Abstract
Dopaminergic medications improve the motor symptoms of Parkinson’s disease (PD), but their effect on response inhibition, a critical executive function, remains unclear. Previous studies primarily enrolled patients in more advanced stages of PD, when dopaminergic medication loses efficacy, and patients were typically on multiple medications. Here, we recruited 21 patients in early-stage PD on levodopa monotherapy and 37 age-matched controls to perform the stop-signal task during functional magnetic resonance imaging. In contrast to previous studies reporting null effects in more advanced PD, levodopa significantly improved response inhibition performance in our sample. No significant group differences were found in brain activations to pure motor inhibition or error processing (stop success vs. error trials). However, relative to controls, the PD group showed weaker striatal activations to salient events (infrequent vs. frequent events: stop vs. go trials) and fronto-striatal task-residual functional connectivity; both were restored with levodopa. Thus, levodopa appears to improve an important executive function in early-stage PD via enhanced salient signal processing, shedding new light on the role of dopaminergic signaling in response inhibition.
Keywords: Cognitive control, Executive function, Dopamine, Stop-signal task, Movement disorders, Basal ganglia
1. Introduction
Parkinson’s disease (PD), a neurodegenerative disorder characterized by severe loss of dopamine in midbrain-striato-thalamocortical circuits, presents with a host of motor and cognitive deficits. Dopamine-replacement therapies improve parkinsonian motor symptoms, but their effects on cognition vary. The variable findings cast doubt on what specific cognitive deficits arise primarily from dopamine loss or instead from other forms of neuropathology (Robbins and Cools, 2014). In particular, the dopaminergic basis of one key cognitive deficit has been repeatedly questioned: response inhibition, the ability to suppress unwanted, habitual behavioral responses (Rae et al., 2016; Ye et al., 2014b). Response inhibition represents a critical therapeutic target in PD, as deficits are thought to be a key factor in freezing of gait (e.g., Shine et al., 2013), and potentially a stronger predictor of future development of dementia than semantic fluency or visual perception (Pedersen et al., 2013).
Although it is well established that individuals with PD consistently display behavioral response inhibition deficits (Gauggel et al., 2004; Kehagia et al., 2014; Ye et al., 2014b), the few studies that directly examined individuals both “on” and “off” dopamine-replacement therapies implied a lack of medication-related improvement (Alegre et al., 2013; Campbell et al., 2008; George et al., 2013; Obeso et al., 2011). Yet a wealth of data in healthy adults implicates a key role of dopamine in response inhibition. For instance, cortical dopamine release (Albrecht et al., 2014), striatal dopamine D1- and D2-receptor availability (Ghahremani et al., 2012; Robertson et al., 2015), and genetic polymorphisms of the dopamine system are strongly associated with individual differences in response inhibition performance and corresponding brain activation during go/no-go and stop-signal tasks (SSTs) (Congdon et al., 2008, 2009; Kasparbauer et al., 2015a,b).
Because the substantial evidence supporting a role of dopamine in response inhibition contradicts the mixed findings in PD, the effects of dopaminergic medication in PD require a closer examination. We recently conducted a meta-analysis on response inhibition studies of PD (Manza et al., 2017) and found that although dopaminergic drugs appear to benefit response inhibition performance in early-stage PD, this effect is missing in advanced PD, ostensibly because there are few remaining dopaminergic cells for the drugs to operate on (Bravi et al., 1994). Furthermore, individuals in previous studies often were taking a mix of medications including dopaminergic agonists, which have different mechanisms of action on the fronto-striatal circuitry and are associated with the development of impulse control disorders (Weintraub et al., 2010). Therefore, we sought to address these concerns by studying individuals in the early stages of PD (Hoehn & Yahr stages I & II) on levodopa monotherapy, using the SSTs during functional magnetic resonance imaging (fMRI).
Previous studies have demonstrated that successful response inhibition performance is dependent on a network of brain regions including right inferior frontal gyrus (IFG), preesupplementary motor area (preSMA), and striatum (Duann et al., 2009). Dopaminergic drugs alter activation in these regions during response inhibition in healthy populations (Kasparbauer et al., 2015b; Nandam et al., 2014), and individuals with PD show deficient stop-related activation and connectivity between these regions (Vriend et al., 2014; Ye et al., 2014a). Although other medications, including atomoxetine and citalopram (noradrenaline and serotonin reuptake inhibitors), have shown some promise in improving response inhibition performance and brain activation/connectivity in mid-stage PD (Ye et al., 2014a,b, 2016), to date, it remains unclear if dopaminergic medications would have similar effects in early-stage PD. We hypothesized that levodopa would influence response inhibition performance and brain activation/connectivity during the SSTs in early-stage PD.
2. Materials and methods
2.1. Participants
Twenty-one individuals (6 females, mean age of 61.9 ± 11.2 years) diagnosed with PD participated in the study. This cohort participated in the experiment after a minimum 12-hour washout of PD medications (“off” medication). Thirty-seven healthy age-matched controls (16 females, mean age of 62.5 ± 8.2 years) participated for 1 session. All patients were free from major medical illnesses other than PD and were diagnosed by a practicing neurologist. They were on a stable dosage of oral carbidopa-levodopa, with no other PD drugs or drugs for cognitive impairment, except 11 participants who were also taking 1 mg/d of the mild monoamine oxidase B inhibitor Azilect. Based on self-report, none had impulse control disorder or hallucinations. One patient demonstrated accuracy on go trials, which was less than 3 SDs from the mean, and another self-reported symptoms of depression (Geriatric Depression Score = 21). These 2 patients were included and indicated by different colors in the plots (individual with self-reported depression shown in brown and individual with low accuracy shown in black in Figs. 1C and 3A, right panel), and additional analyses were conducted without these participants to confirm that the main findings were not changed. Written informed consent was obtained from all participants, and the study was performed under protocols approved by the Stony Brook Human Investigation Review Board.
Fig. 1.
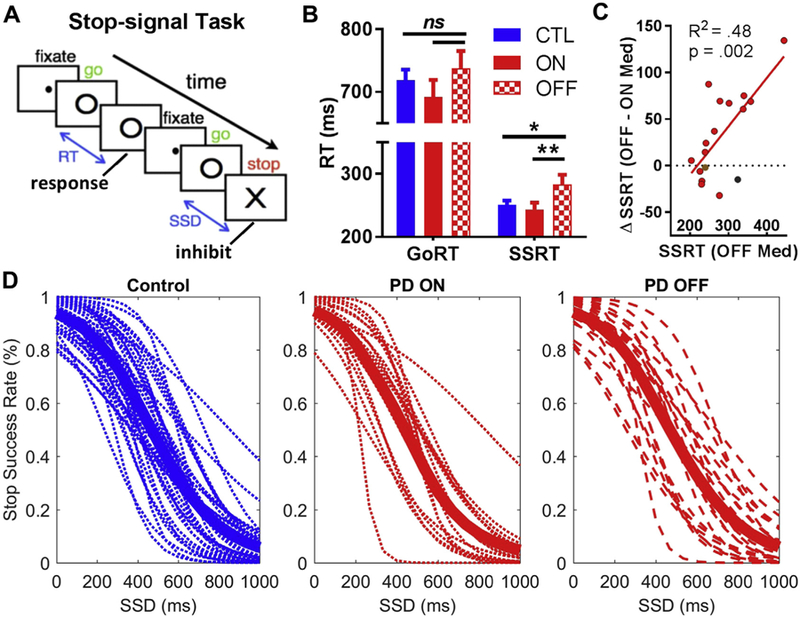
Stop-signal task and behavioral results. (A) A go stimulus “O” appears on each trial, and on 25% of the trials, a stop signal “X” appears shortly after the go signal to instruct participants to withhold the planned response. The SSD, or the time between the go stimulus and the stop signal on a stop trial, is varied based on a staircase procedure. (B) Response time measures on the stop-signal task for all participants. Participants with PD showed significantly longer SSRT in their “off” state (red hatch) compared to their “on” state (red) and the control group (blue), indicating a response inhibition deficit, whereas differences in GoRT were not significant. (C) Greater benefits in response inhibition (i.e., reduction in SSRT from “off” to “on” status) were positively correlated with SSRT “off” medication. The individual with self-reported depression (brown dot) and the individual with low go accuracy (black dot) are shown. (D) Inhibition function for each group, demonstrating that all 3 groups did not violate the basic assumption of the “race model”: as the SSD increases, stop success rate decreases or error rate increases. The thick line in each plot represents the group mean. Note: *, p < 0.05; **, p < 0.01; ns, not significant. Error bars indicate standard error of the mean. Abbreviations: GoRT, go reaction time; PD, Parkinson’s disease; SSD, stop-signal delay; SSRT, stop-signal reaction time. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
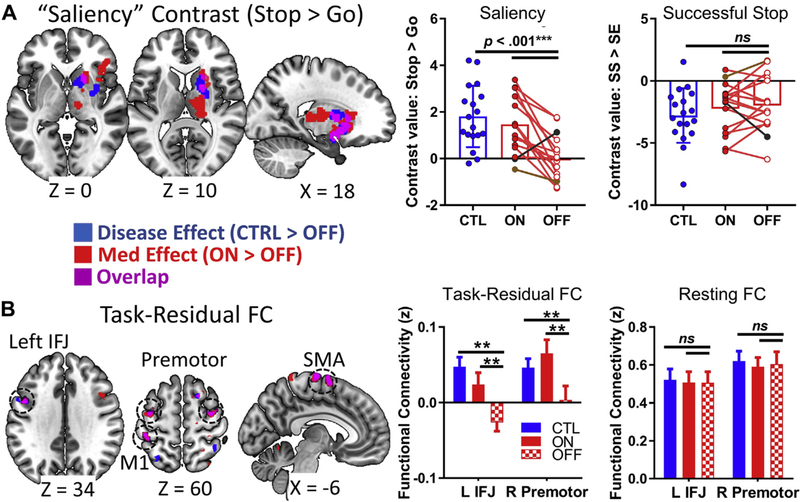
Functional magnetic resonance imaging results: Group differences for SST contrasts and functional connectivity (FC), showing disease effects (controls > PD “off”, shown in blue), medication effects (PD “on” > PD “off”, shown in red), and their overlap (shown in purple). (A) Left: Significant disease and medication effects are shown in the thalamus, striatum, and right insula/inferior frontal gyrus for the saliency contrast, including an overlapping region in the right striatum (purple). Right: Post hoc analysis showing that the PD group had lower right striatum saliency-related activations than when on levodopa or compared to controls; however, there were no consistent group differences in this region between successful versus failed stopping. The individual with self-reported depression (brown dot) and the individual with low go accuracy (black dot) are indicated. (B) Left: In exploratory task-residual functional connectivity analysis using the right striatum region from part (A) as a seed region, there were overlapping disease and medication effects in L IFJ, M1, SMA, and bilateral premotor cortices. Right: Post hoc analysis for 2 example regions (L IFJ and R premotor) showing group differences in task-residual functional connectivity, but not resting-state functional connectivity. Task contrast results have a cluster-forming threshold of p < 0.005, with a nonparametric p < 0.05 FWE cluster correction, using SnPM13. Functional connectivity results were not significant at this nonparametric cluster-level threshold, and so only the conjunction is analyzed at an exploratory threshold of p < 0.0025 uncorrected, with a minimum cluster size k > 20. **p < 0.01; ***p < 0.001. Abbreviations: CTL, control; FWE, family-wise error; IFG, inferior frontal junction; L, left; M1, primary motor cortex; PD, Parkinson’s disease; R, right; SMA, supplementary motor area. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
For fMRI data, 4 of the 21 patients and 19 of the 37 controls completed the experiment on a different scanner with a different MR protocol. Thus, the final sample for fMRI analysis included 17 individuals with PD (6 females, mean age of 61.0 ± 11.9 years) “on” and “off” medications (order of “on” and “off” sessions was counterbalanced), and 18 healthy controls (9 females, mean age of 65.3 ± 7.7 years). Table 1 shows the PD fMRI cohort demographics and clinical characteristics, including PD medication status. The most common non-PD medications were for cholesterol/blood pressure (3 controls and 5 patients) and for thyroid conditions (3 controls and 2 patients). In addition, 1 control and 2 patients were taking an antidepressant.
Table 1.
Demographics and clinical characteristics for the PD cohort included in the fMRI analysis (n = 17)
| Participant number |
Age | Sex | Years of Education |
Hand | Years since diagnosis |
H&Y | Med | LEDD | GDS | STAI | BIS-11 | MoCA | TMT A (on) |
TMT A (off) |
TMT B (on) |
TMT B (off) |
UPDRS III (on) |
UPDR SIII (off) |
Last dose (on) |
Last dose (off) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 16 | R | 2 | 2 | Lev | 338.4 | 21 | 47 | 75 | 29 | 29 | 30 | 48 | 80 | 24 | 25 | 2.5 | 13 |
| 2 | 68 | M | 18 | R | 1 | 1.5 | Lev+A | 325.6 | 2 | 55 | 63 | 30 | 20 | 28 | 36 | 27 | 15 | 17 | 2 | 16 |
| 3 | 67 | F | 21 | R | 0.2 | 1.5 | Lev | 451.1 | 9 | 52 | 63 | 29 | 23 | 31 | 44 | 64 | 14 | 20 | 1 | 17 |
| 4 | 58 | M | 13 | R | 5 | 2 | Lev+A | 889.5 | 4 | 52 | 68 | 28 | 33 | 47 | 87 | 99 | 21 | 29 | 0.5 | 17 |
| 5 | 79 | M | 16 | R | 2 | 1.5 | Lev | 563.9 | 12 | 51 | 73 | 24 | 40 | 52 | 100 | 115 | 16 | 25 | 4 | 12 |
| 6 | 51 | M | 14 | L | 1 | 1 | Lev+A | 663.9 | 4 | 53 | 57 | 29 | 20 | 26 | 32 | 36 | 10 | 15 | 3 | 15 |
| 7 | 45 | M | 16 | R | 0.3 | 1 | Lev+A | 212.8 | 0 | 48 | 63 | 28 | 25 | 26 | 68 | 77 | 10 | 16 | 4 | 18 |
| 8 | 69 | M | 12 | L | 1 | 1 | Lev+A | 325.6 | 4 | 53 | 63 | 25 | 22 | 24 | 39 | 45 | 7 | 13 | 2 | 16 |
| 9 | 68 | M | 20 | L | 2 | 1 | Lev+A | 438.4 | 1 | 52 | 61 | 28 | 39 | 51 | 56 | 85 | 7 | 13 | 1 | 26 |
| 10 | 49 | M | 14 | R | 8 | 2 | Lev+A | 325.6 | 8 | 49 | 81 | 28 | 18 | 25 | 31 | 32 | 22 | 29 | 3 | 26 |
| 11 | 49 | F | 13 | R | 0.25 | 1 | Lev | 225.6 | 6 | 54 | 56 | 28 | 16 | 19 | 40 | 39 | 15 | 18 | 2 | 15 |
| 12 | 80 | F | 21 | R | 6 | 1.5 | Lev | 771.4 | 5 | 52 | 64 | 29 | 36 | 49 | 80 | 87 | 14 | 26 | 1 | 14 |
| 13 | 73 | F | 18 | R | 5 | 2 | Lev+A | 401.4 | 5 | 52 | 67 | 25 | 35 | 48 | 63 | 63 | 14 | 27 | 1 | 16 |
| 14 | 65 | M | 18 | R | 6 | 1 | Lev+A | 425.6 | 5 | 52 | 60 | 26 | 29 | 32 | 73 | 134 | 9 | 14 | 2 | 14 |
| 15 | 48 | M | 16 | R | 4 | 2 | Lev+A | 551.1 | 0 | 47 | 43 | 27 | 22 | 23 | 74 | 47 | 15 | 22 | 1.5 | 15 |
| 16 | 52 | M | 14 | R | 4 | 2 | Lev | 789.5 | 5 | 50 | 62 | 25 | 50 | 31 | 49 | 47 | 21 | 25 | 4 | 16 |
| 17 | 54 | F | 18 | R | 1 | 1 | Lev+A | 400.8 | 1 | 52 | 40 | 30 | 23 | 27 | 51 | 41 | 9 | 16 | 0.5 | 15 |
| PD mean (SD) | 61.1 (11.2) | 11 M,6 F | 16.4 (2.8) | 14 R,3 L | 2.9 (2.4) | 1.5 (0.4) | 476.5 (200.0) | 5.4 (5.1) | 51.2 (2.3) | 62.3 (10.1) | 27.5 (1.9) | 28.2 (9.3) | 33.5 (11.1) | 57.1 (20.4) | 65.8 (31.0) | 14.3 (5.3) | 20.6(5.7) | 2.1 (1.2) | 16.5 (3.9) | |
| CTRLmean(SD) | 65.3 (7.7) | 9 M, 9 F | 15.1 (3.0) | 17 R,1 L | 3.6 (3.9) | 51.4 (3.1) | 57.1 (8.2) | 27.9 (1.3) | 28.9 (10.1) | 64.2 (21.1) |
The last 2 rows show average (mean) and standard deviation (SD) in parentheses for this PD group and the matched control group (n = 18).
Key: BIS-11, Behavioral Inhibition Scale-11; CTRL, control; F, female; fMRI, functional magnetic resonance imaging; GDS, Geriatric Depression Scale; Hand, handedness; H&Y, Hoehn & Yahr; L, left; Last dose, time (in hours) since the last dose of medication; LEDD, levodopa-dose equivalency; Lev, carbidopa-levodopa monotherapy; Lev+A = carbidopa-levodopa and Azilect; M, male; Med, medication profile; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; R, right; STAI, State-Trait Anxiety Inventory (trait score only used here); TMT, Trail Making Test.
2.2. Behavioral task
During fMRI, participants performed the SSTs (Fig. 1A; Logan et al., 1984; Manza et al., 2016a), after a meta-analysis indicated this may be the most sensitive measure for identifying response inhibition deficits in PD, relative to antisaccades, go/no-go, and Stroop tasks (Manza et al., 2017). There were 2 randomly intermixed trial types: go and stop. On “go” trials, a small dot first appeared on the screen signaling the beginning of a trial. After a variable time interval (foreperiod, with a random uniform distribution of 1‒5 seconds), the dot turned into a circle (the go signal), prompting the participant to press a button. The circle vanished with the button press or after 1 second had elapsed, whichever came first, and the trial terminated. A premature button press before the appearance of the circle also terminated the trial. On “stop” trials, an X appeared shortly after the circle and replaced the go signal. The participants were told to withhold their button press on seeing the X, the stop signal. The stop-signal delay (SSD)—the time interval between the go and stop signal—started at 200 ms and varied according to a staircase procedure, increasing or decreasing by 67 ms, respectively, after a successful or failed stop trial (De Jong et al., 1990; Levitt, 1971). The intertrial interval was 2 seconds. Approximately, three-quarters of all trials were go trials and one-quarter were stop trials. Participants were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up occasionally. Participants were not explicitly informed about the ratio of go/stop trials or the SSD staircase procedure. Each participant completed four 10-minute runs of the task during fMRI. Depending on the foreperiod duration and speed of response, the total number of trials varied slightly across participants (mean 283 = 16 ± go trials and 95 ± 10 stop trials). Despite the slight variation in the number of stop trials between participants, our average of 95 stop trials is well above the suggestion of 50 stop trials needed for a stable stop-signal reaction time (SSRT) estimation (Band et al., 2003; Verbruggen and Logan, 2009). With the staircase procedure, participants successfully withhold their response in approximately half of the stop trials. The SSRT of each participant was computed by subtracting the critical SSD (i.e., the estimated SSD required to get half of stop trials correct) from the median go reaction time (GoRT) (Li et al., 2008a; Logan et al., 1984). We used this “maximum likelihood method” so that results were comparable to our previous studies. However, to confirm that the primary findings were not due to inflation of the SSRT using the maximum likelihood method, we also computed SSRT using the integration method (see Verbruggen et al., 2013 for details).
2.3. Imaging protocol and preprocessing of image data
Brain images were acquired using a 3-T Siemens Prisma (64-channel coil). Whole-brain high-resolution T1 anatomical scan was conducted (176 sagittal slices, repetition time = 2400 ms, echo time = 2.24 ms, flip angle = 8°, field of view = 256 × 256 mm, Matrix = 320 × 320, and 0.8 mm3 isotropic voxels). Functional MRI scans were obtained using an echo-planar imaging (EPI) sequence (52 axial-oblique slices, multiband factor = 4, repetition time = 1000 ms, echo time = 33 ms, flip angle = 52°, field of view = 210 × 210 mm, matrix = 84 × 84, and 2.5 mm3 isotropic voxels with no gap; 600 image volumes in each session and 4 task sessions). An additional 10-minute resting-state session was collected a minimum of 10 minutes after completion of the SSTs (identical parameters to the task fMRI sequence), to reduce carryover effects.
Data were analyzed with Statistical Parametric Mapping (SPM12; Wellcome Department of Imaging Neuroscience, University College London, UK). There were no significant group differences in head motion in any of the 4 task scans or in the resting state scan, as assessed by framewise displacement and root mean square variance of % signal intensity (data available upon request; for details of motion calculation, see Section 2.5). Nevertheless, we further minimized sources of motion and physiological noise in the functional images by regressing out signals from the white matter using aCompCor, a principle component analysis‒based cleaning method (Behzadi et al., 2007). The first 10 images of each session were discarded for signal steady-state equilibrium. Functional images were corrected for slice timing and realigned (motion-corrected) to the mean EPI image. Images were then distortion-corrected with FMRIB Software Library v5.0’s “topup” program (Andersson et al., 2003; Smith et al., 2004) by unwarping a pair of spin-echo field maps with reversed phase-encode blips and applying the transformation to all EPI images. A mean functional image volume was constructed for each participant for each run from the distortion-corrected, realigned image volumes and coregistered with the high-resolution structural image, which was segmented. The resulting structural segments were matched to the PD25 EPI Montreal Neurological Institute atlas (Xiao et al., 2015) with affine registration followed by nonlinear transformation. Finally, images were smoothed with 6 mm at full width at half maximum.
2.4. Generalized linear models
Our goal was to identify the effects of Parkinson’s disease and the effects of dopaminergic medications on brain activation, as measured using SST contrasts for response inhibition. There were 4 basic types of SST trial outcomes: go success, go error, stop success, and stop error. A statistical analytical design was constructed for each individual participant, using the general linear model with the onsets of go signal in each of the 4 main trial types (go success, go error, stop success, stop error) convolved with a canonical hemo-dynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al., 1995). Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation of the time series was corrected by the fast model for highetemporal resolution images (Todd et al., 2016). The general linear model estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we constructed 2 separate contrasts that probed distinct aspects of SST performance: “saliency” processing (i.e., stop > go trials, or infrequent [~25%] versus frequently presented [~75%] stimuli) and “successful stopping” (i.e., stop success > stop error trials). The contrast (difference in β) images of the first-level analyses were then used for the second-level group statistics (random effect analysis; Penny and Holmes, 2003). The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
2.5. Task-residual and resting-state functional connectivity analysis
We sought to understand whether group differences in regional task activation were related to changes in functional networks during task and resting state. Therefore, we conducted functional connectivity analysis using similar methods as our previous work (Manza et al., 2015, 2016b). We used seed regions that showed significant and overlapping disease and medication-related effects in the task contrasts. We first “scrubbed” the data (Power et al., 2012) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by , where (dx, dy, dz) and (α, β, γ) are the translational and rotational movements, respectively, and r (= 50 mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transform rotations into displacements. The second head movement metric was the root mean square variance of the differences (DVARS) in % signal intensity I(t) between consecutive time points across all voxels, computed as follows: , where the brackets indicate the mean across brain voxels. We removed every time point that exceeded the head motion limit FD(t) > 0.5 mm or DVARS(t) > 0.5% via regression. In addition, during the task state, HRF-convolved task events and their derivatives were regressed out to remove the influence of transient events on functional connectivity estimates (Fair et al., 2007). Regressing out task events is a critical step, as external stimuli can induce inflated correlations between brain regions (McIntosh and Korostil, 2008). Task-residual signals make a major contribution to task performance, and this approach has been successfully applied in recent work (Alavash et al., 2015; Cole et al., 2014; Rudolph et al., 2017; Zhang and Li, 2010, 2012); furthermore, it is especially useful in sample sizes like the current one that may be underpowered for task event-driven connectivity methods, for example, dynamic causal modeling (Goulden et al., 2012). Thus, after regressing out task events and their derivatives, the fMRI signal time courses were averaged across all voxels for the seed region. We computed the correlation coefficient between the averaged time course of each seed region and the time course of each voxel in the whole brain for each individual. To assess and compare the resting-state correlation maps, we converted the r values, which were not normally distributed, to z scores by Fisher’s z transform (Jenkins and Watts, 1968): .
2.6. Group-level statistical analysis
In the second-level analysis, images were thresholded at a cluster-forming threshold of p < 0.005 and p < 0.05 family-wise error (FWE) cluster-corrected. To ensure cluster-level type I error rates were controlled for, in line with current reporting guidelines (Eklund et al., 2016), we calculated cluster corrections with the Statistical nonparametric mapping toolbox (SnPM13: http://warwick.ac.uk/snpm; 5000 permutations). One-sample t-tests were performed to estimate the group-level effects for each group: the PD “on”, PD “off”, and control groups. Disease effects were determined using 2-sample t-tests between the control and PD “off” groups. Levodopa effects were determined using paired t-tests between the “on” and “off” states in PD.
3. Results
3.1. Behavioral performance
GoRT did not differ significantly between the 3 groups, whereas SSRT was significantly slower in the PD “off” group relative to the other 2 groups [control vs. PD off: t(56) = −2.43, p = 0.018; PD on vs. PD off: t(16) = −2.86, p = 0.008; see Table 2, Fig. 1B], indicating that a response inhibition deficit in the “off” medication state is improved with levodopa in early-stage PD. SSRT results were highly similar between the “maximum likelihood” and “integration” methods (R2 = 0.87), and group differences were significant using either approach (Table 2). Results were also similar when excluding the participant with self-reported depression and the participant who was an outlier by go accuracy [control vs. PD off: t(54) = −2.34, p = 0.023; PD on vs. PD off: t(14) = −2.90, p = 0.012]. Across participants, greater benefits in response inhibition (i.e., reduction in SSRT from “off” to “on” status) was positively correlated with their SSRT “off” medication (R2 = 0.48, p = 0.002, Fig. 1C), indicating that patients with worse response inhibition deficit benefited more from levodopa. A Pitman test (Kelly and Price, 2005) suggested that this was not simply an effect of regression to the mean, as the variance in SSRT was significantly different between the “on” and “off” state [t(16) = 2.49, p = 0.012] (van Wouwe et al., 2016). These differences also did not appear to be due to differences in performance that would violate the assumptions of the race model (i.e., as the SSD increased, stop-error rates increased in all 3 groups, Fig. 1D, and stop-error RT was faster than GoRT in all 3 groups, Table 2). To confirm that medication-based changes in SSRT were not simply due to alleviation of motor symptoms, we conducted an across-participant correlation between change in SSRT (“on” minus “off”) and between change in Unified Parkinson’s disease rating scale III motor ratings (“on” minus “off”). Indeed, SSRT changes between “on” versus “off” state were not significantly associated with changes in UPDRS rating (r = 0.09; p = 0.74), suggesting that medication-based improvement in response inhibition performance was not directly linked to alleviation of motor symptoms as measured by UPDRS. Furthermore, medication-based differences in SSRT were not significantly associated with other clinical features of PD (age, levodopa-dose equivalency, or years from diagnosis; all p’s > 0.35).
Table 2.
Behavioral results for the stop-signal task, presented as mean (standard deviation)
| Group | SSRT (maximum likelihood method) | SSRT (integration method) | GoRT | GoRT-SERT | GoRT CoV | Mean SSD | Go Acc | Stop Acc |
|---|---|---|---|---|---|---|---|---|
| CTL | 251.2 (40.7) | 247.1 (37.2) | 718.6 (106.3) | 94.3 (38.3) | 0.22 (0.05) | 455.16 (98.37) | 0.98 (0.02) | 0.53 (0.03) |
| PD ON | 249.0 (45.1) | 242.4 (47.6) | 686.3 (112.8) | 85.8 (35.3) | 0.22 (0.05) | 432.69 (107.32) | 0.97 (0.05) | 0.53 (0.03) |
| PD OFF | 283.3 (60.0) | 273.9 (63.9) | 734.8 (113.5) | 115.5 (70.1) | 0.24 (0.06) | 444.41 (105.18) | 0.96 (0.03) | 0.54 (0.04) |
| CTL vs. ON | 0.17 (0.864) | 0.40 (0.692) | 1.02 (0.314) | 0.78 (0.442) | −0.02 (0.985) | 0.76 (0.452) | 0.62 (0.539) | 0.88 (0.380) |
| CTL vs. OFF | −2.43 (0.018)a | −2.03 (0.047)a | −0.54 (0.588) | −1.50 (0.140) | −1.70 (0.112) | −0.10 (0.704) | 1.95 (0.093) | −0.10 (0.925) |
| ON vs. OFF | −2.86 (0.008)a | −2.42 (0.016)a | −2.16 (0.064) | −1.64 (0.112) | −1.13 (0.241) | −0.96 (0.443) | 1.20 (0.355) | −1.98 (0.122) |
CTL versus ON and CTL versus OFF are t (p) values for 2-sample t-tests; ON versus OFF is t (p) values for paired t-tests of patients “on” and “off” their medications.
Key: Acc, accuracy; CoV, coefficient of variation; CTL, control; GoRT, go reaction time; SERT, stop-error reaction time; SSD, stop-signal delay; SSRT, stop-signal reaction time.
p < 0.05.
3.2. Task-fMRI results
We examined regional activations in each group that were specific to the “saliency” and “successful stopping” SST contrasts. In 1-sample t-tests, each group showed significant activations in the saliency, stop > go, contrast, including bilateral insula/IFG, bilateral primary and association visual cortices, superior temporal gyrus, dorsal anterior cingulate cortex, and preSMA (Fig. 2A). In the reverse contrast (go > stop), all 3 groups showed significant activation in the sensorimotor cortex. For the “successful stopping”, stop success > stop error, contrast, each group showed significant activations in the lateral occipital cortex, and for the reverse contrast (stop error > stop success), there were widespread activations in medial occipital, superior frontal, sensorimotor, and insular cortices, as well as striatum and thalamus (Fig. 2B).
Fig. 2.
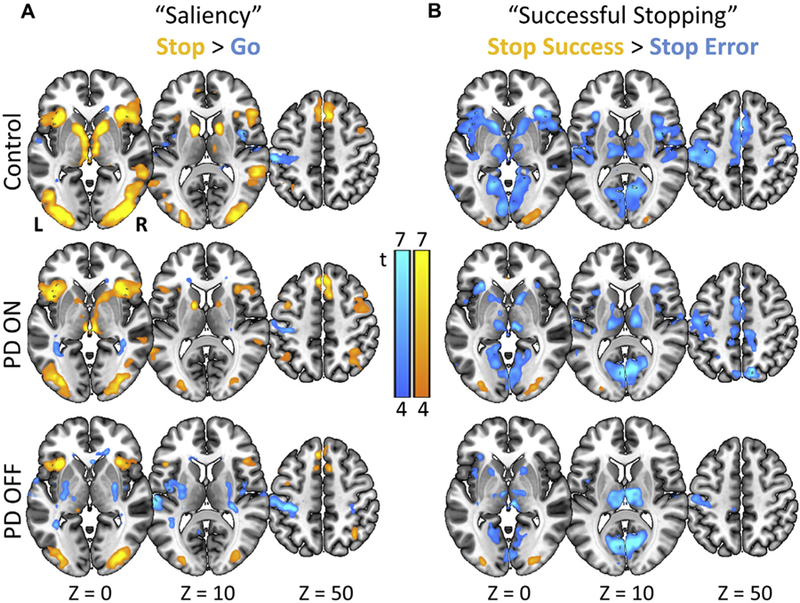
Functional magnetic resonance imaging results: 1-sample t-tests showing SST activations for each group. (A) Activations to “saliency” or all stop > go trials (i.e., infrequent stimuli compared to frequent stimuli). (B) Activations to “successful stopping” or stop success > stop error trials. Maps are thresholded at 4 > t > 7 (where brighter colors indicate higher values), for visualization. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The whole-brain 2-sample t-test between the PD “off” group and controls (i.e., disease effects) revealed significantly lower saliency-related activations in the PD “off” group in the right striatum (pFWE = 0.039; Table 3; Fig. 3A), though the left striatum and regions typically considered part of the “salience” network, such as the cingulate cortex (Seeley et al., 2007), are also evident at a reduced threshold of p < 0.005 uncorrected (Supplementary Fig. 1). There were no significant disease effects for the successful stopping contrast at the corrected threshold or at a reduced threshold of p < 0.005 uncorrected.
Table 3.
Brain activation differences for SST “saliency” contrast analysis (i.e., stop > go trials) between groups, including paired t-test comparison of participants with PD in their “ON” versus “OFF” medication state (Medication effects) and 2-sample t-test comparison of participants with Parkinson’s disease in their “OFF” medication state versus controls (disease effects)
| Contrast | Cluster size (mm3) | p-value (FWE corrected) | t-score | MNI coordinates (mm) |
Identified regions | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| ON > OFF (medication effect) | |||||||
| Stop > Go | 5828 | 0.044 | 6.47 | 20 | 0 | −12 | R striatum, R thalamus, R insula, R IFG |
| 6.08 | 16 | −12 | 10 | ||||
| 5.19 | 16 | −27 | 8 | ||||
| CTL > OFF (disease effect) | |||||||
| Stop > Go | 6764 | 0.039 | 5.07 | 23 | 3 | −18 | R striatum |
| 4.69 | 23 | 8 | −22 | ||||
| 4.55 | 13 | 10 | 0 | ||||
Task contrast results have a cluster-forming threshold of p < 0.005, with a nonparametric p < 0.05 FWE cluster correction, using SnPM13.
Key: CTL, control; FWE, family-wise error; IFG, inferior frontal junction; MNI, Montreal Neurological Institute; R, right.
The whole-brain paired t-tests for PD “on” versus “off” (i.e., medication effects) revealed significantly greater saliency-related activations in the “on” group in the right striatum, thalamus, IFG, and insular cortices (pFWE = 0.044; Table 3; Fig. 3A). When examining the overlap between disease- and medication-related activations, 1 cluster emerged in the right striatum that encompassed the ventral and anterior-dorsal putamen as well as anterior pallidum. Thus, patients “off” medication showed significantly lower saliency-related activation in this striatal cluster that was increased with medication. There were no significant medication effects for the successful stopping contrast (or the reverse contrast, stop error > stop success) at the corrected threshold or at a reduced threshold of p < 0.005 uncorrected. Results were virtually identical when excluding the participant with self-reported depression and the participant who was a go-accuracy outlier. Post hoc analysis of the striatal cluster confirmed that effects were significantly stronger in the stop > go contrast than the stop success > stop error contrast (disease effect: Cohen’s D = 1.62 vs. −0.44; z = 3.97, p < 1 × 10−4; medication effect: Cohen’s D = 1.36 vs. −0.13; z = 2.92, p = 0.004; for analysis of individual trial types contributing to these effects, see Supplementary Fig. 2). Medication-based differences in striatal activations were not significantly associated with clinical features of PD (age, levodopa-dose equivalency, years from diagnosis, or symptom side onset; all p’s > 0.39). The observed disease and medication effects appeared to originate from altered neural processing of a salient stimulus (stop signal) when compared to processing nonsalient, frequent stimuli (go signal), rather than outright stopping compared to impulsive errors. This region was extracted and used for functional connectivity analysis.
3.3. Task-residual functional connectivity results
In task-residual functional connectivity analysis of the right-striatum cluster, no significant disease- or medication-related effects emerged in whole-brain analysis at the more stringent a priori FWE-corrected threshold. At an exploratory, reduced threshold (conjunction p < 0.0025, with a minimum cluster size k > 20), we found an overlap between disease- and medication-related effects in task-residual functional connectivity between right striatum and left inferior frontal junction (IFG), SMA, left primary motor cortex (M1), and bilateral premotor cortices (Table 4; Fig. 3B), regions associated with response inhibition processes in previous studies (Cai et al., 2014; Li et al., 2006). However, these functional connectivity effects were not observed during the resting state (Fig. 3B). Owing to an increased risk for false positives at this lower threshold, we do not interpret the individual disease- and medication-related effects separately, but only the conjunction.
Table 4.
Task-residual functional connectivity differences between groups
| Contrast | Cluster size (mm3) | p-value (FWE corrected) | t-score | MNI coordinates (mm) |
Identified regions | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Conjunction | ||||||||
| Striatal functional connectivity | 406 | <0.001 | 5.25 | −37 | −4 | 62 | L premotor | |
| 313 | <0.001 | 4.62 | −47 | 8 | 32 | L IFJ | ||
| 906 | <0.001 | 4.55 | −24 | −14 | 70 | L M1 | ||
| 4.43 | −24 | −2 | 68 | |||||
| 1328 | <0.001 | 4.46 | −7 | 0 | 68 | L SMA | ||
| 703 | 0.001 | 3.99 | 30 | −7 | 65 | R premotor | ||
| 3.37 | 26 | −10 | 75 | |||||
| 500 | 0.001 | 3.91 | −44 | −32 | 68 | L M1 | ||
The overlapping right striatum region is shown in Fig. 3A, left was used as the seed region in the analysis. Conjunction of disease (CTL vs. PD “OFF”) and medication (“ON” vs. “OFF”) striatal connectivity revealed a set of prefrontal and frontal motor areas at an exploratory threshold of p < 0.0025, with a minimum cluster size k > 20.
Key: CTL, control; IFG, inferior frontal junction; L, left; M1, primary motor cortex; MNI, Montreal Neurological Institute; PD, Parkinson’s disease; R, right; SMA, supplementary motor area.
4. Discussion
We find that levodopa significantly improved SST performance in the early stages of PD. Functional MRI results suggest that levodopa also enhanced the saliency-related activation during SST performance and the functional connectivity of a fronto-striatal network that is critical for goal-directed movement. We discuss these findings in further detail below.
4.1. Impact of levodopa on response inhibition performance
Dopaminergic medication benefited the commonly observed response inhibition deficit in PD, improving SSRT roughly to the level of healthy controls. To our knowledge, this is the first study to show that dopamine-boosting medications improve SSRT in early-stage PD using a within-participant design. We speculate that previous studies did not observe significant dopaminergic effects on SSRT because they typically included participants using mixed medications and in the later stages of PD, when dopaminergic medications are less effective (Bravi et al., 1994). Importantly, the improvements in SSRT observed here were not associated with medication-based recovery of motor symptoms. This supports the intriguing possibility that levodopa benefits cognitive control of motor output independently of its effects on primary motor symptoms in PD, probably through a different dopamine-modulated fronto-striatal mechanism (Rowe et al., 2008).
These data provide further evidence that the specific task and population of interest can have a major impact on how dopamine-boosting medications affect response inhibition. Among healthy young adults, levodopa impaired interference-effect RT on a modified Stroop/Simon task (Onur et al., 2011), and methylphenidate and pramipexole did not significantly improve go/no-go performance (Hester et al., 2012; Kasparbauer et al., 2015b; Nandam et al., 2014; Yang et al., 2016). SST performance may be more amenable to the influence of dopaminergic medications (Manza et al., 2017), as SSRT has improved with methylphenidate (Nandam et al., 2011; but see; Farr et al., 2014), tyrosine (Colzato et al., 2014), and the dopamine agonist cabergoline (Nandam et al., 2013) in healthy adults. The effects of dopamine agonists on SSRT may be strongest in individuals with genetic profiles conferring low basal dopamine transmission (MacDonald et al., 2016). Although levodopa did not significantly improve SST performance in moderate-to-advanced PD (George et al., 2013; Obeso et al., 2011), it did improve Stroop performance in early-stage PD (Fera et al., 2007), particularly among those with poor baseline performance (Costa et al., 2014). The latter finding is in line with our observation that the individuals with the slowest SSRT benefited the most from levodopa. Together, these considerations highlight the need for careful selection and evaluation of study populations when making inferences about the role of dopamine in response inhibition.
4.2. Levodopa boosts deficient striatal activations to salient stimuli in PD
By examining the overlap between disease- and medication-related effects during SST performance, we isolated a striatal region that showed deficient “salience” (i.e., stop > go) activity in the “off” state that was improved with levodopa. This is another demonstration of the critical role that the striatum plays in SST performance (Zandbelt and Vink, 2010). Levodopa also significantly increased salience-related activations in the right thalamus, insula, and IFG. A previous study demonstrated reduced stop-related activation in the right IFG in de novo PD (Vriend et al., 2014), although that was an analysis of stop success > go trials only, and it is unclear if it actually was a direct response suppression effect or it extends to the same stop > go contrast presented here.
Interestingly, the effect here was present for both stop success and stop error trials. This suggests that task-related striatal deficits are not only related to pure motor inhibition but also a deficit in the processing and response to salient, infrequent stimuli. Our work suggests that the striatum may be part of the “ventral attention system” for the detection of infrequent events, in addition to the inferior prefrontal and parietal regions that have been previously identified (Corbetta and Shulman, 2002). These findings build on a body of work demonstrating the strong role of dopamine in processing salient events, regardless of valence or reward (Bromberg-Martin et al., 2010; Horvitz, 2000), and is another example of how striatal dopamine loss impairs executive functions dependent on saliency processing. Individuals with mid-stage PD showed blunted responses to surprising stimuli on an oddball task (Gurvich et al., 2007), which were improved with dopaminergic medication in early-stage PD (Georgiev et al., 2015). Furthermore, PD patients “off” medication showed selective deficits in cognitive control of movement when they had to respond to highly salient, surprising stimuli, and this impairment was ameliorated with levodopa (Galea et al., 2012). This deficit was replicated in healthy adults who were given the D2 receptor blocker haloperidol, confirming a role for dopamine in this behavior (Bestmann et al., 2015). Thus, response inhibition deficits and blunted striatal activations in PD may be a result of impaired salient signal processing, which is improved with levodopa administration.
It is important to clarify what is meant by “salient” processing in the context of this study. In their seminal paper on the “salience network”—which includes the right striatal region identified here—Seeley et al. (2007) note that the salience network performs 2 distinct physiological functions: conflict/infrequency response and also interoception/motivational drive. Our definition of “salience” in this context maps onto the former and should not be confused with the latter function, which may operate in parallel in this system. This type of striatal infrequency response has indeed been observed in the SST previously. Sharp et al. (2010) used a modified SST with a majority of go trials, 20% stop trials, and 20% “continue” trials. The continue trials had identical frequency and timing to stop signals, but the go response was allowed to continue as normal. The contrast “continue (20%) > go (50%)” trials significantly activated the right striatum, whereas the contrast “stop (20%) > continue (20%)” did not. Therefore, this striatal region appears to be critical for the infrequency (saliency) response during the SST, and not necessarily the final motor output of stopping or going. Still, another explanation for stop > go effects (in the absence of stop success > stop error effects) has been posited in previous work. This striatal region could be important for early processing during response inhibition—that is, the initiation of the stop process, which may or may not result in successful stopping on any given trial (Cai et al., 2014; Cai and Leung, 2011; Wessel and Aron, 2013, 2017). The temporal profile of this activation could be dissected with combined electroencephalography (EEG)-fMRI to test this hypothesis in future research. In addition, future studies could examine the nature of the right-lateralized findings here (although the left striatum also emerged at a lower threshold), since a right-lateralized fronto-striatal inhibitory control circuit has been implicated in the literature previously (e.g., Zandbelt et al., 2013).
4.3. Levodopa may increase task functional connectivity between the striatum and a motor planning network
We found that task-residual functional connectivity between the right striatum region (identified from task analysis) and left IFJ was reduced in PD and increased with levodopa. The IFJ is thought to play a critical role in detecting unexpected or salient stimuli, in contrast to a more pure role in response inhibition attributed to the IFG (Corbetta and Shulman, 2002; Leung and Cai, 2007; Verbruggen and Aron, 2010). Although the right IFJ has received relatively more attention than the left IFJ in the response inhibition literature (Cai and Leung, 2011; Levy and Wagner, 2011; Swann et al., 2012), the left IFJ appears to play an important role in maintaining rule sets during tasks that require flexible switching of behavior (Bunge, 2003; Ueltzhöffer et al., 2015). Interestingly, A1 allele carriers of the DRD2-Taq1A polymorphism, who tend to have higher striatal dopamine D2 receptor density, show greater activation of the left IFJ during task switching relative to non-carriers (Stelzel et al., 2010). These findings correspond well with our observations that individuals with PD show reduced brain activation on salient stop trials generally, and not only during successful stopping, which is improved with levodopa administration. Thus, striatal-left IFJ functional connectivity during the task state may be an indicator of rule maintenance and vigilance for salient stimulus detection, which is sensitive to dopaminergic transmission.
We also observed an overlap of disease- and medication-related effects in task-residual functional connectivity between striatum and SMA, left M1, and bilateral premotor cortices. These regions have all been associated with the preparation and execution of goal-directed movement and, critically, are all modulated by dopamine. Prominent theories suggest that dopamine cells support the anticipation of salient stimuli by sending cascading signals through an insula-basal ganglia-SMA network to generate goal-directed motor output during cognitive control (e.g., Uddin, 2015). Recent positron emission tomography and fMRI studies in healthy adults have demonstrated support for these theories. For instance, striatal activations are linked to M1 and SMA output during SST performance (Zandbelt and Vink, 2010). More generally, increased dopamine release in the SMA was associated with faster learning of motor sequence output (Garraux et al., 2007), and depleting dopamine precursors (phenylalanine/tyrosine) decreased brain activation in striatum/SMA along with impaired timing of motor output on a perceptual task (Coull et al., 2012). The premotor cortex is also a critical region for motor planning, as it purportedly generates the “go” signal to basal ganglia to disinhibit the thalamus and excite motor cortex (Li et al., 2008b). While there is little direct evidence for how dopamine modulates premotor function to support response inhibition, some studies have shown hyperactive premotor responses in PD during movements that are associated with Stroop inhibitory control performance (Huang et al., 2007) and normalized with levodopa (Haslinger et al., 2001; Toxopeus et al., 2012). Thus, synchrony between regions critical for the planning and execution of goal-directed movements is disrupted in early-stage PD and responsive to levodopa.
4.4. Limitations
A common limitation of studies that manipulate dopaminergic drug intake in PD, including the present study, is a lack of a true double-blind placebo experimental design. More work is also needed to clarify whether levodopa’s impact on response inhibition is purely due to changes in dopaminergic signaling because levodopa is also a precursor for norepinephrine, which plays a role in response inhibition (Bari et al., 2009; Rae et al., 2016). In addition, while previous studies have concluded that carbidopa-levodopa does not significantly alter cerebral blood flow (Henriksen and Boas, 1985; Hershey et al., 2000, 2003), future studies could include arterial spin labeling sequences to control for any subtle blood flow differences based on medication use. Another potential issue relates to the sample size of the fMRI cohort, which limited our ability to examine PD subgroups, for example, rigid versus tremor dominant, or left- versus right-lateralized symptom groups. However, recent studies suggest that there are no substantial differences in SSRT between these subgroups (Mirabella et al., 2017; Tolleson et al., 2017). Finally, the task-residual functional connectivity results were exploratory and observed at a reduced statistical threshold; replication in a larger cohort with a more stringent thresholding procedure is necessary.
In summary, response inhibition deficits in Parkinson’s disease are associated with reduced striatal activation and connectivity during task performance that appears to be related to deficient salient signal processing. Crucially, these behavioral and neural markers were improved with levodopa in early-stage PD, a finding that was not observed in prior investigations in more advanced PD with more heterogeneous medication profiles. These results highlight the importance of carefully examining cohort characteristics when interpreting dopaminergic medication effects and shed new light on the role of dopamine in response inhibition.
Supplementary Material
Acknowledgements
The authors thank Turhan Canli, Kim Burke, Fred Severino, and Xiang He at the Stony Brook SCAN Center for their help and support in conducting the fMRI experiments. We thank Dardo Tomasi, Ehsan Shokri-Kojori, Şükrü Barış Demiral, Corinde Wiers, Sien Hu, Sheng Zhang, Anna Huang, Thang Le, and Jonathan O’Rawe for their help with data collection, guidance on fMRI analysis, and helpful discussions.
Footnotes
Disclosure statement
The authors thank the Stony Brook Research Foundation for providing funds for participant stipend payment. The study is also supported by grant BCS1309260 (C-sRL) from the National Science Foundation. The authors have no competing interests to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurobiolaging.2018.02.003.
References
- Alavash M, Hilgetag CC, Thiel CM, Gießing C, 2015. Persistency and flexibility of complex brain networks underlie dual-task interference. Hum. Brain Mapp 36, 3542–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Kareken DA, Christian BT, Dzemidzic M, Yoder KK, 2014. Cortical dopamine release during a behavioral response inhibition task. Synapse 68, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, Garcia-Garcia D, Guridi J, Artieda J, Jahanshahi M, Obeso JA, 2013. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp. Neurol 239, 1–12. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD, 2003. Horse-race model simulations of the stop-signal procedure. Acta Psychologica 112, 105–142. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ESJ, Robbins TW, 2009. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology 205, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Ruge D, Rothwell J, Galea J, 2015. The role of dopamine in motor flexibility. J. Cogn. Neurosci 27, 365–376. [DOI] [PubMed] [Google Scholar]
- Bravi D, Mouradian MM, Roberts JW, Davis TL, Sohn YH, Chase TN, 1994. Wearing-off fluctuations in Parkinson’s disease: contribution of postsynaptic mechanisms. Ann. Neurol 36, 27–31. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O, 2010. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, 2003. Neural circuits subserving the retrieval and maintenance of abstract rules. J. Neurophysiol 90, 3419–3428. [DOI] [PubMed] [Google Scholar]
- Cai W, Cannistraci CJ, Gore JC, Leung H-C, 2014. Sensorimotor-independent prefrontal activity during response inhibition. Hum. Brain Mapp 35, 2119–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung H-C, 2011. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PLoS One 6, e20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Karimi M, Weaver PM, Wu J, Perantie DC, Golchin NA, Tabbal SD, Perlmutter JS, Hershey T, 2008. Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia 46, 3162–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE, 2014. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Jongkees BJ, Sellaro R, van den Wildenberg WPM, Hommel B, 2014. Eating to stop: tyrosine supplementation enhances inhibitory control but not response execution. Neuropsychologia 62, 398–402. [DOI] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T, 2009. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol. Psychol 81, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T, 2008. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. Am. J. Med. Genet. B: Neuropsychiatr. Genet 147, 27–32. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Mazzù I, Longarzo M, Caltagirone C, Carlesimo GA, 2014. Dopamine treatment and cognitive functioning in individuals with Parkinson’s disease: the “cognitive flexibility” hypothesis seems to work. Behav. Neurol 2014, 260896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Hwang HJ, Leyton M, Dagher A, 2012. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J. Neurosci 32, 16704–16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G, 1990. In search of the point of no return: the control of response processes. J. Exp. Psychol. Hum. Percept. Perform 16, 164–182. [DOI] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li CR, 2009. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci 29, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE, 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage 35, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Hu S, Matuskey D, Zhang S, Abdelghany O, Li C-SR, 2014. The effects of methylphenidate on cerebral activations to salient stimuli in healthy adults. Exp. Clin. Psychopharmacol 22, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera F, Nicoletti G, Cerasa A, Romeo N, Gallo O, Gioia MC, Arabia G, Pugliese P, Zappia M, Quattrone A, 2007. Dopaminergic modulation of cognitive interference after pharmacological washout in Parkinson’s disease. Brain Res. Bull 74, 75–83. [DOI] [PubMed] [Google Scholar]
- Friston K, Frith C, Frackowiak R, Turner R, 1995. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2, 166–172. [DOI] [PubMed] [Google Scholar]
- Galea JM, Bestmann S, Beigi M, Jahanshahi M, Rothwell JC, 2012. Action reprogramming in Parkinson’s disease: response to prediction error is modulated by levels of dopamine. J. Neurosci 32, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraux G, Peigneux P, Carson RE, Hallett M, 2007. Task-related Interaction between basal ganglia and cortical dopamine release. J. Neurosci 27, 14434–14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff T, 2004. Inhibition of ongoing responses in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 75, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JS, Strunk J, Mak-Mccully R, Houser M, Poizner H, Aron AR, 2013. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. NeuroImage Clin 3, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev D, Jahanshahi M, Dreo J,Čuš A, Pirtošek Z, Repovš G, 2015. Dopaminergic medication alters auditory distractor processing in Parkinson’s disease. Acta Psychologica 156, 45–56. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED, 2012. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J. Neurosci 32, 7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Elliott R, Suckling J, Williams SR, Deakin JFW, McKie S, 2012. Sample size estimation for comparing parameters using dynamic causal modeling. Brain Connect 2, 80–90. [DOI] [PubMed] [Google Scholar]
- Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB, 2007. Inhibitory control and spatial working memory in Parkinson’s disease. Mov. Disord 22, 1444–1450. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO, 2001. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 124, 558–570. [DOI] [PubMed] [Google Scholar]
- Henriksen L, Boas J, 1985. Regional cerebral blood flow in hemiparkinsonian patients. Emission computerized tomography of inhaled 133Xenon before and after levodopa. Acta Neurol. Scand 71, 257–266. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS, 2003. Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 74, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, Perlmutter JS, 2000. Dopa-induced blood flow responses in nonhuman primates. Exp. Neurol 166, 342–349. [DOI] [PubMed] [Google Scholar]
- Hester R, Nandam LS, O’Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA, 2012. Neurochemical enhancement of conscious error awareness. J. Soc. Neurosci 32, 2619–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz J, 2000. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96, 651–656. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D, 2007. Metabolic brain networks associated with cognitive function in Parkinson’s disease. NeuroImage 34, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG, 1968. Spectral Analysis and its Applications Holden-Day, San Francisco. [Google Scholar]
- Kasparbauer A-M, Merten N, Aichert DS, Wöstmann N, Meindl T, Rujescu D, Ettinger U, 2015a. Association of COMT and SLC6A3 polymorphisms with impulsivity, response inhibition and brain function. Cortex 71, 219–231. [DOI] [PubMed] [Google Scholar]
- Kasparbauer A-M, Rujescu D, Riedel M, Pogarell O, Costa A, Meindl T, la Fougère C, Ettinger U, 2015b. Methylphenidate effects on brain activity as a function of SLC6A3 genotype and striatal dopamine transporter availability. Neuropsychopharmacology 40, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Housden CR, Regenthal R, Barker RA, Müller U, Rowe J, Sahakian BJ, Robbins TW, 2014. Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain 137, 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Price TD, 2005. Correcting for regression to the mean in behavior and ecology. Am. Nat 166, 700–707. [DOI] [PubMed] [Google Scholar]
- Leung H-C, Cai W, 2007. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J. Neurosci 27, 9893–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H, 1971. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am 49, 467–477. [PubMed] [Google Scholar]
- Levy BJ, Wagner AD, 2011. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. New York Acad. Sci 1224, 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Chao HHA, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW, 2008a. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience 155, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee T-W, 2008b. Subcortical processes of motor response inhibition during a stop signal task. NeuroImage 41, 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R, 2006. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post response processing. J. Neurosci 26, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA, 1984. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform 10, 276–291. [DOI] [PubMed] [Google Scholar]
- MacDonald HJ, Stinear CM, Ren A, Coxon JP, Kao J, Macdonald L, Snow B, Cramer SC, Byblow WD, 2016. Dopamine gene profiling to predict impulse control and effects of dopamine agonist ropinirole. J. Cogn. Neurosci 28, 909–919. [DOI] [PubMed] [Google Scholar]
- Manza P, Amandola M, Tatenani V, Li CR, Leung H-C, 2017. Response inhibition in Parkinson’s disease: a meta-analysis of dopamine medication and disease duration effects. Parkinson’s Dis 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Hu S, Ide JS, Farr OM, Zhang S, Leung H, Li C-SR, 2016a. The effects of methylphenidate on cerebral responses to conflict anticipation and unsigned prediction error in a stop-signal task. J. Psychopharmacol 3, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Zhang S, Hu S, Chao HH, Leung H-C, Li C-SR, 2015. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. NeuroImage 107, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Zhang S, Li C-SR, Leung H-C, 2016b. Resting-state functional connectivity of the striatum in early-stage Parkinson’s disease: cognitive decline and motor symptomatology. Hum. Brain Mapp 37, 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Korostil M, 2008. Interpretation of neuroimaging data based on network concepts. Brain Imaging Behav 2, 264–269. [Google Scholar]
- Mirabella G, Fragola M, Giannini G, Modugno N, Lakens D, 2017. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia 102, 177–189. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Bellgrove MA, 2014. Dissociable and common effects of methylphenidate, atomoxetine and citalopram on response inhibition neural networks. Neuropsychologia 56, 263–270. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Cummins TDR, Garner K, Dean AJ, Kim BN, Nathan PJ, Mattingley JB, Bellgrove MA, 2011. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol. Psychiatry 69, 902–904. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Dean AJ, Messer C, Honeysett A, Nathan PJ, Bellgrove MA, 2013. Dopamine D2 receptor modulation of human response inhibition and error awareness. J. Cogn. Neurosci 25, 649–656. [DOI] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Jahanshahi M, 2011. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson’s disease. Exp. Brain Res 213, 435–445. [DOI] [PubMed] [Google Scholar]
- Onur ÖA, Piefke M, Lie C-H, Thiel CM, Fink GR, 2011. Modulatory effects of levodopa on cognitive control in young but not in older subjects: a pharmacological fMRI study. J. Cogn. Neurosci 23, 2797–2810. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Tysnes O-B, Alves G, 2013. Prognosis of mild cognitive impairment in early Parkinson disease. JAMA Neurol 70, 580–586. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes A, 2003. Random-effects analysis. In: Human Brain Function Academic Press, Cambridge, Massachusetts, pp. 843–850. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Nombela C, Rodríguez PV, Ye Z, Hughes LE, Jones PS, Ham T, Rittman T, Coyle-Gilchrist I, Regenthal R, Sahakian BJ, Barker RA, Robbins TW, Rowe JB, 2016. Atomoxetine restores the response inhibition network in Parkinson’s disease. Brain 139, 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cools R, 2014. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov. Disord 29, 597–607. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED, 2015. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J. Neurosci 35, 5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BCP, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM, 2008. Parkinson’s disease and dopaminergic therapyedifferential effects on movement, reward and cognition. Brain 131, 2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MD, Miranda-Domínguez O, Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson K, Chein J, Fettich KC, Richeson JA, Dellarco DV, Galván A, Casey BJ, Fair DA, 2017. At risk of being risky: the relationship between “brain age” under emotional states and risk preference. Dev. Cogn. Neurosci 24, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA, 2010. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U. S. A 107, 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Frank MJ, Moustafa A.a, Pearson M, Naismith SL, Lewis SJG, 2013. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 136, 3671–3681. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1, S208–S219. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ, 2010. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J. Neurosci 30, 14205–14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N, 2012. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage 59, 2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd N, Moeller S, Auerbach EJ, Yacoub E, Flandin G, Weiskopf N, 2016. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: sensitivity and slice leakage artifacts. NeuroImage 124, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleson C, Turchan M, van Wouwe N, Isaacs D, Phibbs F, Wylie S, 2017. Parkinson’s disease subtypes show distinct tradeoffs between response initiation and inhibition latencies. J. Int. Neuropsychol. Soc 23, 665–674. [DOI] [PubMed] [Google Scholar]
- Toxopeus CM, Maurits NM, Valsan G, Conway BA, Leenders KL, de Jong BM, 2012. Cerebral activations related to ballistic, stepwise interrupted and gradually modulated movements in Parkinson patients. PLoS One 7, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Ueltzhöffer K, Armbruster-Genç DJN, Fiebach CJ, 2015. Stochastic dynamics underlying cognitive stability and flexibility. PLoS Comput. Biol 11, 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wouwe NC, Kanoff K, Claassen DO, Spears CA, Neimat JS, van den Wildenberg WPM, Wylie SA, 2016. Dissociable effects of dopamine on the initial capture and the reactive inhibition of impulsive actions in Parkinson’s disease. J. Cogn. Neurosci 28, 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron A, 2010. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. U. S. A 107, 13966–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD, 2013. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychol. Sci 24, 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, 2009. Models of response inhibition in the stop signal and stop change paradigms. Neurosci. Biobehav. Rev 33, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend C, Gerrits NJHM, Berendse HW, Veltman DJ, van den Heuvel OA, van der Werf YD, 2014. Failure of stop and go in de novo Parkinson’s disease-a functional magnetic resonance imaging study. Neurobiol. Aging 36, 470–475. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Whetteckey J, 2010. Impulse control disorders in Parkinson disease. Arch. Neurol 67, 589–595. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR, 2017. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron 93, 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Aron AR, 2013. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J. Neurosci 33, 18481–18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fonov V, Bériault S, Al Subaie F, Chakravarty MM, Sadikot AF, Pike GB, Collins DL, 2015. Multi-contrast unbiased MRI atlas of a Parkinson’s disease population. Int. J. Comput. Assist. Radiol. Surg 10, 329–341. [DOI] [PubMed] [Google Scholar]
- Yang XQ, Glizer D, Vo A, Seergobin KN, MacDonald PA, 2016. Pramipexole increases go timeouts but not no-go errors in healthy volunteers. Front. Hum. Neurosci 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ, Barker RA, Robbins TW, Rowe JB, 2014a. Improving response inhibition in Parkinson’s disease with atomoxetine. Biol. Psychiatry 77, 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ, Barker RA, Robbins TW, Rowe JB, 2014b. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson’s disease. Brain 137, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Rae CL, Nombela C, Ham T, Rittman T, Jones PS, Rodríguez PV, Coyle-Gilchrist I, Regenthal R, Altena E, Housden CR, Maxwell H, Sahakian BJ, Barker RA, Robbins TW, Rowe JB, 2016. Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson’s disease with clinical and neuroimaging measures. Hum. Brain Mapp 37, 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Hoogendam JM, Kahn RS, Vink M, 2013. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci 25, 157–174. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M, 2010. On the role of the striatum in response inhibition. PLoS One 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR, 2012. Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front. Psychol 3, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR, 2010. A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. NeuroImage 49, 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


