Abstract
Trauma alters neuroendocrine responses to stress and increases vulnerability to stress-related disorders. Yet, relationships among trauma, stress-induced neural changes and hypothalamic–pituitary–adrenal (HPA) axis activity have not been determined. The present study used functional magnetic resonance imaging to investigate the impact of life trauma on basal cortisol levels and neural responses to acute stress in 73 healthy individuals during brief stress and neutral-relaxing imagery using a well-established, individualized imagery method. We hypothesized that trauma experience would have a negative impact on brain function, resulting in altered basal cortisol levels via dysregulated neural control over the HPA axis system. Results showed that higher life trauma exposure was significantly associated with lower basal cortisol levels. Neuroimaging results indicated that both higher life trauma and low morning cortisol levels were associated with increased response to acute stress in limbic-medial temporal lobe (MTL) regions including the amygdala and hippocampus. A mediation analysis showed that increased limbic-MTL response to stress mediated the relationship between life trauma and low cortisol levels. Findings revealed a significant impact of lifetime trauma on neural responses to acute stress and HPA axis activity. Life trauma may sensitize limbic-MTL regions and its related peripheral systems, which could compromise stress regulation and HPA axis function, and increase risk for negative stress-related health outcomes.
Keywords: trauma, stress, basal cortisol, limbic regions, medial temporal lobe, fMRI
Introduction
Lifetime trauma exposure has been shown to significantly impact physical and psychological well-being, increasing risk for stress-related disorders and negative health outcomes (Bevans, et al., 2008; McEwen, 2002; Sinha, 2008). Studies have identified the neurobiological underpinnings of trauma exposure; this line of studies has largely found altered function associated with trauma in two stress-related pathways, the hypothalamic–pituitary–adrenal (HPA) axis system (Fries, et al., 2005) and limbic brain regions (McEwen, 2001; Williams, et al., 2006).
More specifically, neuroendocrine studies have indicated that HPA axis response is impacted as a result of chronic stress exposure (McEwen, 2002; Sinha, 2008). For example, cortisol typically increases under stressful conditions. However, studies have indicated a negative association between basal cortisol levels and traumatic experience, indicative of a state of hypocortisolism, including in individuals with early trauma (Meinlschmidt and Heim, 2005), repeated trauma exposures (Bevans, et al., 2008; Kolassa, et al., 2007), and in those with various stress-related disorders (Fries, et al., 2005). Researchers have suggested that under chronic stress, a decrease in basal cortisol levels occurs due to overcompensation as accompanied by a highly sensitized HPA axis system (Edwards, et al., 2011). Additional evidence suggests that corticotropin-release-factor (CRF) down-regulation in the hypothalamus generates this response pattern via negative feedback inhibition resulting from excessive cortisol response during repeated stress (Heim, et al., 2000).
Previous neuroimaging studies have demonstrated the impact of prolonged stress and trauma on limbic brain function in regions such as the amygdala (Williams, et al., 2006) and hippocampus (McEwen, 2001). For example, a study incorporating both functional and structural imaging showed that childhood maltreatment was associated with increased amygdalar activity in response to threatening faces and reduced gray matter volumes in the hippocampus (Dannlowski, et al., 2012). This is also consistent with the results of preclinical studies. In rats, an uncontrollable stressor diminished GABA-stimulated chloride uptake in the amygdala (Martijena, et al., 2002), and chronic stress altered synaptic activity in the hippocampus (Karst and Joels, 2003).
These studies provide insights into the neurobiology underlying trauma experience. However, endocrine and neuroimaging studies have been conducted separately, and the impact of repeated trauma on HPA axis alterations and neural responses to stress has not yet been addressed. Recurring trauma exposure may adversely affect neural functioning (e.g., in limbic regions), thereby altering HPA axis function. For example, there is evidence that the amygdala modulates the HPA axis via the hypothalamus (Price, et al., 1987). This suggests that an altered limbic response (e.g., amygdala) might underlie the relationship between trauma and HPA axis activity.
Therefore, to thoroughly understand the neurobiology underlying trauma, the current study sought to identify the link between lifetime trauma exposure, HPA axis activity and neural responses to acute stress using functional magnetic resonance imaging (fMRI). We assessed basal morning cortisol levels and brain activity during acute stress via a well-established and validated, individualized script-driven guided imagery method involving stress vs. neutral-relaxing cues in 73 healthy individuals. Trauma exposure was measured using the Life Trauma subscale of the Cumulative Adversity Interview (CAI; adopted from (Turner, et al., 1995)), a widely used instrument which has been shown to predict psychophysical consequences in a variety of populations (see supplementary information). The fMRI procedure involved the use of a well-validated, individualized script method to provoke stressful and neutral-relaxing situations (see the published manual (Sinha and Tuit, 2012) and a review that summarizes previous evidence supporting the method in (Sinha, 2009)).
Based on prior research, we hypothesized that higher life trauma would be associated with lower basal fasting morning cortisol. We also predicted that greater life trauma and lower cortisol levels would correlate with increased neural activity during stress in limbic regions including the amygdala and hippocampus. Further we expected to find a negative association between hypothalamus activity and cortisol levels, given the crucial role of the hypothalamus in regulating HPA axis function (Edwards, et al., 2011). Finally, because HPA axis regulation at the level of the hypothalamus occurs via afferent limbic projections (Smith and Vale, 2006), we hypothesized that stress-induced limbic response would play a mediating role in the relationship between trauma and basal cortisol levels.
Material and Methods
Seventy-three healthy individuals (right-handed) between the ages of 18 and 50 participated in this study (see Table 1). Participants from the community were recruited via local newspaper advertisements and flyers posted for research participation. All participants completed assessments during 2–3 sessions pertaining to cognitive, demographic, and health status as well as HPA axis function prior to an fMRI scan. Exclusion criteria included history of head trauma, current or past substance use disorder, presence of any mental disorders (as determined by the Structured Clinical Interview for DSM-IV), use of any prescription medications at the time of fMRI testing, pregnancy, and claustrophobia. Women underwent the fMRI session only during the follicular or luteal phases of their menstrual cycle and not the ovulatory or pre-menstrual/menstrual phase in order to avoid any potential influence of steroid hormonal increases on the stress response. Assessment of menstrual cycle was based on self-report. Among 24 women, there were 13 women in the follicular phase (54%) and 11 women in the luteal phase (46%). Prior to study participation, all participants provided informed written consent. All study procedures were approved by the Human Investigation Committee at the Yale University School of Medicine.
Table 1.
Demographics, basal cortisol levels and life trauma scores
| Subject Variable | Total |
|---|---|
| N=73 | |
| Demographics | |
| Age | 27.5 (7.8) |
| Gender - % female | 24 (33%) |
| Race - % Caucasian | 52 (71%) |
| Education | 15.2 (2.1) |
| Body Mass Index (BMI) | 27 (5.0) |
| Morning Fasting Cortisol (ug/dL) | 14.8 (5.8) |
| Life Trauma scoresa | 4.6 (3.4) |
Note: Mean values (standard deviations) are denoted.
For gender and race, frequency (percents) are reported in parenthesis.
Life trauma scores from the Cumulative Adversity Interview (adopted from (Turner, et al., 1995))
Lifetime trauma
Lifetime trauma was measured using the life trauma subscale of the Cumulative Adversity Interview (adapted from (Turner, et al., 1995), a 140- item interview that addresses stressful life experiences and has been well-validated in predicting psychiatric disorders and health conditions (see Supplementary Information). The life trauma scale comprises 34 items and primarily measures traumatic life experience. Trained interviewers inquired about the occurrence and frequency of traumatic events during participants’ lifetime, as previously described. Examples of items comprising the life trauma scale included exposure to violence, witnessing or experiencing serious accident, death or injury, personal trauma involving force or coercion and physical, emotional and /or sexual abuse or assault. Witnessed violence encompasses items that involve being present in dangerous or distressing situations, such as seeing someone get shot or attacked with a weapon. In addition, some items are related to traumatic news involving someone important being killed, injured or abused.
Hormonal evaluation
The current study collected basal morning cortisol under fasting conditions, which is regarded as a reliable biological marker of HPA axis pathology (e.g., (Hagg, et al., 1987)). Cortisol samples were collected and analyzed as described previously (Chao, et al., 2017). On a separate day prior to the fMRI session, participants were asked to visit the Yale Stress Center, for an early morning laboratory session at 7:30 am after fasting overnight. Four repeated samples of cortisol were obtained at 15-minutes intervals for an hour. Cortisol samples were collected in heparinized plasma collection tubes, centrifuged at 4°C within 30 minutes of drawing, aliquoted, and subsequently stored at −80°C. Then, radio-immunoassays for cortisol were conducted by Yale Center for Clinical Investigation Core Laboratories. Basal cortisol was calculated as the mean plasma cortisol value taken from the repeated measurements over the hour. An MRI session was conducted within a week of this laboratory session.
Individualized imagery method
We used a brief guided imagery of individualized stress and neutral-relaxing situations (Sinha and Tuit, 2012; Sinha, 2009), which is a well-validated method in laboratory and neuroimaging studies using emotion and stress induction (see Supplementary Information). Before the fMRI session, imagery scripts were developed for each participant based on the participant’s accounts of two stressful and two neutral-relaxing experiences that occurred in the past year using the standardized Scene Construction Questionnaires (Sinha, 2009). The stress scripts were developed based on participants’ description of a non-traumatic stressful experience that occurred in the past year (e.g., losing a job, family discontent, or relationship troubles). More specifically, they were developed based on participants’ description of a stressful experience that made them sad, mad, and upset in a manner that could not be changed in the moment (e.g., losing a job, family discontent, or relationship troubles). The participants rated the intensity of the distress situations on a 10-point Likert scale (1 = not at all stressful and 10 = most stressful), and only events rated 8 or above were considered for inclusion in the script. In doing so, we were able to control for the self-reported stressfulness of the situation across subjects. This stress provocation method has been shown to have predictive validity of clinical and health symptoms (see Supplementary Information). Participants were also asked to describe experiences evoking neutral-relaxing scenarios (e.g., reading a book or relaxing on the beach) for the development of the neutral-relaxing scripts. These neutral-relaxing scripts were used to compare an individuals’ stress imagery and response to their own non-stress imagery control. Due to the individualized nature of the script, script content was specific to each participant’s experience. However, the script format, style, and length were standardized across conditions and subjects, as detailed in previously published guidelines (Sinha, 2009).
A trained master’s level research associate was audiotaped reading the scripts which were each 2.5 minutes in length and had a tone and valence matching the content of the script. The pre-recorded scripts were then audio played to participants during the fMRI session while they had their eyes closed. The order of scripts was presented in the counterbalanced manner. In order to ensure equal ability to evoke mental images during the imagery process, participants underwent standardized imagery and relaxation training, as described previously (Sinha, 2009). Sample scripts are presented in Table S3 (see Supplementary Information).
fMRI task and acquisition
Each fMRI trial lasted 5 minutes, consisting of a 1.5-minute baseline period followed by a 2.5-minute imagery period (2 minutes of read imagery and 0.5 minute of silent imagery recall) and a 1-minute quiet recovery period. There were two trials per condition (2 stress, 2 neutral) for each participant, and the order of script presentation (stress or neutral) was counterbalanced across subjects. Each script was presented only once without the same condition presented consecutively. Participants were instructed to rate their anxiety prior to and after each trial based on how anxious or nervous they felt on a 10-point Likert scale (1 = not at all and 10 = extremely high). Following each imagery trial, participants rated the vividness of imagery on a 10-point Likert scale (1=cannot visualize the image and 10=extremely clear, ‘as if’ it were happening right now). Heart rate was measured throughout the task using a pulse oximeter placed on the non-dominant forefinger. In between each trial, participants listened to a 2- minute progressive relaxation recording to eliminate any residual effects of previous trials.
MRI data were collected via a 3-T Siemens Trio MRI system with a standard quadrature head coil using a T2*-sensitive gradient-recalled single-shot echo-planar pulse sequence. Anatomical MRI data were collected with spin echo imaging in the axial plane parallel to the AC-PC line (TR=300 msec, TE=2.5 msec, bandwidth=300 Hz/pixel, flip angle=60 degrees, field of view=220×220 mm, matrix=256×256) and 32 slices with slice thickness=4mm with no gap. Functional MRI data were acquired with a single-shot gradient echo planar imaging sequence with thirty-two axial slices parallel to the AC-PC line covering the whole brain (TR=2,000 msec, TE=25 msec, bandwidth=2004 Hz/pixel, flip angle=85 degrees, field of view=220×220 mm, matrix=64×64, 32 slices with slice thickness=4mm and no gap). Sagittal anatomical images were obtained for multi-subject registration using a high-resolution 3D Magnetization Prepared Rapid Gradient Echo sequence (TR=2530 ms; echo time (TE)=3.34 ms; bandwidth=180 Hz/pixel; flip angle (FA)=7°; slice thickness=1mm; field of view=256×256 mm; matrix=256×256).
fMRI analysis
Functional MRI data were converted from Digital Imaging and Communication in Medicine format to Analyze format using XMedCon (Nolfe, 2003). The first 10 images were discarded from each functional run to reach a steady-state equilibrium between radio-frequency pulsing and relaxation. Due to potential carryover effects from the imagery period, the 1-minute recovery period was eliminated from data analysis. fMRI data were preprocessed using MATLAB and Statistical Parametric Mapping (SPM5) and slice time and motion corrected for three translational and three rotational directions. Any trial with linear motion >1.5 mm and a rotation larger than 2° was removed.
For each trial per condition, a general linear model (GLM) was used for individual-level analysis with a regressor comparing time during imagery to baseline (stress–baseline and neutral–baseline) using BioImageSuite (www.bioimagesuite.org., (Duncan, et al., 2004)). Drift correction was also applied in the GLM, such that drift regressors were utilized to remove the mean time course, linear, quadratic, and cubic trends for each run. Each trial was spatially smoothed using a 6-mm Gaussian kernel and normalized to generate β-maps (3.44 mm × 3.44 mm × 4 mm). To account for individual anatomical differences, three sequential registrations were applied to the individual normalized β-maps using BioImageSuite (Duncan, et al., 2004): (1) linear registration between the individual subjects’ functional image to the T1 structural image (within subject), (2) linear registration between the T1 structural image and the 3D MPRAGE image (1 × 1 × 1 mm), and (3) non-linear registration to a reference 3D image. The reference image was the Colin27 Brain (Holmes, et al., 1998), a high-definition anatomical image registered to the Montreal Neurological Institute space.
For group level analysis, a t-test comparing stress and neutral conditions was conducted using BioImageSuite. In addition, to examine the associations between life trauma, basal cortisol levels, and brain activity, whole-brain correlational analyses were conducted using BioImageSuite. To correct for multiple comparisons, cluster-wise correction of family-wise errors was implemented for all analyses. The cluster size was determined by AFNI’s 3dClustSim program (https://afni.nimh.nih.gov (Cox, 1996), version 16.0.09) using Monte Carlo simulation (Xiong, et al., 1995). To best identify specific neural correlates and be appropriately conservative, thresholds were determined based on the type of data analysis using whole brain correction for multiple comparisons; a threshold of 0.05 was used for whole-brain correlation analysis and 0.01 was used for fMRI task results.
Functional connectivity:
To examine connectivity patterns between the hypothalamus (a priori region) and other brain regions, a functional connectivity analysis was implemented using BioImageSuite. Using the inverse transform from the GLM, a reference region was inversely transformed into individual subject space. The time-course of the transformed reference region was calculated for each subject as the average time-course across all pixels within the reference region. Then, the time-course was correlated with the time-courses of all the other voxels in the brain using a whole brain voxel-wise Pearson correlation, fisher transformed to z-values, averaged across runs and then spatially smoothed with a 6mm Gaussian filter.
Mediation analysis
To examine whether brain activity mediates the relationship between trauma scores and basal cortisol levels, we conducted mediation analyses. We tested a, b, c and c′ pathways using the ordinary least squares (OLS) for trauma scores (independent variable) with brain correlates as the mediating variable and basal cortisol levels as the dependent variable. For the mediated effect (a x b) of brain activity in associations between trauma scores and basal cortisol levels, we employed the approach via the SPSS Process macro (Hayes, 2013). Bootstrapping was used to estimate the significance of the indirect effects, as indirect effects do not meet normality assumptions. Bias corrected and accelerated 95% confidence intervals (CI) of the mediated effects were generated using 10,000 bootstrapped re-samples for each indirect effect point estimate. CIs that do not include a zero value indicate a significant indirect effect, as the effect is significantly different from zero at p<0.05 (two-tailed). For consistency with the literature, unstandardized OLS coefficients are reported (MacKinnon and Dwyer, 1993).
Results
Demographics and baseline information
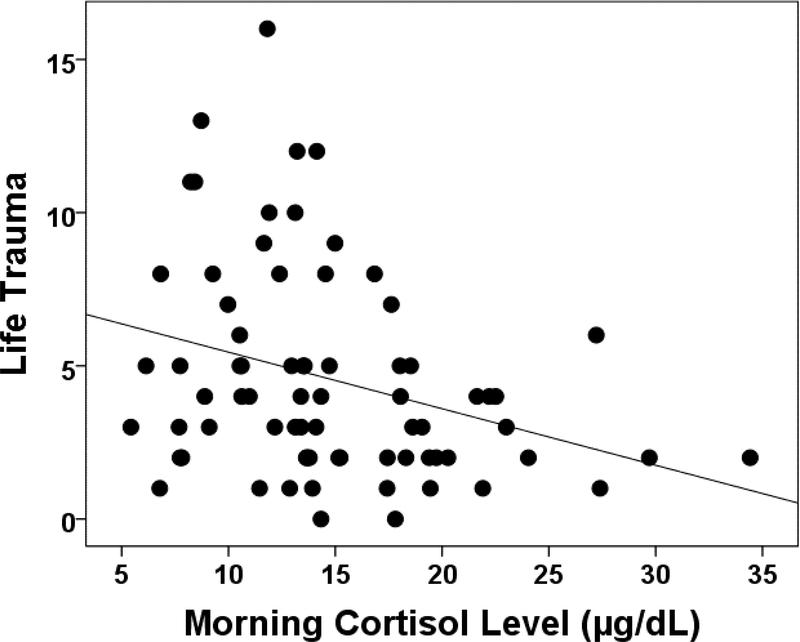
Table 1 presents the demographic characteristics, basal cortisol levels and life trauma scores of all 73 participants. Life trauma scores were negatively correlated with fasting morning cortisol levels in our sample, such that higher levels of trauma were associated with lower cortisol levels (r = −.31, p=0.007; see Figure 1).
Figure 1.
The relationship between life trauma and basal cortisol level. Higher life trauma scores were associated with decreased basal morning cortisol levels (r = −.31, p = 0.007).
Stress versus neutral imagery task effects
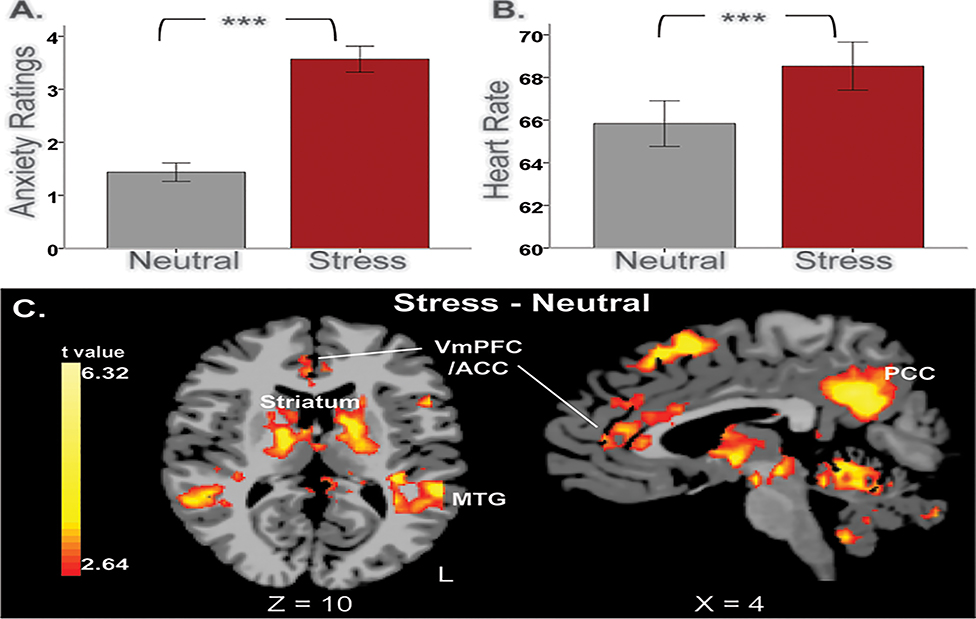
Task-related effects were evident in ratings, heart rate, and brain activity, such that stress exposure elicited greater response in anxiety ratings and heart rate, as well as increased activity in the cortico-striatal regions compared to the neutral condition (Figure 2).
Figure 2.
Task-related effects. During stress exposure, (A) anxiety ratings and (B) heart rate response were significantly increased compared to the neutral condition. *** p < 0.0001. (C) Task-related activity during stress exposure relative to the neutral condition. A whole-brain voxel-based analysis showed that activity in cortico-striatal regions was significantly increased during stress relative to the neutral condition. These areas included the medial prefrontal cortex (PFC), anterior/posterior cingulate cortex (ACC/PCC), superior/middle temporal gyrus, precuneus, striatum, thalamus, mid-brain, and cerebellum (p<0.01, whole-brain FWE corrected). VmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; MTG = middle temporal gyrus; L, left. Coordinates are given in Montreal Neurological Institute (MNI) space.
Ratings and Heart Rate:
Task-related significance in ratings and heart rate response were tested using t-test to examine the effects of stress induction. Anxiety ratings in the stress imagery condition (M = 3.6, SD = 2.1) were significantly elevated compared to those in the neutral-relaxing imagery condition (M = 1.4, SD = 1.5) (t = 9.7, p < 0.0001) (see Figure 2-A). Heart rate response was greater for the stress condition (M=68.5, SD=9.6) than the neutral-relaxing condition (M=65.8, SD=9.1) (t=5.2, p<0.0001) (see Figure 2-B). The means of vividness ratings were 8.2 (1.2) for the stress condition and 7.9 (1.3) for the neutral condition with higher ratings found in the stress compared to the neutral condition (t=2.1, p<0.05).
Task-related brain activity:
The results of the t-test comparing stress vs. neutral conditions showed significant task effects in brain regions including the medial PFC, precuneus, anterior and posterior cingulate cortex (ACC/PCC), left lateral prefrontal cortex (LPFC), left anterior insula, superior/middle temporal gyrus, thalamus, striatum, right amygdala, mid-brain and cerebellum (p<0.01, whole-brain FWE corrected; see Figure 2C). Brain activity in these areas was greater in the stress condition than in the neutral condition. There was no region that was more active in the neutral condition compared to the stress condition.
Correlates of life trauma and fMRI responses to stress
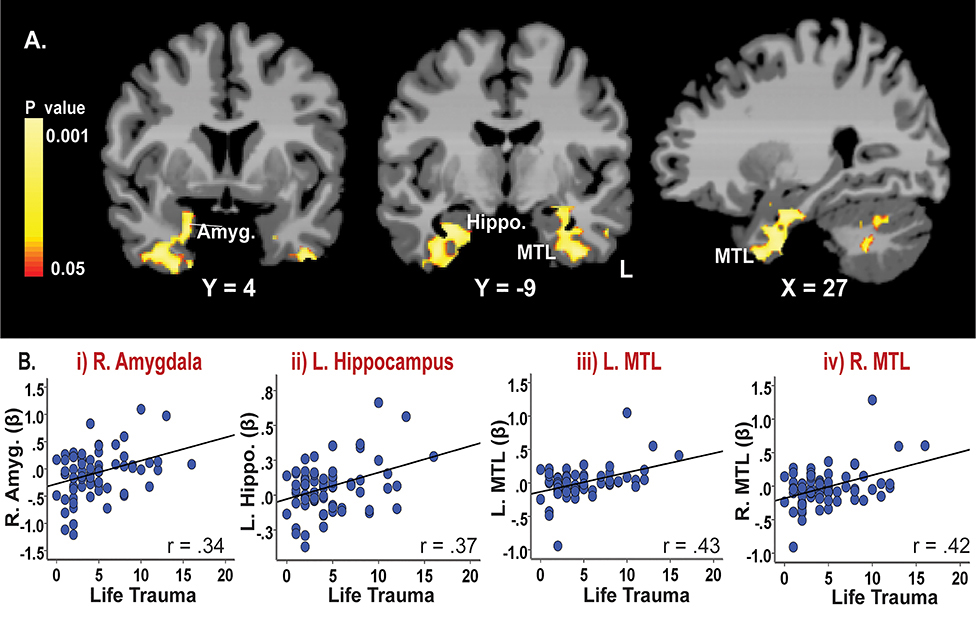
To understand the associations between lifetime trauma scores and brain activity, a whole-brain correlation analysis was conducted separately for the stress and neutral conditions (See Figure 3, Table S1). There was no brain response during the neutral-relaxing condition that was significantly correlated with life trauma. During the stress condition (stress-baseline), the results showed that high life trauma scores were significantly associated with increased neural responses to stress in limbic-medial temporal lobe (MTL) regions including right amygdala, bilateral hippocampus, MTL, and parts of brain stem and cerebellum (p<0.05, whole-brain FWE corrected). Figure 3 shows (A) the whole-brain correlation map with life trauma and (B) corresponding scatterplots showing no outliers in associations with the i) amygdala, ii) hippocampus, iii) left medial temporal lobe and iv) right medial temporal lobe. A whole-brain correlation result between life trauma and brain activity during the Stress-Neutral contrast is presented in the supplemental information (Figure S1). The results show similar findings to Figure 3, suggesting that the stress condition drives the observed effects.
Figure 3.
Neural correlates of lifetime trauma during stress exposure (Stress-Baseline). Results of a whole brain correlation analysis showed that (A) life trauma scores were positively correlated with neural response to stress in limbic-medial temporal regions including the right amygdala, hippocampus, medial temporal lobe and parts of the brain stem/cerebellum (p<0.05, whole-brain corrected). Yellow/red colors = positive correlations. Amyg = amygdala; Hippo. = hippocampus; MTL = medial temporal lobe, L= left. MNI coordinates were used. (B) No outliers were found in associations between life trauma and brain activity, as illustrated by the scatterplots demonstrating correlated patterns in the i) R. amygdala, ii) L. hippocampus, iii) L. medial temporal lobe and iv) R. medial temporal lobe.
Correlates of basal cortisol levels and fMRI response to stress
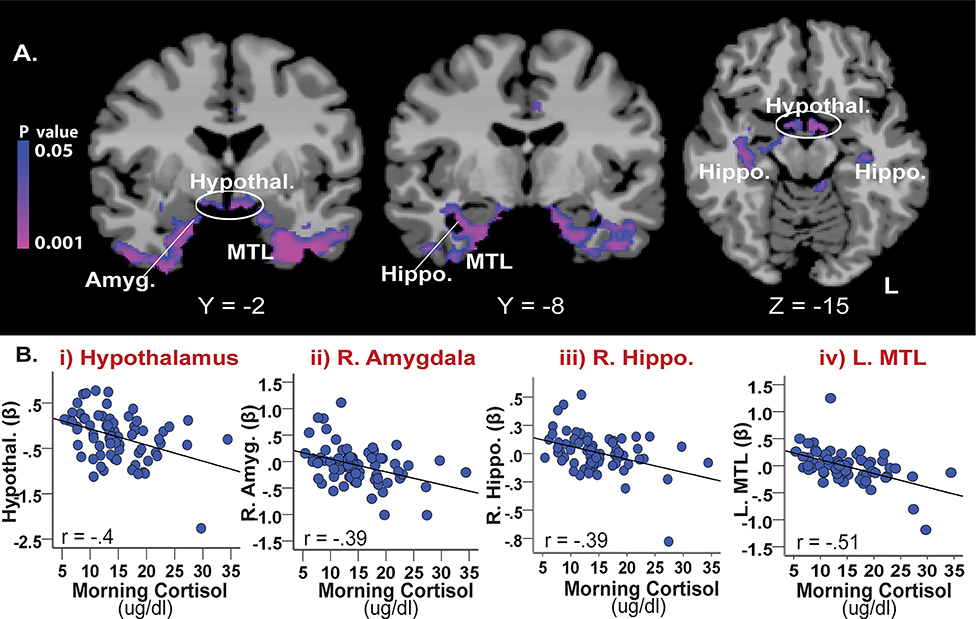
Whole-brain correlation results with basal cortisol levels showed significant negative correlations with brain activity during stress (stress-baseline; see Figure 4, Table S2). The associated regions showed a substantial overlap with regions found in neural correlates of life trauma (described above), which were localized in limbic-MTL regions including the amygdala, hippocampus, and medial temporal lobe and parts of the brain stem and cerebellum. In addition, increased stress-induced activity in the hypothalamus, mid-cingulate gyrus, and precuneus was associated with lower basal cortisol levels (p<0.05, whole-brain FWE corrected). Scatterplots show no outliers in these associations (Figure 4B).
Figure 4.
The association between basal cortisol and stress-related neural activity (Stress-Baseline). (A) Results of a whole brain correlation analysis showed that basal cortisol levels were negatively correlated with stress-related activity in limbic-MTL regions including the amygdala, hippocampus, and medial temporal lobe as well as the hypothalamus (whole-brain FWE corrected, p <0.05). (B) The scatterplots show negative correlated patterns with no outliers between basal cortisol levels and stress-induced activity in the i) hypothalamus, ii) R. amygdala, iii) R. hippocampus, and iv) L. medial temporal lobe. Amyg. = amygdala; Hypothal. = hypothalamus; Hippo. = hippocampus, MTL = medial temporal lobe; R=right; L=left. Blue/purple colors = negative correlations.
Functional connectivity with the hypothalamus
Based on substantial literature indicating the critical role of the hypothalamus in regulating the HPA axis function (e.g., (Sinha, 2008; Smith and Vale, 2006)), hypothalamus activity was defined as a priori region to further examine the relationship between the hypothalamus and other brain regions during stress. To understand a functionally connected network influencing hypothalamus activity associated with low basal cortisol levels, a reference region of the hypothalamus was functionally defined from a cortisol-correlation map (Figure 4). Then a connectivity analysis was conducted with the hypothalamus as a seed region using BioImageSuite. The results showed that the hypothalamus has increased connectivity with the amygdala, ventral striatum and the medial orbitofrontal cortex (See Figure S2; p< 0.001, whole-brain corrected).
Mediation analysis
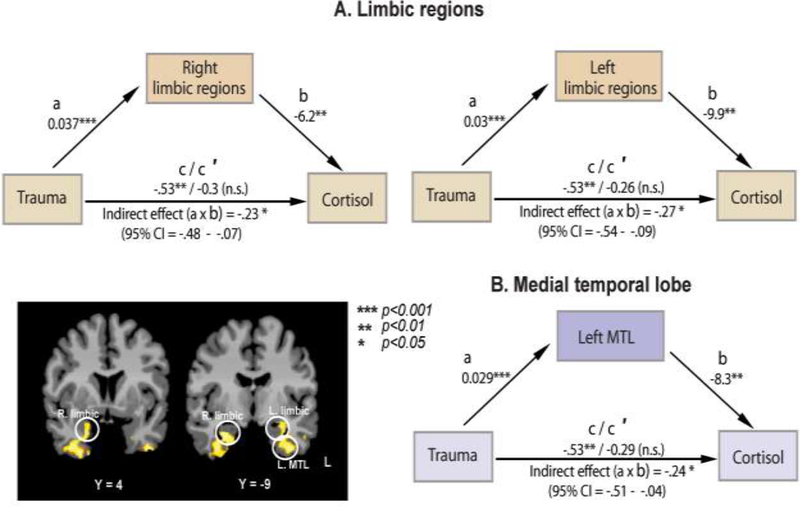
The mediation analysis was conducted to examine whether neural correlates of life trauma (limbic-MTL regions; Figure 3) mediates the relationship between trauma and basal cortisol. The results showed that stress-induced activity in the bilateral limbic and left MTL regions mediated the relationship between trauma scores and basal cortisol levels (see Figure 5, for description of a, b, c, and c′ pathways). The right MTL was not shown to mediate the relationship. Results showed that trauma scores were positively associated with brain activity during stress in bilateral limbic (left, t= 4.3, β =.03, p=.0001; right, t=3.95, β =.037, p<.001) and left MTL (t=3.99, β=.029, p<.001) regions. The effects of the mediators, stress-induced activity in limbic (left, t= −2.9, β= −.9.9, p<.01; right, t= −2.7, β= −6.2, p<.01) and left MTL regions (t=−2.8, β=−8.3, p<.01), on cortisol levels were also significant, after controlling for trauma scores. The effect of trauma on cortisol levels was significant (t=−2.8, β=−.53, p<.01). However, this effect disappeared when the mediators, bilateral limbic (left, t=−1.3, β=−.26, n.s.; right, t=−1.5, β=−.3, n.s.) or left MTL activity (t=−1.4, β= −.29, n.s.), were included in the model. These results indicate that the effect of trauma on basal cortisol levels were mediated by stress-induced activity in limbic-left MTL regions. In addition, the mediation effect of trauma on basal cortisol levels via brain activity was significant for limbic (left, axb=−.27, (95%CI=−.54, −.09); right, axb=−.23 (95%CI= −.48, −.07)) and left MTL (axb= −.24, (95%CI= −.51, −.04)) regions, further confirming statistical mediation effects of brain activity (Figure 5).
Figure 5.
A path diagram showing the mediating effect of brain activity during stress on the relation between trauma and basal cortisol levels. Mediator(s) = beta(s) in limbic-MTL regions during stress (Stress-Baseline); a = Effect of trauma on the mediator; b = Effect of the mediator on basal cortisol levels, controlling for trauma score; c = Effect of the trauma on cortisol levels without brain activity in the model; c′ = Direct effect of trauma on cortisol levels with the effects of brain activity controlled; a x b = Indirect effects of trauma on cortisol levels via the effects of bilateral limbic and left MTL activity; CI = Confidence Interval. The effects of trauma on cortisol levels are not significant when controlling for brain activity, suggesting significant mediating effects of stress-induced limbic-left MTL activity on the relationship between trauma and basal cortisol levels. Note: * p<0.05, **p <.01, *** p<0.001, n.s.=not significant.
Secondary analyses to assess imagery vividness and sex differences
Secondary analyses were conducted to examine the effect of imagery vividness on the associations between basal cortisol, trauma scores, and brain response to stress. The results of these analyses indicated that the main findings were not altered when imagery vividness was statistically controlled, suggesting that individual differences in imagery vividness did not contribute to the observed results. In terms of sex differences, no significant differences were found in our main findings including baseline cortisol levels, trauma scores, and correlations of life trauma and cortisol levels with task-related brain response displayed in Figure 3 and 4. Furthermore, among 24 women, there were no differences between those with follicular vs. luteal phases in our main findings including basal cortisol, trauma scores, and their associations with brain response. This may be due to a small number of women included in our study or indicate that transient menstrual cycle may not affect our findings based on chronic, repeated trauma and associated neurobiological characteristics.
Discussion
The present study examined relationships among life trauma, HPA axis activity, and brain response to stress in healthy community individuals. Our findings showed that greater trauma experience was associated with lower morning cortisol levels, both of which were associated with increased limbic-MTL responses to stress. Decreased cortisol levels were additionally associated with an enhanced response to stress in the hypothalamus, a modulator of the HPA axis system (Smith and Vale, 2006). Functional connectivity was found between the hypothalamus and limbic regions. In addition, a subsequent mediation analysis showed that limbic-MTL activity mediated the relationship between trauma and basal cortisol levels. These findings suggest that repeated trauma exposure sensitizes limbic-MTL regions and their functionally connected network, which may negatively impact the HPA axis system.
Trauma and basal cortisol levels
Our results showed an inverse relationship between life trauma and basal morning cortisol levels. This is consistent with previous studies demonstrating associations between life trauma and reduced basal morning cortisol levels (Bevans, et al., 2008). Another study found decreased baseline saliva cortisol levels in trauma-exposed individuals in the absence of psychopathology (Klaassens, et al., 2010). Our findings along with these studies support the idea that reduced basal cortisol, or a so called state of hypocortisolism (Fries, et al., 2005), is indicative of altered HPA axis activity in individuals with traumatic experiences. It should be noted that we found no sex differences in baseline morning cortisol levels. While it is widely accepted that there are sex differences in HPA axis reactivity, especially in the cortisol response following a stressor (Handa, et al., 1994), there has been some discrepancy in reports of sex differences in baseline morning cortisol levels. Our results are consistent with studies demonstrating similar null findings of sex differences in baseline cortisol levels (e.g., (Barra, et al., 2015)).
A major role of the HPA axis system is to modulate adaptive physiological responses and maintain homeostasis via the regulation of cortisol release (Smith and Vale, 2006). Stress exposure generates corticotropin-releasing factor (CRF) in the hypothalamus which activates the stress response system, initiating the release of cortisol (McEwen, 1998). Prolonged and repeated exposure to high amounts of stress results in an increased ‘allostatic load’, which has deleterious effects on HPA axis function (McEwen, 2002) resulting in long-term biochemical changes leading to decreased basal cortisol response (Clow, et al., 2010). Further, altered cortisol response has been regarded as a symptomatic marker reflecting poor stress and emotion regulation (Compton, et al., 2013), especially in people who have experienced trauma. Together, our findings of low basal cortisol associated with trauma may be indicative of altered HPA-axis activity and associated stress dysregulation in individuals with trauma experiences.
Life trauma and neural response to acute stress
A significant association between life trauma and brain response to acute stress was mainly found in limbic-MTL regions and a portion of the brain stem and cerebellum. Limbic regions including the amygdala and hippocampus have been implicated in emotional processing, acute stress (Roozendaal, et al., 2009), aversive learning (LeDoux, 2000), and trauma exposure (Williams, et al., 2006). Heightened responses in the amygdala and hippocampus were also found in individuals with high cumulative adversity (Seo, et al., 2014), suggesting a crucial role of these regions in adaptive stress responses. In terms of emotional reactivity, the amygdala has been associated with negative emotion, fear, and enhanced vigilance during acute stress (Davis and Whalen, 2001). Specifically, the right amygdala is known to be more involved in aversive aspects of emotional processing (Morris, et al., 1999). Thus, increased activity in the right amygdala, as demonstrated in our study, may be indicative of emotional distress and sensitized limbic function associated with life trauma. The MTL plays a crucial role in emotional memory and consolidation (Dolcos, et al., 2005). The hippocampus is a limbic region located in MTL structure that is critically involved in memory encoding and storage. The hippocampus is known to be vulnerable to damage from chronic stress (McEwen, 2001), and compromised MTL function was also found following stress induction via cortisone administration (de Quervain, et al., 2003).
The limbic and MTL regions are closely associated with each other and play a key role in stress adaptation, as the amygdala interacts with the MTL memory system during stress (Dolcos, et al., 2005; Roozendaal, et al., 2009). It is known that learning and memory is negatively impacted under stress in both quantitative and qualitative ways via stress pathways including the amygdala (Schwabe, et al., 2010). These studies along with our results suggest that stressful and traumatic events may sensitize limbic-MTL regions, which might compromise their cognitive and emotional adaptive functions and result in maladaptive coping with stress.
Neural circuits of basal cortisol
Similar patterns of stress-induced sensitization were observed in brain regions associated with basal cortisol levels. That is, lowered cortisol levels were correlated with greater stress-induced activity in limbic-MTL regions along with the hypothalamus. The association between HPA axis activity and limbic brain regions has been well documented in prior studies showing associations of cortisol levels with amygdala activity during emotion regulation (Urry, et al., 2006), amygdala metabolism (Drevets, et al., 2002) as well as the role of the hippocampus in HPA axis function during stress (Mizoguchi, et al., 2003). It has been suggested that interactions between limbic and HPA axis activity play a crucial role in mediating stress-related adaptive behaviors (Cunningham-Bussel, et al., 2009). Specifically, repeated stress exposure increases vulnerability to allostatic load by adversely impacting limbic-HPA axis regulation of cortisol that is required for stress-related adaptation (McEwen, 2002).
In addition to limbic regions, lowered morning cortisol levels were associated with an increased response to stress in the hypothalamus. In the brain, the paraventricular nucleus of the hypothalamus produces corticotropin-releasing hormone (CRH) that has regulatory control over cortisol release in the adrenal cortex (Dickerson and Kemeny, 2004). It follows that the association between the hypothalamus and low basal cortisol levels observed in our study may reflect disrupted hypothalamic control over HPA axis functioning resulting from trauma-related sensitization.
Limbic mediation between trauma and basal cortisol
Limbic-MTL response to stress was associated with both life trauma and lower basal cortisol levels, suggesting that this circuit may play a mediating role. Consistent with this, a subsequent analysis showed that increased limbic-MTL response to stress mediated the relationship between trauma and low basal cortisol levels. These results suggest that trauma may sensitize limbic-MTL response to stress, which could further compromise HPA axis functions governed by the hypothalamus.
It should be noted that our study did not find direct associations between life trauma and hypothalamic activity, a modulator of the HPA axis system (Smith and Vale, 2006). One possible explanation is that trauma may indirectly impact the hypothalamus via other brain regions, such as the amygdala. There is evidence that the amygdala is involved in the regulation of the hypothalamus and governs adaptive HPA axis response to stress (Herman, et al., 2005). Corroborating this, our connectivity analysis results showed that hypothalamus activity during stress exhibited increased functional connectivity with the medial amygdala. The medial amygdala is known to relay sensory information to the hypothalamus (Keshavarzi, et al., 2014), suggesting the influence of the amygdala on HPA axis activity via the hypothalamus. Consistent with this, we showed that the amygdala mediated the relationship between trauma and decreased cortisol levels. This is in line with findings of preclinical literature. The central nucleus of the amygdala innervates the paraventricular nucleus of the hypothalamus, which allows amygdalar modulation of HPA axis response to stress (Gray, et al., 1989).
In addition to limbic activity, other mechanisms involving more specific pathways within the limbic system may also have contributed to this mediation. For example, repeated stress exposure increases CRF and γ-aminobutyric acid (GABA) release in the amygdala, followed by increased CRF secretion in the paraventricular nucleus (Cook, 2004). Over the long term, hypothalamic CRF increase results in low basal cortisol levels (Fries et al., 2005) via negative feedback inhibition that decreases adrenocorticotropic hormone (ACTH) response to CRF stimulation by down-regulating CRF receptors in the pituitary gland (Edwards, et al., 2011; Heim, et al., 2000). These studies suggest that the amygdalar mediation of the relationship between trauma and low basal cortisol in our study may reflect trauma-related, sensitized pathways (e.g., CRF) between the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus leading to alteration in the HPA axis system. This evidence emphasizes the need to further investigate more specific mechanisms underlying the role of limbic-HPA axis system in trauma-related sensitization.
Conclusion and clinical implications
To summarize, the current study identifies neural correlates of life trauma and associated HPA axis function localized in limbic-MTL regions. It is notable that there is a substantial overlap in brain regions associated with both life trauma and basal cortisol levels. To our knowledge, this is the first study demonstrating similar neural circuits underlying trauma and HPA axis disruption. In particular, the current study shows that the limbic system plays a crucial role in mediating the relationship between life trauma and low cortisol levels, potentially via functional connectivity with the hypothalamus. These results suggest that repeated life trauma may sensitize limbic-MTL circuits and its connected network (e.g., hypothalamus), resulting in compromised HPA axis function in the periphery.
It should be noted that our findings of trauma-related limbic sensitization were observed in healthy community individuals. Similar patterns of brain sensitization were also found in psychiatric samples, such as limbic dysfunction observed in patients with post-traumatic stress disorder (Francati, et al., 2007). These patterns of brain sensitization in healthy individuals may reflect prodromal patterns or susceptibility of these individuals to stress-related clinical disorders. Such a chronic state may lead to increased neurobiological sensitivity to stress, thereby adversely affecting adaptive responses required for regulating high levels of stress. Over time, these alterations may continue to impair adaptive physiological functioning and increase vulnerability to stress-related disorders. However, due to the nature of our sample, caution should be taken when generalizing our findings, and further studies are needed to verify these findings in clinical samples.
Despite the significance of our findings for trauma-related vulnerability, it is important to explore alternative interpretations of our results. Due to the retrospective and self-reported nature of life trauma measurements, it is plausible that individuals with higher limbic-MTL activation may recall past traumas more vividly, resulting in greater self-reports of life trauma experience. However, since vividness ratings were not associated with trauma scores, nor with activity in limbic-MTL regions, it is unlikely that the vividness of stress imagery was a contributing factor in our main findings. Additionally, while life trauma events are reported retrospectively, the imaging data are not based on traumatic situations but rather non-traumatic stressful situations that were reported during script development. Therefore, it is unlikely that an individual’s ability to recall past traumas can explain the observed increases in limbic-MTL activation.
In a similar vein, our study focused only on traumatic life stressors as opposed to more chronic stressors such as neglect or poverty. Therefore, it is unclear whether our results generalize to other types of life stressors. Future studies could address this by exploring how different types of trauma influence HPA axis functioning and brain response to stress.
An additional limitation to our study is the use of morning basal cortisol in interpreting HPA dysfunction related to trauma. We did not collect cortisol samples during the day, nor was it collected during the neuroimaging protocol. While the decision to collect only morning basal cortisol was based on previous research demonstrating its reliability as biological marker of HPA axis condition (e.g., (Hagg, et al., 1987)), it is unclear if diurnal changes in cortisol would affect our results. In order to develop a more complete picture of the effect that trauma has on HPA axis function, future studies should utilize cortisol levels at fixed times over a 24-hour period and during stress challenges. Another direction of research may also involve examining neural correlates of concurrent cortisol response to acute stress, along with morning cortisol levels. To achieve this, research using multimodal neuroimaging techniques (e.g., simultaneous collection of brain and cortisol response to acute stress) is necessary to further clarify neural substrates underlying brain and HPA axis response to acute stress in individuals with trauma. Taken together, future studies should continue to explore whether the limbic-MTL circuit could serve as a neural marker of lifetime trauma and associated HPA disruption, and a target for developing appropriate prevention and treatment strategies.
Supplementary Material
Highlights.
Higher life trauma exposure was significantly associated with lower basal cortisol levels.
Hyperactive response to stress in limbic-medial temporal lobe (MTL) regions was associated with both higher lifetime trauma scores and lower basal cortisol levels.
Hyperactive response to stress in limbic-MTL regions mediated the relationship between trauma and low basal cortisol levels.
Acknowledgement
This research was supported by grants from the National Institutes of Health (R01-AA013892–10; R01–AA20504–0; K08-AA023545–04), Peter F. McManus Foundation Grant, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest pertaining to the aims and results of this study.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barra CB, Silva IN, Rodrigues TM, Santos JL, Colosimo EA (2015) Morning serum Basal cortisol levels are affected by age and pubertal maturation in school-aged children and adolescents. Horm Res Paediatr, 83:55–61. [DOI] [PubMed] [Google Scholar]
- Bevans K, Cerbone A, Overstreet S (2008) Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Dev Psychopathol, 20:257–72. [DOI] [PubMed] [Google Scholar]
- Chao AM, Jastreboff AM, White MA, Grilo CM, Sinha R (2017) Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity (Silver Spring), 25:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L (2010) The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews, 35:97–103. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Hofheimer J, Kazinka R (2013) Stress regulation and cognitive control: evidence relating cortisol reactivity and neural responses to errors. Cogn Affect Behav Neurosci, 13:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CJ (2004) Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav, 82:751–62. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29:162–73. [DOI] [PubMed] [Google Scholar]
- Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, Weisholtz DS, Pavony M, Silverman ME, Goldstein MS, Altemus M, Cloitre M, LeDoux J, McEwen B, Stern E, Silbersweig D (2009) Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology, 34:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry, 71:286–93. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001) The amygdala: vigilance and emotion. Mol Psychiatry, 6:13–34. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C (2003) Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci, 17:1296–302. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME (2004) Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin, 130:355–91. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R (2005) Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A, 102:2626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME (2002) Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav, 71:431–47. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH (2004) Geometric strategies for neuroanatomic analysis from MRI. Neuroimage, 23 Suppl 1:S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LD, Heyman AH, Swidan S (2011) Hypocorticolism: An Evidence-based Review. Integrative Medicine, 10:26–33. [Google Scholar]
- Francati V, Vermetten E, Bremner JD (2007) Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depression and Anxiety, 24:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH (2005) A new view on hypocortisolism. Psychoneuroendocrinology, 30:1010–6. [DOI] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ (1989) Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology, 50:433–46. [DOI] [PubMed] [Google Scholar]
- Hagg E, Asplund K, Lithner F (1987) Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf), 26:221–6. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav, 28:464–76. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis, First Edition: A Regression-Based Approach New York: The Guilford Press. [Google Scholar]
- Heim C, Ehlert U, Helhammer DH (2000) The potential role of hypocorticolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25:1–35. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry, 29:1201–13. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr, 22:324–33. [DOI] [PubMed] [Google Scholar]
- Karst H, Joels M (2003) Effect of chronic stress on synaptic currents in rat hippocampal dentate gyrus neurons. J Neurophysiol, 89:625–33. [DOI] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RKP, Ianno DJ, Sah P (2014) Functional Properties and Projections of Neurons in the Medial Amygdala. The Journal of Neuroscience, 34:8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassens ER, Giltay EJ, van Veen T, Veen G, Zitman FG (2010) Trauma exposure in relation to basal salivary cortisol and the hormone response to the dexamethasone/CRH test in male railway employees without lifetime psychopathology. Psychoneuroendocrinology, 35:878–86. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Eckart C, Ruf M, Neuner F, de Quervain DJ, Elbert T (2007) Lack of cortisol response in patients with posttraumatic stress disorder (PTSD) undergoing a diagnostic interview. BMC Psychiatry, 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci, 23:155–84. [DOI] [PubMed] [Google Scholar]
- MacKinnon D, Dwyer J (1993) Estimating mediating effects in prevention studies. Eval. Rev, 17:144–158. [Google Scholar]
- Martijena ID, Rodriguez Manzanares PA, Lacerra C, Molina VA (2002) Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse, 45:86–94. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998) Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840:33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2001) Plasticity of the Hippocampus: Adaptation to Chronic Stress and Allostatic Load. Annals of the New York Academy of Sciences, 933:265–277. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2002) Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging, 23:921–39. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C (2005) Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology, 30:568–76. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T (2003) Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience, 119:887–97. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999) A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci U S A, 96:1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolfe E (2003) XMedCon- An open-source medical image conversion toolkit. European journal of nuclear medicine, 30 (Supp.2). [Google Scholar]
- Price JL, Russchen FT, Amaral DG (1987) The Limbic Region. II. The amygdaloid complex In: Bjorkland A, T.H.L.W.S., editor. Handbook of Chemical Neuroanatomy: Integrated Systems of the CNS, Part I. Amsterdam: Elsevier. [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci, 10:423–433. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS (2010) Memory formation under stress: quantity and quality. Neurosci Biobehav Rev, 34:584–91. [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R (2014) Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology, 39:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K, Tuit K (2012) Imagery Script Development Procedures Manual. New Haven, CT: CreateSpace Independent Publishing Platform; 122 p. [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci, 1141:105–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2009) Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol, 14:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R, Wheaton B, Lloyd D (1995) The Epidemiology of Social Stress American Sociological Review 60:104–125 [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ (2006) Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci, 26:4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA (2006) Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage, 29:347–57. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J-H, Lancaster J, PT F (1995) Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping, 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.