Abstract
Background:
Veterans with hepatitis C infection (HCV) may face geographic obstacles to obtaining treatment.
Objective:
We studied the influence of region and rural versus urban residence on receipt of direct acting anti-viral medications (DAAs) for HCV.
Subjects:
Veterans receiving care within Veterans Affairs Healthcare System born between 1945-65.
Research Design:
Observational study using national electronic health record data.
Measures:
Receipt of DAAs was defined as ≥1 filled prescription from 1/1/2014 to 12/31/2016. Region (South, Northeast, Midwest, and West) and residence (urban, rural-micropolitan, small rural towns, and isolated rural towns) variables were created using residential ZIP codes and rural urban commuting area (RUCA) codes. Multivariable models were adjusted for age, race, gender, severity of liver disease, comorbidities, and prior treatment experience.
Results:
Among 166,353 eligible patients 64,854 received, DAAs. Variation by rural-urban residence depended on region. In unadjusted analyses, receipt varied by rural-urban designations within Midwest, and West regions (p<0.05) but did not vary within the South (p=0.12). Southern rural small town had the lowest incidence of DAA receipt (40.1%) whereas the incidence was 52.9% in Midwestern isolated rural towns. In adjusted logistic analyses, compared to southern urban residents (the largest single group), southern rural small town residents had the lowest odds, OR 0.85: 95% CI 0.75, 0.93, and Midwestern residents from isolated and small rural towns had the highest odds (ORs both 1.27) to receive treatment.
Conclusions:
Substantial geographic variation exists in receipt of curative HCV treatment. Efforts are needed to provide more equitable access to DAAs.
Keywords: Hepatitis C, Direct Acting Antiretroviral, geographic variation, rural-urban variation and veterans
INTRODUCTION
Untreated hepatitis C virus (HCV) infection is associated with substantial resource utilization, morbidity and mortality.1-3 In the United States, 2.7-3.9 million persons are estimated to be chronically infected with HCV and 75-80% of these individuals were born between 1945-1965.4-6 With cure rates greater than 90% and few reported side effects, second-generation (all oral) direct acting anti-virals (DAAs) make cure an attainable goal for nearly all patients.7-12
The Veterans Healthcare Administration System (VA) has made a major commitment to treating hepatitis C including provider training and unrestricted access to DAAs.13 However, if experience with dissemination of antiretroviral treatment for human immunodeficiency virus (HIV) within VA is a guide,14 these steps may not be sufficient to overcome rural-urban and regional disparities in care. On a patient-level, such barriers likely include travel burden, access to transportation, rural/geography barriers and social isolation.15-20 System level barriers may include limited availability of providers experienced in treating patients with HCV.18,19,21 For example, studies conducted within VA and other healthcare systems find that patients with HCV who have not received a gastroenterology (GI) or hepatology visit are less likely to receive HCV treatment.22 A study in the pre-DAA era found that rural patients had less access to HCV specialists.22-24 We previously documented higher HCV testing rates (54%) in urban VA centers compared to rural centers (47%) and modest regional variation in HCV testing based on Veteran Integrated Service Networks (VISNs).25 However, our prior paper did not consider HCV treatment or interactions between region and rural-urban status.
From January 2014 until December 2016, over 60,000 individuals have been treated with DAAs in the VA.8 Despite prior evidence of geographic variation in dissemination of new treatments within VA, little is known about regional and rural-urban variation in provision of DAAs in VA. We hypothesized that DAA adoption would vary by region and rural-urban residence and that these two factors would interact.
METHODS
This analysis is focused on HCV treatment with DAAs in individuals with a positive HCV antibody or quantifiable HCV viral load.
Data Source
We used electronic health record (EHR) data available through the VA national Corporate Data Warehouse (CDW). CDW is a data repository of over 8 million veterans in care starting on October 1, 1999, with at least one VA outpatient visit. It includes all laboratory test results as well as inpatient and outpatient utilization as indicated by procedure (Current Procedural Terminology codes) and diagnosis by International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9) codes. The database also includes patient demographics, vital status, and pharmacy utilization. To ensure complete identification of all relevant HCV tests, trends in completed HCV tests for each VA laboratory were reviewed. Test results were standardized by previously published methods.26
Study Cohort
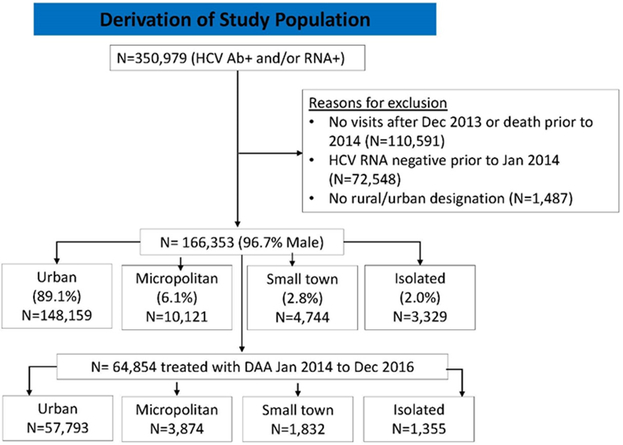
January 1, 2014 was chosen as the index date at which clinicians began prescribing all-oral combination DAA therapies.27-29 Patients with a positive HCV RNA and/or HCV antibody who had at least one VA visit from January 2014 forward were considered eligible. Patients with a negative HCV RNA prior to the index date, or patients with no urban/rural designation (see below) were excluded (Figure 1). VINCI (VA informatics and computing infrastructure) approval using DART (data access request tracker) was also obtained for access and use of CDW electronic data. Information extracted included baseline patient characteristics, and factors that potentially predict or act as barriers to DAA HCV treatment for veterans born between 1945 and 1965.
Figure 1:
Study Flow Chart
Primary Measures
Outcome:
Receipt of second-generation DAA was defined as ≥1 filled prescription of sofosbuvir, ledipasvir, simeprevir, daclatasvir or paritaprevir/ritonavir/ombitasvir plus dasabuvir (PrOD) from January 1, 2014 to December 31, 2016.
Primary Exposures:
Residential status was determined by US Postal Service zone improvement plan (ZIP) codes. Two geographic exposures were considered—region and rural-urban status.
Region:
Using state of residence as indicated by zip codes and standard regional groupings, residence was divided into census regions: Northeast (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont, New Jersey, New York, Pennsylvania), Midwest (Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota), South (Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, District of Columbia, West Virginia, Alabama, Kentucky, Mississippi, Tennessee, Arkansas, Louisiana, Oklahoma, Texas), and West (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming, Alaska, California, Hawaii, Oregon, Washington).
Rural-Urban Status:
ZIP codes were linked to rural urban commuting area (RUCA) codes using the ZIP code/RUCA code crosswalk file available from the University of Washington (http://depts.washington.edu/uwruca/uses.html). Briefly, RUCA codes are frequently used in studies of rural-urban variation in healthcare delivery and health behaviors. RUCA takes into account primary and secondary commuting patterns to Urbanized Areas, Urban Clusters, or smaller population centers to classify census tracts into 33 distinct categories, which typically are combined into fewer and larger categories for data analyses. We applied a commonly-used algorithm to collapse the 33 RUCA codes into a four level geographic residence variable. 30
-
(1)
Urban: have metropolitan cores (identified by the Census Bureau as having populations of at least 50,000, as well as adjacent counties that are economically and socially integrated with that core) and substantial primary or secondary commuting flow patterns to Urbanized Areas.31
-
(2)
Rural-Micropolitan: have micropolitan cores (urban clusters of 10,000–49,999 residents).
-
(3)
Small Rural Towns: have primary commuting flows to or within population centers of between 2,500 and 9,999 residents.
-
(4)
Isolated Rural Towns: less populated rural areas with no primary commuting flows to Urbanized Areas or Urban Clusters.
Secondary Variables
Other variables collected from the EHR and chosen a priori included age, gender, race, prescription fills for specific DAAs, body mass index (BMI), HCV genotype, fibrosis-4 (FIB 4, a composite of aspartate transaminase, alanine transaminase, platelets and age), and Alcohol Use Disorders Identification Test (AUDIT-C, hazardous alcohol use defined as AUDIT-C>=4). ICD-9 administrative codes were used to determine cirrhosis, hepatocellular cancer, liver transplantation, diabetes, HIV, alcohol use disorder, substance use disorder, and severe mental health diagnoses (Appendix).
Statistical Analysis
Urban and rural residents (by RUCA definition) were characterized using descriptive statistics (frequencies and percentages or mean and standard deviation (SD)). We determined associations between region and rural-urban residence and DAA adoption using three logistic regression analyses: 1) unadjusted models; 2) models adjusted for demographics (age, gender, race) only; and 3) models adjusted for demographics, severity of liver disease (FIB4>3.25, cirrhosis, hepatocellular carcinoma, and liver transplantation), and relevant comorbid disease (diabetes, obesity, HIV infection, hazardous alcohol use, alcohol use disorder, substance use disorder, and severe mental illness). Cumulative incidence rates were estimated using Gray’s method for accounting for competing risk (i.e. death).32 Analyses were done using SAS 9.4 (Cary, NC) and R version 3.4.2. Missing data were imputed using multiple imputation (10 imputations) under the missing at random assumption.
RESULTS
Cohort Characteristics
From January 2014 to December 2016, we identified 166,353 eligible persons. The mean age of patients was 60.8 years (SD=4.9 years), 55.4% were white, and 96.7% were men. Overall, 24.8% had FIB-4>3.25, 16.6% of patients had a diagnosis of cirrhosis, 1.5% had hepatocellular cancer, and 0.8% had undergone liver transplant. The majority of patients lived in the south (Table 1, n=76,851 or 46.2%) and/or in an urban setting (n= 148,159 or 89.1%). Only 10.9% (n=18,194) lived in any rural setting. The largest proportion of patients living in a rural setting in any region was in the Midwest (15.6%, data not otherwise shown). During our observation period, 64,854 (39.0% of eligible patients) received at least one prescription of DAA therapy.
Table 1:
Characteristics of 166,353 Direct Acting Antiretroviral (DAA) Eligible Patients, By Rural-Urban Residence
| Characteristic | Total | DAAa Receipt (col %) |
Urbanb (col %) |
Rural (col %) | p-valueg | p-valueh | |||
|---|---|---|---|---|---|---|---|---|---|
| n | col % | Micro- politan |
Small Town |
Isolated | |||||
| n | 166,353 | 100.0% | 64,854 | 148,159 | 10,121 | 4,744 | 3,329 | ||
| Gender | 0.03 | <0.01 | |||||||
| Female | 5,473 | 3.3% | 37.7% | 3.3% | 3.0% | 2.8% | 2.9% | ||
| Male | 160,880 | 96.7% | 39.0% | 96.7% | 97.0% | 97.2% | 97.1% | ||
| Mean Agec in years (SD) | 60.7 (4.5) | NA | 60.8 (4.4) | 60.7 (4.5) | 60.6 (4.6) | 60.7 (4.6) | 61.0 (4.6) | ||
| Race | <0.01 | <0.01 | |||||||
| White | 92,210 | 55.4% | 38.8% | 52.8% | 73.9% | 77.6% | 82.9% | ||
| Black | 64,041 | 38.5% | 40.3% | 41.2% | 19.5% | 15.4% | 9.6% | ||
| Native Hawaiian/Pacific Isl. | 660 | 0.4% | 36.1% | 0.4% | 0.4% | 0.3% | 0.5% | ||
| American Indian/AK Native | 1,343 | 0.8% | 37.6% | 0.7% | 1.2% | 1.3% | 1.9% | ||
| Asian | 332 | 0.2% | 34.6% | 0.2% | 0.2% | 0.1% | 0.1% | ||
| Mixed Race | 1,034 | 0.6% | 40.5% | 0.6% | 0.6% | 0.7% | 0.6% | ||
| Unknown/Other | 6,733 | 4.1% | 29.5% | 4.0% | 4.3% | 4.6% | 4.4% | ||
| Census Region | <0.01 | <0.01 | |||||||
| South | 76,851 | 46.2% | 38.8% | 46.7% | 43.7% | 44.7% | 33.3% | ||
| West | 37,306 | 22.4% | 37.7% | 22.2% | 25.2% | 22.6% | 24.5% | ||
| Midwest | 30,646 | 18.4% | 41.7% | 17.5% | 24.5% | 25.4% | 32.4% | ||
| Northeast | 21,550 | 13.0% | 38.1% | 13.6% | 6.6% | 7.3% | 9.8% | ||
| HCV Genotype | <0.01 | <0.01 | |||||||
| 1 | 104,453 | 62.8% | 51.8% | 63.5% | 58.1% | 57.0% | 55.4% | ||
| 2 | 11,688 | 7.0% | 47.8% | 6.8% | 8.5% | 8.8% | 10.5% | ||
| 3 | 7,423 | 4.5% | 43.2% | 4.3% | 5.8% | 5.9% | 5.4% | ||
| 4 | 1,180 | 0.7% | 46.5% | 0.7% | 0.6% | 0.5% | 0.5% | ||
| 5 or 6 | 25 | 0.0% | 60.0% | 0.01% | 0.01% | 0% | 0% | ||
| Multiple Genotypes | 458 | 0.3% | 56.6% | 0.3% | 0.3% | 0.3% | 0.2% | ||
| Unknown | 41,126 | 24.7% | 2.9% | 24.4% | 26.7% | 27.5% | 28.2% | ||
| HCV Complications | |||||||||
| FIB4>3.25d | 26,656 | 24.8% | 45.8% | 24.8% | 24.7% | 25.4% | 22.8% | <0.01 | <0.01 |
| Cirrhosis | 27,568 | 16.6% | 44.4% | 16.7% | 15.4% | 15.9% | 13.5% | <0.01 | <0.01 |
| Hepatocellular Carcinoma | 2,523 | 1.5% | 30.1% | 1.5% | 1.4% | 1.4% | 1.5% | 0.65 | 0.25 |
| Liver Transplant | 1,325 | 0.8% | 56.3% | 0.8% | 0.9% | 0.8% | 1.0% | 0.32 | 0.13 |
| Treatment Experience | 10,156 | 6.1% | 60.1% | 6.1% | 6.1% | 6.2% | 5.9% | 0.94 | 0.97 |
| Prior Interferon | 9,127 | 5.5% | 58.7% | 5.5% | 5.5% | 5.7% | 5.3% | ||
| Prior Boceprevir | 839 | 0.5% | 73.1% | 0.5% | 0.5% | 0.5% | 0.4% | ||
| Prior Telaprevir | 190 | 0.1% | 73.2% | 0.1% | 0.1% | 0.04% | 0.2% | ||
| Specialist Clinic Visit | |||||||||
| Gastroenterology | 103,159 | 62.0% | 45.3% | 62.4% | 59.6% | 59.0% | 57.1% | <0.01 | <0.01 |
| Infectious Disease | 36,803 | 22.1% | 46.3% | 22.4% | 17.5% | 16.4% | 16.9% | <0.01 | <0.01 |
| Comorbid Disease | |||||||||
| Diabetes | 48,627 | 29.2% | 40.8% | 29.6% | 26.7% | 27.2% | 24.2% | <0.01 | <0.01 |
| BMI>30 kg/m2 d | 37,787 | 30.1% | 46.1% | 29.9% | 31.1% | 30.8% | 33.1% | <0.01 | <0.01 |
| HIV | 4,481 | 2.7% | 46.8% | 2.9% | 1.1% | 0.8% | 0.7% | <0.01 | <0.01 |
| AUDIT C >=4d | 18,114 | 16.7% | 35.3% | 16.6% | 17.6% | 17.7% | 18.3% | <0.01 | <0.01 |
| Alcohol Use Disorder | 65,635 | 39.4% | 37.0% | 40.1% | 35.0% | 35.5% | 28.8% | <0.01 | <0.01 |
| Substance Use Disorder | 68,364 | 41.1% | 36.9% | 42.2% | 33.1% | 32.4% | 27.5% | <0.01 | <0.01 |
| Severe Mental Illnesse | 66,021 | 39.7% | 39.8% | 40.0% | 38.1% | 37.8% | 35.3% | <0.01 | <0.01 |
| Mortalityf | 20,293 | 12.2% | 8.4% | 12.2% | 12.9% | 12.2% | 10.9% | 0.02 | 0.54 |
| After Treatment | 1,697 | 1.0% | NA | 1.0% | 1.2% | 1.1% | 1.0% | 0.56 | 0.23 |
Raw percentages of the individuals with the given characteristic
Categorize rural vs. urban Veteran residence by RUCA (Rural-urban commuting area) codes linked to residential ZIP.
Age on January 1, 2014
Percentages of missing data: 35.3%(N=58,790) missing FIB-4; 24.5% (N=40,741) missing BMI; 34.9% (N=57,975) missing AUDIT-C
Includes major depression, bipolar disorder, post-traumatic stress disorder, and schizophrenia
Between January 1, 2014 and December 31, 2016
Comparison of 4 categories of Region using Chi-square test of association
Comparions of Urban and Rural using Chi-square test of association
Abbreviations: DAA, direct acting anti-retroviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; SD, standard deviation; BMI, body mass index; AUDIT-C, alcohol use disorders identification test; FIB4, Fibrosis 4
Baseline Laboratory data and AUDIT-C score: Closest to January 2014, and restricted to within one year prior to baseline. FIB-4 score = [age × aspartate aminotransferase] / [platelets × alanine aminotransferase1/2 ]
Rural persons were more likely than urban to be white (76.5% vs. 52.8%, P < 0.01 for overall race comparison) and less likely to have a substance or alcohol use diagnosis (31.9% vs. 42.2%, p < 0.01 and 34.0% vs. 40.1%, p < 0.01 respectively).
Predictors of DAA Receipt
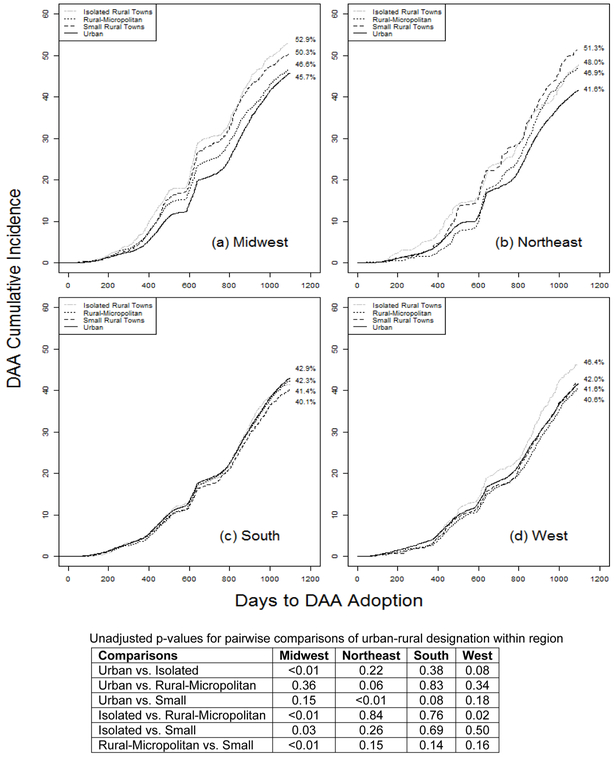
Urban-rural variation in incident DAA prescription depended upon region (Figure 2 a-d). Midwestern and Northeastern veterans experienced the greatest variation in receipt of DAAs by rural-urban residence (Figure 2a-2b). Given the results of Figure 2 and the pairwise comparisons within region (Figure 2 table), we created a 16 level variable that addresses the interaction between region and urban/rural status.
Figure 2 a-d:
Cumulative Incidence of Adoption of Direct Acting Antiretroviral (DAA) Treatment by Region and Rurality from index date (1/1/2014) to cohort end (12/31/2016) accounting for competing risk of death. Values in the table represent unadjusted p-values for pairwise comparisons of urban-rural designation within region (i.e. comparison of the curves within a region) demonstrating the interaction between region and rural-urban designation.
Before and after adjustment (Table 2) DAA receipt varied by region and rural-urban status. Compared to southern urban dwellers, those living in southern small towns had 0.85 times the odds (95% CI 0.77, 0.93) of receiving DAAs and those living in all areas of the Midwest (OR range from 1.11 to 1.27)) had greater odds of receiving DAAs. Other independent positive predictors of DAA receipt included: being male, severe liver disease, a diagnosis of cirrhosis, receipt of a liver transplant, prior treatment for HCV, being obese, having a diagnosis of HIV, and having a diagnosis of severe mental illness. Presence of Hepatocellular Cancer, a diagnosis of substance use, or a diagnosis of alcohol use disorder were associated with lower odds of treatment.
Table 2:
Association of Patient Characteristics with Direct Acting Antiretroviral (DAA) Adoption
| Unadjusted | Adjusted for Demographics | Fully Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Region | <0.01 | <0.01 | <0.01 | |||||||||
| South-Urban | Ref | Ref | Ref | |||||||||
| Isolated Rural Towns | 0.92 | 0.82 | 1.04 | 0.94 | 0.83 | 1.06 | 0.94 | 0.83 | 1.06 | |||
| Rural Micropolitan | 0.95 | 0.89 | 1.01 | 0.95 | 0.90 | 1.02 | 0.95 | 0.89 | 1.01 | |||
| Small Rural Towns | 0.85 | 0.78 | 0.93 | 0.87 | 0.79 | 0.95 | 0.85 | 0.77 | 0.93 | |||
| Midwest-Urban | 1.12 | 1.08 | 1.15 | 1.12 | 1.08 | 1.15 | 1.15 | 1.12 | 1.19 | |||
| Isolated Rural Towns | 1.24 | 1.10 | 1.40 | 1.28 | 1.13 | 1.44 | 1.27 | 1.12 | 1.44 | |||
| Rural Micropolitan | 1.07 | 0.98 | 1.16 | 1.10 | 1.01 | 1.19 | 1.11 | 1.02 | 1.19 | |||
| Small Rural Towns | 1.22 | 1.09 | 1.37 | 1.26 | 1.13 | 1.42 | 1.27 | 1.13 | 1.43 | |||
| Northeast-Urban | 0.96 | 0.92 | 0.99 | 0.95 | 0.92 | 0.98 | 0.93 | 0.90 | 0.96 | |||
| Isolated Rural Towns | 1.04 | 0.83 | 1.30 | 1.06 | 0.85 | 1.32 | 1.00 | 0.79 | 1.25 | |||
| Rural Micropolitan | 1.10 | 0.94 | 1.28 | 1.12 | 0.96 | 1.31 | 1.07 | 0.91 | 1.25 | |||
| Small Rural Towns | 1.22 | 0.99 | 1.52 | 1.26 | 1.01 | 1.55 | 1.24 | 1.00 | 1.54 | |||
| West-Urban | 0.95 | 0.92 | 0.97 | 0.93 | 0.86 | 1.01 | 1.00 | 0.97 | 1.03 | |||
| Isolated Rural Towns | 1.10 | 0.96 | 1.27 | 1.15 | 1.00 | 1.33 | 1.21 | 1.05 | 1.40 | |||
| Rural Micropolitan | 0.89 | 0.82 | 0.97 | 0.93 | 0.86 | 1.01 | 0.94 | 0.87 | 1.03 | |||
| Small Rural Towns | 0.94 | 0.83 | 1.07 | 0.98 | 0.87 | 1.12 | 1.02 | 0.90 | 1.16 | |||
| Age | 1.005 | 1.00 | 1.01 | <0.01 | 0.999 | 0.997 | 1.001 | 0.42 | ||||
| Female | 0.97 | 0.92 | 1.03 | 0.33 | 0.91 | 0.86 | 0.96 | <0.01 | ||||
| Race | <0.01 | <0.01 | ||||||||||
| White | Ref | Ref | ||||||||||
| Black | 1.07 | 1.04 | 1.09 | 1.09 | 1.07 | 1.12 | ||||||
| Native Hawaiian/Pacific Isl. | 0.90 | 0.77 | 1.05 | 0.87 | 0.74 | 1.03 | ||||||
| American Indian/AK Native | 0.95 | 0.85 | 1.07 | 0.96 | 0.86 | 1.08 | ||||||
| Asian | 0.85 | 0.68 | 1.07 | 0.82 | 0.65 | 1.03 | ||||||
| Mixed Race | 1.08 | 0.95 | 1.22 | 1.08 | 0.95 | 1.22 | ||||||
| Unknown/Other | 0.66 | 0.63 | 0.70 | 0.66 | 0.62 | 0.70 | ||||||
| HCV Complications | ||||||||||||
| FIB4>3.25 | 1.16 | 1.13 | 1.20 | <0.01 | ||||||||
| Cirrhosis | 1.18 | 1.15 | 1.22 | <0.01 | ||||||||
| Hepatocellular Carcinoma | 0.46 | 0.42 | 0.50 | <0.01 | ||||||||
| Liver Transplant | 1.70 | 1.52 | 1.92 | <0.01 | ||||||||
| Prior Treatment Experience | 2.29 | 2.20 | 2.39 | <0.01 | ||||||||
| Comorbid Disease | ||||||||||||
| Diabetes | 0.99 | 0.97 | 1.02 | 0.62 | ||||||||
| BMI>30 kg/m2 | 1.15 | 1.12 | 1.18 | <0.01 | ||||||||
| HIV | 1.37 | 1.29 | 1.46 | <0.01 | ||||||||
| AUDIT C >=4 | 0.72 | 0.70 | 0.74 | <0.01 | ||||||||
| Alcohol Use Dependence | 0.95 | 0.92 | 0.97 | <0.01 | ||||||||
| Substance Use Dependence | 0.83 | 0.81 | 0.85 | <0.01 | ||||||||
| Severe Mental Illness | 1.08 | 1.05 | 1.10 | <0.01 | ||||||||
DISCUSSION
Second-generation DAA therapies have ushered in an era of safer, better-tolerated treatments for HCV. Our analyses suggest that substantial geographic disparities exist within the first 3 years of approval of second-generation DAAs, and those disparities differ by urban/rural status (i.e. interaction between urban/rural status and region), with the incidence of DAA treatment receipt ranging from 40.1% to 52.9% (Figure 2). After adjustment, in models examining the odds of receiving a DAA, and compared to the largest group (southern residents living in an urban setting), Midwestern residents in any setting had greater odds of receiving DAAs. Residents of the rural south had lower odds of receiving DAAs compared to their urban southern counterparts.
It is not surprising that variation in utilization by rural settings is influenced by region 33. Not all rural environments are the same across the United States. For example, rural communities in Vermont, Alabama, Iowa, and Alaska are likely to vary substantially in their physical environments, access to healthcare, and cultural contexts. This was apparent in our study, which found significant variation in DAA use in rural compared to urban communities, depending on the geographic region examined. The variations in DAA use between rural and urban residence observed in the Northeast and Midwest were much less pronounced in the South.
A previous study in the pre-DAA era found that although rural patients had less access to HCV specialists, this did not translate to lower HCV treatment rates.22-24 The lack of difference in treatment rates for urban and rural veterans in prior studies during the interferon-era may reflect the very small number of treated patients overall in that era. Treatment rates have increased by over 12-fold with the wide availability of all-oral DAA’s, enhancing statistical power to detect a significant difference between treatment subgroups.8
While the VA is one of the few healthcare systems that has treated enough patients with DAAs to support a study addressing this question, it is important to keep in mind that the VA differs in important ways from US non-Veteran health care. VA patients have few insurance-associated barriers to care, many of which are inherent to privatized, insurance-based health systems. Non-VA rural populations possess more elderly patients and children, higher unemployment and underemployment, as well as higher percentages of poor, uninsured, and underinsured residents, less affected by government-operated medical care through the VA.34,35 Health literacy and education is also not directly assessed which may impact important decisions to treat and may lead to differences in DAA adoption. Therefore, our results may not be generalizable to a system where cost and insurance are paramount determinants of healthcare utilization.36,37
Furthermore, due to the low percentage of women in our study, as well as the VA caring for a largely male population, examinations of gender disparities or gaps in practice patterns cannot be fully assessed. Disparities in health care can also be influenced by physician availability, clinical judgment and patient-level factors such as knowledge and willingness to seek treatment and travel burden. We did not have the data to evaluate these factors.
Despite these limitations, our study has several strengths. Most importantly, our analyses use a national database summarizing all veterans’ prescribed DAA therapy from 2014 to December 2016. The VA health system is America’s largest integrated health care system providing comprehensive services to over 8 million Veterans each year via over 1,700 sites of care. Since VA funding for HCV is centralized nationally 13, it seems unlikely that variations in funding support within VA would act as a confounder for analyses of geographic disparities. Additionally, although most patients included were from an urban setting, this study illustrates a substantial number of patients are DAA adopters that live in rural settings, suggesting a potential to expand treatment efforts directed at this population. Additional strengths include the ability to track multiple DAA therapies over time including newer DAA treatments such as daclatasvir or PrOD.
In conclusion, Midwestern and Northeastern veterans are more likely to be early adopters of second-generation DAA treatments for HCV. The south census region had the lowest national treatment rates. Urban-rural differences in DAA treatment varied by region. Within the south, rural veterans were the least likely to receive treatment. In contrast, within the Midwest and Northeast, rural residence was associated with an increased likelihood of treatment. Future studies are needed to explore provider specific characteristics and prescribing habits within these settings in addition to patient knowledge assessments to explain these disparities. With these data, dedicated, government-sponsored interventions should target Southern-dwelling veterans in an effort to improve overall adoption of HCV treatment.
Supplementary Material
Acknowledgments
Funding:
NIH/NIAAA (U24 AA020794, U01 AA027090, U10 AA013566), VA-ORH, NIH/NCATS (UL1TR001863)
Abbreviations:
- DAA
direct acting anti-viral
- HCV
hepatitis C virus
- VA
Veterans Administration
- HIV
human immunodeficiency virus
- SD
standard deviation
- AUDIT-C
Alcohol Use Disorders Identification Test
Footnotes
There are no conflicts of interest
Contributor Information
Basile Njei, Department of Gastroenterology and Hepatology, Yale University School of Medicine, VA Connecticut Healthcare System, 333 Cedar Street, New Haven, CT, 06516, (312) 415-7525, basile.njei@yale.edu.
Denise Esserman, Department of Biostatistics, Yale University School of Public Health, VA Connecticut Healthcare System, 300 George Street, New Haven, CT 06510-3210, (203)785-4297,denise.esserman@yale.edu.
Supriya Krishnan, Department of Internal Medicine, Yale University School of Medicine, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, CT, 06516, (203) 932-5711 ×5306, Supriya.Krishnan@va.gov.
Michael Ohl, University of Iowa Carver College of Medicine, Veterans Rural Health Resource Iowa City VA Medical Center, 601 Highway 6 West Iowa City, IA, 52246, (319)338-0581 ext. 3534, Michael.ohl@va.gov.
Janet P. Tate, Department of Internal Medicine, Yale University School of Medicine, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, CT,06516, (203) 932-5711 ×5371, Janet.Tate2@va.gov.
George Hauser, Center for Biomedical Data Science, Yale University School of Medicine, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, CT, 06516 (203)932-5711 ×7140, ronald.hauser@yale.edu.
Tamar Taddei, Section of Digestive Diseases, Department of Internal Medicine, Yale University School of Medicine, VA Connecticut Healthcare System, 950 Campbell Ave., West Haven, CT, 06516, (203) 932-5711 ×4696, tamar.taddei@yale.edu.
Joseph Lim, Department of Internal Medicine Yale University School of Medicine, VA Connecticut Healthcare System, 333 Cedar Street, New Haven CT, 06510, New Haven CT, (203)737-6063, joseph.lim@yale.edu.
References:
- 1.Leigh JP, Bowlus CL, Leistikow BN, Schenker M. Costs of hepatitis C. Arch Intern Med 2001;161:2231–7. [DOI] [PubMed] [Google Scholar]
- 2.Stahmeyer JT, Krauth C, Bert F, et al. Costs and outcomes of treating chronic hepatitis C patients in routine care - results from a nationwide multicenter trial. J Viral Hepat 2016;23:105–15. [DOI] [PubMed] [Google Scholar]
- 3.Stahmeyer JT, Rossol S, Bert F, Liersch S, Krauth C. [Costs of a guideline-based treatment of patients with chronic hepatitis C in the era of interferon-free treatment]. Z Gastroenterol 2016;54:760–9. [DOI] [PubMed] [Google Scholar]
- 4.Daniels D, Grytdal S, Wasley A, Centers for Disease C, Prevention. Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ 2009;58:1–27. [PubMed] [Google Scholar]
- 5.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis 2013;56:40–50. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012;61:1–32. [PubMed] [Google Scholar]
- 7.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509–16 e1. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016;151:457–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology 2015;61:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012;366:216–24. [DOI] [PubMed] [Google Scholar]
- 11.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015;162:407–19. [DOI] [PubMed] [Google Scholar]
- 12.Njei B, McCarty TR, Fortune BE, Lim JK. Optimal timing for hepatitis C therapy in US patients eligible for liver transplantation: a cost-effectiveness analysis. Aliment Pharmacol Ther 2016;44:1090–101. [DOI] [PubMed] [Google Scholar]
- 13.Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med 2017;167:499–504. [DOI] [PubMed] [Google Scholar]
- 14.Ohl M, Lund B, Belperio PS, et al. Rural Residence and Adoption of a Novel HIV Therapy in a National, Equal-Access Healthcare System. AIDS Behav 2013;17:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckman TG, Somlai AM, Kalichman SC, Franzoi SL, Kelly JA. Psychosocial differences between urban and rural people living with HIV/AIDS. J Rural Health 1998;14:138–45. [DOI] [PubMed] [Google Scholar]
- 16.Reif S, Golin CE, Smith SR. Barriers to accessing HIV/AIDS care in North Carolina: rural and urban differences. AIDS Care 2005;17:558–65. [DOI] [PubMed] [Google Scholar]
- 17.Schur CL, Berk ML, Dunbar JR, Shapiro MF, Cohn SE, Bozzette SA. Where to seek care: an examination of people in rural areas with HIV/AIDS. J Rural Health 2002;18:337–47. [DOI] [PubMed] [Google Scholar]
- 18.Ohl M, Lund B, Belperio PS, et al. Rural residence and adoption of a novel HIV therapy in a national, equal-access healthcare system. AIDS Behav 2013;17:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahan V, Almashhrawi A, Mutrux R, Ibdah JA. Show Me ECHO-Hepatitis C: A telemedicine mentoring program for patients with hepatitis C in underserved and rural areas in Missouri as a model in developing countries. Turk J Gastroenterol 2015;26:447–9. [DOI] [PubMed] [Google Scholar]
- 20.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health 2006;22:140–6. [DOI] [PubMed] [Google Scholar]
- 21.West AN, Weeks WB, Wallace AE. Rural veterans and access to high-quality care for high-risk surgeries. Health Serv Res 2008;43:1737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwal F, Kramer JR, El-Serag HB, et al. Race and Gender Differences in the Use of Direct Acting Antiviral Agents for Hepatitis C Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016;63:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellinger JL, Moser S, Welsh DE, et al. Access to Subspecialty Care And Survival Among Patients With Liver Disease. Am J Gastroenterol 2016;111:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PloS one 2013;8:e84826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Esserman DA, Skanderson M, Levin FL, Justice AC, Lim JK. Disparities in hepatitis C testing in U.S. veterans born 1945-1965. Journal of hepatology 2016;65:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser RG, Quine DB, Ryder A. LabRS: A Rosetta stone for retrospective standardization of clinical laboratory test results. J Am Med Inform Assoc 2018;25:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson LP, Kirby AJ, Polk S, et al. Changes in survival after acquired immunodeficiency syndrome (AIDS):1984-1991. American journal of epidemiology 1993;138:952–64. [DOI] [PubMed] [Google Scholar]
- 28.Seligman M, Warrell DA, Aaboulker JP, et al. Concorde: MRC/ANRS randomized double blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. The Lancet 1994. [PubMed] [Google Scholar]
- 29.European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392–420. [DOI] [PubMed] [Google Scholar]
- 30.Rural-Urban Commuting Area Codes. 2016. at https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.)
- 31.Research. AfHP. Evaluation and management of early HIV infection1994 1994. January Report No.: 94–0572. [Google Scholar]
- 32.Scitovsky A The economic impact of the HIV epidemic in the United States. AIDS Updates 1991;4. [Google Scholar]
- 33.Bennett KJ. Health Disparities: A Rural - Urban Chartbook Columbia, South Carolina: Rural Health Research & Policy Centers; 2008 June 2008. Report No.: 20093189673. [Google Scholar]
- 34.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health 2005;95:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Veterans Analysis and Statistics. Characteristics of Rural Veterans: 2010 - Data from the American Community Survey. United States Department of Veteran Affairs. http://www1.va.gov/vetdata/docs/SpecialReports/Rural_Veterans_ACS2010_FINAL.pdf. Accessed December 4, 2016.
- 36.Cohen J Pharmaceuticals. Advocates protest the cost of a hepatitis C cure. Science 2013;342:1302–3. [DOI] [PubMed] [Google Scholar]
- 37.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat 2013;20:847–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.