10%) | 1 (20%) |
| Solid or almost completely solid | 13 (87%) | 9 (90%) | 4 (80%) |
| Echogenicity | |||
| Hyperechoic or isoechoic | 11 (73%) | 8 (80%) | 3 (60%) |
| Hypoechoic | 2 (13%) | 1 (10%) | 1 (20%) |
| Very hypoechoic | 2 (13%) | 1 (10%) | 1 (20%) |
| Shape | |||
| Wider-than-tall | 15 (100%) | 10 (100%) | 5 (100%) |
| Taller-than-wide | 0 | 0 | 0 |
| Margin | |||
| Smooth | 6 (40%) | 4 (40%) | 2 (40%) |
| Ill-defined | 1 (7%) | 1 (10%) | 0 |
| Lobulated or irregular | 8 (53%) | 5 (50%) | 3 (60%) |
| ETE | 0 | 0 | 0 |
| Echogenic foci | |||
| None or large comet-tail artifacts | 10 (67%) | 8 (80%) | 2 (40%) |
| Macrocalcifications | 1 (7%) | 1 (10%) | 0 |
| Peripheral (rim) calcifications | 0 | 0 | 0 |
| Punctate echogenic foci | 4 (27%) | 1 (10%) | 3 (60%) |
| Cervical lymph nodes | 5.1 (2–8) | 4.6 (2–7) | 6.2 (5–8) |
| Abnormal lymph nodes seen | 0 | 0 | 0 |
| FNA Bethesda Classificationb | (n = 16) | (n = 12) | (n = 4) |
|---|---|---|---|
| Unsatisfactory | 0 | 0 | 0 |
| Benign | 0 | 0 | 0 |
| Atypia/Follicular lesion of undetermined significance | 3 (19%) | 3 (25%) | 0 |
| Follicular neoplasm | 10 (63%) | 7 (58%) | 3 (75%) |
| Suspicious for malignancy | 3 (19%) | 2 (17%) | 1 (25%) |
| Malignant | 0 | 0 | 0 |
Ultrasonographic data are presented for 15 of 18 patients for whom preoperative images were available.
FNA data are presented for 16 of 18 patients for whom FNA was completed and results were available.
At US, the majority of nodules had solid or almost completely solid composition (13 of 15; 87%), hyperechoic or isoechoic echogenicity (11 of 15; 73%), wider-than-tall shape on transverse imaging (15 of 15; 100%), and no echogenic foci (10 of 15; 67%). ETE and abnormal lymph nodes were not noted during review of US images or reports for any patients in the cohort.
FNA results were available for 16 of 18 patients for whom FNA was completed before surgery. FNA for most patients (13 of 16; 81%) was reported in the indeterminate categories of the Bethesda Classification System for Reporting Thyroid Cytopathology, either atypia or follicular lesion of undetermined significance (AUS/FLUS; three of 16; 19%) or follicular neoplasm (FN; 10 of 16; 81%). Three patients (three of 16; 19%) had FNA results suspicious for malignancy, and none were classified as malignant. Nine of the 13 patients with indeterminate cytology (AUS/FLUS or FN) underwent total thyroidectomy as initial surgery because of additional US features of bilateral nodules or the presence of diffuse Hashimoto thyroiditis.
Postsurgical characteristics
Postsurgical characteristics for the total cohort and for those with unilateral vs bilateral disease on final histopathology review are shown in Table 3. Most patients had tumors <4 cm in the largest dimension (median, 2.3 cm; range, 1.1 to 4.5 cm) and were therefore classified as T1a (one of 18; 6%), T1b (seven of 18; 39%), or T2 (eight of 18; 44%). Two patients were classified as T3, one on the basis of size >4 cm and one on the basis of histopathologic evidence of microscopic ETE (per AJCC 7th edition criteria). A majority of patients underwent central neck lymph node resection (13 of 18; 72%), with a median of four lymph nodes (range, one to nine lymph nodes) removed to confirm N0. Lymph node metastases (N1a or N1b) were absent in all patients in our cohort who underwent central neck lymph node resection. However, one patient who had not undergone central neck lymph node dissection (Nx) had evidence of a mediastinal lymph node and distant pulmonary metastases (M1) on postoperative radioiodine whole-body scan. This patient, who had no predisposing risk factors, had bilateral angioinvasive FVPTC as well as multifocal PTMC.
Table 3.
Comparison of Postsurgical Characteristics by Histologic Laterality
| Characteristic | Total Cohort (n = 18) | Unilateral Disease (n = 13) | Bilateral Disease (n = 5) |
|---|---|---|---|
| AJCC TNM classificationa | |||
| Primary tumor (T) | |||
| T1a | 1 (6%) | 0 | 1 (20%) |
| T1b | 7 (39%) | 5 (39%) | 2 (40%) |
| T2 | 8 (44%) | 7 (54%) | 1 (20%) |
| T3 | 2 (11%) | 1 (8%) | 1 (20%) |
| T4a or T4b | 0 | 0 | 0 |
| Lymph nodes (N) | |||
| NX | 5 (28%) | 4 (31%) | 1 (20%) |
| N0 | 13 (72%) | 9 (69%) | 4 (80%) |
| N1a or N1b | 0 | 0 | 0 |
| Distant metastasis (M) | |||
| MX | 2 (11%) | 2 (15%) | 0 |
| M0 | 15 (83%) | 11 (85%) | 4 (80%) |
| M1b | 1 (6%) | 0 | 1 (20%) |
| Histopathology characteristics (primary tumor) | |||
| Tumor size in largest dimension, median (range), cm | 2.3 (1.1–4.5) | 2.3 (1.3–4.5) | 2.0 (1.1–2.9) |
| Ipsilateral multifocality | 4 (28%) | 0 | 4 (80%) |
| Histopathology | |||
| Uniform histology (follicular architecture only) | 14 (78%) | 10 (76%) | 4 (80%) |
| Mixed histology (follicular + solid/trabecular) | 4 (22%) | 3 (23%) | 1 (20%) |
| Encapsulation | |||
| Complete encapsulation | 14 (78%) | 9 (69%) | 5 (100%) |
| Capsular invasion | 9/14 | 7/9 | 2/5 |
| No capsular invasion | 5/14 | 2/9 | 3/5 |
| Partial encapsulation | 2 (11%) | 2 (15%) | 0 |
| No encapsulation | 2 (11%) | 2 (15%) | 0 |
| Vascular invasion | |||
| Extensive vascular invasion (≥4 vessels) | 2 (11%) | 1 (8%) | 1 (20%) |
| Minimal vascular invasion (<4 vessels) | 4 (22%) | 3 (23%) | 1 (20%) |
| No vascular invasion | 12 (67%) | 9 (69%) | 3 (60%) |
| Mitotic activity | |||
| High | 0 | 0 | 0 |
| Low | 5 (28%) | 4 (31%) | 1 (20%) |
| None | 13 (72%) | 9 (69%) | 4 (80%) |
| ATA pediatric risk category | |||
| Low | 16 (89%) | 13 (100%) | 3 (60%) |
| Intermediate or high | 2 (11%) | 0 | 2 (40%) |
Abbreviation: TNM, tumor node metastasis.
AJCC Cancer Staging Manual, 7th edition (2010).
One patient had pulmonary metastases detected postoperatively on whole-body scan. This FVPTC was classified as NX because cervical lymph node dissection was not performed with thyroidectomy.
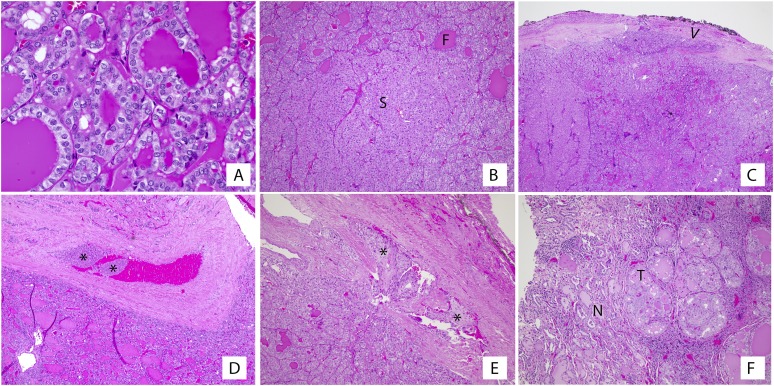
Visual depiction of representative histopathology characteristics from our patient cohort is shown in Fig. 2. None of the patients with unilateral disease had multifocal disease in the ipsilateral lobe on histopathologic review. Uniform histology was seen in most patients with both unilateral disease (10 of 13; 76%) and bilateral disease (four of five; 80%). The mixed histology cases were characterized by a follicular pattern with a solid component comprising <50% of tumor cellularity. All tumors exhibited low or no mitotic activity. Most patients had completely encapsulated (14 of 18; 78%) or partially encapsulated (two of 18; 11%) FVPTC. For those with complete encapsulation, capsule invasion was seen frequently (nine of 14; 64%), and vascular invasion was found in one-third of patients (six of 18; 33%). For two of these patients, vascular invasion was described as extensive (more than four vessels involved). Two of the six patients (33%) with vascular invasion had multifocal bilateral disease (PTMC), one with three vessels involved and the other with five vessels involved. One of these patients (described previously) had mediastinal lymph node and pulmonary metastases (M1). The other patient had evidence of ETE. Others with vascular invasion (four of six; 67%) ultimately had unifocal disease with no evidence of ETE or metastases.
Figure 2.
Histopathology characteristics of FVPTC. Tumors were composed of predominantly follicular architecture with the following histologic features: (A) nuclear features of PTC, such as clearing, membrane irregularity, overlap, and grooves, were present at least focally in all tumors; (B) foci of solid growth intermixed with the predominant follicular architecture; (C) evidence of tumor invasion into (arrowhead) or through the capsule; (D, E) foci of angioinvasion, with tumor present within vascular spaces of the tumor capsule (asterisks); and (F) unencapsulated tumors showed an invasive growth pattern with tumor intermingled with non-tumor parenchyma. F, follicular; N, non-tumor; S, solid; T, tumor.
Two patients, both of whom had vascular invasion, met criteria for pediatric ATA intermediate or high risk—one with pulmonary metastases (M1) and the other because of the presence of minimal ETE on histology, though there was no evidence of lymph node or distant metastases in the latter patient. All other patients (16 of 18; 89%) were classified as pediatric ATA low risk.
Follow-up
Sixteen patients (16 of 18; 89%) achieved both biochemical and anatomic remission, and all had continued evidence of remission after a median follow-up of 4.5 years (range, 1.5 to 6.6 years). Two of 18 patients (11%) achieved anatomic remission, but definitive biochemical remission was not achieved because of the presence of detectable Tg along with low-titer but stable antithyroglobulin antibodies.
Discussion
In keeping with current pediatric guidelines and historical standard of care, all 18 patients with FVPTC in our case cohort underwent total or completion thyroidectomy (8). Most patients had solitary, unilateral FVPTC with complete or partial encapsulation and no evidence of ETE or vascular invasion. None of the 13 patients who underwent prophylactic central neck lymph node dissection had lymph node metastases. One patient (6%) who had PTMC on pathology and persistent cervical lymph node disease on radioiodine whole-body scan had distant (pulmonary) metastases. On review, this patient’s tumor was characterized as mixed histology including focal solid PTC, multifocal capsular invasion, and vascular invasion. Overall, these findings are consistent with adult data showing low risk of invasive disease and metastases in patients with E-FVPTC (17, 31, 32).
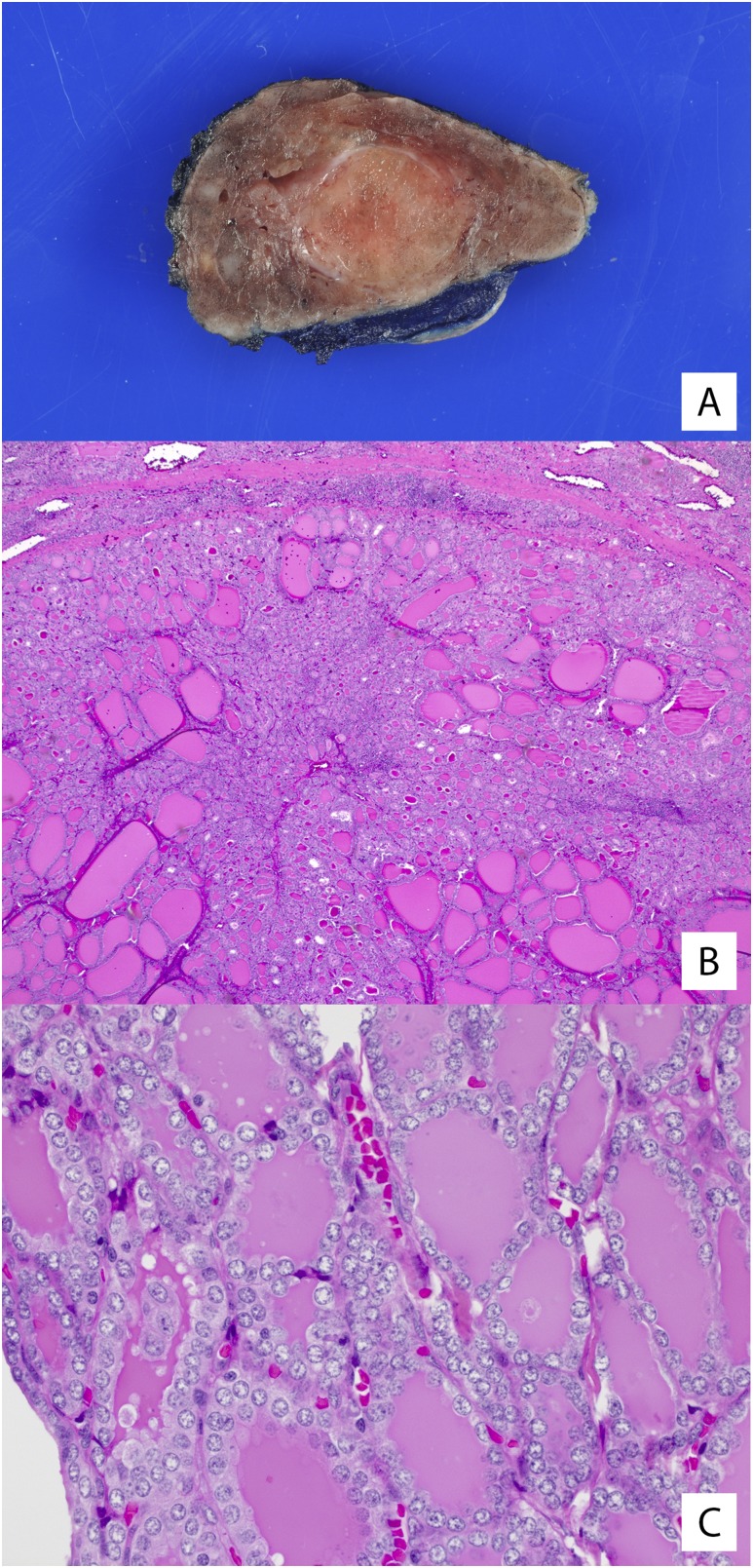
According to recent literature in the adult population, tumors classified as E-FVPTC without capsular or vascular invasion may be reclassified as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” when they meet strict histologic criteria (27, 33). This change in terminology, which aims to remove the designation of “carcinoma” from this noninvasive neoplasm, has not been validated in a pediatric population. If we apply the same strict criteria to our cohort of patients, two of 18 (11%) tumors would have met the criteria for reclassification to noninvasive follicular thyroid neoplasm with papillary-like nuclear features instead of FVPTC (Fig. 3).
Figure 3.
Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. (A, B) The two cases in our cohort with dominant nodules that met criteria for the diagnosis of neoplasm with papillary-like nuclear features showed completely encapsulated nodules with a thin, fibrous tumor capsule. The tumors had entirely follicular architecture with microfollicular growth. (C) Nuclear features of PTC, such as clearing, elongation, overlapping, and grooves, were present in both cases.
As with histologic and postoperative findings, our data from preoperative evaluation (US and FNA results) are generally consistent with those from adult studies of FVPTC. Previous studies in adults showed that FVPTC with ovoid to round shape, a smooth margin (anechoic or hypoechoic rim), and isoechoic echogenicity without evidence of ETE or lymph node metastasis is more likely to display indolent behavior (34). With the exception of margin, for which 40% of patients in our cohort demonstrated a smooth margin, the remaining US features in our cohort—including hyperechoic or isoechoic echogenicity, wider-than-tall shape, and absence of echogenic foci—were typical of adult FVPTC (35). In addition, none of our patients had abnormal cervical lymph nodes or evidence of ETE on US review. In general, FNA has not been reliable in identifying the subgroup of patients with FVPTC with invasion, as a major proportion of FVPTCs had indeterminate (AUS/FLUS or FN) cytology in the Bethesda classification system (35). Our experience was similar to that in previous reports, with 81% of tumors that underwent FNA being classified as either AUS/FLUS or FN.
On the basis of this limited case cohort, we suggest that surgical lobectomy could be considered in pediatric patients with thyroid nodules and the following criteria: (1) no history of radiation or familial tumor predisposition syndrome and (2) preoperative US showing a unifocal nodule with no evidence of gross ETE or lymph node metastases. After lobectomy, active surveillance rather than completion thyroidectomy may then be considered for patients with FVPTC meeting the following criteria: (1) ATA pediatric low risk (N0 or incidental microscopic N1a if a central neck lymph node dissection was performed); (2) unifocal disease; (3) complete or near-complete encapsulation; (4) absence of high mitotic activity; (5) no vascular invasion; (6) no capsular invasion; and (7) no ETE. Applying this suggested approach to the patients in this study would not have missed any cases of bilateral disease or metastases. This approach would also be consistent with care for adult patients for whom noninvasive, E-FVPTC is treated similarly to minimally invasive follicular thyroid carcinoma (17, 36, 37). Future prospective studies are needed to assess whether lobectomy with active surveillance rather than total thyroidectomy is sufficient to achieve remission in pediatric patients with FVPTC with low risk features.
Although our study was among the largest studies of pediatric FVPTC reported, it is limited by a small sample size of 18 patients. However, our rationale for the previously suggested algorithm is strengthened by two recent single-center studies. A retrospective review of 62 patients with pediatric DTC confirmed overall excellent prognosis after a median of 5 years’ follow-up and noted that all five patients in the sample with E-FVPTC lacking vascular invasion remained in remission; two of these patients had been treated with partial thyroidectomy (38). Another retrospective analysis defined a set of “very low‒risk” histopathologic criteria for pediatric PTC (39). Although this study did not limit the review to FVPTC cases, it included nine patients with FVPTC found to have low-risk features similar to those in our cohort.
The addition of molecular genotyping may provide additional information to stratify the surgical approach in patients with thyroid tumors that appear to have low-invasive potential on the basis of preoperative US imaging (40). In adult patients with FVPTC, the presence of a RAS or THADA mutation or a PPARG fusion correlates with E-FVPTC and lower invasive potential, whereas a BRAF mutation or RET-PTC fusion is associated with infiltrative FVPTC (27, 41). To date, similar data exploring the genotype-phenotype correlation in pediatric patients with FVPTC has not been published. This information may be useful in continuing efforts to stratify surgical management and help accurately guide clinical decision making for lobectomy vs completion/total thyroidectomy in pediatric patients with FVPTC.
In summary, our findings suggest that a selected subset of pediatric patients with FVPTC may be considered for lobectomy with surveillance rather than total thyroidectomy. As with recent efforts to limit RAI to pediatric patients with higher-risk PTC, stratification of surgical management may help decrease overly aggressive treatment of patients with indolent DTC. In particular, the associated risks of thyroidectomy, including recurrent laryngeal nerve palsy, hypoparathyroidism, and need for lifelong thyroid hormone replacement, would be eliminated or minimized in patients undergoing lobectomy. Future multicenter studies are needed to determine whether lobectomy suffices to achieve remission in this low-risk subset of pediatric patients diagnosed with FVPTC.
Acknowledgments
Financial Support: This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award TL1TR001880 (to S.L.S.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AJCC
American Joint Commission on Cancer
- ATA
American Thyroid Association
- AUS
atypia
- CHOP
Children’s Hospital of Philadelphia
- DTC
differentiated thyroid carcinoma
- E-FVPTC
encapsulated follicular variant of papillary thyroid carcinoma
- ETE
extrathyroidal extension
- FLUS
follicular lesion of undetermined significance
- FN
follicular neoplasm
- FNA
fine-needle aspiration
- FVPTC
follicular variant of papillary thyroid carcinoma
- PTC
papillary thyroid carcinoma
- PTMC
papillary thyroid microcarcinoma
- RAI
radioactive iodine
- Tg
thyroglobulin
- TI-RADS
Thyroid Imaging, Reporting, and Data System
- TSH
thyrotropin
- US
ultrasonography
References
- 1. Raval MV, Bentrem DJ, Stewart AK, Ko CY, Reynolds M. Utilization of total thyroidectomy for differentiated thyroid cancer in children. Ann Surg Oncol. 2010;17(10):2545–2553. [DOI] [PubMed] [Google Scholar]
- 2. Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164(6):1481–1485. [DOI] [PubMed] [Google Scholar]
- 3. Wu X, Groves FD, McLaughlin CC, Jemal A, Martin J, Chen VW. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control. 2005;16(3):309–320. [DOI] [PubMed] [Google Scholar]
- 4. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4):e945–e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, Yamashita S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243(4):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EK, Barletta J, Doubilet PM, Peters HE, Webb S, Modi BP, Paltiel HJ, Kozakewich H, Cibas ES, Moore FD Jr, Shamberger RC, Larsen PR, Huang SA. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98(8):3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halac I, Zimmerman D. Thyroid nodules and cancers in children. Endocrinol Metab Clin North Am. 2005;34(3):725–744, x. [DOI] [PubMed] [Google Scholar]
- 8. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force . Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol. 2008;20(1):59–65. [DOI] [PubMed] [Google Scholar]
- 10. Popovtzer A, Shpitzer T, Bahar G, Feinmesser R, Segal K. Thyroid cancer in children: management and outcome experience of a referral center. Otolaryngol Head Neck Surg. 2006;135(4):581–584. [DOI] [PubMed] [Google Scholar]
- 11. Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Francis GL. Extensive surgery improves recurrence-free survival for children and young patients with class I papillary thyroid carcinoma. J Pediatr Surg. 1999;34(12):1799–1804. [DOI] [PubMed] [Google Scholar]
- 12. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, Kukulska A, Prokurat A, Wygoda Z, Jarzab B. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007;48(6):879–888. [DOI] [PubMed] [Google Scholar]
- 13. Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34(6):1192–1202. [DOI] [PubMed] [Google Scholar]
- 14. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid. 2013;23(10):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu B, Ghossein R. Encapsulated thyroid carcinoma of follicular cell origin. Endocr Pathol. 2015;26(3):191–199. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–1264. [DOI] [PubMed] [Google Scholar]
- 18. Rosario PW, Penna GC, Calsolari MR. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: is lobectomy sufficient for tumours ≥1 cm? Clin Endocrinol (Oxf). 2014;81(4):630–632. [DOI] [PubMed] [Google Scholar]
- 19. Ganly I, Wang L, Tuttle RM, Katabi N, Ceballos GA, Harach HR, Ghossein R. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol. 2015;46(5):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta S, Ajise O, Dultz L, Wang B, Nonaka D, Ogilvie J, Heller KS, Patel KN. Follicular variant of papillary thyroid cancer: encapsulated, nonencapsulated, and diffuse: distinct biologic and clinical entities. Arch Otolaryngol Head Neck Surg. 2012;138(3):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schneider DF, Elfenbein D, Lloyd RV, Chen H, Sippel RS. Lymph node metastases do not impact survival in follicular variant papillary thyroid cancer. Ann Surg Oncol. 2015;22(1):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baloch ZW, LiVolsi VA. Encapsulated follicular variant of papillary thyroid carcinoma with bone metastases. Mod Pathol. 2000;13(8):861–865. [DOI] [PubMed] [Google Scholar]
- 23. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference . The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665. [DOI] [PubMed] [Google Scholar]
- 24. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–595. [DOI] [PubMed] [Google Scholar]
- 25. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92(9):3590–3594. [DOI] [PubMed] [Google Scholar]
- 26. DeLellis RA. Pathology and genetics of tumours of endocrine organs. Vol 8 Lyon, France: International Agency for Research on Cancer;; 2004. [Google Scholar]
- 27. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nosé V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosai J. Papillary thyroid carcinoma. In: Ricardo V, Lloyd RYO, Gunter Kloppel, Juan Rosai, eds. World Health Organization Classification of Tumours of Endocrine Organs. 4th ed Lyon, France: International Agency for Research on Cancer; 2017:81–91. [Google Scholar]
- 29. Jeon M, Kim YN, Sung TY, Hong SJ, Cho YY, Kim TY, Shong YK, Kim WB, Kim SW, Chung JH, Kim TH, Kim WG. Practical initial risk stratification based on lymph node metastases in pediatric and adolescent differentiated thyroid cancer [published online ahead of print January 13, 2018]. Thyroid. [DOI] [PubMed]
- 30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vivero M, Kraft S, Barletta JA. Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid. 2013;23(3):273–279. [DOI] [PubMed] [Google Scholar]
- 32. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ, Al-Kuraya K, Fugazzola L, Colombo C, Kebebew E, Jarzab B, Czarniecka A, Bendlova B, Sykorova V, Sobrinho-Simões M, Soares P, Shong YK, Kim TY, Cheng S, Asa SL, Viola D, Elisei R, Yip L, Mian C, Vianello F, Wang Y, Zhao S, Oler G, Cerutti JM, Puxeddu E, Qu S, Wei Q, Xu H, O’Neill CJ, Sywak MS, Clifton-Bligh R, Lam AK, Riesco-Eizaguirre G, Santisteban P, Yu H, Tallini G, Holt EH, Vasko V, Xing M. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016;101(1):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, Mandel SJ, Morris JC, Nassar A, Pacini F, Schlumberger M, Schuff K, Sherman SI, Somerset H, Sosa JA, Steward DL, Wartofsky L, Williams MD. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481–483. [DOI] [PubMed] [Google Scholar]
- 34. Rhee SJ, Hahn SY, Ko ES, Ryu JW, Ko EY, Shin JH. Follicular variant of papillary thyroid carcinoma: distinct biologic behavior based on ultrasonographic features. Thyroid. 2014;24(4):683–688. [DOI] [PubMed] [Google Scholar]
- 35. Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, Sohn CH, Choi YH. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009;28(12):1685–1692. [DOI] [PubMed] [Google Scholar]
- 36. Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern). Thyroid. 2009;19(2):119–127. [DOI] [PubMed] [Google Scholar]
- 37. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balachandar S, La Quaglia M, Tuttle RM, Heller G, Ghossein RA, Sklar CA. Pediatric differentiated thyroid carcinoma of follicular cell origin: prognostic significance of histologic subtypes. Thyroid. 2016;26(2):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kluijfhout WP, Pasternak JD, van der Kaay D, Vriens MR, Propst EJ, Wasserman JD. Is it time to reconsider lobectomy in low-risk paediatric thyroid cancer? Clin Endocrinol (Oxf). 2017;86(4):591–596. [DOI] [PubMed] [Google Scholar]
- 40. Hahn SY, Shin JH, Oh YL, Kim TH, Lim Y, Choi JS. Role of ultrasound in predicting tumor invasiveness in follicular variant of papillary thyroid carcinoma. Thyroid. 2017;27(9):1177–1184. [DOI] [PubMed] [Google Scholar]
- 41. Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]