Abstract
The serine/threonine phosphatase PP2A regulates a vast portion of the phosphoproteome including pathways involved in apoptosis, proliferation and DNA damage response and PP2A inactivation is a vital step in malignant transformation. Many groups have explored the therapeutic venue of combining PP2A reactivation with kinase inhibition to counteract the very changes in tumor suppressors and oncogenes that lead to cancer development. Conversely, inhibition of PP2A to complement chemotherapy and radiation-induced cancer cell death is also an area of active investigation. Here we review the studies that utilize PP2A targeted agents as combination therapy in cancer. A potential role for PP2A in tumor immunity is also highlighted.
I. Introduction
Protein Phosphatase 2A (PP2A) is a serine/threonine phosphatase with functions that counter-balance kinase-mediated phosphorylation throughout cell signaling networks. Its activity is critical to maintaining physiologic, ‘healthy’ cellular function. PP2A is frequently inactivated in human cancers as a means to removing its tumor suppressive activity, thereby allowing for unregulated growth that is a hallmark of malignancy. While cancer remains the most researched disease context for PP2A disruption, PP2A inhibition also contributes to pathogenesis in cardiovascular disease [1-3], diabetes [4-7], neurodegenerative disease (e.g. Alzheimer’s and Parkinson’s disease) [8-12] and developmental conditions involving intellectual disability [13, 14]. Consequently, therapeutic targeting of PP2A has become an exciting area of research with promising potential for clinical impact across fields.
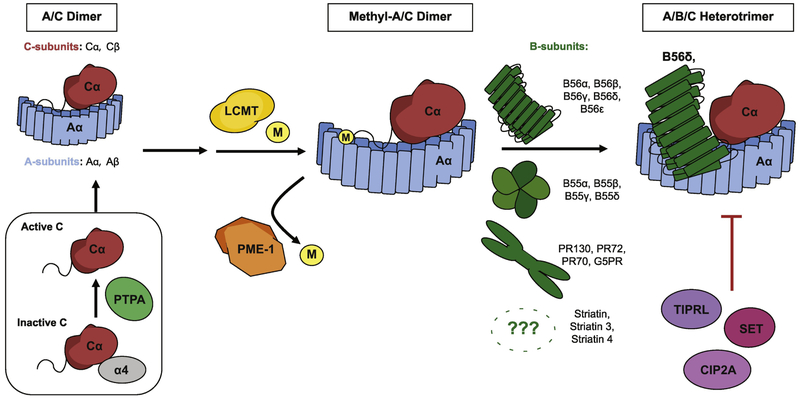
PP2A is a heterotrimeric enzyme comprised of a scaffolding subunit (PP2A-A), a catalytic subunit (PP2A-C), and a regulatory subunit (PP2A-B). Subunits assemble into A/B/C heterotrimers (Figure 1) that dephosphorylate substrates. Multiple isoforms exist for each subunit: 2 isoforms each for PP2A-A and PP2A-C, and 16 isoforms grouped into four families for PP2A-B (Figure 1). Importantly, the multi-subunit nature and variety of potential isoforms allows for PP2A’s notable diversity of substrates, as greater than 60 unique holoenzyme combinations may assemble. In addition to the core PP2A protein subunits, endogenous regulatory proteins for PP2A (e.g. CIP2A, SET, PME-1) provide further points of pharmacologic targeting to modulate PP2A function. PP2A is functionally impaired in cancer through inactivating mutations, suppression of individual subunits and up-regulation of endogenous regulators [15-19].
Figure 1.
Protein Phosphatase 2A (PP2A) is a heterotrimeric enzyme complex composed of an ‘A’ scaffolding subunit, a ‘B’ regulatory subunit, and a ‘C’ catalytic subunit. Multiple isoforms exist for each. B-subunits demonstrate the greatest diversity with 16 identified isoforms grouped into 4 families. During PP2A biogenesis, monomeric C-subunit interaction with PTPA induces conformational and biochemical changes that activate the C-subunit prior to A-subunit binding and dimer formation. Methylation of the C-subunit at the carboxyl terminus by LCMT then takes place to facilitate binding of specific methyl-sensitive B-subunits. Demethylation carried out by PME-1 may conversely alter or reduce holoenzyme assembly. Endogenous inhibitors of PP2A such as TIPRL, SET, and CIP2A may further regulate PP2A physiologic activity.
A number of recent reviews provide comprehensive summaries of PP2A-targeting compounds in development (please see: [20-23] also, Table 1). Here, we will review and discuss the potential for PP2A therapeutics to complement and enhance existing treatment strategies through combination therapy.
Table 1:
PP2A targeting agents
| PP2A Activating Agent |
Class | Target Protein | Reference(s) |
|---|---|---|---|
| Phenothiazine | Tricyclic neuroleptic | PP2A-A scaffolding subunit | Gutierrez et al. JCI 2014 |
| SMAP | Phenothiazine derivative | PP2A-A scaffolding subunit | Sangodkar et al. JCI 2017 |
| OP449 | SET-targeting peptide | SET | Christensen et al. Blood 2014 |
| FTY720 | Sphingosine analog | SET | Neviani et al. JCI 2007 |
| Forskolin | Diterpene | Unknown | Feschenko et al. J Pharmacol Exp Ther 2002 |
| PP2A Inhibiting Agent | Class | Target Protein | Reference(s) |
| LB100 (LB1.2) | Norcantharidin derivative | PP2A – binding partner unknown | Lu et al. PNAS 2009 |
II. PP2A Activation
The last decade has seen the emergence of a large number of PP2A activation strategies as a therapeutic venue in cancer (Table 1). Certain strategies directly target PP2A by using small molecules that bind to the scaffolding subunit resulting in conformational changes which lead to activation of the holoenzyme. These small molecules include perphenazine, a tricyclic neuroleptic, and SMAP, a re-engineered tricyclic sulfonamide [24-26]. More commonly, PP2A endogenous inhibitors are targeted for inhibition (Figure 1). OP449 is a peptide that binds antagonistically to the PP2A inhibitor SET, resulting in increased PP2A activity [27]. SET is also targeted using FTY720 (Fingolimod), which disrupts the SET-PP2A interaction [28]. FTY720 was originally developed for its immunomodulatory properties in the treatment of multiple sclerosis. Phosphorylation of FTY720 allows it to act as a functional antagonist of the sphingosine-1-phosphate receptors (S1PRs), leading to sequestration of lymphocytes and immune suppression [29, 30]. Analogs of FTY720 that are incapable of being phosphorylated by sphingosine kinase-2 lack these immunosuppressive properties but retain their ability to activate PP2A and induce apoptosis [31-33], suggesting that the effect on PP2A is independent of S1PR related signaling.
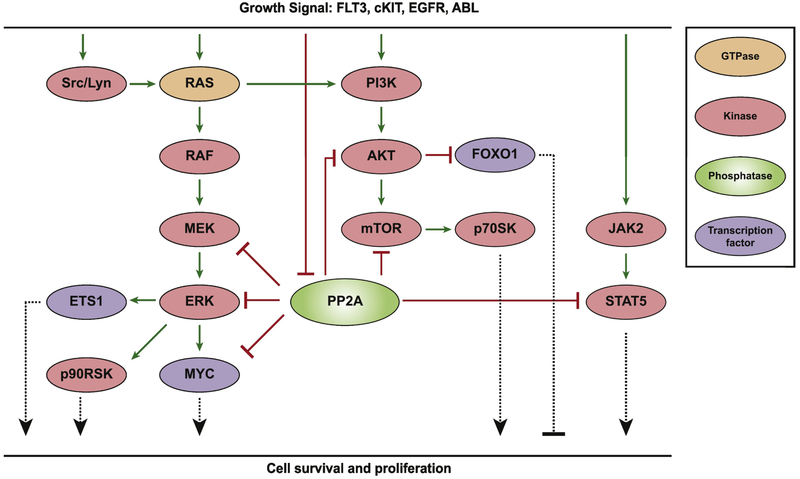
Early genetic models of malignant transformation described the role of PP2A as a tumor suppressor, showing that PP2A inhibition was a requirement for the initiation of carcinogenesis [34]. PP2A is now known to inhibit numerous growth and survival pathways [35, 36] (Figure 2), suggesting that the ability to activate PP2A in cancer may suppress the development of resistance at multiple nodes and provide enhanced efficacy at reducing tumor burden when combined with specific inhibitors of pro-proliferative pathways. Multiple groups have utilized this combinatorial approach (summarized in Table 2). Here, we highlight some of the studies that have investigated these potential therapies.
Figure 2.
Simplified signaling pathways downstream of growth factor signaling. PP2A negatively regulates proliferation and cell survival at multiple nodes. However, PP2A may be inhibited in cells with aberrantly active growth signaling resulting from activating mutations or oncogenic fusion proteins of the receptor tyrosine kinases shown, thereby allowing increased signaling through these pathways and tumorigenic progression. In this setting, simultaneously inhibition of oncogenic kinases and reactivation of PP2A harbors the potential for synergistic antineoplastic effects.
Table 2:
PP2A activation in combination with other targeted therapies
| Reference | Cancer | PP2A activating agent |
Combination Therapy |
Major Findings In addition to enhanced cell death in vitro with combination therapy vs. single agent |
|---|---|---|---|---|
| Alinari et al. Blood 2011 | Mantle cell lymphoma | FTY720 | Milatuzumab (mAb against CD74) | FTY720 increases sensitivity to milatuzumab by blocking CD74 lysosomal degradation resulting in increased CD74 abundance Combination treatment significantly increased overall survival in a SCID murine xenograft model of systemic MCL |
| Agarwal et al. Clin Cancer Res 2014 | Chronic myeloid leukemia/ acute myeloid leukemia | OP449 | Imatinib, nilotinib, ponatinib, dasatinib, AC220 (FLT3 inhibitor), JAK Inhibitor I, AraC | OP449 leads to decreased BCR-ABL phosphorylation and protein levels in CML cells OP449 treatment decreased STAT5, AKT and ERK phosphorylation and is selectively toxic to patient derived AML cells and has no effect on normal CD34+ cells OP449 enhances apoptotic response from targeted therapy in AML driven by FLT3 and JAK3 and increases AraC-induced cell death in AML cells with NRAS mutations |
| Gutierrez et al. JCI 2014 | T cell acute lymphoblastic leukemia | Perphenazine | Compound E (Gamma secretase inhibitor) | Combination treatment leads to synergy only in GSI sensitive lines. Perphanazine also has single agent activity in GSI-resistant T-ALL lines Perphanazine causes regression in a zebrafish model of T-ALL. The effect is lost by overexpression of BCL2 |
| Chien et al. Molecular Oncology 2015 | Pancreatic cancer | Penfluridol, FTY720 | Dasatinib | Phenothiazines identified in silico as class of molecules that can reverse dasatinib-resistance in pancreatic cancer cell lines Confirmed synergy with the structurally unrelated PP2A activator FTY720 |
| Zonta et al. Blood 2015 | Chronic lymphocytic leukemia | MP07-66 (FTY720 analog) | Dasatinib | Cell death induced by both dasatinib alone and combination treatment is reversible by okadaic acid Combination treatment increases dephosphorylation of AKT, GSK3b and SHP-1 over single agent |
| Richard et al. Oncotarget 2016 | T cell acute lymphoblastic leukemia | OP449 | Dovitinib (multikinase inhibitor) | Dovitinib identified in a screen for small molecules that synergize with OP449 in T-ALL cell lines Synergy observed in both NOTCH-dependent and NOTCH-independent lines |
| Smith et al. Oncotarget 2016 | FLT3+ acute myeloid leukemia | FTY720 and related analog AAL(S) | Multiple FLT3 inhibitors | PP2A activity is reduced in primary AML cells and FLT3+ cell lines Both FTY720 and FLT3 inhibitors alone restore PP2A activity and in some cases the effect is additive |
| Shu et al. Nature 2016 | Triple-negative breast cancer | Perphenazine (PPZ) | JQ1 | Acquired resistance to JQ1 resulted from BRD4 hyperphosphorylation and increased association with MED1 leading to bromodomain independent binding of BRD4 to new super-enhancers PPZ treatment decreased BRD4 phosphorylation and restored sensitivity to JQ1 |
| Martin et al. Breast Cancer Res 2017 | Triple-negative breast cancer | FTY720 | Gefitinib | Combination treatment enhanced tumor growth inhibition in 2 xenograft models 4T1 cells grown in immune-competent mice responded to combination treatment while no significant response was seen in 4T1 grown in nude mice |
| Kauko et al Sci Transl Med 2018 | Non-small cell lung carcinoma | SMAP | AZD6244 (MEK inhibitor) | Reduced PP2A activity contributes to MEKi resistance due to enhanced mTOR signaling Combination treatment is significantly better than either agent, causing regression in 2 xenograft models of RAS-driven NSCLC |
| Hayashi et al Oncotarget 2018 | ER+ breast cancer | Forskolin | Everolimus (mTORC1 inhibitor) | Sensitivity to EVE is correlated to the ability of EVE to decrease CIP2A levels CIP2A knockdown restores sensitivity to EVE in resistance clones |
a. PP2A activation in combination with targeted therapy
i). Reactivation of PP2A downstream of oncogene-induced inhibition
Recently, PP2A inhibition has been reported in cancer cells downstream of aberrantly active oncogenic pathways driven by receptor tyrosine kinases. Tumors with activating mutations of both cKIT and FLT3 have reduced expression of the PP2A scaffolding subunit (PP2A-A) and in some cases, of specific B subunits. In addition, amplification and increased expression of EGFR are correlated with overexpression of the PP2A inhibitor CIP2A [37-39]. PP2A is known to negatively regulate the pathways downstream of these tyrosine kinases at multiple nodes (Figure 2), suggesting that PP2A inhibition strategies may have been selected for during early stages of malignant transformation. It follows that combining a therapy that specifically inhibits the driving oncogene in these cancers with one that allows reactivation of PP2A to further inhibit their survival and proliferative signals provides an exciting potential therapy with increased efficacy and reduced side effects. Multiple groups have attempted to harness this therapeutic opportunity. Agarwal et al. reported that primary AML cells inhibit PP2A by overexpressing SET, and investigated the reactivation of PP2A with OP449 combined with rationally chosen kinase inhibitors targeting the diving oncogenes [40]. Specifically, the AML cell lines MOLM-14 (FLT3-ITD driven) and CMK (JAK3A527V driven) were treated with OP449 and either FLT3 or JAK inhibitors respectively. Combination therapy in both cases resulted in synergistic cell death measured by cell viability. Similarly, Smith et al. reported PP2A inhibition downstream of FLT3-ITD expressing AML via decreased expression of PP2A-A and investigated the ability of PP2A reactivation in sensitizing response to FLT3 inhibitors [38]. This study utilized FTY720 and AAL(S), a related analog that lacks immunomodulatory effects, in combination with multiple FLT3 inhibitors. Combination treatment resulted in synergistic cell death, measured by cell viability and methylcellulose colony formation, in two FLT3-ITD-driven cell lines and one primary AML line. Importantly, treatment with PKC412 (a FLT3 inhibitor) alone resulted in increased PP2A activity, while combination with FTY720 further increased PP2A activity. This is consistent with the earlier observation that FLT3-ITD causes decreased active PP2A by reducing expression of the scaffolding subunit, while FLT3 inhibition allows for PP2A reactivation. Since 30% of AML is FLT3 driven and FLT3 inhibitors have not proven to be efficacious as monotherapy [41], these studies provide preliminary data for the use of PP2A activation strategies in combination with FLT3 inhibitors in AML. The inclusion of relevant in vivo models would have greatly strengthened this data and provided a pre-clinical basis for future trials in humans.
An additional example of oncogene-induced PP2A inactivation was proposed in a model of chronic lymphocytic leukemia (CLL) [33]. The authors demonstrated increased phosphorylation of PP2A-C at Tyr307 in CLL cells but not normal B cells, and conclude that PP2A-C hyperphosphorylation is a result of LYN (a SRC family kinase) overexpression and leads to PP2A inhibition via increased association of PP2A-C with SET. Due to the issues surrounding the phospho-Y307 PP2A-C antibody [42], and the fact that the authors do not provide any antibody validation for the unknown ‘Santa Cruz’ antibody used in this paper, it is difficult to interpret their Tyr307 phosphorylation data. Nonetheless, the authors also demonstrate increased phosphatase activity upon treatment with multiple SRC family kinase inhibitors (SFKi) including dasatinib, and confirm that the activity is reversible with the PP2/PP4/PP6 family inhibitor okadaic acid. Furthermore, combination of SFKi with an FTY720 analog led to enhanced dephosphorylation of AKT and increased apoptosis over either treatment alone, as measured by Annexin V staining. Cell death as a result of combination treatment was completely reversible by okadaic acid. Although the mechanism of PP2A inhibition downstream of LYN may be unclear, combination of PP2A activation and SFKi should be further explored in in vivo models of CLL to determine if this strategy can yield a new therapy for this common form of leukemia.
ii). PP2A activation as a strategy to combat resistance
Development of resistant clones downstream of both targeted therapy and cytotoxic chemotherapy is a critical problem in the treatment of cancer. The BCR-ABL targeting small molecule imatinib, perhaps the most well-known example of targeted therapy, represented a breakthrough in the treatment of Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) [43]. However, despite an impressive initial clinical response, resistance inevitably develops in a subset of patients, despite the availability of 2nd and 3rd generation compounds nilotinib and ponatinib to combat this [44]. SET overexpression has been reported in CML and is associated with poor prognosis and PP2A activation via FTY720 has shown efficacy in pre-clinical models of this disease [45, 46]. Agarwal et al. investigated the ability of PP2A activation using OP449 to enhance response to ABL kinase inhibitors [40]. The authors reported marked synergy in cell death of K562 cells when OP449 was combined with ABL kinase inhibitors (imatinib, nilotinib, dasatinib, ponatinib). Furthermore, the combination of OP449 and nilotinib synergized to reduce colony formation of primary CML CD34+ cells, but not normal CD34+ cells. Importantly, OP449 displayed single agent activity against nilotinib and ponatinib resistant cell lines, harboring the BCR-ABL T135I and BCR-ABL E255V/T315I mutations. Mechanistically, OP449 treatment reduced the total levels of BCR-ABL, which may explain why it is agnostic to mutation status. This suggests that upfront therapy with both nilotinib and OP449 may suppress the emergence of refractory CML arising from these mutant clones.
Another example of the use of PP2A activating therapy to combat resistance was recently described in cellular and mouse models of lung cancer [47]. In non-small cell lung carcinoma (NSCLC), constitutive activation of KRAS occurs most commonly via mutation at the G12 residue [48], resulting in hyperactive signaling to growth and survival pathways, including the mitogen activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) pathways [49]. Currently, there are no targeted therapies for the subset of patients harboring this mutation [50]. Although multiple inhibitors of the downstream PI3K and MAPK signaling pathway have been developed, they have largely been unsuccessful in clinical trials due to high toxicity and limited efficacy [51, 52]. Since loss of PP2A regulatory subunits and overexpression of SET and CIP2A have all been reported in lung cancer [53-55], the Westermarck group investigated the role of PP2A suppression in driving resistance to kinase inhibitors in KRAS-driven lung cancer cell lines. This was done using a high-throughput drug screen in which two KRAS-driven NSCLC cell lines were treated with 230 different kinase inhibitors. The response of these cell lines was compared to cells in which PP2A was inhibited via siRNA against PP2A-A. PP2A-A knockdown in these cells significantly increased resistance to inhibitors in the MAPK pathway targeting MEK and ERK. Furthermore, activation of PP2A via siRNA against CIP2A sensitized cells to these MAPK inhibitors, suggesting that pharmacological activation of PP2A may overcome MEK inhibitor (MEKi) resistance. They utilized a phenothiazine derivative (SMAP) known to bind and activate PP2A and showed that this compound synergized with the MEK inhibitor trametinib in vitro. Importantly, combination of SMAP and selumetinib (a MEKi used in clinical trials) caused significant tumor regression in two KRAS-driven xenograft mouse models over either agent alone. Mechanistically, single agent treatment with the MEKi trametinib resulted in a reciprocal increase in the PI3K-AKT-mTORC signaling pathway. This feedback loop was further enhanced in PP2A inhibited cells. The activation of the PI3K pathway upon MAPK inhibition is well described [56] but previous attempts to combine MAPK and AKT inhibitors in patients have been unsuccessful due to dose-limiting toxicity [57]. This study suggests that an alternate approach to combat feedback mechanisms may be to combine MEKi with PP2A activators, thereby suppressing the PI3K pathway while simultaneously inhibiting other potential mechanisms of resistance such as c-MYC stabilization [58] (Figure 2).
In a separate study, PP2A inhibition was also responsible for acquired resistance to the BET bromodomain inhibitor (BBI) JQ1 [59]. The use of BBIs for the treatment of multiple cancer types [60-63] has recently been described and some studies have progressed to phase I clinical trials [64, 65]. These small molecules mimic the acetyl lysine-binding domain of BET (bromo- and extra-terminal domain) family proteins such as BRD4 and displace them from binding and activating transcription regulatory regions upstream of a large number of genes, including those involved in mitotic progression [60]. Shu et al. demonstrated that the BBI JQ1 displayed pre-clinical efficacy in triple negative breast cancer models. In order to predict resistance mechanisms that may arise in patients treated with BBIs, JQ1-resistant cell lines were developed. It was found that resistance arose as a result of PP2A inhibition by an unknown mechanism, resulting in hyperphosphorylation of BRD4 at Ser7. This resulted in bromodomain-independent binding of BRD4 to new super-enhancers, including the one that regulates BCLXL. This allowed cells to escape apoptosis by overexpression of the BH3 family protein BCLXL. Resistance in these lines was overcome by combining JQ1 with perphenazine to reactivate PP2A, resulting in synergistic cell death in vitro. Similar results were observed when JQ1 was combined with an inhibitor of CK2, the kinase that phosphorylates this site on BRD4. Hyperphosphorylation at this site was also detected in breast cancer tumor microarrays and correlated with decreased progression free survival. This suggests that a subset of patients with hyperphosphorylation at this site may have de novo resistance to BBI treatment, and future trials using BBIs should consider the inclusion of a combination arm with PP2A activators.
iii). Identification of PP2A activation via high throughput drug screens as a strategy to enhance the efficacy of existing therapy
The phenothiazine class of PP2A activators has also been identified in unbiased drug screens for molecules that enhance efficacy of dasatinib and γ-secretase inhibitors [24, 66]. In an initial drug screen across 14 pancreatic cancer cell lines, the SRC family kinase inhibitor dasatinib emerged as the most potent small molecule tested. Dasatinib has previously been tested in clinical trials of locally advanced and metastatic pancreatic cancer, both as monotherapy and in combination with gemcitabine. In both trials, dasatinib did not display significant clinical activity, possibly due to de novo resistance against this agent [67, 68]. The authors used gene signatures from dasatinib-sensitive and dasatinib-resistant cell lines and used an in silico method to determine which drugs would re-sensitize cells to dasatinib. The top hit from this query was thioridazine, a small molecule belonging to the phenothiazine group known to directly bind and activate PP2A. The authors tested multiple phenothiazines against a panel of pancreatic cancer lines and chose to proceed with penfluridol, as this displayed the most potency. Penfluridol treatment decreased phosphorylation at PP2A targets c-MYC, AKT and GSK3β and caused cell death in a PP2A dependent manner. Importantly, penfluridol and the structurally unrelated PP2A activator FTY720 both displayed synergy with dasatinib in multiple pancreatic cancer lines. PP2A activation using OP449 has previously shown to have efficacy in pancreatic cancer in in vivo xenograft models [69], and the overexpression of SET and CIP2A in this malignancy suggests that PP2A inhibition may be driving some of the de novo resistance against dasatinib, although further experiments are required to test this.
In a study by Gutierrez et al., multiple members of the phenothiazine class were identified in a drug screen to find compounds that would enhance the efficacy of gamma secretase inhibitors in the NOTCH-dependent T cell acute lymphoblastic leukemia (T-ALL) cell line KOPT-K1 [24]. About 60% of T-ALL is driven by activated NOTCH signaling [70]. This transmembrane receptor is only active after cleavage by gamma secretase, which releases the NOTCH intracellular domain to act as a transcription factor that drives the expression of MYC, BCL2 and other oncogenes [71]. However, treatment with gamma secretase inhibitors (GSI) only leads to a modest cytostatic response in cell line models of NOTCH-activated T-ALL [72]. From the drug screen described above, Gutierrez discovered that PP2A activators in the phenothiazine class could synergize with GSIs. One of the top hits, perphenazine (PPZ), displayed both single agent and additive or synergistic activity with GSI in multiple viability and apoptosis assays, both in T-ALL cell lines and primary cells. However this was only true for cell lines that were already sensitive to GSI. PPZ is unable to re-sensitize cells that are already GSI resistant; in these cases combining the two treatments was only as efficacious as PPZ alone. While the authors did not attempt to explore the mechanistic basis for this, they postulate that PPZ-induced PP2A activation and subsequent apoptosis occur via signaling pathways that are NOTCH-independent. This combination may be therapeutic in T-ALL patients with NOTCH1 activating mutations known to be GSI sensitive.
Given the specificity with which PP2A activation strategies cause cell death in malignant but not normal cell types, and that PP2A is frequently inactivated in cancer [22], PP2A activation strategies in combination with many of the potent inhibitors that already exist to inhibit driver oncogenes should be further explored for the treatment of cancer.
b. PP2A activation in combination with cytotoxic chemotherapy
Several studies have explored the utilization of drugs that activate PP2A, specifically FTY720, in combination with standard chemotherapies such as anthracyclines and platinum-based agents. Some of the most common drugs used to treat patients with breast cancer include anthracyclines, such as doxorubicin. While several therapeutics have been tested in combination with doxorubicin, targeting PP2A is of particular interest because of findings showing that expression of endogenous inhibitors of PP2A such as CIP2A modulate sensitivity to doxorubicin [73]. Furthermore, breast cancer patients with PP2A inhibition have a significantly worse clinical outcome. In vitro studies showed that PP2A activation by FTY720 reduced cell viability, induced caspase-dependent apoptosis and decreased phosphorylation of AKT and ERK in breast cancer cell lines. Combination of FTY720 with doxorubicin enhanced the antitumor activity in vitro and in vivo [74]. Given these results, further studies exploring PP2A activating drugs in combination with doxorubicin could lead to potential treatment strategies for breast cancer patients.
PP2A has been found to be frequently inactivated in patients with colorectal cancer as well. Restoration of PP2A activity using FTY720 reduced proliferation and colony formation, induced caspase-dependent apoptosis and inhibited AKT and ERK signaling in colorectal cancer cells [75]. SET was also found to be overexpressed in colorectal cancer cell lines. SET overexpression increased cellular proliferation whereas PP2A overexpression or SET silencing decreased cell growth in colorectal cancer cells [76]. SET silencing also increased the efficacy of treatment with oxaliplatin as well as 5-Fluorouracil. Both oxaliplatin and 5-Fluorouracil are chemotherapeutic agents used to treat colorectal cancer. Since SET expression modulated the response to these chemotherapeutic agents, the authors tested the combination of FTY720 with oxaliplatin or 5-Fluorouracil which resulted in enhanced efficacy in colorectal cancer cells [76].
The efficacy of FTY720 in combination with cisplatin has been explored in several cancers with opposing results. Combination of cisplatin with FTY720 has been found to antagonize the cytotoxicity of cisplatin in ovarian cancer cells [77]. In melanoma however, it was found that FTY720 treatment in combination with cisplatin resulted in decreased cell viability, increased expression of apoptosis-associated cleaved poly (ADP-ribose) polymerase (PARP) and reduced phosphorylation of PI3K, AKT and mTOR [78]. Similarly, in lung cancer, treatment of a mouse model of lung cancer with FTY720 in combination with cisplatin resulted in enhanced anti-tumor activity. Molecular analysis showed that treatment with this combination resulted in decreased expression of ATG7 and Ki67 [79]. Further examination of these molecular pathways will lead to a better understanding of which cancers will benefit from PP2A activating drugs in combination with cisplatin.
FTY720 has also been shown to be important in treating obesity-related breast cancer.In obesity-related cancers, inflammation increases the malignant potential of cancer cells [80, 81]. Sphingosine-1-phosphate plays a significant role in inflammation, which leads to an increase in cancer progression. Doxorubicin treatment of a murine breast cancer model resulted in an increase of sphingosine-kinase-1 (SPK1), sphingosine-1-phosphate receptor 1 (S1PR1), interleukin 6 (IL-6), and STAT3. FTY720 is a functional antagonist of S1PR1. Thus, treatment of the murine breast cancer model with FTY720 resulted in suppression of SPK1, S1PR1, IL-6 and STAT3. Combination of doxorubicin with FTY720 resulted in reduced inflammation and synergistic suppression of cancer growth in vitro and in vivo. Furthermore, in an obesity breast cancer model where mice were fed a high-fat diet, this combination therapy was also efficacious [82].
Modulation of the sphingosine-1-phosphate pathway by FTY720 has been reported to be efficacious in clear-cell renal carcinoma as well. FTY720 was found to inhibit HIF1α and HIF2α, which are hypoxia-inducible factors shown to induce chemoresistance in clear-cell renal carcinoma [83]. Treatment of a heterotopic xenograft model of clear cell renal carcinoma with FTY720 resulted in a decrease in HIF1α and HIF2α expression and vascular normalization. Combination of FTY720 with a gemcitabine-based chemotherapy resulted in significant decrease in tumor size [83].
While these studies investigated several signaling pathways in response to FTY720, they did not directly evaluate the role of PP2A. Thus, it is unclear whether PP2A carried out a role in the additive or synergistic response seen in these studies.
III. PP2A Inhibition
While pharmacologic activation has been the primary therapeutic approach for targeting PP2A given its inactivation in many cancers, PP2A inhibition has become an area of recent interest following demonstrated efficacy of the compound LB100. LB100 (also published as LB1) is a water-soluble homolog of the lead compound, LB102 (LB1.2), developed through norcantharidin derivation [84, 85]. Cantharidin and norcantharidin are PP2A inhibitors used in traditional Chinese medicine whose clinical potential is reduced by significant toxicity. Derivation was performed to overcome this limitation [86]. Although the exact mechanism through which LB100 binds to PP2A and inhibits its activity has not been established, the parent compound cantharidin has been suggested to bind the PP2A C-subunit [87, 88]. Research on LB100 has largely focused on and highlighted its potential as a potent chemo- and radio-sensitizer. A 2016 Phase I clinical trial was completed for LB100 in combination with Docetaxol for the treatment of solid tumors [89]. The compound was found to have minimal adverse toxicities with efficacy potential that merits further clinical investigation.
a. PP2A inhibition in combination with DNA Damage Inducers
DNA damage response (DDR) entails a highly coordinated sequence of signaling events that are triggered upon detection of damage to ensure maintenance of genome integrity. Critically, PP2A participates at multiple steps in DDR, including damage identification, activation of proteins for damage repair, and checkpoints to halt cell cycle progression [90-94] (Figure 3). It was hypothesized that direct impairment of PP2A could knock out critical defense pathways and render cells susceptible to lethal damage accumulation in the face of DNA damage induction. With the latter being readily accomplished through ionizing radiation or select chemo-toxins already used in cancer therapy, LB100 was predicted to further enhance their effects.
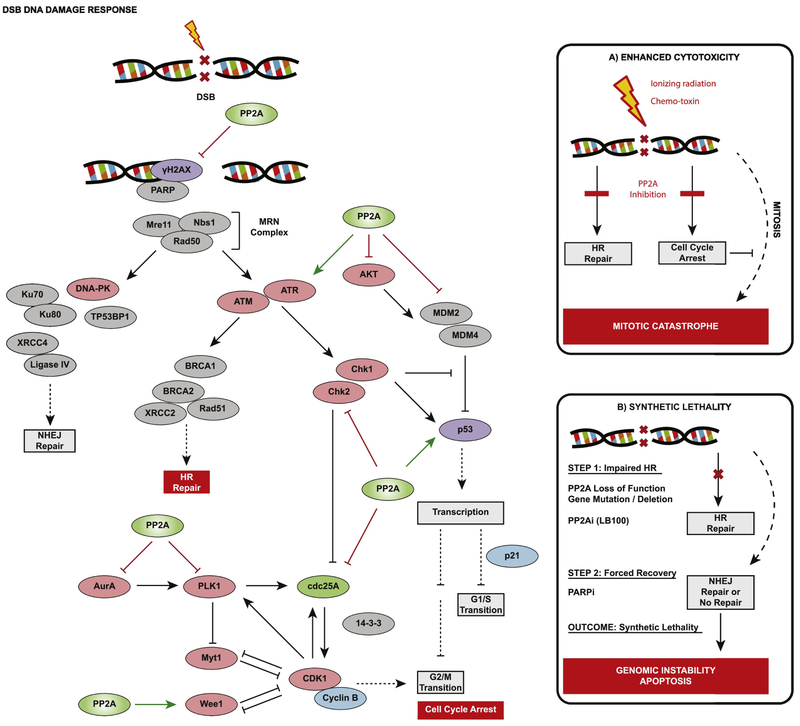
Figure 3.
PP2A exerts regulatory activity against multiple substrates within the DNA damage response (DDR) pathways. Shown is a simplified schematic of reported PP2A targets; a complete picture of PP2A’s role would involve added timing and contextual dynamics. Critically, PP2A activity against ATM and Chk1 / Chk2 promotes the high-integrity homologous recombination (HR) repair of damaged DNA and resolution of γH2AX foci marking sites of DNA strand break. In parallel, cell cycle progression is halted due to PP2A inhibition of MDM2 / activation of p53, as well as PP2A activity against PLK1, Aurora Kinase A (AurA), Wee1, and cdc25 that altogether inhibits CDK1/CyclinB. (A) In the face of significant DNA damage induced by radiation or a chemotherapeutic compound, PP2A inhibition impairs damage response and repair, and appropriate cell cycle arrest. Damage persists and cells that attempt to divide experience mitotic catastrophe due to a lethal loss of genome integrity. (B) Similar to BRCA1/2, PP2A has multiple key roles in facilitating HR repair that may allow it to be a candidate for synthetic lethality when its inactivation is coupled with PARP inhibition.
In the ground-breaking report, Lu et al. evaluated LB100 combination therapy with Doxorubicin (DOX) or Temozolomide (TMZ) for tumor response in Glioblastoma and Neuroblastoma model systems [84]. They found that treatment with LB100 alone results in signs of mitotic catastrophe, such as disordered microtubules and abnormal mitotic figures, as well as hyperphosphorylated AKT1, PLK1, and MDM2 resulting in the improper activation of these major cell cycle and DDR regulatory proteins. P53 was also decreased, presumably as a consequence of MDM2 activation. In contrast, treatment with cytotoxic compounds DOX or TMZ alone led to increased p53 coupled with S-phase arrest, consistent with appropriate checkpoint triggering and cell cycle halting following DNA damage. Combination treatment with DOX or TMZ and LB100 led to phosphorylation patterns consistent with LB100 treatment alone, highlighting a central role for PP2A in response to DOX/TMZ-induced damage that when overridden, leads to accelerated, inappropriate entry into mitosis and accentuated cell death induction. In vivo, combination therapy with DOX or TMZ demonstrated greater tumor response—growth inhibition or regression—than either agent alone, and in some animals prevented tumor recurrence that otherwise occurs in this glioblastoma model.
LB100 has since been studied in multiple cancer model systems in combination with radiation or cytotoxic compounds (Table 3). Efficacy is found across classes of cytotoxic compounds: DNA-intercalating agents (e.g. Doxorubicin), DNA-alkylating agents (e.g. Temozolomide) and DNA-crosslinking agents (e.g. Cisplatin). In aggregate, research reports provide a largely consistent picture of the signaling mechanisms that underlie increased cytotoxin efficacy with LB100 adjuvant therapy: (1) AKT1 and MDM2 are activated while p53 is inhibited to prevent checkpoint activation in G1/S [84, 95, 96]. (2) Hyperphosphorylated PLK1 is activated and inhibits Chk1/2, which is no longer able to inhibit CDK1 and trigger G2 arrest [84, 95-98]. (3) This is compounded by removal of PP2A inhibition of CDC25c, which is also able to maintain CDK1 activity via de-phosphorylation. The PP2A substrate and CDK1 inhibitor Wee1 is also inactive. Cells progress to mitosis, but spindle formation is disordered and mitotic catastrophe occurs [84, 95, 96, 98, 99]. Disrupted cell cycle progression was further evident in cell cycle spread analyses, where treatment with a DNA damage inducer led to predominant S-phase arrest (more cells in G1/S), but when combined with PP2A inhibition by LB100, cells progress through S-phase and accumulated in G2/M due to disordered mitosis [84, 95, 96, 98-100]. PLK1 inhibition of TCTP, a protein that stabilizes microtubules and has anti-apoptotic functions, may contribute to abnormal mitotic figures and mitotic catastrophe [84, 96].
Table 3:
PP2A inhibition by LB100 in combination with cytotoxic chemotherapy or radiation.
| Reference | Cancer | Combination Therapy |
Major Findings In addition to enhanced tumor growth inhibition with combination therapy vs. radiation / chemotherapy alone |
|---|---|---|---|
| Lu et al. PCNA 2009 | Glioblastoma Neuroblastoma | Temozolamide Doxorubicin | Combination therapy induced tumor regression and prevented recurrence in 50% of animals Prevented DNA damage-induced S-phase arrest; cells progress to mitosis and are susceptible to irregular replication and mitotic catastrophe |
| Zhang et al. Biomaterials 2010 | Sarcoma | Doxorubicin | Reduced incidence of lung metastasis |
| Martiniova et al. PLoS One 2011 | Pheochromocytoma | Temozolamide | Delayed tumor recurrence, prolonged disease-free survival in 40% of animals that had developed intra-hepatic metastases |
| Wei et al. Clin Cancer Res 2013 | Pancreatic Cancer | Radiation | LB100-mediated radio-sensitization was specific to pancreatic cancer cells vs. normal colonic epithelial cells HRR in response to DNA damage was also inhibited LB100-induced signaling alterations were consistent with those induced by si-PPP2R1A |
| Bai et al. Mol Cancer Ther 2014 | Hepatocellular Carcinoma | Doxorubicin Cisplatin | Reported increased angiogenesis and vascular permeability, which enhanced drug penetration but may also create potential for metastasis |
| Lv et al. Oncotarget 2014 | Nasopharyngeal Carcinoma | Radiation | LB100 was capable of radio-sensitizing a previously resistant cell line |
| Bai et al. Cancer Letters 2014 | Pancreatic Cancer | Doxorubicin | Increased blood vessel density allowed for enhanced blood perfusion and doxorubicin drug concentration in tumor tissue; was linked to HIF-1α expression and increased VEGF secretion |
| Zhang et al. Cell Cycle 2015 | Osteosarcoma | Cisplatin | Reduced incidence of lung metastases |
| Chang et al. Mol Cancer Ther 2015 | Ovarian Carcinoma | Cisplatin | Delayed tumor burden in an intraperitoneal metastasis model and prevented tumor relapse PP2A-C knockdown also sensitized cells to cisplatin |
| Gordon et al. Mol Cancer Ther 2015 | Glioblastoma | Radiation | In contrast to some reports, mitotic catastrophe was a major cell death mechanism with combination therapy, while apoptosis was minimally observed |
| Ho et al. Oncotarget 2016 | Medulloblastoma | Cisplatin | LB100 decreased cell motility and in vivo invasiveness, tumors for both combination and LB100 monotherapy displayed well-demarcated borders LB100 has anti-tumor activity through STAT3 inhibition and altered subcellular localization |
| Fu et al. Tumor Biol 2016 | Hepatocellular Carcinoma | Sorafenib | LB100 activity was linked to hypoxic environments, where apoptosis occurred subsequent to Smad3 hyperphosphorylation and Bcl2 inhibition |
| Hu et al. Sci Rep 2017 | Acute Myeloid Leukemia | Daunorubicin | Bcl2 upregulation is a hallmark of the syndrome. LB100 increased miR-181b which inhibited Bcl2 to induce apoptosis |
| Hao et al. Neuro Oncol 2017 | Chordoma | Radiation | LB100 reduced tumor cell mobility and invasiveness both without and with radiation co-treatment |
| Lai et al. Sci Transl Med 2018 | BCR-ABL+ Leukemia | Imatinib | PP2A inhibition was able to re-sensitize TKI non-responding cells, and also demonstrated success in targeting stem cell populations |
| Ho et al. Cancer Letters 2018 | Meningioma | Radiation | Also linked LB100 anti-tumor growth activity to inhibited STAT3 signaling |
In addition to irregular cell cycle regulation, a few studies reported persistence of DNA damage with LB100 treatment, which was indicated by presence of γ-H2AX foci [96, 98-100]; likewise, LB100 inhibited Rad51 foci formation as part of DDR [97]. This suggests that homologous recombination (HR) repair may also be impaired by LB100, which is unsurprising given PP2A’s additional roles in regulating damage repair.
Altered cell motility and/or tumor invasiveness in the context of LB100 was also revealed through the experiments of two research groups [101, 102]; elucidation of the mechanistic underpinnings could reveal the potential of this compound for invasive disease management
Finally, some investigations suggest that PP2A inhibition by LB100 treatment may modulate stem cell state to promote differentiation [100, 103]. Triggering stem cells, which are often quiescent, to re-enter cell cycle may help to render them more sensitive to radio- or chemotherapy [100]. This could present an additional anti-neoplastic function for LB100. However, data to support LB100 effects on stem cell or quiescent cell state is limited and requires more investigation.
b. Combination with PP2A inhibition to achieve synthetic lethality
The concept of ‘synthetic lethality’ —in which either of two alterations is viable alone, but become lethal when present in combination—has garnered interest in cancer research as a treatment strategy to exploit the very genetic alterations that are favorable to cancer development. Loss or mutation of DNA damage sensors, such as p53, BRCA1/2, ATM/ATR and PLK1 allow cancer cells to escape checkpoints, survive and divide, despite loss of genomic integrity. However, there is a limit to tolerated genome disruption beyond which damage is too severe, and mitotic catastrophe or apoptosis may be induced.
Given PP2A’s multiple roles in DDR, synthetic lethality may be achieved through LB100 treatment of susceptible tumors with genomic disruption of a complementary DDR protein. For example, studies have suggested that cancer cells overexpressing Mad2, a mitotic spindle checkpoint protein, or PLK1, a DDR kinase with multiple identified roles, may be vulnerable to synthetic lethality upon pharmacologic PP2A inhibition [104, 105]. These combinations resulted in severely impaired maintenance of genome integrity to the point of cell death induction. This is an avenue yet unexplored, but may highlight target patient populations for whom genomic susceptibility in combination with LB100 achieves a synthetically lethal therapeutic response.
Finally, synthetic lethality has been of great interest for patients with BRCA1/2 mutations, for whom PARP inhibitors have shown promising therapeutic benefit [106, 107]. BRCA1/2 facilitate HR repair of double-strand breaks (DSB) and are functionally impaired by cancer-associated mutations. Meanwhile, PARP is involved in single-strand break (SSB) repair and PARP inhibition appears—through mechanisms still being investigated—to result in degeneration of SSB into DSB whose resolution requires competent HR. When HR is deficient, the outcome is lethal for the rapidly dividing cancer cells [108, 109]. Given its own ability to disrupt HR repair, inhibition of PP2A may similarly synergize with compounds such as PARP inhibitors to achieve a pharmacologic synthetic lethality (Figure 3). In support of this, genetic studies have highlighted cancer-acquired genetic perturbations in DDR-dependent PP2A B-subunits that may prime cells for PARP inhibitor sensitivity [55].
c. PP2A inhibition in combination with kinase inhibitors
Two papers report on the efficacy of LB100 combination therapy with kinase inhibitors: (1) Sorafenib in the treatment of hepatocellular carcinoma, and (2) Imatinib for BCL-ABL+ leukemia [110, 111]. Fu et al. showed that resistance to sorafenib is partially due to a decrease in phosphorylated SMAD3 in hypoxic microenvironments. Their studies showed that inactivation of PP2A by LB100 resulted in increased phosphorylation of SMAD3 and subsequent apoptosis. Combination of LB100 with sorafenib enhanced the treatment of hepatocellular carcinoma during hypoxia. In a study by Lai et al., a drug screen found that cantharidin (CAN) in combination with imatinib resulted in 93% growth inhibition in AHI-1 (Abelson helper integration site-1) transduced K562 cells whereas treatment with CAN or imatinib alone resulted in 30% and 15% growth inhibition respectively. Subsequent treatment studies with the combination of LB100 and a tyrosine kinase inhibitor, such as dasatinib showed synergistic response in BCR-ABL positive blast cells and drug insensitive leukemic stem cells in vivo. Mechanistically, this occurred through the inhibition of AHI-1 mediated signaling molecules and PP2A-mediated protein degradation of β-catenin and inhibition of its downstream targets. However, toxicity studies for the combination treatment in higher-order mammals are required to determine if the cell death observed in normal CD34+ cells translates to severe adverse events in organisms, in which case the treatment is unlikely to progress to clinical trial in CML.
d. PP2A inhibition in the treatment of other diseases
PP2A plays a role in the pathologies of several human diseases beyond cancer, such as cardiovascular disease and neuro-psychiatric conditions, suggesting that PP2A-targeting therapeutics under development for cancer may find use in these clinical fields as well. One report utilized LB100 treatment to inhibit PP2A activity in a mouse model of depression [112]. The authors found that PP2A suppresses inhibitory GABA-B receptor and GIRK channel activity, leading to hyper-active neuronal signaling in lateral habenula circuits (LHb). LHb activity correlated with expression of depression-like symptoms when animals experienced aversive stimuli, but was significantly reduced upon PP2A inhibition by LB100. Importantly, LHb hyperactivity is also seen in fMRI data collected from depressed patients. With further experimentation, the study findings may be expanded upon to determine the translation potential of this approach for human patients suffering from clinical depression. Therapeutic potential for PP2A inhibition in non-cancer diseases has otherwise remained minimally explored.
Discussion and future perspectives
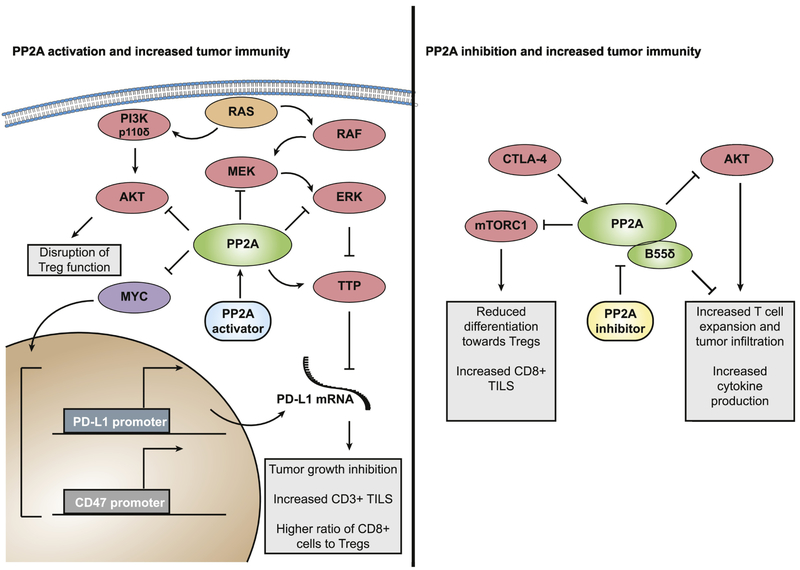
The role of PP2A in negatively regulating multiple oncogenic pathways makes the activation of this phosphatase in cancer a promising strategy, especially when combined with potent inhibitors of the driver oncogene. Furthermore, PP2A appears to be commonly inhibited in cancer by a variety of mechanisms [22]. This may provide a greater therapeutic window for PP2A reactivation strategies, as cancer cells are more likely to be dependent on reduced PP2A levels for survival, while healthy tissue that already has high PP2A activity, remains relatively unaffected. In contrast, the role of PP2A in cell homeostasis and particularly DNA damage repair have revealed a different vulnerability in cancer cells, where the inhibition of PP2A coupled with additional DNA damaging strategies may be therapeutically beneficial. Chemotoxic compounds are associated with significant off-target toxicity that results in morbidity for patients and/or restricts treatment options. The use of LB100 as a chemo- or radio-sensitizer, may allow for use of less toxic doses while achieving equivalent clinical response. Another area that remains relatively unexplored is the role of PP2A in tumor immunity. PP2A has been reported to function as a regulator of immune checkpoint signaling, in particular by the immune checkpoint inhibitors programmed death-1 (PD-1) and cytotoxic T lymphocyte associated protein 4 (CTLA-4). The PP2A target c-MYC is a transactivator for the PD-1 ligand PD-L1 [113], suggesting that PP2A activation and subsequent dephosphorylation and degradation of c-MYC may result in suppression of these factors involved in immune evasion. In addition PP2A dephosphorylates the mRNA binding protein Tristetraprolin (TTP), allowing its dissociation from 14-3-3 [114]. This allows TTP to bind AU-rich elements on PD-L1 mRNA, leading to PD-L1 mRNA degradation (Figure 4). Furthermore, PP2A inhibits the MAPK signaling pathways responsible for phosphorylation of TTP at its inhibitory site [115], suggesting that PP2A regulates TTP, and by extension PD-L1 stability at multiple nodes. Finally, PIK3CD (p110δ) signaling was recently shown to be essential for normal regulatory T cell (Tregs) function [116] and negative regulation of this pathway by PP2A may disrupt Tregs and allow cytotoxic T cell expansion. Since the majority of PP2A activators are tested in immune-deficient murine models, the role of PP2A in tumor immunity has remained unexplored and warrants increased investigation. Recently immune checkpoint therapy has seen great success in the treatment of cancer, but a subset of patients demonstrate de novo resistance to this approach. Given the roles of PP2A in suppressing PD-L1 and Tregs (Figure 4), future studies should determine whether the combination of immune checkpoint inhibitors and PP2A activators could overcome this resistance. By contrast PP2A has also been shown to function downstream of CTLA-4 in lymphocytes to inhibit signaling by the co-stimulatory molecule CD28 [117], suggesting PP2A itself may play a role in immune co-inhibition in Tregs, which express high levels of CTLA-4. Indeed genetic knockout of PP2A in murine Tregs phenocopies CTLA-4 loss, resulting in multi-organ autoimmunity and death [118]. In addition, the PP2A regulatory subunit B55δ was identified in an in vivo shRNA screen whereby loss of B55δ resulted in T cell expansion, increased tumor infiltrating lymphocyte (TILs) and enhanced production of IFN-γ and granzyme [119]. A recent study demonstrated that PP2A inhibition with LB100 enhanced the efficacy of anti-PD-1 treatment, potentially through activation of mTORC1 which resulted in reduced differentiation toward Tregs and increased tumor infiltrating CD8+ T cells [120]. A better understanding of which PP2A-B regulatory subunits contribute to these functions combined with additional studies that utilize PP2A modulating strategies in in vivo immune-competent settings may help to elucidate the seemingly contrasting roles of PP2A in tumor immunity. Overall, PP2A represents an intriguing drug target in cancer and a better understanding of its roles in normal and tumorigenic signaling will lead to the development of promising therapeutics for multiple malignancies.
Figure 4.
The complex roles of PP2A in immune checkpoint signaling. PP2A activation may increase tumor immunity via dephosphorylation and subsequent degradation of MYC as well as inhibition of MAPK and PI3K pathways. In contrast, loss of PP2A is linked to reduced Treg function, and specific depletion of the regulatory subunit B55δ was shown to increase tumor immunity.
Highlights.
PP2A is a tumor suppressor that exists as a heterotrimer comprised of multiple possible regulatory subunits, allowing it to negatively regulate numerous proliferative and survival pathways.
PP2A inactivation is a frequent event in cancer and its reactivation in combination with oncogenic kinase inhibition harbors therapeutic potential.
Paradoxically, PP2A inhibition has also been shown to have tumoricidal effects, particularly when combined with DNA damaging agents.
Acknowledgments
The authors would like to thank Eric Yuan for valuable discussions regarding the role of PP2A in tumor immunity. This work was supported by an R01 (CA181654) granted to G. Narla.
Abbreviations
- AML
Acute myeloid leukemia
- BBI
BET bromodomain inhibitor
- CIP2A
Cancerous inhibitor of PP2A
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myelogenous leukemia
- DDR
DNA damage response
- DOX
Doxorubicin
- DSB
Double-strand break
- EGFR
Epidermal growth factor receptor
- FLT3-ITD
FLT3 internal tandem duplications
- fMRI
Functional magnetic resonance imaging
- GSI
Gamma secretase inhibitor
- HR
Homologous recombination
- IL-6
Interleukin 6
- LHb
Lateral habenula
- MAPK
Mitogen activated protein kinase
- MEKi
MEK inhibitor
- NSCLC
Non-small cell lung carcinoma
- PARP
Poly (ADP-ribose) polymerase
- Ph+
Philadelphia chromosome positive
- PI3K
Phosphatidylinositol-3-kinase
- PP2A-A
PP2A scaffolding subunit
- PP2A-B
PP2A regulatory subunit
- PP2A-C
PP2A catalytic subunit
- PP2A
Protein Phosphatase 2A
- PPZ
Perphenazine
- S1PR
Sphingosine-1-phosphate receptor
- SFKi
SRC family kinase inhibitor
- siRNA
small interfering RNA
- SMAP
Small molecule activator of PP2A
- SPK1
Sphingosine-kinase-1
- SSB
Single-strand break
- T-ALL
T cell acute lymphoblastic leukemia
- TIL
Tumor infiltrating lymphocyte
- TMZ
Temozolomide
- Treg
Regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The Icahn School of Medicine at Mount Sinai, on behalf of G. Narla, has filed patents covering composition of matter on the small molecules disclosed herein for the treatment of human cancer and other diseases (International Application Numbers: PCT/US15/19770, PCT/US15/19764; and US Patent: US 9,540,358 B2). RAPPTA Therapeutics LLC has licensed this intellectual property for the clinical and commercial development of this series of small molecule PP2A activators. G. Narla, has an ownership interest in RAPPTA Therapeutics LLC.
References
- [1].DeGrande ST, Little SC, Nixon DJ, Wright P, Snyder J, Dun W, Murphy N, Kilic A, Higgins R, Binkley PF, Boyden PA, Carnes CA, Anderson ME, Hund TJ, Mohler PJ, Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart, J Biol Chem, 288 (2013) 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lubbers ER, Mohler PJ, Roles and regulation of protein phosphatase 2A (PP2A) in the heart, J Mol Cell Cardiol, 101 (2016) 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kirchhefer U, Brekle C, Eskandar J, Isensee G, Kucerova D, Muller FU, Pinet F, Schulte JS, Seidl MD, Boknik P, Cardiac function is regulated by B56alpha-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates, J Biol Chem, 289 (2014) 33862–33873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Galbo T, Olsen GS, Quistorff B, Nishimura E, Free fatty acid-induced PP2A hyperactivity selectively impairs hepatic insulin action on glucose metabolism, PLoS One, 6 (2011) e27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kowluru A, Matti A, Hyperactivation of protein phosphatase 2A in models of glucolipotoxicity and diabetes: potential mechanisms and functional consequences, Biochem Pharmacol, 84 (2012) 591–597. [DOI] [PubMed] [Google Scholar]
- [6].Arora DK, Machhadieh B, Matti A, Wadzinski BE, Ramanadham S, Kowluru A, High glucose exposure promotes activation of protein phosphatase 2A in rodent islets and INS-1 832/13 beta-cells by increasing the posttranslational carboxylmethylation of its catalytic subunit, Endocrinology, 155 (2014) 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yadav H, Devalaraja S, Chung ST, Rane SG, TGF-beta1/Smad3 Pathway Targets PP2A-AMPK-FoxO1 Signaling to Regulate Hepatic Gluconeogenesis, J Biol Chem, 292 (2017) 3420–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL 3rd, Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology, J Neuropathol Exp Neurol, 63 (2004) 287–301. [DOI] [PubMed] [Google Scholar]
- [9].Taymans JM, Baekelandt V, Phosphatases of alpha-synuclein, LRRK2, and tau: important players in the phosphorylation-dependent pathology of Parkinsonism, Front Genet, 5 (2014) 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu J, Lou H, Alerte TN, Stachowski EK, Chen J, Singleton AB, Hamilton RL, Perez RG, Lewy-like aggregation of alpha-synuclein reduces protein phosphatase 2A activity in vitro and in vivo, Neuroscience, 207 (2012) 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lou H, Montoya SE, Alerte TN, Wang J, Wu J, Peng X, Hong CS, Friedrich EE, Mader SA, Pedersen CJ, Marcus BS, McCormack AL, Di Monte DA, Daubner SC, Perez RG, Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo, J Biol Chem, 285 (2010) 17648–17661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X, Ray N, Fernandez JR, Chao Y, Masliah E, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM, Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model, J Neurosci, 31 (2011) 6963–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Houge G, Haesen D, Vissers LE, Mehta S, Parker MJ, Wright M, Vogt J, McKee S, Tolmie JL, Cordeiro N, Kleefstra T, Willemsen MH, Reijnders MR, Berland S, Hayman E, Lahat E, Brilstra EH, van Gassen KL, Zonneveld-Huijssoon E, de Bie CI, Hoischen A, Eichler EE, Holdhus R, Steen VM, Doskeland SO, Hurles ME, FitzPatrick DR, Janssens V, B56delta-related protein phosphatase 2A dysfunction identified in patients with intellectual disability, J Clin Invest, 125 (2015) 3051–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loveday C, Tatton-Brown K, Clarke M, Westwood I, Renwick A, Ramsay E, Nemeth A, Campbell J, Joss S, Gardner M, Zachariou A, Elliott A, Ruark E, van Montfort R, Childhood Overgrowth C, Rahman N, Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth, Hum Mol Genet, 24 (2015) 4775–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC, Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity, Cancer Res, 65 (2005) 8183–8192. [DOI] [PubMed] [Google Scholar]
- [16].Cheng Y, Liu W, Kim S-T, Sun J, Lu L, Sun J, Zheng SL, Isaacs WB, Xu J, Evaluation of PPP2R2A as a prostate cancer susceptibility gene: a comprehensive germline and somatic study, Cancer Genetics, 204 (2011) 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shouse GP, Nobumori Y, Liu X, A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A, Oncogene, 29 (2010) 3933–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haesen D, Abbasi Asbagh L, Derua R, Hubert A, Schrauwen S, Hoorne Y, Amant F, Waelkens E, Sablina A, Janssens V, Recurrent PPP2R1A Mutations in Uterine Cancer Act through a Dominant-Negative Mechanism to Promote Malignant Cell Growth, Cancer Res, 76 (2016) 5719–5731. [DOI] [PubMed] [Google Scholar]
- [19].Tamaki M, Goi T, Hirono Y, Katayama K, Yamaguchi A, PPP2R1B gene alterations inhibit interaction of PP2A-Abeta and PP2A-C proteins in colorectal cancers, Oncol Rep, 11 (2004) 655–659. [PubMed] [Google Scholar]
- [20].O'Connor CM, Perl A, Leonard D, Sangodkar J, Narla G, Therapeutic targeting of PP2A, Int J Biochem Cell Biol, 96 (2018) 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grech G, Baldacchino S, Saliba C, Grixti MP, Gauci R, Petroni V, Fenech AG, Scerri C, Deregulation of the protein phosphatase 2A, PP2A in cancer: complexity and therapeutic options, Tumour Biol, 37 (2016) 11691–11700. [DOI] [PubMed] [Google Scholar]
- [22].Sangodkar J, Farrington CC, McClinch K, Galsky MD, Kastrinsky DB, Narla G, All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase, FEBS J, 283 (2016) 1004–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perrotti D, Neviani P, Protein phosphatase 2A: a target for anticancer therapy, Lancet Oncol, 14 (2013) e229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gutierrez A, Pan L, Groen RW, Baleydier F, Kentsis A, Marineau J, Grebliunaite R, Kozakewich E, Reed C, Pflumio F, Poglio S, Uzan B, Clemons P, VerPlank L, An F, Burbank J, Norton S, Tolliday N, Steen H, Weng AP, Yuan H, Bradner JE, Mitsiades C, Look AT, Aster JC, Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia, J Clin Invest, 124 (2014) 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sangodkar J, Perl A, Tohme R, Kiselar J, Kastrinsky DB, Zaware N, Izadmehr S, Mazhar S, Wiredja DD, O'Connor CM, Hoon D, Dhawan NS, Schlatzer D, Yao S, Leonard D, Borczuk AC, Gokulrangan G, Wang L, Svenson E, Farrington CC, Yuan E, Avelar RA, Stachnik A, Smith B, Gidwani V, Giannini HM, McQuaid D, McClinch K, Wang Z, Levine AC, Sears RC, Chen EY, Duan Q, Datt M, Haider S, Ma'ayan A, DiFeo A, Sharma N, Galsky MD, Brautigan DL, Ioannou YA, Xu W, Chance MR, Ohlmeyer M, Narla G, Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth, J Clin Invest, 127 (2017) 2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kastrinsky DB, Sangodkar J, Zaware N, Izadmehr S, Dhawan NS, Narla G, Ohlmeyer M, Reengineered tricyclic anti-cancer agents, Bioorg Med Chem, 23 (2015) 6528–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christensen DJ, Chen Y, Oddo J, Matta KM, Neil J, Davis ED, Volkheimer AD, Lanasa MC, Friedman DR, Goodman BK, Gockerman JP, Diehl LF, de Castro CM, Moore JO, Vitek MP, Weinberg JB, SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target, Blood, 118 (2011) 4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D, FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia, J Clin Invest, 117 (2007) 2408–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H, Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists, Science, 296 (2002) 346–349. [DOI] [PubMed] [Google Scholar]
- [30].Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR, The immune modulator FTY720 targets sphingosine 1-phosphate receptors, J Biol Chem, 277 (2002) 21453–21457. [DOI] [PubMed] [Google Scholar]
- [31].Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, Kogure T, Patel T, Terracciano L, Muthusamy N, Byrd JC, Kulp SK, Chen CS, Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma, Hepatology, 53 (2011) 1943–1958. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [32].Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, Carpenter H, Don AS, Morris JC, Zimmermann N, Bartlett NW, Rothenberg ME, Johnston SL, Foster PS, Mattes J, The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity, Nat Med, 19 (2013) 232–237. [DOI] [PubMed] [Google Scholar]
- [33].Zonta F, Pagano MA, Trentin L, Tibaldi E, Frezzato F, Trimarco V, Facco M, Zagotto G, Pavan V, Ribaudo G, Bordin L, Semenzato G, Brunati AM, Lyn sustains oncogenic signaling in chronic lymphocytic leukemia by strengthening SET-mediated inhibition of PP2A, Blood, 125 (2015) 3747–3755. [DOI] [PubMed] [Google Scholar]
- [34].Zhao JJ, Roberts TM, Hahn WC, Functional genetics and experimental models of human cancer, Trends Mol Med, 10 (2004) 344–350. [DOI] [PubMed] [Google Scholar]
- [35].Meeusen B, Janssens V, Tumor suppressive protein phosphatases in human cancer: Emerging targets for therapeutic intervention and tumor stratification, Int J Biochem Cell Biol, 96 (2018) 98–134. [DOI] [PubMed] [Google Scholar]
- [36].Narla G, Sangodkar J, Ryder CB, The impact of phosphatases on proliferative and survival signaling in cancer, Cell Mol Life Sci, 75 (2018) 2695–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, Sim AT, Ashman LK, Verrills NM, Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers, Cancer Res, 70 (2010) 5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smith AM, Dun MD, Lee EM, Harrison C, Kahl R, Flanagan H, Panicker N, Mashkani B, Don AS, Morris J, Toop H, Lock RB, Powell JA, Thomas D, Guthridge MA, Moore A, Ashman LK, Skelding KA, Enjeti A, Verrills NM, Activation of protein phosphatase 2A in FLT3+ acute myeloid leukemia cells enhances the cytotoxicity of FLT3 tyrosine kinase inhibitors, Oncotarget, 7 (2016) 47465–47478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bockelman C, Lassus H, Hemmes A, Leminen A, Westermarck J, Haglund C, Butzow R, Ristimaki A, Prognostic role of CIP2A expression in serous ovarian cancer, Br J Cancer, 105 (2011) 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Agarwal A, MacKenzie RJ, Pippa R, Eide CA, Oddo J, Tyner JW, Sears R, Vitek MP, Odero MD, Christensen DJ, Druker BJ, Antagonism of SET using OP449 enhances the efficacy of tyrosine kinase inhibitors and overcomes drug resistance in myeloid leukemia, Clin Cancer Res, 20 (2014) 2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takahashi S, Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications, J Hematol Oncol, 4 (2011) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ogris E, Sontag E, Wadzinski B, Narla G, Specificity of research antibodies: "trust is good, validation is better", Hum Pathol, 72 (2018) 199–201. [DOI] [PubMed] [Google Scholar]
- [43].Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL, Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia, N Engl J Med, 344 (2001) 1031–1037. [DOI] [PubMed] [Google Scholar]
- [44].Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, Khoury HJ, Larson RA, Konopleva M, Cortes JE, Kantarjian H, Jabbour EJ, Kornblau SM, Lipton JH, Rea D, Stenke L, Barbany G, Lange T, Hernandez-Boluda JC, Ossenkoppele GJ, Press RD, Chuah C, Goldberg SL, Wetzler M, Mahon FX, Etienne G, Baccarani M, Soverini S, Rosti G, Rousselot P, Friedman R, Deininger M, Reynolds KR, Heaton WL, Eiring AM, Pomicter AD, Khorashad JS, Kelley TW, Baron R, Druker BJ, Deininger MW, O'Hare T, BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia, Cancer Cell, 26 (2014) 428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D, The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein, Cancer Cell, 8 (2005) 355–368. [DOI] [PubMed] [Google Scholar]
- [46].Neviani P, Harb JG, Oaks JJ, Santhanam R, Walker CJ, Ellis JJ, Ferenchak G, Dorrance AM, Paisie CA, Eiring AM, Ma Y, Mao HC, Zhang B, Wunderlich M, May PC, Sun C, Saddoughi SA, Bielawski J, Blum W, Klisovic RB, Solt JA, Byrd JC, Volinia S, Cortes J, Huettner CS, Koschmieder S, Holyoake TL, Devine S, Caligiuri MA, Croce CM, Garzon R, Ogretmen A, Arlinghaus RB, Chen CS, Bittman R, Hokland P, Roy DC, Milojkovic D, Apperley J, Goldman JM, Reid A, Mulloy JC, Bhatia R, Marcucci G, Perrotti D, PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells, J Clin Invest, 123 (2013) 4144–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kauko O, O'Connor CM, Kulesskiy E, Sangodkar J, Aakula A, Izadmehr S, Yetukuri L, Yadav B Padzik A, Laajala TD, Haapaniem P, Momeny M, Varila T, Ohlmeyer M, Aittokallio T, Wennerberg K, Narla G, Westermarck J, PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells, Sci Transl Med, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Prior IA, Lewis PD, Mattos C, A comprehensive survey of Ras mutations in cancer, Cancer Res, 72 (2012) 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Downward J, Targeting RAS signalling pathways in cancer therapy, Nat Rev Cancer, 3 (2003) 11–22. [DOI] [PubMed] [Google Scholar]
- [50].Papke B, Der CJ, Drugging RAS: Know the enemy, Science, 355 (2017) 1158–1163. [DOI] [PubMed] [Google Scholar]
- [51].Do K, Speranza G, Bishop R, Khin S, Rubinstein L, Kinders RJ, Datiles M, Eugeni M, Lam MH, Doyle LA, Doroshow JH, Kummar S, Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer, Invest New Drugs, 33 (2015) 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Papadimitrakopoulou V, Lee JJ, Wistuba II, Tsao AS, Fossella FV, Kalhor N, Gupta S, Byers LA, Izzo JG, Gettinger SN, Goldberg SB, Tang X, Miller VA, Skoulidis F, Gibbons DL, Shen L, Wei C, Diao L, Peng SA, Wang J, Tam AL, Coombes KR, Koo JS, Mauro DJ, Rubin EH, Heymach JV, Hong WK, Herbst RS, The BATTLE-2 Study: A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer, J Clin Oncol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hung MH, Wang CY, Chen YL, Chu PY, Hsiao YJ, Tai WT, Chao TT, Yu HC, Shiau CW, Chen KF, SET antagonist enhances the chemosensitivity of non-small cell lung cancer cells by reactivating protein phosphatase 2A, Oncotarget, 7 (2016) 638–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cha G, Xu J, Xu X, Li B, Lu S, Nanding A, Hu S, Liu S, High expression of CIP2A protein is associated with tumor aggressiveness in stage I-III NSCLC and correlates with poor prognosis, Onco Targets Ther, 10 (2017) 5907–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kalev P, Simicek M, Vazquez I, Munck S, Chen L, Soin T, Danda N, Chen W, Sablina A, Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition, Cancer Res, 72 (2012) 6414–6424. [DOI] [PubMed] [Google Scholar]
- [56].Roper J, Sinnamon MJ, Coffee EM, Belmont P, Keung L, Georgeon-Richard L, Wang WV, Faber AC, Yun J, Yilmaz OH, Bronson RT, Martin ES, Tsichlis PN, Hung KE, Combination PI3K/MEK inhibition promotes tumor apoptosis and regression in PIK3CA wild-type, KRAS mutant colorectal cancer, Cancer Lett, 347 (2014) 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B, Wick MJ, Alvarez C, Cavazos A, Mangold GL, Patnaik A, The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer, Clin Cancer Res, 18 (2012) 2316–2325. [DOI] [PubMed] [Google Scholar]
- [58].Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, Counter CM, Nevins JR, Means AR, Sears R, A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells, Nat Cell Biol, 6 (2004) 308–318. [DOI] [PubMed] [Google Scholar]
- [59].Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, Janiszewska M, Huh SJ, Liang Y, Ryan J, Doherty E, Mohammed H, Guo H, Stover DG, Ekram MB, Brown J, D'Santos C Krop IE, Dillon D, McKeown M, Ott C, Qi J, Ni M, Rao PK, Duarte M, Wu SY, Chiang AM, Anders L, Young RA, Winer E, Letai A, Barry WT, Carroll JS, Long H, Brown M, Liu XS, Meyer CA, Bradner JE, Polyak K Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer, Nature, 529 (2016) 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE, Selective inhibition of BET bromodomains, Nature, 468 (2010) 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Trabucco SE, Gerstein RM, Evens AM, Bradner JE, Shultz LD, Greiner DL, Zhang H, Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma, Clin Cancer Res, 21 (2015) 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sakaguchi T, Yoshino H, Sugita S, Miyamoto K, Yonemori M, Osako Y, Meguro-Horike M, Horike SI, Nakagawa M, Enokida H, Bromodomain protein BRD4 inhibitor JQ1 regulates potential prognostic molecules in advanced renal cell carcinoma, Oncotarget, 9 (2018) 23003–23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shahbazi J, Liu PY, Atmadibrata B, Bradner JE, Marshall GM, Lock RB, Liu T, The Bromodomain Inhibitor JQ1 and the Histone Deacetylase Inhibitor Panobinostat Synergistically Reduce N-Myc Expression and Induce Anticancer Effects, Clin Cancer Res, 22 (2016) 2534–2544. [DOI] [PubMed] [Google Scholar]
- [64].Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, Taussig DC, Rezai K, Roumier C, Herait P, Kahatt C, Quesnel B, Michallet M, Recher C, Lokiec F, Preudhomme C, Dombret H, Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study, Lancet Haematol, 3 (2016) e186–195. [DOI] [PubMed] [Google Scholar]
- [65].Hottinger AF, Sanson M, Moyal E, Delord J-P, Micheli RD, Rezai K, Leung ACF, Perez S, Bekradda M, Lachaux N, Lokiec FM, Chinot OL, Dose optimization of MK-8628 (OTX015), a small molecule inhibitor of bromodomain and extra-terminal (BET) proteins, in patients with recurrent glioblastoma, Journal of Clinical Oncology, 34 (2016) e14123–e14123. [Google Scholar]
- [66].Chien W, Sun QY, Lee KL, Ding LW, Wuensche P, Torres-Fernandez LA, Tan SZ, Tokatly I, Zaiden N, Poellinger L, Mori S, Yang H, Tyner JW, Koeffler HP, Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer, Mol Oncol, 9 (2015) 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Evans TRJ, Van Cutsem E, Moore MJ, Bazin IS, Rosemurgy A, Bodoky G, Deplanque G, Harrison M, Melichar B, Pezet D, Elekes A, Rock E, Lin C, Strauss L, O'Dwyer PJ, Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer, Ann Oncol, 28 (2017) 354–361. [DOI] [PubMed] [Google Scholar]
- [68].Chee CE, Krishnamurthi S, Nock CJ, Meropol NJ, Gibbons J, Fu P, Bokar J, Teston L, O'Brien T, Gudena V, Reese A, Bergman M, Saltzman J, Wright JJ, Dowlati A, Brell J, Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas, Oncologist, 18 (2013) 1091–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Farrell AS, Allen-Petersen B, Daniel CJ, Wang X, Wang Z, Rodriguez S, Impey S, Oddo J, Vitek MP, Lopez C, Christensen DJ, Sheppard B, Sears RC, Targeting inhibitors of the tumor suppressor PP2A for the treatment of pancreatic cancer, Mol Cancer Res, 12 (2014) 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ferrando AA, The role of NOTCH1 signaling in T-ALL, Hematology Am Soc Hematol Educ Program, (2009) 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Olsauskas-Kuprys R, Zlobin A, Osipo C, Gamma secretase inhibitors of Notch signaling, Onco Targets Ther, 6 (2013) 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Weng AP, Ferrando AA, Lee W, Morris J.P.t., Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC, Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia, Science, 306 (2004) 269–271. [DOI] [PubMed] [Google Scholar]
- [73].Choi YA, Park JS, Park MY, Oh KS, Lee MS, Lim JS, Kim KI, Kim KY, Kwon J, Yoon DY, Moon EY, Yang Y, Increase in CIP2A expression is associated with doxorubicin resistance, FEBS Lett, 585 (2011) 755–760. [DOI] [PubMed] [Google Scholar]
- [74].Rincon R, Cristobal I, Zazo S, Arpi O, Menendez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, Garcia-Foncillas J, Madoz-Gurpide J, Rojo F, PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects, Oncotarget, 6 (2015) 4299–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cristobal I, Manso R, Rincon R, Carames C, Senin C, Borrero A, Martinez-Useros J, Rodriguez M, Zazo S, Aguilera O, Madoz-Gurpide J, Rojo F, Garcia-Foncillas J, PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential, Mol Cancer Ther, 13 (2014) 938–947. [DOI] [PubMed] [Google Scholar]
- [76].Cristobal I, Rincon R, Manso R, Carames C, Zazo S, Madoz-Gurpide J, Rojo F, Garcia-Foncillas J, Deregulation of the PP2A inhibitor SET shows promising therapeutic implications and determines poor clinical outcome in patients with metastatic colorectal cancer, Clin Cancer Res, 21 (2015) 347–356. [DOI] [PubMed] [Google Scholar]
- [77].Zhang N, Dai L, Qi Y, Di W, Xia P, Combination of FTY720 with cisplatin exhibits antagonistic effects in ovarian cancer cells: role of autophagy, Int J Oncol, 42 (2013) 2053–2059. [DOI] [PubMed] [Google Scholar]
- [78].Ishitsuka A, Fujine E, Mizutani Y, Tawada C, Kanoh H, Banno Y, Seishima M, FTY720 and cisplatin synergistically induce the death of cisplatin-resistant melanoma cells through the downregulation of the PI3K pathway and the decrease in epidermal growth factor receptor expression, Int J Mol Med, 34 (2014) 1169–1174. [DOI] [PubMed] [Google Scholar]
- [79].Li Y, Hu T, Chen T, Yang T, Ren H, Chen M, Combination treatment of FTY720 and cisplatin exhibits enhanced antitumour effects on cisplatin-resistant non-small lung cancer cells, Oncol Rep, 39 (2018) 565–572. [DOI] [PubMed] [Google Scholar]
- [80].Sinicrope FA, Dannenberg AJ, Obesity and breast cancer prognosis: weight of the evidence, J Clin Oncol, 29 (2011) 4–7. [DOI] [PubMed] [Google Scholar]
- [81].Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thurlimann B, Bonnefoi H, Forbes JF, Paridaens RJ, Rabaglio M, Gelber RD, Colleoni M, Lang I, Smith IE, Coates AS, Goldhirsch A, Mouridsen HT, Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1-98 trial, J Clin Oncol, 30 (2012) 3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Katsuta E, Yan L, Nagahashi M, Raza A, Sturgill JL, Lyon DE, Rashid OM, Hait NC, Takabe K, Doxorubicin effect is enhanced by sphingosine-1-phosphate signaling antagonist in breast cancer, J Surg Res, 219 (2017) 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gstalder C, Ader I, Cuvillier O, FTY720 (Fingolimod) Inhibits HIF1 and HIF2 Signaling, Promotes Vascular Remodeling, and Chemosensitizes in Renal Cell Carcinoma Animal Model, Mol Cancer Ther, 15 (2016) 2465–2474. [DOI] [PubMed] [Google Scholar]
- [84].Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, Zhuang Z, Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms, Proc Natl Acad Sci U S A, 106 (2009) 11697–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang C, Peng Y, Wang F, Tan X, Liu N, Fan S, Wang D, Zhang L, Liu D, Wang T, Wang S, Zhou Y, Su Y, Cheng T, Zhuang Z, Shi C, A synthetic cantharidin analog for the enhancement of doxorubicin suppression of stem cell-derived aggressive sarcoma, Biomaterials, 31 (2010) 9535–9543. [DOI] [PubMed] [Google Scholar]
- [86].Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y, Xu Z, Han X, Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis, Cancer Sci, 101 (2010) 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kadioglu O, Kermani NS, Kelter G, Schumacher U, Fiebig HH, Greten HJ, Efferth T, Pharmacogenomics of cantharidin in tumor cells, Biochem Pharmacol, 87 (2014) 399–409. [DOI] [PubMed] [Google Scholar]
- [88].Chen X, Liu J, Zhang Y, Cantharidin impedes the activity of protein serine/threonine phosphatase in Plutella xylostella, Mol Biosyst, 10 (2014) 240–250. [DOI] [PubMed] [Google Scholar]
- [89].Chung V, Mansfield AS, Braiteh F, Richards D, Durivage H, Ungerleider RS, Johnson F, Kovach JS, Safety, Tolerability, and Preliminary Activity of LB-100, an Inhibitor of Protein Phosphatase 2A, in Patients with Relapsed Solid Tumors: An Open-Label, Dose Escalation, First-in-Human, Phase I Trial, Clin Cancer Res, 23 (2017) 3277–3284. [DOI] [PubMed] [Google Scholar]
- [90].Mi J, Bolesta E, Brautigan DL, Larner JM, PP2A regulates ionizing radiation-induced apoptosis through Ser46 phosphorylation of p53, Mol Cancer Ther, 8 (2009) 135–140. [DOI] [PubMed] [Google Scholar]
- [91].Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J, gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair, Mol Cell, 20 (2005) 801–809. [DOI] [PubMed] [Google Scholar]
- [92].Wang Q, Gao F, Wang T, Flagg T, Deng X, A nonhomologous end-joining pathway is required for protein phosphatase 2A promotion of DNA double-strand break repair, Neoplasia, 11 (2009) 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yan Y, Cao PT, Greer PM, Nagengast ES, Kolb RH, Mumby MC, Cowan KH, Protein phosphatase 2A has an essential role in the activation of gamma-irradiation-induced G2/M checkpoint response, Oncogene, 29 (2010) 4317–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Forester CM, Maddox J, Louis JV, Goris J, Virshup DM, Control of mitotic exit by PP2A regulation of Cdc25C and Cdk1, Proc Natl Acad Sci U S A, 104 (2007) 19867–19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang C, Hong CS, Hu X, Yang C, Wang H, Zhu D, Moon S, Dmitriev P, Lu J, Chiang J, Zhuang Z, Zhou Y, Inhibition of protein phosphatase 2A with the small molecule LB100 overcomes cell cycle arrest in osteosarcoma after cisplatin treatment, Cell Cycle, 14 (2015) 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lv P, Wang Y, Ma J, Wang Z, Li JL, Hong CS, Zhuang Z, Zeng YX, Inhibition of protein phosphatase 2A with a small molecule LB100 radiosensitizes nasopharyngeal carcinoma xenografts by inducing mitotic catastrophe and blocking DNA damage repair, Oncotarget, 5 (2014) 7512–7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, Morgan MA, Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair, Clin Cancer Res, 19 (2013) 4422–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chang KE, Wei BR, Madigan JP, Hall MD, Simpson RM, Zhuang Z, Gottesman MM, The protein phosphatase 2A inhibitor LB100 sensitizes ovarian carcinoma cells to cisplatin-mediated cytotoxicity, Mol Cancer Ther, 14 (2015) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ho WS, Sizdahkhani S, Hao S, Song H, Seldomridge A, Tandle A, Maric D, Kramp T, Lu R, Heiss JD, Camphausen K, Gilbert MR, Zhuang Z, Park DM, LB-100, a novel Protein Phosphatase 2A (PP2A) inhibitor, sensitizes malignant meningioma cells to the therapeutic effects of radiation, Cancer Lett, 415 (2018) 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gordon IK, Lu J, Graves CA, Huntoon K, Frerich JM, Hanson RH, Wang X, Hong CS, Ho W, Feldman MJ, Ikejiri B, Bisht K, Chen XS, Tandle A, Yang C, Arscott WT, Ye D, Heiss JD, Lonser RR, Camphausen K, Zhuang Z, Protein Phosphatase 2A Inhibition with LB100 Enhances Radiation-Induced Mitotic Catastrophe and Tumor Growth Delay in Glioblastoma, Mol Cancer Ther, 14 (2015) 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ho WS, Feldman MJ, Maric D, Amable L, Hall MD, Feldman GM, Ray-Chaudhury A, Lizak MJ, Vera JC, Robison RA, Zhuang Z, Heiss JD, PP2A inhibition with LB100 enhances cisplatin cytotoxicity and overcomes cisplatin resistance in medulloblastoma cells, Oncotarget, 7 (2016) 12447–12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hao S, Song H, Zhang W, Seldomridge A, Jung J, Giles AJ, Hutchinson MK, Cao X, Colwell N, Lita A, Larion M, Maric D, Abu-Asab M, Quezado M, Kramp T, Camphausen K, Zhuang Z, Gilbert MR, Park DM, Protein phosphatase 2A inhibition enhances radiation sensitivity and reduces tumor growth in chordoma, Neuro Oncol, 20 (2018) 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lu J, Zhuang Z, Song DK, Mehta GU, Ikejiri B, Mushlin H, Park DM, Lonser RR, The effect of a PP2A inhibitor on the nuclear receptor corepressor pathway in glioma, J Neurosurg, 113 (2010) 225–233. [DOI] [PubMed] [Google Scholar]
- [104].Bian Y, Kitagawa R, Bansal PK, Fujii Y, Stepanov A, Kitagawa K, Synthetic genetic array screen identifies PP2A as a therapeutic target in Mad2-overexpressing tumors, Proc Natl Acad Sci U S A, 111 (2014) 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cunningham CE, Li S, Vizeacoumar FS, Bhanumathy KK, Lee JS, Parameswaran S, Furber L, Abuhussein O, Paul JM, McDonald M, Templeton SD, Shukla H, El Zawily AM, Boyd F, Alli N, Mousseau DD, Geyer R, Bonham K, Anderson DH, Yan J, Yu-Lee LY, Weaver BA, Uppalapati M, Ruppin E, Sablina A, Freywald A, Vizeacoumar FJ, Therapeutic relevance of the protein phosphatase 2A in cancer, Oncotarget, 7 (2016) 61544–61561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lord CJ, Tutt AN, Ashworth A, Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors, Annu Rev Med, 66 (2015) 455–470. [DOI] [PubMed] [Google Scholar]
- [107].Papadimitriou M, Mountzios G, Papadimitriou CA, The role of PARP inhibition in triple-negative breast cancer: Unraveling the wide spectrum of synthetic lethality, Cancer Treat Rev, 67 (2018) 34–44. [DOI] [PubMed] [Google Scholar]
- [108].Helleday T, The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings, Mol Oncol, 5 (2011) 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lord CJ, Ashworth A, PARP inhibitors: Synthetic lethality in the clinic, Science, 355 (2017) 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fu QH, Zhang Q, Zhang JY, Sun X, Lou Y, Li GG, Chen ZL, Bai XL, Liang TB, LB-100 sensitizes hepatocellular carcinoma cells to the effects of sorafenib during hypoxia by activation of Smad3 phosphorylation, Tumour Biol, 37 (2016) 7277–7286. [DOI] [PubMed] [Google Scholar]
- [111].Lai D, Chen M, Su J, Liu X, Rothe K, Hu K, Forrest DL, Eaves CJ, Morin GB, Jiang X, PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL(+) human leukemia, Sci Transl Med, 10 (2018). [DOI] [PubMed] [Google Scholar]
- [112].Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, Mameli M, Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice, Nat Med, 22 (2016) 254–261. [DOI] [PubMed] [Google Scholar]
- [113].Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gutgemann I, Eilers M, Felsher DW, MYC regulates the antitumor immune response through CD47 and PD-L1, Science, 352 (2016) 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP, Tristetraprolin (TTP)-14–3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA, J Biol Chem, 282 (2007) 3766–3777. [DOI] [PubMed] [Google Scholar]
- [115].Coelho MA, de Carne Trecesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, East P, Spencer-Dene B, Nye E, Barnouin K, Snijders AP, Lai WS, Blackshear PJ, Downward J, Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA, Immunity, 47 (2017) 1083–1099 e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H, Friedman L, Turner M, Okkenhaug K, Vanhaesebroeck B, Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer, Nature, 510 (2014) 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL, CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms, Mol Cell Biol, 25 (2005) 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Apostolidis SA, Rodriguez-Rodriguez N, Suarez-Fueyo A, Dioufa N, Ozcan E, Crispin JC, Tsokos MG, Tsokos GC, Phosphatase PP2A is requisite for the function of regulatory T cells, Nat Immunol, 17 (2016) 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, Brogdon J, Lamb J, Turley SJ, Ploegh HL, Root DE, Love JC, Dranoff G, Hacohen N, Cantor H, Wucherpfennig KW, In vivo discovery of immunotherapy targets in the tumour microenvironment, Nature, 506 (2014) 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ho WS, Wang H, Maggio D, Kovach JS, Zhang Q, Song Q, Marincola FM, Heiss JD, Gilbert MR, Lu R, Zhuang Z, Pharmacologic inhibition of protein phosphatase-2A achieves durable immune-mediated antitumor activity when combined with PD-1 blockade, Nat Commun, 9 (2018) 2126. [DOI] [PMC free article] [PubMed] [Google Scholar]