SUMMARY
Background
Based on the encouraging activity and manageable safety profile observed in a phase 1 study, the ECHELON-2 trial was initiated to compare the efficacy and safety of brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone (A+CHP) versus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for the treatment of CD30-positive peripheral T-cell lymphomas (PTCL).
Methods
ECHELON-2 (ClinicalTrials.gov No. NCT01777152) is a double-blind, double-dummy, randomised, placebo-controlled, active-comparator phase 3 study. Eligible adults with previously-untreated CD30-positive PTCL (targeting 75% with systemic anaplastic large cell lymphoma [sALCL]) were randomised 1:1 to receive either A+CHP or CHOP for six or eight 21-day cycles. All subjects received cyclophosphamide 750 mg/m2 and doxorubicin 50 mg/m2 on Day 1 of each cycle intravenously (IV) and prednisone 100 mg daily on Days 1 to 5 of each cycle orally, followed by either brentuximab vedotin 1·8 mg/kg and placebo form of vincristine on Day 1 of each cycle IV (A+CHP arm) or vincristine 1·4 mg/m2 and placebo form of brentuximab vedotin IV (CHOP arm). Randomization was stratified by histological subtype as per local pathology assessment and by international prognostic index (IPI) score. The primary endpoint, progression-free survival (PFS) per blinded independent central review, was analysed by the intent-to-treat.
Findings
A total of 452 subjects were enrolled between January 24, 2013 and November 07, 2016 and 226 subjects were randomly assigned to each arm. The hazard ratios of both the PFS (0·71 [95% CI: 0·54, 0·93], p=0·0110) and overall survival (OS) (0·66 [95% confidence interval {CI}: 0·46, 0·95], p=0·0244) favoured A+CHP over CHOP. The median PFS was 48·2 months (95% CI: 35·2, not evaluable) versus 20·8 months (95% CI: 12·7, 47·6), for A+CHP and CHOP, respectively. The median was not met for OS. Adverse events (AEs), including incidence and severity of febrile neutropenia (in 41 subjects (18%) in A+CHP arm and 33 (15%) in CHOP) and peripheral neuropathy (in 117 subjects (52%) in A+CHP arm and 124 (55%) in CHOP), were similar between arms. Fatal AEs occurred in 7 subjects (3%) in the A+CHP arm and 9 subjects (4%) in the CHOP arm.
Interpretation
Frontline treatment with A+CHP is superior to CHOP for subjects with CD30-positive PTCL as demonstrated by a statistically significant improvement in PFS and OS with a manageable safety profile.
Funding
Supported by Seattle Genetics, Inc. and Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
INTRODUCTION
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of aggressive non-Hodgkin lymphoma (NHL) accounting for approximately 10% of all NHL cases in the United States and Europe, and as high as 24% in parts of Asia.1 The most common PTCLs are the so-called nodal PTCLs which include PTCL-not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic lymphoma kinase (ALK)-positive or negative systemic anaplastic large cell lymphoma (sALCL). These subtypes are usually treated similarly with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens.2,3 However, anthracycline-containing regimens result in low complete remission (CR) rates and poor rates of progression-free survival (PFS) and overall survival (OS).4-6 Even with the relatively more favourable ALK-positive sALCL, 5-year OS is less than 50% for older patients and those with adverse prognostic factors (International Prognostic Index [IPI] ≥2).7 With intensified approaches in frontline therapy, such as the addition of etoposide to CHOP and/or consolidation with stem cell transplantation (SCT), there still remains a considerable risk for disease relapse or early progression,8,9 underscoring the high unmet need in these patients. Moreover, randomised studies to guide therapy are lacking in PTCL, with management approaches primarily derived from phase 2 studies, retrospective series, and clinical experience.4,7,8,10-12
CD30 is universally expressed and is pathognomonic in sALCL. Among non-sALCL subtypes, CD30 expression is variable, with estimates from approximately 58% to 64% in PTCL-NOS, 43% to 63% in AITL, 55% in adult T-cell leukaemia/lymphoma (ATLL), and 0% to 100% in enteropathy-associated T-cell lymphoma (EATL).13,14 Brentuximab vedotin is an antibody–drug conjugate composed of an anti-CD30 monoclonal antibody conjugated by a protease-cleavable linker to the microtubule-disrupting agent monomethyl auristatin E. It is presently approved for several indications, including the treatment of adults with relapsed sALCL.15 Based on the encouraging activity and manageable safety profile observed in a phase 1 trial16 combining brentuximab vedotin with cyclophosphamide, doxorubicin, and prednisone (CHP [CHOP without vincristine to eliminate the risk of overlapping neurotoxicity that could be worsened by delivering two microtubule-disrupting agents]), the double-blinded phase 3 ECHELON-2 trial was initiated to compare the efficacy and safety of brentuximab vedotin in combination with CHP (A+CHP) with standard CHOP for the treatment of previously-untreated subjects with CD30-positive PTCL.
RESEARCH IN CONTEXT
Evidence before this study
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of rare, aggressive lymphoproliferative disorders that represent approximately 10% to 15% of non-Hodgkin’s lymphoma (NHL) cases worldwide.
Clinical outcomes for patients with previously untreated PTCL depend upon histologic subtype, but are typically poor. Most subtypes of PTCL are treated similarly with combination chemotherapy, most commonly: cyclophosphamide [C], doxorubicin [H], vincristine [O], and prednisone [P] (CHOP) or CHOP-like regimens.
Several of the PTCL subtypes express CD30, most notably systemic anaplastic large cell lymphoma (sALCL), for which CD30 expression is a hallmark of the diagnosis. Brentuximab vedotin is an antibody-drug conjugate with demonstrated efficacy in the treatment of relapsed or refractory sALCL. In addition, combination treatment of brentuximab vedotin with cyclophosphamide, doxorubicin, and prednisone (CHP [A+CHP]) in a phase 1 trial, demonstrated encouraging activity and a manageable safety profile. Given the results of brentuximab vedotin monotherapy in the relapsed and refractory sALCL setting, and its demonstrated tolerability when combined with CHP, the ECHELON-2 trial was designed to evaluate the efficacy and safety of A+CHP versus CHOP in subjects with previously untreated, CD30-positive PTCL.
We searched the scientific literature to identify reports of patients with PTCL treated with brentuximab vedotin or CHOP chemotherapy. A PubMed search in June 2012 using the terms (ADCETRIS or “Brentuximab vedotin” or BV) AND (CHOP OR CHP) AND (PTCL or MTCL) identified no other clinical trials of brentuximab vedotin in combination with CHP. Additionally, there were no published reports from randomised, prospective, phase 3 clinical trials establishing the superiority of any regimen over CHOP in untreated patients with PTCL.
Added value of this study
Prior trials that have attempted to improve upon CHOP have demonstrated either no or only modest improvements in response rates and/or PFS with often high rates of toxicity. To our knowledge, this is the first randomised, double-blinded study of a targeted agent combination treatment against standard therapy for this indication and the first reported phase 3 prospective trial in previously untreated patients with PTCL to show an overall survival benefit over CHOP chemotherapy. Our results show that A+CHP improved PFS and overall survival compared with CHOP alone in patients with CD30-positive PTCL. Importantly, these improvements in survival came without an apparent increase in toxicity.
Implications of all the available evidence
We consider these results to be potentially practice changing and regulatory approval is being sought from health authorities worldwide for the use of A+CHP in the treatment of patients with previously untreated CD30-positive PTCL.
METHODS
Study design and participants
ECHELON-2 is a double-blind, double-dummy, randomised, placebo-controlled, active-comparator phase 3 study conducted at 132 sites (including four satellite sites) in 17 countries across North America, Europe, Asia Pacific, and the Middle East. Subjects were enrolled between January 24, 2013 and November 07, 2016. The data cutoff date for this primary analysis was August 15, 2018. Eligible subjects were aged 18 years or older and had previously-untreated, CD30-positive (≥10% of cells by local review; criteria in appendix Text S1) PTCL per the World Health Organization 2008 classification system17 by local assessment. Eligible histologies were limited to the following: ALK-positive sALCL with an International Prognostic Index (IPI) score ≥2, ALK-negative sALCL, PTCL-NOS, AITL, ATLL, EATL, and hepatosplenic T-cell lymphoma. Histologies were assessed by a central pathology laboratory after enrolment. Full eligibility criteria are provided in appendix Text S1.
The trial was conducted in accordance with regulatory requirements and the protocol was approved by institutional review boards and ethics committees at individual sites. All subjects provided written informed consent. Additional trial design details are provided in the protocol.
Randomisation and masking
Subjects were randomly assigned (1:1) to the A+CHP or CHOP arm. Randomisation was performed centrally using an interactive web response system (IWRS) that assigned a unique subject randomisation number and did not specify the actual treatment assignment. Randomisation numbers and their corresponding treatment assignments were assigned to subjects per the randomisation list by sequential ascending block number, and by sequential ascending randomisation numbers within the appropriate strata. The randomisation list was generated by the IWRS vendor, Bracket. Brentuximab vedotin and vincristine were dispensed in a double-blinded, double-dummy manner. Brentuximab vedotin, vincristine, and their placebo replacements were prepared by the pharmacist at each study site, and a pharmacy blind was enforced. The investigators, subjects, Blinded Independent Central Review (BICR), and the sponsor were blinded to treatment assignments. Randomisation was stratified by histologic subtype as per local pathology assessment (ALK-positive sALCL vs. all other histologies) and baseline IPI score (0-1 vs. 2-3 vs. 4-5).
Procedures
Subjects received 21-day cycles of either A+CHP or CHOP. The number of cycles (six or eight) was determined by investigator discretion at registration. All subjects received the CHP components of the CHOP regimen (cyclophosphamide 750 mg/m2 and doxorubicin 50 mg/m2 administered IV on Day 1 of each cycle; prednisone 100 mg daily administered orally on Days 1 to 5 of each cycle). The study utilized a double-dummy design with brentuximab vedotin and placebo form of vincristine (A+CHP arm; brentuximab vedotin 1·8 mg/kg administered IV on Day 1 of each cycle) or vincristine and placebo form of brentuximab vedotin (CHOP arm; vincristine 1·4 mg/m2 [maximum 2·0 mg] administered IV on Day 1 of each cycle) administered after CHP to subjects in a double-blind, active-controlled manner. Post treatment consolidative SCT or radiotherapy was permitted at the investigator’s discretion (SCT intent was prespecified prior to the first cycle of chemotherapy). Details regarding concomitant therapy and permitted dose modifications (appendix Table S1) are provided in appendix Text S1.
Outcomes
The primary endpoint was PFS per BICR, defined as the time from the date of randomisation to the date of first documentation of relapse or progressive disease,18 death due to any cause, or receipt of subsequent systemic chemotherapy to treat residual or progressive PTCL as determined by the investigator, whichever came first. The latter outcome was considered an event because it represents a failure of frontline treatment to achieve a cure. In the absence of progressive disease, receipt of radiotherapy to consolidate response to initial treatment, chemotherapy for the purpose of mobilizing hematopoietic stem cells, or consolidative autologous or allogeneic SCT were not considered events. The key alpha-controlled secondary endpoints were PFS per BICR for subjects with centrally-confirmed sALCL, CR rate per BICR following the completion of study treatment, OS, and objective response rate (ORR) per BICR. Other secondary and exploratory endpoints are described in the protocol.
Lymphoma response and progression were assessed using the 2007 Revised Response Criteria for Malignant Lymphoma.18 Radiographic disease evaluations were submitted to an independent third-party imaging facility for blinded review. Computed tomographic (CT) and positron emission tomography (PET) scans were performed at screening, after Cycle 4, and at the end of treatment (EOT). In long-term follow-up, CT scans were required at nine, 12, 15, 18, 21, and 24 months after initiation of study treatment, and every six months thereafter until the subject experienced disease progression, death, or analysis of the primary endpoint, whichever came first. Subjects were followed for survival. Safety outcomes were the surveillance and recording of adverse events (defined according to the Medical Dictionary for Regulatory Activities [MedDRA], version 21·0, and the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4·03). Additional long-term follow-up and safety assessments are described in the protocol.
Statistical Analysis
The trial was powered on the following assumption: a median PFS of 23·9 months for the A+CHP arm and 16·5 months for the CHOP arm. An estimated 238 PFS events would give the trial approximately 80% power to detect a hazard ratio for disease progression or death due to any cause of 0·6895 at a one-sided significance level of 0·025. Enrolment of 450 subjects was planned, targeting 75% (±5%) of subjects with a diagnosis of sALCL per central pathology assessment to ensure the secondary endpoint of PFS in sALCL could be appropriately evaluated. Important changes to the targeted enrolment and primary analysis timing are described in the Supplementary Appendix (appendix Text S2).
Formal statistical tests were performed for PFS per BICR and for the key alpha-controlled secondary endpoints. Following the statistically significant test of the primary analysis of PFS in favour of the A+CHP arm, a fixed sequence testing procedure, used to ensure type 1 error control, was performed for the key secondary endpoints at an unadjusted alpha level until the preceding null hypothesis was not rejected. Testing was carried out in the following order: 1) PFS per BICR for subjects with centrally-confirmed sALCL; 2) CR rate per BICR; 3) OS; and 4) ORR per BICR. All statistical tests were performed using a two-sided alpha of 0·05. Confidence intervals (CIs) were calculated at a two-sided 95% level. Results favouring the treatment group with P<0·05 are statistically significant at the one-sided 0·025 level.
For the primary efficacy analysis, the stratified log-rank test (by the randomisation stratification factors) was used to compare the difference in PFS between the treatment groups. Estimation of the hazard ratio (HR) was based upon the stratified Cox regression model. PFS was also summarised using the Kaplan-Meier method. Similar methods were used for the key secondary efficacy endpoints of PFS in subjects with sALCL and OS. The PFS and OS median follow-up were calculated using reverse Kaplan Meier method.19 The ORR and CR rate between the experimental arm and the standard-of-care arm were tested using the Cochran-Mantel-Haenszel (CMH) test, stratified by the randomisation stratification factors.
An Independent Data Monitoring Committee monitored safety and assessed the results of an interim analyses for futility (appendix Text S2). All efficacy evaluations were performed in the intention-to-treat (ITT) population unless otherwise specified. Safety was analysed in subjects who received any amount of brentuximab vedotin or any component of CHOP (the safety population). Analyses were done with SAS version 9·4.
This study was registered with ClinicalTrials.gov number NCT01777152.
Role of the Funding Source
Research was funded by Seattle Genetics, Inc. and Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. This research was supported in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. The funders and ECHELON-2 steering committee members jointly designed the trial. The investigators and funders collected and interpreted the data, and the funders analysed the data. Medical writing assistance was funded by Seattle Genetics, Inc., and provided by Seattle Genetics, Inc. and MMS Holdings, Inc. All authors had access to the data, contributed to the manuscript development, approved the manuscript for submission, and vouch for its integrity. Steven Horwitz had final authority over the manuscript and the decision to submit for publication.
RESULTS
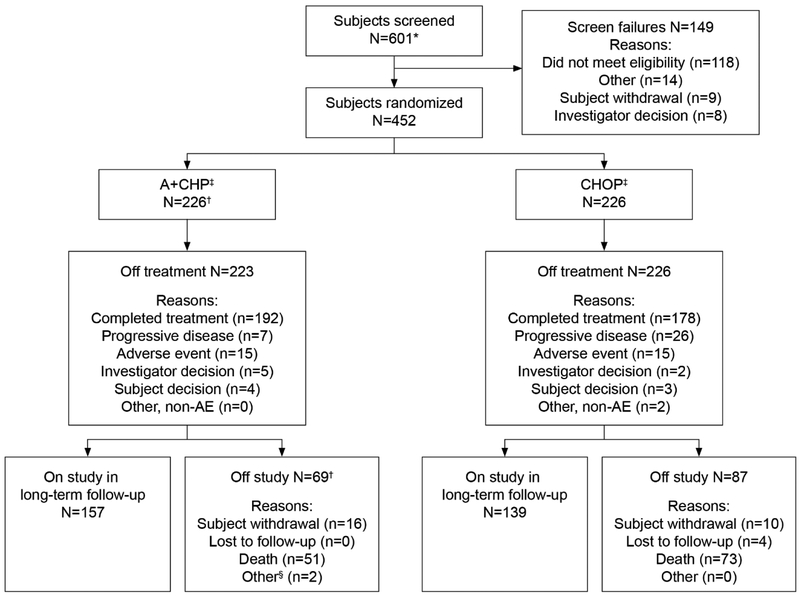
A total of 452 subjects across 17 countries were recruited and randomised to receive either A+CHP (N=226) or CHOP (N=226) between January 24, 2013 and November 07, 2016 (Figure 1: CONSORT diagram). Baseline characteristics were generally balanced between the two treatment arms (Table 1 and appendix Table S2). Overall, the median age was 58 years (range, 18 to 85). The study enrolled subjects with advanced disease (Stage III, 124 subjects [27%] and Stage IV, 240 subjects [53%]; IPI ≥2, 351 subjects [78%]) and most subjects (316 subjects [70%]) had sALCL (218 subjects [48%] ALK-negative and 98 subjects [22%] ALK-positive). Consolidative SCT was delivered in 50 subjects (22%) in the A+CHP arm and 39 subjects (17%) in the CHOP arm following EOT at the discretion of the investigator (appendix Table S3).
Figure 1: CONSORT diagram.
A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone. *Screening informed consents were obtained for seven subjects to allow sites to perform screening activities that were not considered standard of care at their sites. The remaining 594 subjects signed the full informed consent for the study. †Includes three subjects who were randomised to the A+CHP arm but did not receive study treatment. ‡A total of 89 subjects in the A+CHP arm and 81 subjects in the CHOP arm were prespecified by the investigator at baseline to receive consolidative stem cell transplantation. §Other reasons for study discontinuation were change in diagnosis for one subject and one subject who was found to be ineligible after randomization and who did not receive any study treatment.
Table 1:
Baseline subject demographic and disease characteristics*
| Characteristic | A+CHP (N=226) |
CHOP (N=226) |
|---|---|---|
| Male sex | 133 (59%) | 151 (67%) |
| Age (years) | ||
| Median | 58·0 | 58·0 |
| Min, Max | 18, 85 | 18, 83 |
| Race | ||
| Asian | 45 (20%) | 54 (24%) |
| Black or African American | 12 (5%) | 6 (3%) |
| White | 139 (62%) | 142 (63%) |
| Native Hawaiian or Other Pacific Islander | 1 (0%) | 0 |
| Other/Unknown | 29 (13%) | 24 (11%) |
| ECOG performance† | ||
| 0 | 84 (37%) | 93 (41%) |
| 1 | 90 (40%) | 86 (38%) |
| 2 | 51 (23%) | 47 (21%) |
| Diagnosis‡ | ||
| sALCL | 162 (72%) | 154 (68%) |
| ALK-positive | 49 (22%) | 49 (22%) |
| ALK-negative | 113 (50%) | 105 (46%) |
| PTCL-NOS | 29 (13%) | 43 (19%) |
| AITL | 30 (13%) | 24 (11%) |
| ATLL | 4 (2%) | 3 (1%) |
| EATL | 1 (0%) | 2 (1%) |
| Disease stage at diagnosis§ | ||
| Stage I | 12 (5%) | 9 (4%) |
| Stage II | 30 (13%) | 37 (16%) |
| Stage III | 57 (25%) | 67 (30%) |
| Stage IV | 127 (56%) | 113 (50%) |
| Baseline IPI scored¶ | ||
| 0 | 8 (4%) | 16 (7%) |
| 1 | 45 (20%) | 32 (14%) |
| 2 | 74 (33%) | 78 (35%) |
| 3 | 66 (29%) | 66 (29%) |
| 4 | 29 (13%) | 25 (11%) |
| 5 | 4 (2%) | 9 (4%) |
Data are n (%), unless stated otherwise. Data shown are for the intention-to-treat population. Percentages may not total 100 because of rounding. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; AITL=angioimmunoblastic T-cell lymphoma; ALK=anaplastic lymphoma kinase; ATLL=adult T-cell leukaemia/lymphoma; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; EATL=enteropathy-associated T-cell lymphoma; ECOG=eastern cooperative oncology group; IPI=international prognostic index; max=maximum; min=minimum; PTCL-NOS=peripheral T-cell lymphoma-not otherwise specified; sALCL=systemic anaplastic large cell lymphoma.
A full description of baseline characteristics can be found in appendix.
Values for ECOG performance status range from 0 to 5, with higher scores indicating greater disability.
Diagnosis per local assessment.
The Ann Arbor staging system ranges from I to IV, with higher stages indicating more widespread disease.
The IPI score is determined based on a subject’s disease characteristics and represents increasing degrees of risk.
The data cutoff for the primary analysis was performed after a total of 219 PFS events had occurred (appendix Table S4). The PFS hazard ratio was 0·71 (95% confidence interval [CI]: 0·54, 0·93; p=0·0110), equating to a 29% reduction in the risk of a PFS event for A+CHP versus CHOP (Figure 2A). After a median follow-up of 36·2 months (95% CI: 35·9, 41·8), the median PFS in the A+CHP arm was longer than that of CHOP (48·2 months [95% CI: 35·2, -] versus 20·8 months [95% CI: 12·7, 47·6], respectively). The 3-year PFS was 57·1% (95% CI: 49·9, 63·7) for A+CHP compared to 44·4% with CHOP (95% CI: 37·6, 50·9) (appendix Table S4).
Figure 2: Progression-free survival as assessed per Blinded Independent Central Review.
Figure 2A: Kaplan–Meier estimates of progression-free survival, by treatment arm, according to Blinded Independent Central Review for the intent-to-treat population. The hazard ratio for treatment with A+CHP versus CHOP and the 95% CIs were computed from log-rank test using stratification factors (ALK-positive sALCL: yes/no and IPI score: 0-1/2-3/4-5) at randomization. Tick marks indicate censored data. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; ALK=anaplastic lymphoma kinase; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; CI=confidence interval; IPI=international prognostic index; sALCL=systemic anaplastic large cell lymphoma.
Figure 2B: Forest-Plot analysis of progression-free survival This forest plot shows progression-free survival according to the Blinded Independent Central Review in key prespecified subgroups. The hazard ratio for treatment with A+CHP versus CHOP and the 95% CIs were based on the cox regression model considering stratification factors at randomization. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; AITL=angioimmunoblastic T-cell lymphoma; ALK=anaplastic lymphoma kinase; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; CI=contidence interval; ECOG=eastern cooperative oncology group; IPI=international prognostic index; ITT=intention-to-treat; PTCL-NOS=peripheral T-cell lymphoma-not otherwise specified; sALCL=systemic anaplastic large cell lymphoma.
Prespecified analyses of PFS were similar to the primary analysis of PFS: investigator-assessed PFS (hazard ratio, 0·70 [95% CI: 0·53, 0·92], appendix), BICR-assessed PFS where events were limited to progression and death (hazard ratio 0·75 [95% CI: 0·56, 1·00], appendix), and BICR-assessed PFS where consolidative SCT or consolidative radiotherapy were censored (hazard ratio 0·71 [95% CI: 0·53, 0·94]).
The PFS analyses for important subgroups were generally consistent with the overall study results. Among the different histologic subtypes, ALK-positive sALCL had the lowest hazard ratio point estimate, ALK-negative sALCL and PTCL-NOS were similar to the ITT population, and the AITL hazard ratio point estimate for PFS was above unity. Importantly, this study was not powered to compare efficacy between individual histologic subtypes (Figure 2B).
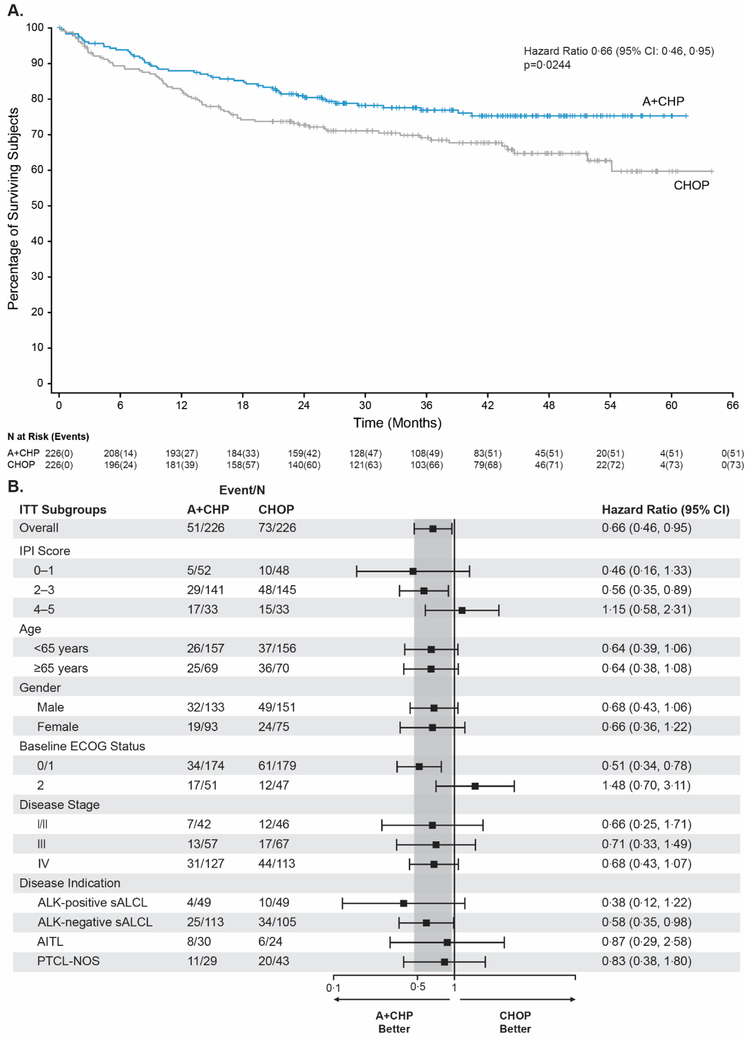
Treatment with A+CHP reduced the risk of death by 34% compared to CHOP (hazard ratio, 0·66 [95% CI: 0·46, 0·95], p=0·0244) (Figure 3A; appendix Table S5). As of the data cutoff date 124 deaths occurred, 51 deaths (23%) in the A+CHP arm and 73 deaths (32%) in the CHOP arm. After a median follow-up of 42·1 months (95% CI: 40·4, 43·8), the median OS was not reached for either arm. Furthermore, the 75th percentile OS was not reached for the A+CHP arm but was 17·5 months for the CHOP arm. OS was numerically in favour of A+CHP for key subgroups, including both non-sALCL histologic subtypes, PTCL-NOS, and AITL (Figure 3B). The confidence intervals for all histologic subtypes and the ITT population again overlapped (Figure 3B).
Figure 3: Overall survival for the ITT population.
Figure 3A: Kaplan-Meier analysis of overall survival, by treatment arm, in the intent-to-treat population. Tick marks indicate censored data. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; CI=confidence interval; ITT=intent-to-treat.
Figure 3B: Forest-Plot analysis of overall survival. This forest plot shows overall survival in key prespecified subgroups. The hazard ratio for treatment with A+CHP versus CHOP and the 95% CIs were based on the cox regression model considering stratification factors at randomization. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; AITL=angioimmunoblastic T-cell lymphoma; ALK=anaplastic lymphoma kinase; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; CI=confidence interval; ECOG=eastern cooperative oncology group; IPI=international prognostic index; ITT=intention-to-treat; PTCL-NOS=peripheral T-cell lymphoma-not otherwise specified; sALCL=systemic anaplastic large cell lymphoma.
Analysis of PFS per BICR for the subset of subjects with centrally-confirmed sALCL was consistent with the results of the primary analysis. There was a 41% reduction in risk of PFS events per BICR for the subset of subjects with sALCL on the A+CHP arm compared to the CHOP arm (hazard ratio, 0·59 [95% CI: 0·42, 0·84], p=0·0031). The CR rate and ORR for subjects treated with A+CHP were significantly higher than those treated with CHOP (p=0·0066 and p=0·0032 for CR rate and ORR, respectively) (Table 2). Similar results were obtained when the CR rate and ORR were evaluated by investigator assessment (p=0·0043 and p=0·0018, respectively).
Table 2:
Summary of response at end of treatment according to the Blinded Independent Central Review
| A+CHP (N=226) |
CHOP (N=226) |
||
|---|---|---|---|
| Response* | |||
| Complete remission | 153 (68%) | 126 (56%) | |
| Partial remission | 35 (15%) | 37 (16%) | |
| Stable disease | 5 (2%) | 11 (5%) | |
| Progressive disease | 15 (7%) | 31 (14%) | |
| Not evaluable† | 18 (8%) | 21 (9%) | |
| Objective response rate | 188 (83%) | 163 (72%) | |
| 95% CI | (77·7, 87·8) | (65·8, 77·9) | |
| Response rate difference (95% CI) | 11·1 (3·4, 18·7) | ||
| P value | 0·0032 | ||
| Complete remission rate | 153 (68%) | 126 (56%) | |
| 95% CI | (61·2, 73·7) | (49·0, 62·.3) | |
| Response rate difference (95% CI) | 11·9 (3·1, 20·8) | ||
| p value | 0·0066 |
Data are n (%).Data shown are for the intention-to-treat population. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; CI=confidence interval.
Best response at End of Treatment was evaluated in accordance with the Revised Response Criteria for Malignant Lymphoma (Cheson, 2007). Complete remission, partial remission, stable disease, progressive disease, and not evaluable are mutually exclusive.
Subjects with no post-baseline response assessments were not evaluable.
Excluding SCT or radiotherapy for consolidation of response to initial therapy, 59 subjects (25%) on the A+CHP arm and 94 subjects (40%) on the CHOP arm received subsequent anticancer therapies for residual or progressive disease (appendix Table S6); 23 subjects (10%) and 49 subjects (22%) received brentuximab vedotin-containing subsequent therapy, respectively.
Most subjects completed treatment as intended with 198 subjects (89%) and 184 subjects (81%) receiving ≥6 cycles in the A+CHP and CHOP arms, respectively (appendix Table S7). The proportion of subjects receiving >6 cycles of treatment was 19% in both the A+CHP and CHOP arms (42 and 44 subjects, respectively) (appendix Table S7). The median relative dose intensity was 99·2% (range, 49 to 104) for brentuximab vedotin in the A+CHP arm and 99·1% (range, 42 to 116) for vincristine in the CHOP arm.
The incidence and severity of treatment-emergent adverse-events (TEAEs) were comparable between arms (Table 3). A higher incidence of diarrhoea (any grade) was reported in the A+CHP arm (85 subjects [38%]) than in the CHOP arm (46 subjects [20%]). The majority (49 of 85 subjects [58%]) of diarrhoea in the A+CHP arm was Grade 1; 23 of 85 subjects (27%) had maximum Grade 2, and 13 of 85 subjects (15%) had maximum Grade 3. Other TEAEs of any grade reported in ≥20% of subjects on the A+CHP arm (versus the CHOP arm) were nausea (103 subjects [46%] vs. 87 subjects [38%]), peripheral sensory neuropathy (100 subjects [45%] vs. 92 subjects [41%]), neutropenia (85 subjects [38%] in both), constipation (64 subjects [29%] vs. 67 subjects [30%]), alopecia (58 subjects [26%] vs. 56 subjects [25%]), pyrexia (58 subjects [26%] vs. 42 subjects [19%]), vomiting (57 subjects [26%] vs. 39 subjects [17%)], fatigue (54 subjects [24%] vs. 46 subjects [20%]), and anaemia (46 subjects [21%] vs. 36 subjects [16%]). Grade 3 or higher events were generally similar between arms. Treatment discontinuations due to adverse events (AEs) occurred in 14 subjects (6%) and 15 subjects (7%) in the A+CHP and CHOP arms, respectively. AEs leading to death occurred in 7 subjects (3%) in the A+CHP arm and 9 subjects (4%) in the CHOP arm; causes of deaths are summarised in appendix Table S8.
Table 3:
Summary of adverse events*
| A+CHP (N=223) |
CHOP (N=226) |
|||
|---|---|---|---|---|
| Adverse events | ||||
| Any adverse events | 221 (99%) | 221 (98%) | ||
| Grade ≥3 adverse events | 147 (66%) | 146 (65%) | ||
| Serious adverse event | 87 (39%) | 87 (38%) | ||
| Discontinued treatment due to adverse event | 14 (6%) | 15 (7%) | ||
| Death due to adverse events | 7 (3%) | 9 (4%) | ||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| Common adverse events | ||||
| Nausea | 103 (46%) | 5 (2%) | 87 (38%) | 4 (2%) |
| Peripheral sensory neuropathy | 100 (45%) | 8 (4%) | 92 (41%) | 6 (3%) |
| Neutropenia | 85 (38%) | 77 (35%) | 85 (38%) | 76 (34%) |
| Diarrhoea | 85 (38%) | 13 (6%) | 46 (20%) | 2 (1%) |
| Constipation | 64 (29%) | 2 (1%) | 67 (30%) | 3 (1%) |
| Alopecia | 58 (26%) | 0 | 56 (25%) | 3 (1%) |
| Pyrexia | 58 (26%) | 4 (2%) | 42 (19%) | 0 |
| Vomiting | 57 (26%) | 2 (1%) | 39 (17%) | 4 (2%) |
| Fatigue | 54 (24%) | 2 (1%) | 46 (20%) | 4 (2%) |
| Anaemia | 46 (21%) | 30 (13%) | 36 (16%) | 23 (10%) |
Data are n (%). Common adverse events are shown for those occurring in ≥20% of subjects in the safety population. A+CHP=brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone.
Adverse events are presented and defined as newly occurring (not present at baseline) or worsening after first dose of any component of A+CHP and CHOP.
The incidence and severity of neutropenia were similar between arms, and lower in the subset of subjects receiving primary prophylaxis with granulocyte-colony stimulating factor (appendix Table S9). Febrile neutropenia was reported in 41 subjects (18%) in the A+CHP arm versus 33 subjects (15%) in the CHOP arm including one Grade 5 event in the CHOP arm. Grade 3 or higher infections occurred in 42 subjects (19%) in the A+CHP arm and 31 subjects (14%) in the CHOP arm.
Peripheral neuropathy events were determined on the basis of a standardized MedDRA query (SMQ) and summarised by event in appendix Table S10. Treatment-emergent peripheral neuropathy events occurred in 117 subjects (52%) in the A+CHP arm and 124 subjects (55%) in the CHOP arm; the majority had a maximum severity of Grade 1 (75 of 117 subjects [64%] and 88 of 124 subjects [71%], respectively). Peripheral neuropathy events returned to baseline or lower in 58 of 117 subjects (50%) in the A+CHP arm and 79 of 124 subjects (64%) in the CHOP arm (appendix Table S10) and the median time to resolution was 17·0 weeks and 11·4 weeks, respectively. Of the subjects with ongoing events at last follow-up, most were Grade 1 (44 of the 61 [72%] subjects in the A+CHP arm and 32 of the 45 [71%] subjects in the CHOP arm). Two subjects in the A+CHP arm and one subject in the CHOP arm had ongoing Grade 3 peripheral neuropathy events.
DISCUSSION
ECHELON-2 is the first prospective trial in PTCL to show an OS benefit over an established standard therapy, CHOP. In this double-blind, double-dummy, randomised, placebo-controlled, active-comparator phase 3 study enrolling 452 subjects with previously-untreated CD30-positive PTCL, A+CHP demonstrated superior PFS and significantly longer OS compared with CHOP. Treatment with A+CHP led to a 29% reduction in the risk of a PFS event and a 34% lower risk of death, with a 77% probability of survival at 36 months. Importantly, these improvements in survival came without an observed increase in toxicity. A+CHP was well-tolerated, with a manageable safety profile compared to CHOP, although the median time to resolution of peripheral neuropathy was longer with A+CHP compared with CHOP (17·0 weeks and 11·4 weeks, respectively). The rates of neutropenia, febrile neutropenia, and neuropathy were similar between A+CHP and CHOP.
For decades CHOP has remained the most commonly used frontline regimen for previously-untreated patients with PTCL.4,20,21 With the exception of low IPI score (<2) ALK-positive sALCL, PTCLs are aggressive neoplasms with poor prognosis. Attempts to improve upon CHOP, primarily in single arm or phase 2 studies, have been largely unsuccessful. Single agents such as alemtuzumab, pralatrexate, and denileukin diftitox have been added to CHOP or a CHOP-like backbone without any clear benefit and often excess toxicity.22-24 Romidepsin plus CHOP has been evaluated in a phase 1b/2 trial, but with a higher rate of toxicity than would be anticipated with CHOP alone.25 A phase 3 randomised trial comparing this regimen to CHOP is ongoing. Similarly, alternate or more intensive combination chemotherapy regimens have failed to demonstrate superiority over CHOP alone.5,26,27
The main frontline therapy thought to offer a potential benefit over CHOP is CHOP plus etoposide (CHOEP).9,20 This assessment is based on a retrospective subset analysis of completed prospective studies, which found a 3-year event-free survival advantage of 75·4% vs. 51% for a subset of younger (≤60 years), more favourable patients with the greatest benefit seen in patients with ALK-positive sALCL. However, CHOEP provided no improvement in OS and older patients experienced greater toxicity, with more pronounced rates of Grades 3/4 leukocytopenia, thrombocytopenia, anaemia, compared with the CHOP.20,28 To date, the superiority of CHOEP remains untested in a prospective randomised trial.
As detailed above, previous attempts to improve upon CHOP have generally followed a one-size-fits-all approach, applying non-targeted therapy to heterogeneous PTCL subtypes. ECHELON-2 capitalizes on the documented single-agent activity of brentuximab vedotin in CD30-positive relapsed/refractory sALCL29,30 and other CD30-positive PTCL subtypes, to improve efficacy in a patient population most likely to benefit. To ensure the key secondary endpoint of PFS in sALCL subtype could be appropriately evaluated, the trial was designed to enrol a target of 75% (±5%) sALCL subjects. As such, the majority (70%) of the ITT population was made up of subjects with sALCL. A limitation of this study was that it was not powered to compare efficacy between individual histologic subtypes and small subgroup sizes preclude definitive determination of the treatment effect in the non-ALCL population. In subjects with PTCL-NOS, the hazard ratio point estimates for PFS and OS were both below 1, while AITL exhibited wide confidence intervals and the hazard ratio point estimates for PFS and OS were 1·4 and 0·87, respectively. It is possible that a future study with a larger number of AITL or non-sALCL subjects could increase the precision by which benefit can be assessed. Nevertheless, the PFS and OS benefits for the study, while most clearly demonstrated for sALCL, are generally consistent across all evaluable histologic subtypes with overlapping confidence intervals.
In addition to superior survival, this trial represents an elevation in the quality of data for studies in PTCL. Our knowledge of the expected outcomes for patients with PTCL is largely based on single-arm phase 2 studies or retrospective analyses.4,6,8,20,28,31 In the prospective, randomised ECHELON-2 trial, the CHOP arm performed better than these historical controls with a median PFS of 20·8 months and a median OS not reached. Possible explanations for these superior outcomes may be attributed to the greater number of subjects with sALCL, including those with ALK-positive sALCL (albeit with an IPI requirement ≥2), enrolment on a clinical trial, and the relatively young age of the subjects (median 58 years). In addition, it remains unknown if CD30 is prognostic among PTCL subtypes such as PTCL-NOS and AITL.7 Despite the higher than expected efficacy in the CHOP arm, A+CHP was statistically superior for all primary and secondary endpoints.
The high rate of subsequent disease progression in previously untreated PTCL has led to the use of consolidation with ASCT as a means of improving long-term outcomes. While phase 2 studies have suggested higher rates of PFS with frontline consolidation using high dose chemotherapy and ASCT,32 no randomised studies have been conducted. Post-treatment consolidation with ASCT has nevertheless become part of the standard treatment plan at many centres, particularly for patients with high risk disease and histologic subtypes other than ALK-positive sALCL. In ECHELON-2, consolidative therapy was permitted, but did not affect the results of the primary or secondary endpoints of PFS and OS as the benefits of A+CHP were seen both with and without censoring the subjects on both arms who received consolidative therapy.
In conclusion, the ECHELON-2 trial has demonstrated that the addition of brentuximab vedotin to CHP resulted in higher rates of progression-free and overall survival without added toxicity and supports the potential for A+CHP to become a new standard of care for many patients with CD30-positive PTCL.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients who participated in this trial and their families, as well as the investigators and staff at all ECHELON-2 clinical sites; the members of the independent data and safety monitoring committee and the independent review committee; and Dana Kennedy, Pharm.D., Eric Sievers, M.D. and Jonathan Drachman, M.D. for their contributions to the design of the study. Medical writing assistance was funded by Seattle Genetics, Inc., and provided by Katie Hutchinson, Ph.D. of Seattle Genetics, Inc. and Elizabeth O’Connor, M.S. of MMS Holdings, Inc.
Footnotes
All ECHELON-2 investigators are listed in the Supplementary Appendix.
DECLARATION OF INTERESTS
Dr. Horwitz reports receiving grant support from Spectrum, grant support and personal fees from Seattle Genetics, Takeda, Kyowa Hakka Kirin, Verastem Oncology, Aileron, ADC Therapeutics, Celgene, and Forty Seven, and personal fees from Portola, Corvus, Miragen, and Innate; Dr. O’Connor, receiving support from Seattle Genetics to conduct the study; Dr. Pro, receiving grant support and personal fees from Seattle Genetics and personal fees from Takeda; Dr. Illidge, receiving personal fees from Takeda; Dr. Fanale, receiving grant support and personal fees from Seattle Genetics during the conduct of the study, grant support, personal fees, and being employed by and holding shares in Seattle Genetics, grant support and personal fees from Takeda, Celgene, Bristol-Myers Squibb, and Merck, grant support from ADC Therapeutics, MedImmune, Gilead, Molecular Templates, and Genentech, and personal fees from Spectrum and Bayer; Dr. Advani, receiving grant support from Agensys, Celgene, Forty Seven, Infinity, Janssen, Kura Oncology, Merck, Millennium, and Regeneron, grant support and consulting and advisory fees from Bristol Myers Squibb, Genentech/Roche, Pharmacyclics, and Seattle Genetics, consulting and advisory fees from Astra Zeneca, Autolus, Bayer Healthcare Pharmaceuticals, Gilead, Juno, Kite, Kyowa Hakko Kirin, NanoString, Spectrum, Sutro Biopharma, and Takeda, and Data Safety Monitoring Board fees from Cell Medica; Dr. Bartlett, receiving research funding from Celgene, Seattle Genetics, Genentech, Kite, Merck, Bristol-Meyers Squibb, Immune Designs, Forty Seven, Affimed, Janssen, Pharmacyclics, Millennium, and Gilead, and advisory board fees from Acerta and Pfizer; Dr. Christensen has nothing to disclose; Dr. Morschhauser, receiving honoraria from Takeda, advisory board fees from Bristol- Myers Squibb, lecture fees from Janssen, advisory board and lecture fees from Celgene and Roche, consultant fees from Epizyme, and consultant, advisory board, and lecture fees from Gilead; Dr. Domingo-Domenech, receiving non-financial support from Seattle Genetics and personal fees from Bristol-Myers Squibb and Takeda; Dr. Rossi, receiving personal fees from Roche, Celgene, Janssen, Amgen, Gilead, Sanofi, Pfizer, AbbVie, Jazz Pharmaceuticals, Novartis, Bristol-Myers Squibb, and Sandoz; Dr. Kim has nothing to disclose; Dr. Feldman, receiving consultant fees from Bristol-Myers Squibb, speaker’s bureau fees and honoraria from Celgene, Pharmacyclis, Janssen, Kite, and AbbVie, and sponsor support, consultant and speaker’s bureau fees, and honoraria from Seattle Genetics; Dr. Lennard has nothing to disclose; Dr. Belada, receiving research support from Seattle Genetics and consultant and advisory board fees from Takeda; Dr. Illés has nothing to disclose; Dr. Tobinai, receiving grant support from GlaxoSmithKline, Servier, and AbbVie, honoraria from Zenyaku Kogyo, and grant support and honoraria from Takeda, Eisai, Celgene, Mundipharma, Janssen, HUYA Bioscience International, Kyowa Hakko Kirin, Chugai Pharma, and Ono Pharma; Dr. Tsukasaki, receiving grant support from Seattle Genetics and Eisai, consultant fees from Ono Pharma and Daiichi-Sankyo, honoraria from Kyowa Hakko Kirin, grant support and honoraria from Celgene and Chugai Pharma, and grant support, consultant fees, and honoraria from HUYA Bioscience International; Dr. Yeh, has nothing to disclose; Dr. Shustov, receiving research funding from Seattle Genetics; Dr. Hüttmann, receiving grant support, honoraria and drug supply for study conduct from Takeda; Dr. Savage, receiving honoraria and advisory board fees from Seattle Genetics, and honoraria from Takeda during the conduct of the study, honoraria and advisory board frees from Bristol-Myers Squibb, Merck, Verastem, Abbvie, and consulting fees from Servier; Dr. Yuen, has nothing to disclose; Dr. Iyer, receiving grant support from Seattle Genetics, Takeda, Roche, Rhizen, Spectrum, Celgene, Gilead, Novarits, Amgen, and Trillium; Dr. Zinzani, receiving advisory board fees and honoraria from Gilead, Sandoz, Johnson & Johnson, Bristol-Myers Squibb, Servier, Takeda, Celtrion, Roche, and Celgene; Dr. Hua has nothing to disclose; Ms. Little, holding shares in and employment with Takeda; Dr. Rao, being employed by and holding shares in Seattle Genetics; Dr. Woolery, being employed by and holding shares in Seattle Genetics; Dr. Manley, being employed by and holding shares in Seattle Genetics and has a patents 62/580,261, 62/739,631, and 62/739,635 licensed to Takeda (all ex-US, except Canada); and Dr. Trümper, receiving grant support from Seattle Genetics and the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung) and grant and non-financial support from Genzyme. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Tang T, Tay K, Quek R, et al. Peripheral T-cell lymphoma: review and updates of current management strategies. Advances in hematology 2010; 2010: 624040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz SM, Zelenetz AD, Gordon LI, et al. NCCN Guidelines® insights: Non-Hodgkin's lymphomas, version 3.2016 featured updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive Cancer Network 2016; 14(9): 1067–79. [DOI] [PubMed] [Google Scholar]
- 3.d'Amore F, Gaulard P, Trumper L, et al. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 Suppl 5: v108–15. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004; 15(10): 1467–75. [DOI] [PubMed] [Google Scholar]
- 5.Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol 2010; 151(2): 159–66. [DOI] [PubMed] [Google Scholar]
- 6.Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26(25): 4124–30. [DOI] [PubMed] [Google Scholar]
- 7.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008; 111(12): 5496–504. [DOI] [PubMed] [Google Scholar]
- 8.Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 2009; 27(1): 106–13. [DOI] [PubMed] [Google Scholar]
- 9.d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012; 30(25): 3093–9. [DOI] [PubMed] [Google Scholar]
- 10.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011; 117(12): 3402–8. [DOI] [PubMed] [Google Scholar]
- 11.Jantunen E, Boumendil A, Finel H, et al. Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: a retrospective study by the EBMT. Blood 2013; 121(13): 2529–32. [DOI] [PubMed] [Google Scholar]
- 12.Perrone G, Corradini P. Autologous stem cell transplantation for T-cell lymphomas. Seminars in hematology 2014; 51(1): 59–66. [DOI] [PubMed] [Google Scholar]
- 13.Bossard C, Dobay MP, Parrens M, et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels. Blood 2014; 124(19): 2983–6. [DOI] [PubMed] [Google Scholar]
- 14.Sabattini E, Pizzi M, Tabanelli V, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica 2013; 98(8): e81–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pro B, Advani RH, Brice P, et al. Five-year survival data from a pivotal Phase 2 study of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2016; 128(22): Abstract 4144. [Google Scholar]
- 16.Fanale MA, Horwitz SM, Forero-Torres A, et al. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 2018; 131(19): 2120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.: IARC; 2008. [Google Scholar]
- 18.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25(5): 579–86. [DOI] [PubMed] [Google Scholar]
- 19.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled clinical trials 1996; 17(4): 343–6. [DOI] [PubMed] [Google Scholar]
- 20.Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 2014; 124(10): 1570–7. [DOI] [PubMed] [Google Scholar]
- 21.Federico M, Bellei M, Marcheselli L, et al. Peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). A new prognostic model developed by the International T cell Project Network. Br J Haematol 2018; 181(6): 760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol 2011; 22(7): 1595–600. [DOI] [PubMed] [Google Scholar]
- 23.Advani RH, Ansell SM, Lechowicz MJ, et al. A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) Alternating with Pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): final results from the T- cell consortium trial. Br J Haematol 2016; 172(4): 535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foss FM, Sjak-Shie N, Goy A, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma 2013; 54(7): 1373–9. [DOI] [PubMed] [Google Scholar]
- 25.Dupuis J, Morschhauser F, Ghesquieres H, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. The Lancet Haematology 2015; 2(4): e160–5. [DOI] [PubMed] [Google Scholar]
- 26.Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer 2013; 119(2): 371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleeson M, Peckitt C, To YM, et al. CHOP versus GEM-P in previously untreated patients with peripheral T-cell lymphoma (CHEMO-T): a phase 2, multicentre, randomised, open-label trial. The Lancet Haematology 2018; 5(5): e190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010; 116(18): 3418–25. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014; 123(20): 3095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pro B, Advani R, Brice P, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017; 130(25): 2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson KR, Horwitz SM, Pinter-Brown LC, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 2017; 123(7): 1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amore F, Relander T, Lauritzen GF, et al. Dose-dense induction followed by autologous stem cell transplant (ASCT) leads to sustained remissions in a large fraction of patients with previously untreated peripheral T-cell lymphomas (PTCLs) - overall and subtype-specific results of a phase II study from the nordic lymphoma group. Haematologica 2009; 94(Suppl 2): 437.19176364 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.