Abstract
Age-related hearing loss, which affects roughly 35% of those over the age of 70, is the second most common disorder among the elderly. The severity of age related hearing loss may actually be worse if assessments are made under more realistic conditions, such as communicating in noise. Emerging data from humans and animal models suggest that damage to the inner hair cells and/or type I neurons, that relay sound information to the brain may contribute to hearing deficits in a noisy background. Data obtained from carboplatin-treated chinchillas suggest that tone-in-noise thresholds are a sensitive and frequency dependent method of detecting damage to the IHC/type I system. Therefore, tone detection thresholds measured in broadband noise may provide an efficient method of detecting the deficits in specific frequency regions. Preliminary data obtained in elderly subject with normal thresholds in quiet compared to young subjects illustrate the importance of repeating these measurements in broadband noise because thresholds in noise were worse for our elderly subjects than young subjects, even though both groups had similar hearing thresholds in quiet. N-acetyl cysteine supplementation which protects against inner hair cell loss in animal models, may represent a viable therapy for protecting the inner hair cell/type I neurons.
Keywords: Age-related hearing loss, hidden hearing loss, hearing disorders, presbycusis, clinical audiogram, inner hair cells
Introduction
The ability to communicate and interact with friends and family is crucially dependent one’s ability to hear and process speech and environmental sounds. When individuals develop a moderate to severe hearing loss from aging, they are hampered in their ability to converse by phone or face to face. To make matters worse, severe hearing loss has been linked to depression, cognitive decline and dementia [1–3]. Hearing loss can be caused by many factors, but aging is by far the most prevalent cause.
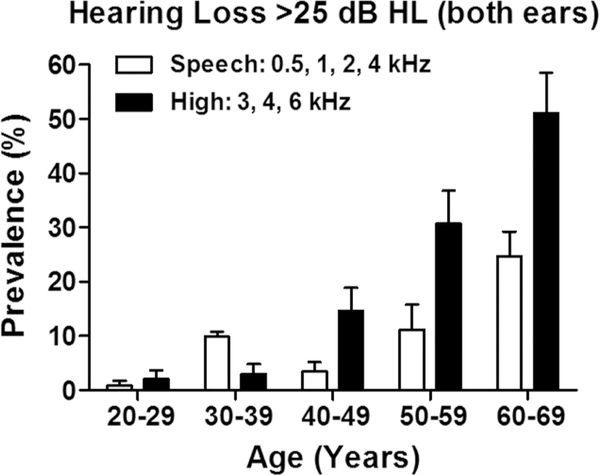
Age-Related Hearing Loss (ARHL) is a more significant problem than currently recognized. The Center for Disease Control in 2003 ranked ARHL or presbycusis as the second most common health problem of the elderly after arthritis. The distribution of ARHL from 18–80 years of age is documented in the International Standards Organization document ISO 7029:2017 (0.25–8 kHz) and ISO 7029:2017 (9–12. kHz; 22–80 years). The standard shows the deviation of hearing thresholds for a population of otologically normal subjects at various ages relative median hearing thresholds at 18 years of age. A recent hearing assessment based on the US National Health and Nutrition Examination Survey from 2011–2012 documented the prevalence of ARHL for males and females [4]. Figure 1 shows the prevalence of bilateral ARHL (Plotted from Table 1 of Hoffman et al., 2017) where impairment was defined as the pure tone average in both ears > 25 dB HL. The data were organized by “speech frequencies” (mean of 0.5, 1, 2 and 4 kHz) and “high frequencies” (mean of 3, 4 and 6 kHz). The prevalence of ARHL in the speech frequencies increased noticeably from 60–69 years of age; in this age range, .roughly 27% were affected. For the high frequencies, a noticeable increase was first seen from 40–49 years, at least a decade earlier than for the speech frequencies. High frequency hearing loss increased to ~50 by 60–69 years of age.
Figure 1:
Prevalence of Speech-Frequency and High-Frequency, bilateral hearing impairment in United States women and men from US National Health and Nutrition Examination Survey, 2011–2012. Bilateral impairment defined as pure tone average in both ears > 25 dB HL. Data replotted from Table 1 of Hoffman et al. (4).
What’s Missing in the Clinical Audiogram for detecting ARHL?
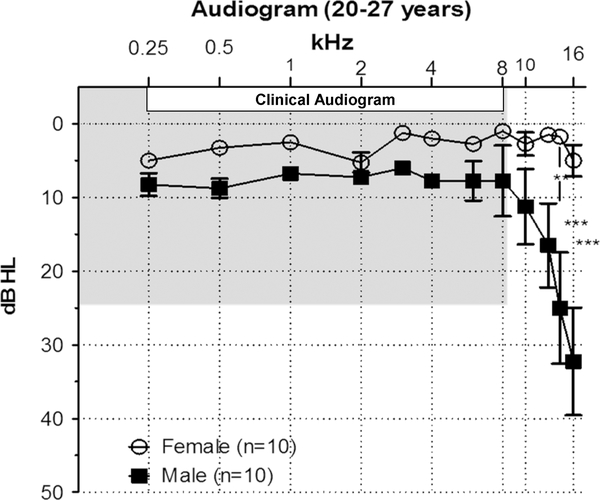
The “gold standard” for assessing hearing loss is the clinical audiogram which measures the minimum sound intensity needed to detect a tone (0.125, 0.5, 1, 2, 4 or 8 kHz) in quiet. The clinical audiogram provides useful information regarding a listener’s ability to detect sounds in the speech-frequency range. However, it fails to identify the very early signs of hearing loss in the extended high frequencies (10–20 kHz) which are the first to deteriorate with age or other ototraumatic insults presumably due reduced levels of antioxidant enzymes in the basal, high frequency region of the cochlea [5–8]. The first signs of hearing loss appear in the high frequency range [9]. This is consistent with the data we obtained from a group of 20–27 males we tested (Figure 2).
Figure 2:
Mean (+/−SEM) audiogram from 10 young females and 10 young males ages 20–27. Mean thresholds in the 0.25–8 kHz range of the clinical audiogram (shaded region) were clinically normal (<25 dB HL) for both males and females. Although male thresholds were slightly higher than females, the gender differences were not significantly different. However, thresholds in the extended high frequency range (10–16 kHz) increased with frequency in males and exceeded the 25 dB HL cutoff for normal at 14 and 16 kHz. Male thresholds were significantly higher than females at 12.5 kHz (p<0.01), 14 kHz (p<0.001) and 16 kHz (p<0.001).
Hearing thresholds for our males and females in the 0.25–8 kHz range were within the clinically normal range (≤25 dB HL). However, there was clear evidence of hidden hearing loss; male thresholds were significantly higher than females at 12.5, 14 and 16 kHz and thresholds at 14 and 16 kHz were above the 25 dB HL cutoff for normal hearing. Testing at the extended high frequencies allows the clinician to identify subject at risk for developing early onset ARHL, potentially linked to genetic or environmental factors [10, 11]. Clinicians also encounter elderly patients with normal hearing in the 0.25–8 kHz range that present with tinnitus, a phantom buzzing or ringing sensation. Because tinnitus is generally associated with hearing loss, the use of ototoxic drugs or other conditions [12–17], it is puzzling to find a tinnitus patient with normal hearing. This paradoxical finding is often resolved by testing in the extended frequency range. In such cases, the pitch of the tinnitus often matches the hearing loss in the extended high frequency region.
Selective Destruction of Inner Hair Cells and type I Auditory Nerve Fibers
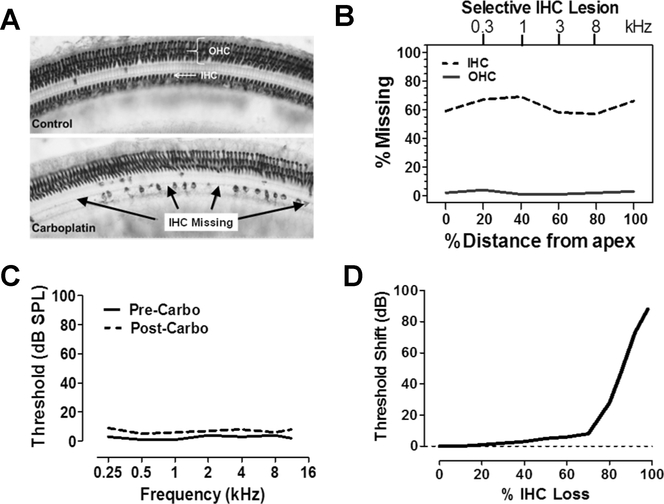
There is a growing recognition that the audiogram may not detect certain types of degenerative processes in the cochlea. In most cases, hair cell degeneration due to aging, noise exposure and most ototoxic drugs begins with outer hair cell (OHC) loss in the high-frequency base of the cochlea followed by a progression of OHC towards the low-frequency apex of the cochlea [18–20]. When the OHC are completely destroyed, but inner hair cells (IHC) remain intact, audiometric thresholds increase by 40–50 dB [21]. A unique lesion can be induced in chinchillas treated carboplatin, an anti-cancer drug that preferentially damages the IHC and their type I auditory nerve fibers along the entire length of the cochlea. A moderate dose of carboplatin (40–50 mg/kg) selectively damages IHC/type I neurons, but leave the OHC intact [22–24]. Figure 3A (top) shows a surface view of the sensory epithelium of a normal chinchilla labeled with succinate dehydrogenase that stains the three parallel rows of OHC and single row of OHC. The lower panel of Figure 3 A illustrates selective damage to IHC following carboplatin treatment; a few IHC were present among large patches of missing IHC [23]. Hair cells lesions can by quantified in the form of a cochleogram that shows the % missing OHC or IHC as a function of percent distance from the apical end of the cochlea (Figure 3B) [23]. In this schematic representation, 60–70% of the IHC are missing along the cochlea whereas OHC losses are negligible. The upper x-axis in Figure 3B shows the relationship between stimulus frequency and cochlear location. Figure 3C is a schematic that shows the behavioral thresholds of a typical chinchilla pre-carboplatin treatment and several months post-carboplatin [23,25]. In cases where carboplatin caused <75% IHC loss with full retention of OHC, there was little increase in threshold. Figure 3D is a schematic that illustrates the change in quiet thresholds (i.e., threshold shift) as a function of IHC loss. There was a negligible increase in threshold shift until the IHC lesions exceeded 75%; lesions greater than this were associated with a precipitous increase in threshold shift. Thus, thresholds in quiet remained nearly normal as long as the OHC were intact and IHC losses were <75% [23]. Apparently, a sound in presented in quiet can be detected with only a few IHC.
Figure 3:
(A) Photomicrographs of a surface preparation of the chinchilla organ of Corti stained with succinate dehydrogenase, a metabolic enzyme highly expressed in OHC and IHC. Control (upper panel) shows strong staining of all OHC and IHC. When chinchillas were treated with carboplatin, large patches of IHC were missing interspersed among surviving IHC (lower panel); all the OHC by contrast were present. (B) Schematic cochleogram from a carboplatin treated chinchilla in which 60–70% of the IHC were missing from the apex (0%) toward the base of the cochlear (100%); there was virtually no loss of OHC. (C) Schematic showing the thresholds of a typical chinchilla pre- and post-carboplatin treatment that resulted in selective loss of roughly 50% of the IHC. Post-carboplatin treated thresholds were nearly identical to those measured pre-carboplatin. In this example, the large IHC lesion had minimal effect on threshold. (D) Schematic illustrating the change in threshold (i.e., threshold shift) plotted as a function of % IHC loss. Threshold shifts were minimal until the IHC lesion exceeded 75% after which thresholds increased precipitously. Data schematized from Salvi et al. (23).
Synaptopathy and IHC Loss
OHC have traditionally been considered the most vulnerable structures to various cochlear insults including aging, noise and ototoxic drugs. In the past decade work by Liberman and Kujawa have highlighted the extreme vulnerability of the afferent synapse and type I spiral ganglion neurons that contact IHC. Their data now suggest that the most vulnerable structures may be the afferent terminals beneath the IHC, which connect to type I auditory nerve fibers and spiral ganglion neurons [26–30]. Amongst the population of type I nerve fibers the most vulnerable are the neurons with low to medium spontaneous rate and high thresholds neurons. The high threshold type I neurons are believed to play a major role in detecting and processing sounds in a noisy environment, that is processing sounds roughly 20 dB or more above the threshold measured in quiet.
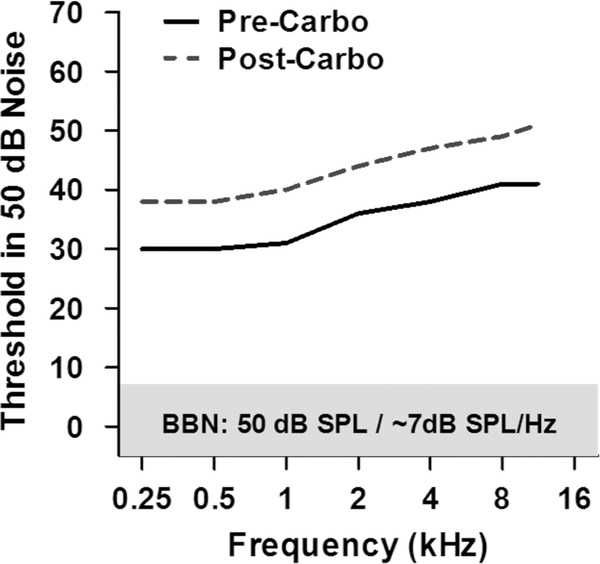
Ototoxic drugs preferentially damage the synapses of high thresholds type I neurons [26]. Although carboplatin damage to IHC and type I neurons in the chinchilla may not be identical to synaptopathy, insights into the perceptual deficits associated with synaptopathy might be gleaned from the hearing deficits observed in carboplatin treated chinchilla. Moderate lesions confined to IHC and type I neurons have little effect on the audiogram measured in quiet (Figure 3C) [23]. However, the lesions had a profound effect on sound detection in the presence of noise. When thresholds were measured in broadband noise (BBN, 100–16,000 Hz) with an overall level of 50 dB SPL and a spectrum level of ~7 dB/Hz, pre-carboplatin thresholds increased from approximately 30 dB at low frequencies to roughly 40 dB at 8–16 kHz (Figure 4) [31]. This increase is consistent with increase in the critical band with increasing frequency. After these baseline measures were obtained, chinchillas were treated with a dose of carboplatin that induced a moderate IHC lesion along the length of the cochlea. Post-carboplatin behavioral thresholds in BBN increased roughly 8–10 dB across the entire frequency range (Figure 4), consistent with the moderate loss of IHC along the length of the cochlea [31]. Thus, tone thresholds measured in BBN appears to be a sensitive technique to detect IHC/type I lesions in contrast to tone thresholds in quiet, which are insensitive to IHC/type I loss [31].
Figure 4:
Schematic illustrating chinchilla behavioral threshold obtained in the presence of 50 dB SPL broadband noise (BBN) with a spectrum level of ~7 dB SPL/Hz. Prior to carboplatin treatment, behavioral thresholds measured in BBN increased from approximately 30 dB at low frequency to roughly 40 dB at 8–16 kHz. After carboplatin treatment that induced a moderate IHC lesion along the length of the cochlea, the behavioral thresholds in BBN increased roughly 8–10 dB. Data schematized from Lobarinas et al. (31).
Detecting Hidden ARHL in Humans
Ask an elderly person with so-called normal hearing if they have they difficulty hearing. Most will report that conversing with friends in a noisy environment such as a restaurant is extremely difficult even though they have no trouble carrying on a conversation in a quiet setting. Data obtained from carboplatin-treated chinchillas suggest that tone-in-noise thresholds are a sensitive and frequency dependent method of detecting damage to the IHC/type I system. To determine if this approach might be useful for detecting hidden ARHL in elderly subjects, we carried out a pilot study with 10 young normal hearing subjects (mean age 20.09 years, range: 19–24 years) and six elderly subjects (mean age 62.66 years, range: 54–71 years) with audiometric thresholds within the normal range (≤25 dB HL) (Figure 5).
Figure 5:
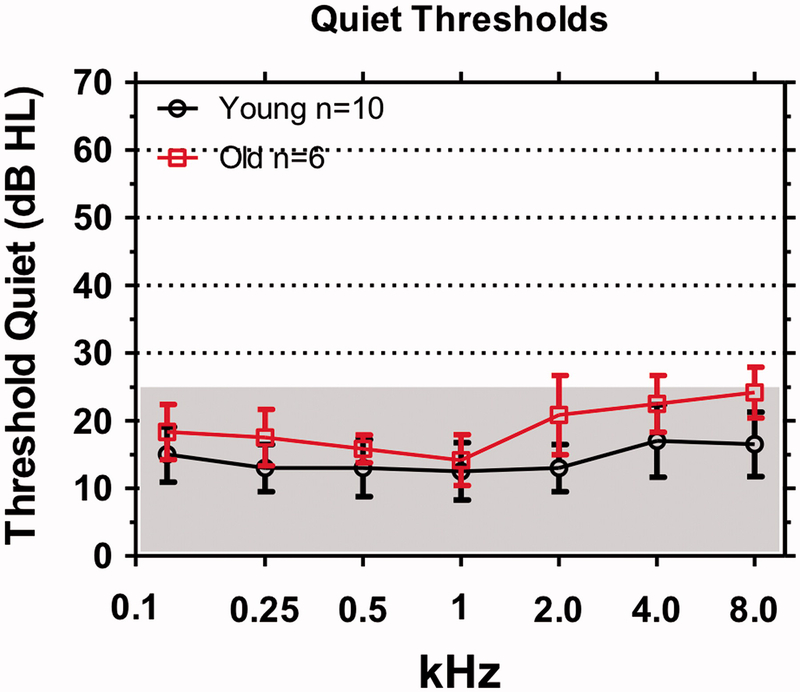
Clinical audiograms in which hearing thresholds in dB HL were measured in quiet from 0.125 to 8 kHz. Shaded areas define range of clinically normal thresholds (<25 dB HL). Mean thresholds (+/− SD) shown for 10 young subjects and six, elderly subjects.
Figure 6 shows the tone-in-noise thresholds for the young and elderly subjects measured in the presence of BBN presented at 20 dB HL and 30 dB HL. The mean data for the young subjects are shown along with the 95% confidence interval. Data for each of the six elderly subjects with ostensibly normal hearing are shown by the symbols. For the 20 dB HL noise condition (Figure 6A), nearly all the individual tone-in-noise thresholds from the elderly subjects were above the mean and 95% confidence interval of the young subjects except at 1 kHz. For the 30 dB HL BBN, a similar trend was evident with most of the individual data points for the elderly above the mean and 95% confidence interval of the young subjects except at 1 kHz. These results suggest that elderly subjects are worse than young subjects at detecting tones in noise particularly at the high frequencies. One interpretation of the tone-in-noise data from elderly with “normal hearing” is that their IHC or high-thresholds type I fibers are damaged except near 1 kHz region. Histological examination of the temporal bones from elderly subjects such as these would be needed to confirm the hypothesized structure-function correlations.
Figure 6:
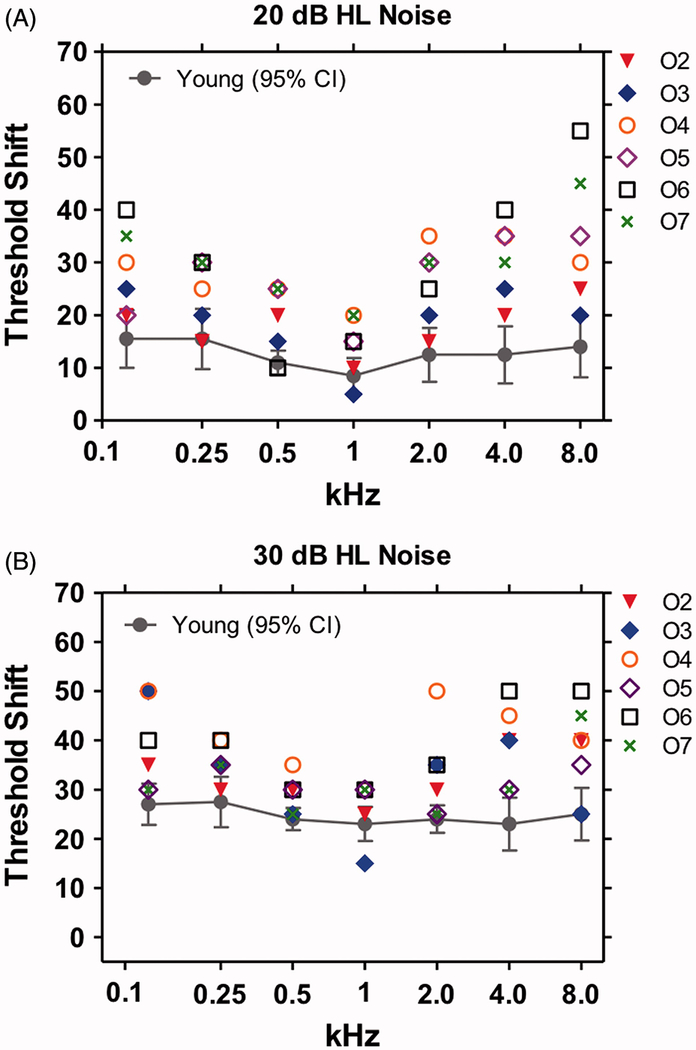
Tone thresholds as a function of frequency in broadband background noise of (A) 20 dB HL and (B) 30 dB HL. Mean +/− 95% confidence interval shown for 10 young normal hearing subject s (mean age 20.09 years). Means for young subjects increased from 20 dB HL to 30 dB HL noises. Symbols show data for each of six elderly (mean age 62.66 years) subjects (O2-O7) in the 20 and 30 dB HL noise. Most of tone-in-noise thresholds for the elderly subjects were above the mean thresholds of the young subjects for the 20 and 30 dB HL noise conditions; these differences were most pronounced from 2–8 kHz. Little difference observed between the old and young at 1 kHz.
Genetics of ARHL and Oxidative Stress
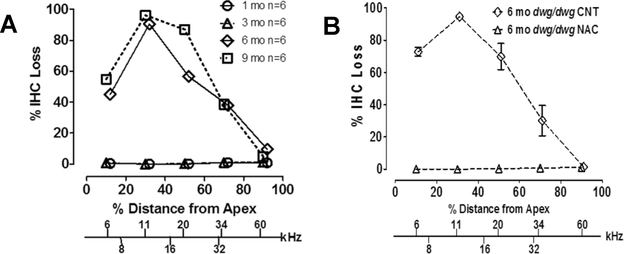
ARHL is a complex disorder involving both genetic and environmental factors [32–35]. Some have argued that 25–75% of the variance in ARHL has a genetic basis [36, 37]. Studies of ARHL using inbred mouse strains raised under controlled laboratory conditions obviate many of the problems that plague human epidemiological studies such as genetic and environmental heterogeneity. Hearing and histological assessments of numerous laboratory mice have identified strains that develop hearing loss early in life as well as other strains that retain normal hearing well into later life [38, 39]. Several excellent manuscripts list strains that develop hearing loss as a function of age [38, 40]. In addition, a growing number of genes that accelerate or slow ARHL have been identified [41–44]. The CBA/CaJ mouse is one of the most widely used to investigate ARHL because its hearing and cochlea remain reasonably normal until much later in life [11, 45]. CBA/CaJ mice exhibit little IHC or OHC loss at 8 months of age (Figure 7A) and show little evidence of hearing loss [19, 46]. At 26 months of age (Figure 7B), moderate OHC losses appear near the low frequency apex (10% location) and high frequency base of the cochlea (90% location), resulting in a U-shaped lesion. Smaller IHC lesions occur in similar cochlear locations.
Figure 7:
Cochleograms showing the percent outer hair cell (OHC) and inner hair cell (IHC) loss as a function of percent distance from the apex of the cochlea in CBA and C57BL/6 mice at 8 months and 26 months of age. Frequency vs. place map shown on lower x-axis. Data schematized from Spongr et al. (19).
CBA/CaJ mice have been used extensively in studies of noise-induced hearing loss [29, 47]; they also have been used to test for age-related deficits in the central auditory pathway [48, 49]. C57BL/6J mice are also widely used in hearing research. This strain begins to develop progressive hearing loss and cochlear pathology around 5–6 months of age [19, 46]. At 8 months of age, large OHC and IHC losses are evident in the high frequency basal third of the cochlea (70–100% location). At 26 months of age nearly all the OHC are missing throughout the cochlea whereas the IHC lesion decreases from 100% loss at the high frequency base of the cochlea to a 20% loss at the low-frequency apex. The growth of hair cell loss is consistent with progression of hearing loss [46]. This strain and its variants have been used to study the genetic [42] and biological variables [50, 51] that contribute to ARHL and to evaluate therapies to slow the progression of ARHL [52]. A major genetic factor contributing to ARHL in C57BL/6J mice is a splice variant of the cadherin 23 gen on the ahl locus on Chromosome 10 t [53]. The cadherin 23 protein is a component of the tip links on hair cell stereocilia, which regulate the opening and closing of the transduction channels that control the influx of cations into the hair cells. A single nucleotide variant of the cadherin 23 gene, Cdh23c.753A, which is common to many inbred strains of mice, accelerates ARHL [54, 55].
A major theory of aging involves oxidative stress from reactive oxygen species (ROS) [56, 57]. The importance of ROS in ARHL in humans is ambiguous [58–60]. Inconsistencies in the human literature could be due to genetic diversity in the human population and/or environmental factors. Studies in mouse models in which oxidative stress can be genetically altered provide strong support for the oxidative stress tin ARHL. Glutathione (GSH), which is one of the most important antioxidants for protecting cells, is heavily expressed in the cochlea. Intracellular GSH is resynthesized through the gamma-glutamyl cycle. The Ggt1gene codes for gamma-glutamyl transferase 1 (GGT1); GGT1 transfers the glutamyl moiety of GSH to an acceptor molecule facilitating its transport into the cell [61]. Loss of GGT1 depletes intracellular GSH resulting in increased oxidative stress. We recently found that dwg/dwg mice with a spontaneous loss of function mutation of the Ggt1 gene develop an unusual form of progressive ARHL that involves the selective loss of IHC in the apical half of the cochlea [62]. As shown by cochleograms in Figure 8A, the IHC were intact up to 3 months of age, but by 6 months of age, large IHC lesions were present in the low-frequency apical half of the cochlea and the lesions increased at 9 months of age. Importantly, the OHC remained in intact in dwg/dwg mice and there did not appear to be any loss of auditory nerve fibers. No lesions were observed in the heterozygous dwg mice suggesting that 50% of the GGT1 protein was sufficient to prevent oxidative stress and preserve the integrity of the IHC. N-acetyl cysteine (NAC) promotes the synthesis of glutathione and has been found to ameliorate several forms of hearing loss Since NAC promotes the synthesis of glutathione and protects against several forms of hearing loss [63–67], we added NAC to the drinking water of dwg/dwg mice from 3 weeks to 6 months of age. NAC treatment completely prevented the age-related loss of IHC (Figure 8B). When NAC treatment was discontinued from 6–9 months, the IHC lesion reappeared confirming the importance of NAC supplementation in preventing oxidative stress by replenishing the intracellular stores of GSH.
Figure 8:
Cochleograms showing the percent IHC loss as a function of percent distance from the apex of the cochlear. Lower x-axis shows the relationship between frequency and cochlear place. (A) % IHC loss in dwg/dwg mice at 1, 3, 6 and 9 months of age. (B) % IHC loss at 6 months of age in dwg/dwg control mice and dwg/dwg mice treated with N-acetyl cysteine from 3 weeks to 6 months of age. Data schematized from Ding et al. (62).
Conclusions
ARHL, one of the most common disabilities of the elderly, will likely continue to increase as the average lifespan increases in developing countries. The traditional metric for quantifying ARHL, the clinical audiogram is inadequate for detecting the early onset of ARHL in the extended high frequency region. A simple solution is to evaluate thresholds above 8 kHz, since hearing losses at the extended high frequency region is the “canary in the coal mine”. There is growing recognition that the IHC/type I neurons, which transfer auditory information to the central auditory system, may be extremely vulnerable to many ototraumatic agents, particularly information relayed by the high-threshold type I neurons. Damage to the IHC/type I neurons cannot be detected by the conventional audiogram measurements in quiet. As demonstrated in the chinchilla, a simple measurement to detect IHC/type I cochlear pathologies involves measuring tone thresholds in BBN noise. Preliminary data from our elderly subject with normal thresholds in quiet illustrate the importance of repeating these measurements in BBN because thresholds in noise were worse for our elderly subjects than young subjects, even though both groups had similar hearing thresholds in quiet. An added benefit of tone-in-noise threshold detection is that it pinpoints the frequencies most affected (high frequencies) while identifying those where noise had little effect (1 kHz). Our results indicate that NAC protect the IHC in dwg/dwg mice and raise the possibility that it may protect the IHC/type I neurons in normal mice or humans.
Acknowledgments:
Research supported in part ty NIH grants (R01DC011808; R01DC014452 and R01DC014693)
Footnotes
Declaration of Interest: The authors report no conflicts of interest
References
- 1.Lee KY. Pathophysiology of age-related hearing loss (peripheral and central). Korean J Audiol. 2013;17(2):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parham K, Lin FR, Coelho DH, et al. Comprehensive management of presbycusis: central and peripheral. Otolaryngol Head Neck Surg. 2013;148(4):537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman HJ, Dobie RA, Losonczy KG, et al. Declining Prevalence of Hearing Loss in US Adults Aged 20 to 69 Years. JAMA Otolaryngol Head Neck Surg. 2017;143(3):274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sha SH, Taylor R, Forge A, et al. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122(6):828–33. [DOI] [PubMed] [Google Scholar]
- 7.Fausti SA, Henry JA, Schaffer HI, et al. High-frequency monitoring for early detection of cisplatin ototoxicity. Arch Otolaryngol Head Neck Surg. 1993;119(6):661–6. [DOI] [PubMed] [Google Scholar]
- 8.Al-Malky G, Dawson SJ, Sirimanna T, et al. High-frequency audiometry reveals high prevalence of aminoglycoside ototoxicity in children with cystic fibrosis. J Cyst Fibros. 2015;14(2):248–54. [DOI] [PubMed] [Google Scholar]
- 9.Matthews LJ, Lee FS, Mills JH, et al. Extended high-frequency thresholds in older adults. J Speech Lang Hear Res. 1997;40(1):208–14. [DOI] [PubMed] [Google Scholar]
- 10.Kumar P, Upadhyay P, Kumar A, et al. Extended high frequency audiometry in users of personal listening devices. Am J Otolaryngol. 2017;38(2):163–7. [DOI] [PubMed] [Google Scholar]
- 11.Han C, Ding D, Lopez MC, et al. Effects of Long-Term Exercise on Age-Related Hearing Loss in Mice. J Neurosci. 2016;36(44):11308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralli M, Troiani D, Podda MV, et al. The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol Ital. 2014. June;34(3):198–204. [PMC free article] [PubMed] [Google Scholar]
- 13.Shekhawat GS, Searchfield GD, Stinear CM. The relationship between tinnitus pitch and hearing sensitivity. Eur Arch Otorhinolaryngol. 2014;271(1):41–8. [DOI] [PubMed] [Google Scholar]
- 14.Sereda M, Hall DA, Bosnyak DJ, et al. Re-examining the relationship between audiometric profile and tinnitus pitch. Int J Audiol. 2011;50(5):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan T, Tyler RS, Ji H, et al. The relationship between tinnitus pitch and the audiogram. Int J Audiol. 2009;48(5):277–94. [DOI] [PubMed] [Google Scholar]
- 16.Ralli M, Greco A, Turchetta R, et al. Somatosensory tinnitus: Current evidence and future perspectives. J Int Med Res. 2017. June;45(3):933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralli M, Altissimi G, Turchetta R, et al. Somatosensory Tinnitus: Correlation between Cranio-Cervico-Mandibular Disorder History and Somatic Modulation. Audiol Neurootol. 2016;21(6):372–382. [DOI] [PubMed] [Google Scholar]
- 18.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5(1):3–22. [DOI] [PubMed] [Google Scholar]
- 19.Spongr VP, Flood DG, Frisina RD, et al. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. Journal of the Acoustical Society of America. 1997;101(6):3546–53. [DOI] [PubMed] [Google Scholar]
- 20.Chen GD, Decker B, Krishnan Muthaiah VP, et al. Prolonged noise exposure-induced auditory threshold shifts in rats. Hear Res. 2014;317:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallos P, Harris D, Ozdamar O, et al. Behavioral, compound action potential, and single unit thresholds: relationship in normal and abnormal ears. The Journal of the Acoustical Society of America. 1978;64(1):151–7. [DOI] [PubMed] [Google Scholar]
- 22.Takeno S, Harrison RV, Ibrahim D, et al. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75(1–2):93–102. [DOI] [PubMed] [Google Scholar]
- 23.Salvi R, Sun W, Ding D, et al. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front Neurosci. 2016;10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36(6):301–11. [DOI] [PubMed] [Google Scholar]
- 25.Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez KA, Jeffers PW, Lall K, et al. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sergeyenko Y, Lall K, Liberman MC, et al. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobarinas E, Salvi R, Ding D. Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. J. Assoc. Res. Otolaryngol 2016. 17, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huyghe JR, Van Laer L, Hendrickx JJ, et al. Genome-wide SNP-based linkage scan identifies a locus on 8q24 for an age-related hearing impairment trait. American journal of human genetics. 2008;83(3):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopinath B, Flood VM, McMahon CM, et al. Dietary antioxidant intake is associated with the prevalence but not incidence of age-related hearing loss. J Nutr Health Aging. 2011;15(10):896–900. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan N, James K, McDermott D, et al. A 5-year prospective study of diabetes and hearing loss in a veteran population. Otol Neurotol. 2006;27(1):37–43. [DOI] [PubMed] [Google Scholar]
- 35.Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12(6):345–58. [DOI] [PubMed] [Google Scholar]
- 36.Viljanen A, Era P, Kaprio J, et al. Genetic and environmental influences on hearing in older women. J Gerontol A Biol Sci Med Sci. 2007;62(4):447–52. [DOI] [PubMed] [Google Scholar]
- 37.Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch Otolaryngol Head Neck Surg. 1999;125(6):654–9. [DOI] [PubMed] [Google Scholar]
- 38.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130(1–2):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Q-Y, Johnson KR, Erway LC. Screening mice at The Jackson Laboratory for hereditary hearing impairment. Abstract of Association for Research in Otolaryngology. 1997;882:221. [Google Scholar]
- 40.Willott JF, Erway LC, Archer JR, et al. Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res. 1995;88(1–2):143–55. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KR, Yu H, Ding D, et al. Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hear Res. 2010;268(1–2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kane KL, Longo-Guess CM, Gagnon LH, et al. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 2012;283(1–2):80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFadden SL, Burkard RF, Ohlemiller KK, et al. Copper/zinc superoxide dismutase deficiency potentiates age related and noise-hearing loss in SOD-1 mice1999: Assoc. for Res. in Otolaryngology. [Google Scholar]
- 44.Ohlemiller KK, McFadden SL, Ding DL, et al. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. Journal of the Association for Research in Otolaryngology : JARO. 2000;1(3):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111(5):827–34. [DOI] [PubMed] [Google Scholar]
- 46.Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hearing Research. 1987;30(2–3):207–18. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hear Res. 2002;165(1–2):96–102. [DOI] [PubMed] [Google Scholar]
- 48.Williamson TT, Zhu X, Walton JP, et al. Auditory brainstem gap responses start to decline in mice in middle age: a novel physiological biomarker for age-related hearing loss. Cell Tissue Res. 2015;361(1):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowe AS, Walton JP. Alterations in peripheral and central components of the auditory brainstem response: a neural assay of tinnitus. PLoS One. 2015;10(2):e0117228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40(6):313–21. [PubMed] [Google Scholar]
- 51.Ding DL, McFadden SL, Wang J, et al. Age- and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiology and Neuro-Otology. 1999;4(2):55–63. [DOI] [PubMed] [Google Scholar]
- 52.Someya S, Xu J, Kondo K, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35(1):21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mianne J, Chessum L, Kumar S, et al. Correction of the auditory phenotype in C57BL/6N mice via CRISPR/Cas9-mediated homology directed repair. Genome Med. 2016;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson KR, Tian C, Gagnon LH, et al. Effects of Cdh23 single nucleotide substitutions on age-related hearing loss in C57BL/6 and 129S1/Sv mice and comparisons with congenic strains. Scientific Reports. 2017;7:44450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buffenstein R, Edrey YH, Yang T, et al. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordr). 2008;30(2–3):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790(10):1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bared A, Ouyang X, Angeli S, et al. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol Head Neck Surg. 2010;143(2):263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unal M, Tamer L, Dogruer ZN, et al. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope. 2005;115(12):2238–41. [DOI] [PubMed] [Google Scholar]
- 60.Ates NA, Unal M, Tamer L, et al. Glutathione S-transferase gene polymorphisms in presbycusis. Otol Neurotol. 2005;26(3):392–7. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji T, Yamada K, Kunieda T. Characterization of the dwg mutations: dwg and dwg(Bayer) are new mutant alleles of the Ggt1 gene. Mamm Genome. 2009;20(11–12):711–9. [DOI] [PubMed] [Google Scholar]
- 62.Ding D, Jiang H, Chen GD, et al. N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in γ-glutamyl transferase 1 deficient mice. Aging (Albany NY). 2016;8(4):730–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pena-Llopis S, Ferrando MD, Pena JB. Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol. 2003;65(4):337–60. [DOI] [PubMed] [Google Scholar]
- 64.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009. April;29(2):70–5. [PMC free article] [PubMed] [Google Scholar]
- 65.James SJ, Slikker W, Melnyk S, et al. Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. Neurotoxicology. 2005;26(1):1–8. [DOI] [PubMed] [Google Scholar]
- 66.Wu HP, Hsu CJ, Cheng TJ, et al. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res. 2010;267(1–2):71–7. [DOI] [PubMed] [Google Scholar]
- 67.Ohinata Y, Yamasoba T, Schacht J, et al. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146(1–2):28–34. [DOI] [PubMed] [Google Scholar]